To the Editor
Ves v 5 (antigen 5) is a 23-kDa protein from Vespula venom, and it is recognized as the most potent allergen in venoms of the Vespidae family.1 There is a high sequence similarity of Ves v 5 within species of the same genus, such as yellow jacket, that is, Vespula (>95%); however, when it is compared with other genera such as Dolichovespula or Polistes, the sequence identity is much lower (about 60%).2 Another potential Vespula allergen is a 37-kDa phospholipase A1, known as Ves v 1.1 Neither Ves v 5 nor Ves v 1 is found in honeybee venom. Ves v 5 and Ves v 1 recombinant allergen components, both expressed in insect cells, became available in 2010 and 2011, respectively, for analyses on the ImmunoCAP solid-phase IgE assay (CAP-FEIA; Phadia, Uppsala, Sweden).
Very recently we demonstrated that the current CAP-FEIA recombinant major honeybee venom allergen rApi m 1 (i208) has a limited clinical usefulness for the detection of honeybee venom allergy because of its low diagnostic sensitivity, which was about 60%.3 Similarly, low sensitivity for CAP-FEIA rApi m 1 was recently also demonstrated by another group.4 For that reason we wanted to evaluate the diagnostic sensitivity of novel recombinant Ves v 5 (rVes v 5) and rVes v 1 in a routine clinical laboratory setting by analyzing a group of Vespula venom allergic patients.
In total, 200 subjects (mean age, 42 years; range, 16-81 years; 99 women) with established Vespula venom allergy (19 with large local reactions and 17, 42, 68, and 54 with Mueller grade I, II, III, and IV reactions, respectively) were recruited during a 4-year period. We included only those subjects in which the culprit insect was the yellow jacket. In all subjects, honeybee and yellow jacket (Vespula species) venom-specific IgE levels were measured with CAP-FEIA. Tests for CAP-FEIA rVes v 5 (i209) and rVes v 1 (i211) were performed in 2011 from serum samples stored at −40°C. We first tested the samples for rVes v 5, and if they were negative (<0.35 kUA/L), we tested them with rVes v 1. In addition, samples that were negative for rVes v 5 and rVes v 1 in the CAP were also tested for IgE reactivity to rVes v 2b (Vespula hyaluronidase), rVes v 5, and glycosylated nPhl p 4 by IgE dot-blotting as described previously.5 rVes v 2b was produced by baculovirus-infected insect cells.6
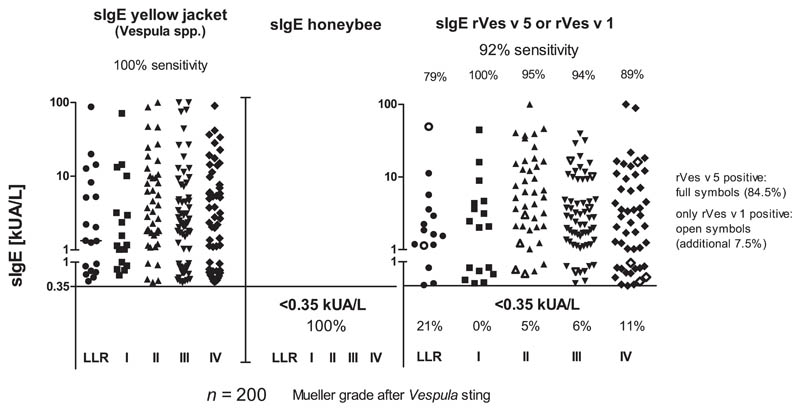
In all study subjects, we demonstrated a positive specific IgE response (2.24 kUA/L [range, 0.41 to >100]) to yellow jacket venom and a negative specific IgE response (<0.35 kUA/L) to honeybee venom (Fig 1). The median concentration of specific IgE antibodies to yellow jacket venom was 1.34 (range, 0.44-87.4) in large local reactions and 1.17 (range, 0.57-71.2), 3.5 (range, 0.42 to >100), 2.16 (range, 0.41 to >100), and 2.51 kUA/L (range, 0.43-90.3) in Mueller grade I, II, III, or IV subgroup, respectively. Next, we examined the subjects for the presence of specific IgE antibodies to rVes v 5 or rVes v 1. We found that 84.5% of the subjects (169 of 200) had positive specific IgE for rVes v 5. Of 31 negative subjects, 15 subjects were positive for rVes v 1 (additional 7.5%). Thus, altogether 184 of 200 subjects (92%) were positive for either rVes v 5 or rVes v 1. The median concentration of rVes v 5 or rVes v 1 in all positive subjects was 2.78 kUA/L (range, 0.36 to >100) and 1.63 (range, 0.36-49.4), 2.08 (range, 0.39-44.7), 5.7 (range, 0.59 to >100), 2.58 (range, 0.39-39.6), and 2.24 kUA/L (range, 0.36 to >100) with sensitivities of 79% (15 of 19), 100% (all 17), 95% (40 of 42), 94% (64 of 68), and 89% (48 of 54) in large local reactions and Mueller grade I, II, III, or IV subgroup, respectively. Consequently, the diagnostic sensitivity of Ves recombinants was comparably high in all subjects who had experienced systemic reactions following a yellow jacket sting and also in those who experienced very severe reactions of Mueller grades III and IV. It was slightly lower (79%) only in those patients who experienced local reactions. Of the 16 sera that were negative to rVes v 5 and rVes v 1 in the CAP, 2 sera were positive to dot-blotted rVes v 2b (additional 1%). Both subjects experienced severe reactions of Mueller grade III or IV. In the dot blot, an additional 2 sera were positive to rVes v 5 and an additional 4 sera contained IgE antibodies specific for carbohydrates on Phl p 4.
Fig. 1.
Wasp and honeybee venom, recombinant vesp v 5 and vesp v 1 CAP-FEIA specific IgE measurement in wasp venom–allergic subjects.
These results are comparable with the previous rVes v 5 diagnostic evaluations by commercially available liquid phase detection system (ADVIA Centaur; Siemens Medical Solution Diagnostics, Deerfield, Ill).7 In this report, specific IgE to rVes v 5 were demonstrated in 87% of 100 Vespula allergic subjects. An even higher ratio of identification (100%) was observed in our previous report by using in-house IgE immunoblotting and/or ELISA rVes v 5, but on limited number on Vespula allergic 20 patients.5
In this study, we demonstrated that additional use of Ves v 1 significantly enhances the diagnostic sensitivity (for almost 8%) of diagnostic tests based on recombinant yellow jacket venom allergens. Nevertheless, rVes v 5 and rVes v 1 had missed 8% of subjects with established allergy. Testing for rVes v 2b added only a minor contribution to diagnostic sensitivity. Primarily, 3 allergens were recognized as responsible for Vespula venom allergy, beyond Ves v 5 and Ves v 1, also Ves v 2, which occurs in isoforms.1 Recently, a novel 100-kDa glycosylated protein with homology to dipeptidyl peptidases with allergenic potential, namely, Ves v 3, was reported as a yellow jacket allergen.7 rVes v 3 showed IgE reactivity in approximately 50% of Vespula allergic subjects (in overall 54 tested)8 and might be useful to diagnose the few patients who are not identified with rVes v 5, 1, and 2.
Clinically, we cannot afford to miss a patient who is sensitive to insect venom; thus, the whole venom (that contains all the venom allergens as a single test) needs to be the first line of laboratory evaluation. However, the identification of the disease-causing insect venom in venom allergy is often difficult. In such cases, commercially available CAP-FEIA tests based on recombinant rVes v 5 and rVes v 1 allergens should be helpful for the serological dissection of Vespula venom allergy.
Acknowledgments
We thank Lucas Mach, Daniel Kolarich, and Friedrich Altmann, Department of Applied Genetics and Cell Biology, University of Natural Resources and Life Science, Vienna, Austria, for recombinant Ves v 2b.
This study was supported in part by the Slovenian research agency (ARRS), Christian Doppler Research Association, and the Austrian Science Fund (FWF).
Footnotes
Disclosure of potential conflict of interest: R.Valenta has received research support from the Austrian Science Fund, CDG, Biomas, and Phadia. The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Hoffman DR. Allergens in Hymenoptera venom, XXV: the amino acid sequences of antigen 5 molecules and the structural basis of antigenic cross-reactivity. J Allergy Clin Immunol. 1993;92:707–16. doi: 10.1016/0091-6749(93)90014-7. [DOI] [PubMed] [Google Scholar]
- 2.King TP, Lu G, Gonzalez M, Qian N, Soldatova L. Yellow jacket venom allergens, hyaluronidase and phospholipase: sequence similarity and antigenic cross-reactivity with their hornet and wasp homologs and possible implications for clinical allergy. J Allergy Clin Immunol. 1996;98:588–600. doi: 10.1016/s0091-6749(96)70093-3. [DOI] [PubMed] [Google Scholar]
- 3.Korošec P, Valenta R, Mittermann I, Celesnik N, Eržen R, Zidarn M, et al. Low sensitivity of commercially available rApi m 1 for diagnosis of honeybee venom allergy. J Allergy Clin Immunol. 2011;128:671–3. doi: 10.1016/j.jaci.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Sturm GJ, Hemmer W, Hawranek T, Lang R, Ollert M, Spillner E, et al. Detection of IgE to recombinant Api m 1 and rVes v 5 is valuable but not sufficient to distinguish bee from wasp venom allergy. J Allergy Clin Immunol. 2011;128:247–8. doi: 10.1016/j.jaci.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Mittermann I, Zidarn M, Silar M, Markovic-Housley Z, Aberer W, Korosec P, et al. Recombinant allergen-based IgE testing to distinguish honeybee and wasp allergy. J Allergy Clin Immunol. 2010;125:1300–7. doi: 10.1016/j.jaci.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Kolarich D, Loos A, Leonard R, Mach L, Marzban G, Hemmer W, et al. A proteomic study of the major allergens from yellow jacket venoms. Proteomics. 2007;7:1615–23. doi: 10.1002/pmic.200600800. [DOI] [PubMed] [Google Scholar]
- 7.Müller U, Johansen N, Petersen AB, Fromberg-Nielsen J, Haeberli G. Hymenoptera venom allergy: analysis of double positivity to honey bee and Vespula venom by estimation of IgE antibodies to species-specific major allergens Api m 1 and Ves v 5. Allergy. 2009;64:543–8. doi: 10.1111/j.1398-9995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 8.Blank S, Seismann H, Bockisch B, Braren I, Cifuentes L, McIntyre M, et al. Identification, recombinant expression, and characterization of the 100 kDa high molecular weight Hymenoptera venom allergens Api m 5 and Ves v 3. J Immunol. 2010;184:5403–13. doi: 10.4049/jimmunol.0803709. [DOI] [PubMed] [Google Scholar]