Abstract
Background
Allergen-specific immunotherapy is clinically effective for the treatment of cat allergy but shows a high rate of side effects.
Objective
We sought to engineer recombinant fusion proteins for cat immunotherapy that allow reducing both IgE-mediated and T cell–mediated side effects.
Methods
Fusion proteins consisting of the hepatitis B virus–derived PreS domain and 2 nonallergenic Fel d 1–derived peptides were expressed in Escherichia coli and purified. IgE reactivity and allergenic activity of Fel d 1 and the fusion proteins were compared by using IgE-binding assays and basophil activation tests in patients with cat allergy. Mice and rabbits were immunized subcutaneously with Fel d 1 and the fusion proteins to investigate the allergenicity of the vaccines and the development of Fel d 1–specific IgG antibodies.
Results
The recombinant fusion proteins showed no relevant IgE reactivity and exhibited more than 1000-fold reduced allergenic activity in basophil activation tests. On immunization of mice and rabbits, the fusion proteins induced Fel d 1–specific IgG antibodies that inhibited the binding of allergic patients’ IgE to the allergen without allergic sensitization to Fel d 1.
Conclusion
The described recombinant fusion proteins exhibit strongly reduced IgE-mediated allergenic activity, contain less than 40% of the Fel d 1 sequence, and thus lack many of the specific T-cell epitopes. Therefore they should represent safe vaccines for the treatment of cat allergy.
Keywords: Recombinant allergen, cat allergy, Fel d 1, peptide, hypoallergenic vaccine, immunotherapy
The domestic cat (Felis domesticus) is one of the most common causes of IgE-mediated allergic diseases.1–3 The severity of symptoms ranges from relatively mild rhinitis to life-threatening asthmatic responses. Allergen-specific immunotherapy (SIT) is the only disease-modifying treatment for IgE-mediated allergies that leads to long-lasting relief of symptoms.4,5 Several studies have demonstrated the clinical efficacy of SIT for cat-induced asthma. In these patients SIT is associated with the induction of IgG antibodies specific for the major cat allergen Fel d 1 and reduced cutaneous and respiratory symptoms.6–10 However, SIT with cat allergen extracts is often associated with a high rate of severe side effects that limit its broad applicability.11 Because the majority of patients with cat allergy are almost exclusively sensitized to the major allergen Fel d 1, several strategies have been developed for the reduction of side effects during SIT.3 One approach toward side effect–free SIT for cat allergy is based on the use of Fel d 1–derived T cell epitope–containing peptides without IgE reactivity.12 The administration of these peptides has been thought to induce T-cell tolerance. Several clinical trials have been performed with Fel d 1–derived peptides. In these studies IgE-mediated immediate-type side effects could be eliminated, but a considerable number of patients experienced T cell–dependent late-phase side effects that preceded the suppression of chronic inflammation.13–18 Another recently studied approach involves the coadministration of an anti-IgE antibody in the course of SIT to reduce IgE-mediated side effects.19
Here we report the construction of a novel type of vaccine for the treatment of cat allergy that should eliminate both IgE-mediated and T cell–mediated side effects. This approach is based on the selection of allergen-derived peptides that lack IgE reactivity and IgE-mediated allergenic activity and exhibit reduced T-cell reactivity. Coupled to a non–allergen-related carrier, they should then lead to a vaccine that induces allergen-specific IgG with T-cell help from carrier-derived epitopes.20
Recombinant fusion proteins consisting of the PreS domain of hepatitis B virus (HBV) containing nonallergenic Fel d 1 peptides at the N- and C-termini were expressed in Escherichia coli and purified. The PreS domain is a part of the large surface protein, which together with the middle and small surface proteins comprises the HBV envelope containing important antigenic sites for both B and T cells in the PreS sequence.21–23 We report the engineering, expression, purification, and immunologic characterization of 3 different fusion molecules for vaccination against cat allergy. Furthermore, we demonstrate more than 1000-fold reduced allergenic activity of the fusion proteins and their ability to induce, on immunization, IgG antibodies that inhibit IgE binding of patients with cat allergy to Fel d 1.
Methods
Allergic patients, recombinant allergens, synthetic peptides and antibodies
Patients with cat allergy (n = 23) and nonallergic control subjects (n = 4) were characterized by case history, skin prick test responses, and measurements of specific IgE antibodies.24 Blood and serum samples were used after informed consent was obtained with approval of the local ethics committee. rFel d 1 was expressed in E coli and purified.25 The rFel d 1–derived peptides P1 (EICPAVKRDVDLFLTGTPDEYVEQVAQYKALPVV) and P5 (MTTISSSKDCMGEAVQNTVEDLKLNTLGR) were synthesized.26 Peptide-specific rabbit antibodies were obtained by immunizing rabbits with the keyhole limpet hemocyanin–coupled peptides (Charles River, Kissleg, Germany). For more information, see the Methods section in this article’s Online Repository at www.jacionline.org.
Expression and purification of recombinant PreS fusion proteins
The amino acid sequence of the PreS region of the HBV subtype adw was obtained from the National Center for Biotechnology Information database (GenBank: AAT28735.1). Genes (codon use optimized for E coli expression) coding for the PreS protein containing Fel d 1–derived peptides fused to the N- and C-termini (Fig 1) were synthesized (ATG:biosynthetics, Merzhausen, Germany) and inserted into the NdeI/XhoI sites of either pET-17b (PreS protein) or pET-27b (PreS fusion proteins; Novagen, Darmstadt, Germany). The DNA sequences were confirmed by means of restriction enzyme analysis of midi-prep DNA (Promega, Madison, Wish) with NdeI/XhoI (Roche, Mannheim, Germany) and by means of automated sequencing of both DNA strands.
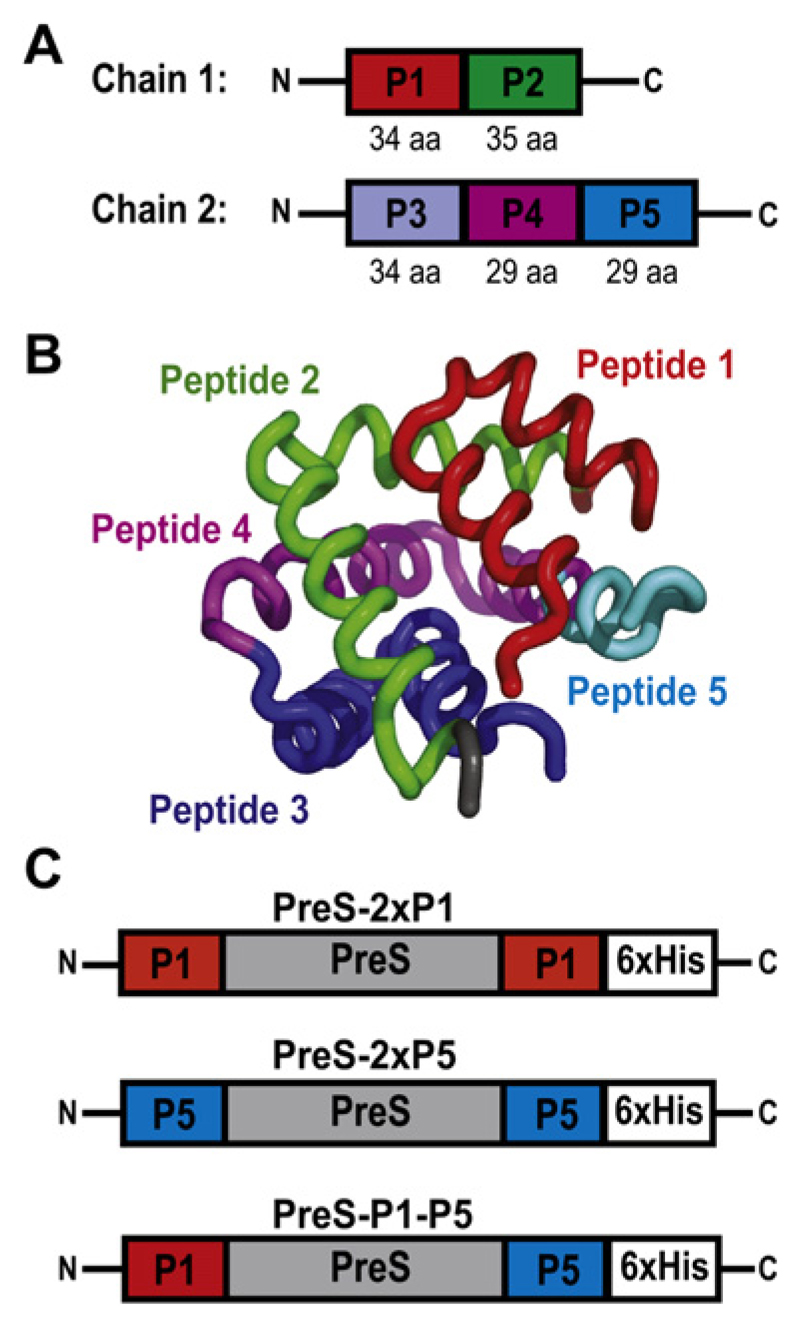
Fig 1.
Construction of PreS fusion proteins. A, Chains 1 and 2 of Fel d 1 with peptides indicated. aa, Amino acid. B, Ribbon representation of Fel d 1 with peptides highlighted in different colors. C, PreS fusion proteins containing peptide 1 (P1; PreS-2xP1) or peptide 5 (P5; PreS-2xP5) at the N- and C-termini, as well as PreS-P1-P5 with an N-terminal P1 and C-terminal P5 peptide. Hexahistidine tags (6xHis) are at the C-terminal end.
Recombinant PreS and PreS fusion proteins were expressed in E coli strain BL21 (DE3; Stratagene, La Jolla, Calif). The protein expression was induced by adding isopropyl-β-D-thiogalactopyranoside to a final concentration of 1 mmol/L for 4 hours at 37°C. Cells were harvested by means of centrifugation at 3500 rpm for 10 minutes. The inclusion bodies containing the recombinant proteins were purified as follows. Cells were resuspended in 25 mmol/L imidazole and 0.1% Triton X-100 (pH 7.4; Buffer A), lysed with Ultra-Turrax (Janke & Kunkel-IKA Labortechnik, Staufen, Germany), and incubated for 30 minutes with 10 μg/mL DNaseI (Roche) at room temperature. After addition of 6% Triton X-100, 60 mmol/L EDTA, and 1.5 mol/L NaCl pH 7.0 (Buffer B), the pellets were collected by means of centrifugation at 18,000 rpm for 20 minutes at 4°C and washed again with Buffer B and subsequently with Buffer A. The inclusion bodies were solubilized in 6 mol/L guanidine hydrochloride, 100 mmol/L NaH2PO4, and 10 mmol/L Tris-HCl (pH 8.0) overnight at room temperature. The lysates were cleared by means of centrifugation and incubated with 2 mL of a previously equilibrated Ni-NTA resin for 2 hours (Qiagen, Hilden, Germany). The suspensions were then loaded onto a column, washed with 2 column volumes of washing buffer (8 mol/L urea, 100 mmol/L NaH2PO4, and 10 mmol/L Tris-HCl [pH 6.1]), and eluted with the same buffer (pH 3.5). Urea was removed by means of dialysis against 10 mmol/L NaH2PO4 (pH 4.8). The purity of recombinant proteins was analyzed by means of SDS-PAGE. The identity of the fusion proteins was confirmed with rabbit anti-P1, anti-P5, anti–rFel d 1, and anti-PreS anti-sera diluted 1:1000 by means of immunoblotting. Bound rabbit IgG was detected with iodine 125–labeled donkey anti-rabbit IgG antibodies (GE Healthcare, Buckinghamshire, United Kingdom) diluted 1:2000 and visualized by means of autoradiography. Protein concentrations were determined by using the BCA Protein Assay Kit (Novagen).
IgE reactivity and allergenic activity of Fel d 1 and PreS fusion proteins
Allergic patients’ IgE reactivity to recombinant PreS, PreS-2xP1, PreS-2xP5, PreS-P1-P5, and human serum albumin was determined with sera from 23 patients with cat allergy and 4 nonallergic subjects by means of ELISA with antigens bound to the solid phase or in the liquid phase (see the Methods section in this article’s Online Repository).27 In vitro basophil activation tests were performed with heparinized peripheral blood from 7 patients with cat allergy after informed consent was given (Table I, nos. 4, 7, 13, 14, 17, 18, and 23). Blood samples (100 μL) were incubated with serial dilutions of rFel d 1, PreS fusion proteins (0.01-0.00001 mg/mL), a monoclonal anti-IgE antibody (1 μg/mL; Immunotech, Marseille, France), or PBS for 15 minutes at 37°C. CD203c expression on basophils was measured by means of flow cytometry with the phycoerythrin-conjugated mAb 97A6 (CD203c). Allergen-induced upregulation of CD203c on basophils was expressed as the stimulation index, as described previously.28
Table I. IgE reactivity of sera from patients with cat allergy to Fel d 1 and PreS fusion proteins.
| PreS fusion proteins | Controls | |||||
|---|---|---|---|---|---|---|
| Patient no. | 2xP1 | 2xP5 | P1-P5 | rFeld1 | PreS | HSA |
| 1 | 0.03 | 0.03 | 0.03 | 0.48 | 0.03 | 0.03 |
| 2 | 0.03 | 0.03 | 0.03 | 0.69 | 0.03 | 0.03 |
| 3 | 0.02 | 0.03 | 0.03 | 0.95 | 0.03 | 0.02 |
| 4 | 0.87 | 0.03 | 0.28 | 1.83 | 0.03 | 0.02 |
| 5 | 0.02 | 0.04 | 0.03 | 2.60 | 0.03 | 0.02 |
| 6 | 0.03 | 0.05 | 0.04 | 0.46 | 0.03 | 0.03 |
| 7 | 0.02 | 0.02 | 0.03 | 1.49 | 0.02 | 0.02 |
| 8 | 0.06 | 0.03 | 0.03 | 2.63 | 0.03 | 0.03 |
| 9 | 0.03 | 0.03 | 0.02 | 0.71 | 0.03 | 0.03 |
| 10 | 0.02 | 0.02 | 0.02 | 0.52 | 0.03 | 0.02 |
| 11 | 0.02 | 0.02 | 0.02 | 0.70 | 0.02 | 0.02 |
| 12 | 0.02 | 0.06 | 0.13 | 0.68 | 0.03 | 0.02 |
| 13 | 0.02 | 0.09 | 0.02 | 1.06 | 0.03 | 0.02 |
| 14 | 0.02 | 0.02 | 0.02 | 0.23 | 0.03 | 0.02 |
| 15 | 0.02 | 0.02 | 0.02 | 0.15 | 0.03 | 0.02 |
| 16 | 0.02 | 0.02 | 0.02 | 0.47 | 0.04 | 0.02 |
| 17 | 0.02 | 0.02 | 0.03 | 1.56 | 0.03 | 0.02 |
| 18 | 0.03 | 0.66 | 0.61 | 2.65 | 0.03 | 0.02 |
| 19 | 0.02 | 0.02 | 0.02 | 0.14 | 0.03 | 0.02 |
| 20 | 0.02 | 0.02 | 0.02 | 1.15 | 0.03 | 0.02 |
| 21 | 0.03 | 0.03 | 0.03 | 0.13 | 0.03 | 0.03 |
| 22 | 0.03 | 0.03 | 0.03 | 1.04 | 0.03 | 0.03 |
| 23 | 0.03 | 0.03 | 0.04 | 3.21 | 0.04 | 0.03 |
| Means | 0.06 | 0.06 | 0.07 | 1.11 | 0.03 | 0.02 |
| 24 | 0.02 | 0.03 | 0.02 | 0.03 | 0.03 | 0.02 |
| 25 | 0.02 | 0.02 | 0.03 | 0.03 | 0.03 | 0.02 |
| 26 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.02 |
| 27 | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.02 |
| Means | 0.02 | 0.03 | 0.02 | 0.03 | 0.03 | 0.02 |
Displayed are mean OD values of duplicate measurements corresponding to the levels of IgE for sera from 23 patients with cat allergy and 4 nonallergic subjects. Mean OD values for each antigen are indicated in boldface (cutoff level, OD = 0.06).
HSA, Human serum albumin.
Peptide-specific lymphoproliferation was assessed in PBMCs from patients with cat allergy (see the Methods section in this article’s Online Repository).
Inhibition of allergic patients’ IgE binding to rFel d 1 and CD203c expression on basophils by rabbit IgG antibodies
The inhibition of allergic patients’ IgE binding to rFel d 1 by rabbit IgG antibodies raised against rFel d 1, PreS-2xP1, PreS-2xP5, and PreS-P1-P5 was measured by using the ELISA competition assay.29–31 ELISA plates were coated with 1 μg/mL rFel d 1 and incubated with rabbit anti-sera specific for Fel d 1 or the PreS fusion proteins or the corresponding preimmune sera diluted 1:50. After washing, sera from 23 patients with cat allergy or 4 nonallergic subjects were diluted 1:10 and allowed to react with Fel d 1. Bound human IgE was detected with horseradish peroxidase (HRP)–coupled goat anti-human IgE antibodies diluted 1:2500 (KPL, Gaithersburg, Md). The OD corresponding to bound IgE was measured at 405 and 490 nm. The percentage of inhibition of IgE binding was calculated as follows:
where ODs and ODp represent the optical density values after preincubation with specific rabbit IgG or the preimmune immunoglobulin, respectively. Increasing concentrations of rFel d 1 (0.00001-0.001 μg/mL) were preincubated with a 1:10 dilution of the rabbit anti–rFel d 1, anti–PreS-P1-P5, and the corresponding preimmune serum overnight at 4°C to test whether rabbit IgG antibodies induced by PreS-P1-P5 can inhibit allergen-induced upregulation of CD203c expression. Thereafter, blood samples from patients with cat allergy (Table I, nos. 4 and 7) were incubated with these mixtures, and CD203c expression was assessed as described above.
Rat basophil leukemia assay
The allergenic activity of IgE antibodies induced in mice by means of immunization with Fel d 1 and the PreS fusion proteins was determined with the rat basophil leukemia (RBL) cell mediator release assay.32 RBL-2H3 cells were cultivated in 96-well tissue-culture plates (4 × 104 cells/well) for 24 hours at 37°C in 7% CO2 and then loaded with different serum dilutions (1:10, 1:25, and 1:50) from mice immunized with rFel d 1, PreS-2xP1, PreS-P1-P5, PreS, or alum alone (PBS). Cells were washed twice with 200 μL of Tyrode/BSA buffer (137 mmol/L NaCl, 2.7 mmol/L KCl, 0.5 mmol/L MgCl2, 1.8 mmol/L CaCl2, 0.4 mmol/L NaH2PO4, 5.6 mmol/L D-glucose, 12 mmol/L NaHCO3, 10 mmol/L HEPES, and 0.1% BSA [pH 7.2]; Sigma-Aldrich, Vienna, Austria) and stimulated with different concentrations (0.0001-10 μg/mL) of rFel d 1. The dependence of degranulation on IgE antibodies was investigated by using murine sera that had been heated at 56°C for 30 minutes to 4 hours before loading onto the cells. A serum dilution of 1:50 and an allergen concentration of 0.03 μg/mL were used in the experiments. Total β-hexosaminidase release was induced by the addition of 10 μL of 10% vol/vol Triton X-100 (Merck, Darmstadt, Germany). To analyze β-hexosaminidase release, cells were incubated with assay solution (80 μmol/L 4-methylumbelliferyl-N-acetyl-β-D-glucosaminide in 0.1 mol/L citrate buffer, pH 4.5) for 1 hour at 37°C in 7% CO2, and the reaction was stopped with 100 μL of glycine buffer (0.2 mol/L glycine and 0.2 mol/L NaCl [pH 10.7]). The fluorescence was measured at λex of 360 nm and λem of 465 nm by using a fluorescence microplate reader (Dynatech MR 7000; Dynatech Laboratories, Chantilly, Va). Results are shown as mean percentages of total β-hexosaminidase release and represent the mean of triplicate determinations.
Results
Engineering, expression, and purification of recombinant fusion proteins consisting of hepatitis B–derived PreS and hypoallergenic Fel d 1 peptides
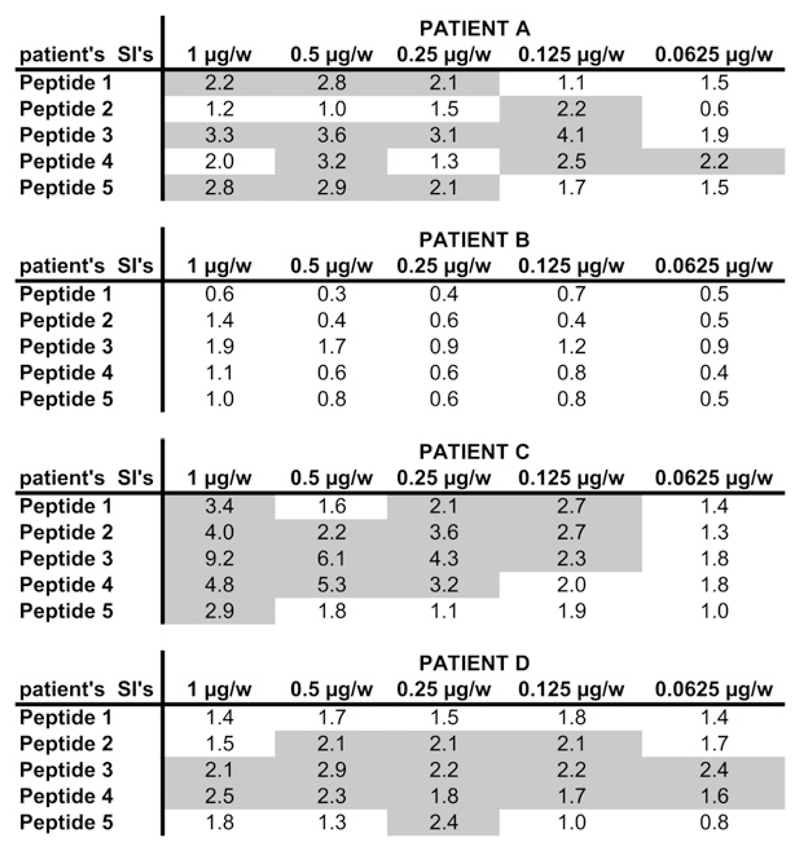
Testing of Fel d 1–derived peptides (ie, P1-P5; Fig 1, A) revealed that P1 and P5 lacked allergenic activity but, when coupled to keyhole limpet hemocyanin, induced IgG antibodies that strongly blocked the binding of IgE in patients with cat allergy to Fel d 1 (Focke-Tejkl and Valenta, unpublished data). Interestingly, P1 and P5 appear in close vicinity in the 3-dimensional structure of Fel d 1, although they are derived from the N- and C-terminal ends of chains 1 and 2 of Fel d 1, respectively (Fig 1, B). Because P1 and P5 represent less than 40% of the Fel d 1 sequence, a large portion of Fel d 1–derived epitopes has been eliminated that have induced T cell–dependent late-phase side effects in immunotherapy trials performed with a mix of Fel d 1–derived T cell epitope–containing peptides.16 Fig E1 (available in this article’s Online Repository at www.jacionline.org) shows that peptides 2, 3, and 4, which had been eliminated from the vaccine, induced T-cell proliferation in PBMCs from patients with cat allergy.
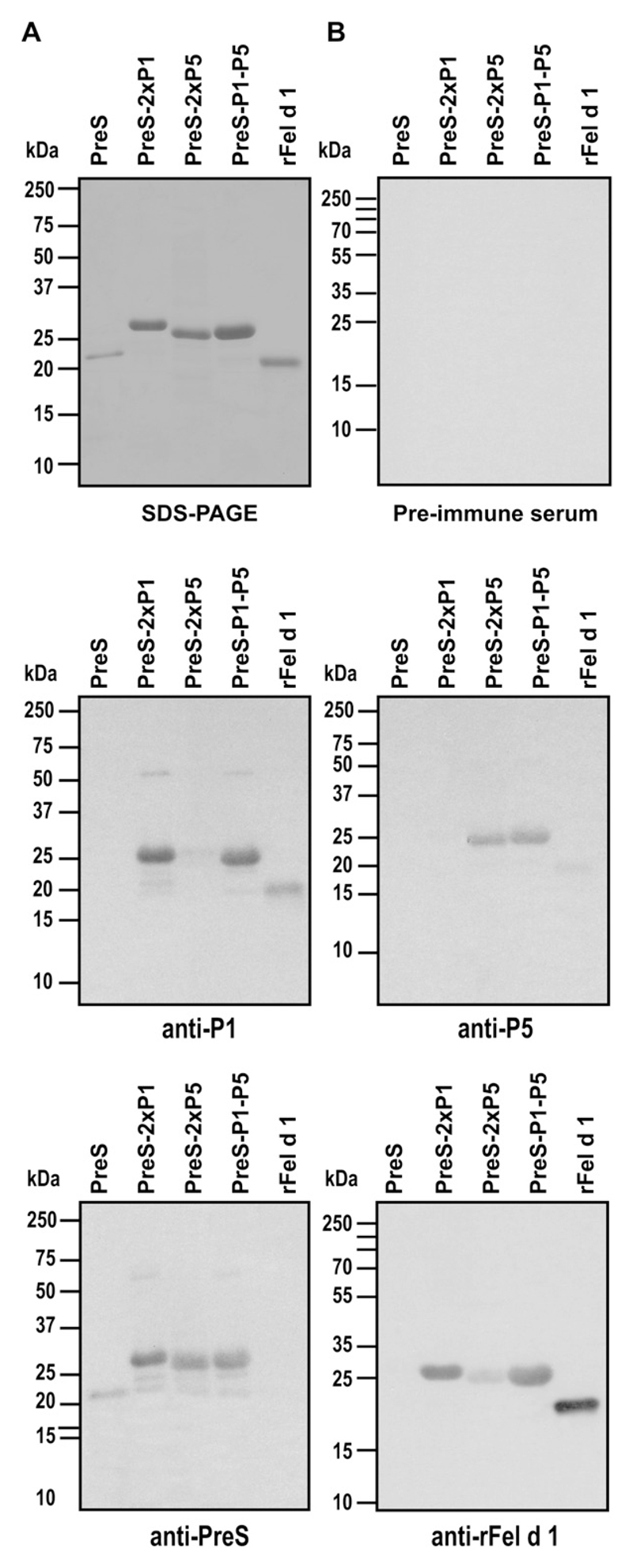
In contrast to the approach of using Fel d 1–derived T cell epitope–containing peptides for inducing Fel d 1–specific T-cell tolerance, we aimed at constructing a vaccine that induces Fel d 1–specific IgG antibodies that block allergic patients’ IgE recognition of Fel d 1 with T-cell help derived from a Fel d 1–unrelated carrier protein. For this purpose, we used the PreS domain of HBV surface antigen as a carrier protein for P1 and P5. Three recombinant fusion proteins consisting of PreS with P1 at the N- and C-termini (PreS-2xP1), P5 at the N- and C-termini (PreS-2xP5), and P1 at the N-terminus and P5 at the C-terminus (PreS-P1-P5; Fig 1, C) were expressed in E coli and purified to homogeneity by means of nickel affinity chromatography. The recombinant fusion proteins could be purified as soluble proteins with high yields (20-25 mg/L culture; Fig 2, A). Recombinant PreS, PreS-2xP1, PreS-2xP5, and PreS-P1-P5 migrated at approximately 19, 26, 25, and 26 kd, respectively, in Coomassie-stained SDS-PAGE (Fig 2, A). Matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry of the recombinant fusion proteins confirmed the masses deduced from their sequences without methionine (data not shown). Fig 2, B, shows that anti-sera specific for P1 and P5 reacted with the fusion proteins containing the respective peptides and with Fel d 1, although to a lower degree. Fel d 1–specific antibodies reacted specifically with Fel d 1 and the fusion proteins. The fusion proteins and recombinant PreS were recognized by anti-PreS rabbit IgG antibodies (Fig 2, B). No reactivity was observed when a nitrocellulose blot was probed with rabbit preimmune antibodies (Fig 2, B).
Fig 2.
Purity and immune reactivity of recombinant proteins. Coomassie-stained SDS-PAGE (A) and nitrocellulose blots (B) containing PreS (lane 1), PreS-2xP1 (lane 2), PreS-2xP5 (lane 3), PreS-P1-P5 (lane 4), and rFel d 1 (lane 5) were probed with rabbit preimmune serum (Fig 2, B, right, upper part), anti-P1, anti-P5, anti-PreS (Fig 2, B, left, bottom part), or anti–rFel d 1 (Fig 2, B, right, bottom part) antibodies. Molecular weights in kilodaltons are indicated in the left margin.
Recombinant PreS fusion proteins show reduced IgE reactivity and more than 1000-fold reduced allergenic activity
Serum IgE reactivity to the purified fusion proteins was determined by means of direct ELISA (Table I). Four of the 23 patients with cat allergy showed some residual IgE reactivity to the fusion proteins (Table I, nos. 4, 12, 13, and 18). All of the 23 patients with cat allergy showed IgE reactivity to Fel d 1. No IgE reactivity to human serum albumin or PreS was observed (Table I). Sera from 4 nonallergic subjects showed no IgE reactivity to any of the tested antigens. In liquid-phase ELISA competition experiments, rFel d 1 inhibited almost completely IgE reactivity to rFel d 1 (ie, 92% mean inhibition), whereas preincubation of sera with the fusion proteins caused less than 13% mean inhibition of IgE reactivity to Fel d 1 (see Table E1 in this article’s Online Repository at www.jacionline.org).
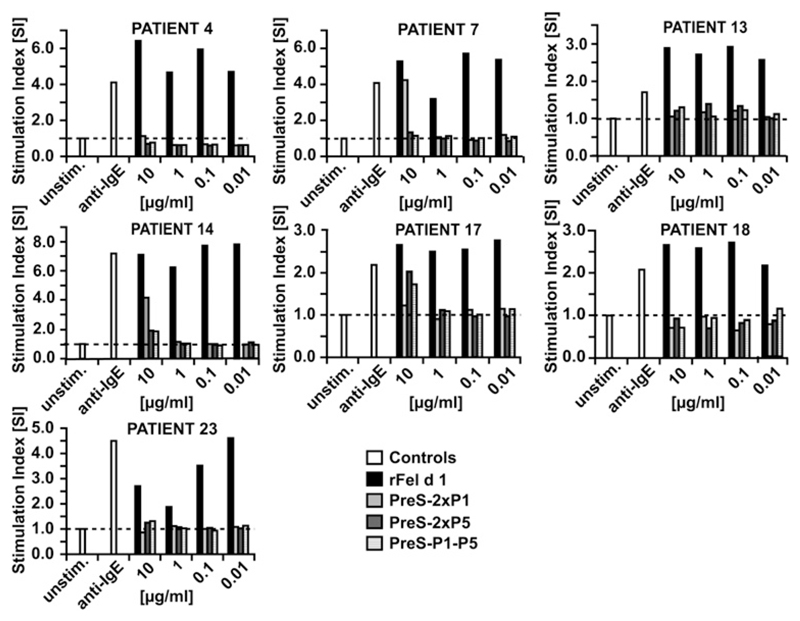
Next, the allergenic activity of the peptide vaccines was compared with that of the rFel d 1 allergen in basophil activation tests (Fig 3). Basophils from 7 patients with cat allergy with high Fel d 1–specific IgE levels and in particular from those 2 patients who had shown residual IgE reactivity to the fusion proteins (ie, Table I, nos. 4 and 18) were incubated with serial dilutions of rFel d 1, PreS-2xP1, PreS-2xP5, or PreS-P1-P5. Already the lowest concentration of Fel d 1 (ie, 0.01 μg/mL) induced a strong upregulation of CD203c expression on basophils in each of the tested patients, whereas PreS fusion proteins showed in each of the tested patients an at least 1000-fold reduction of allergenic activity compared with the rFel d 1 allergen (Fig 3). Anti-IgE (positive control) induced CD203c upregulation on basophils in all patients.
Fig 3.
Comparison of allergenic activity of rFel d 1 and PreS fusion proteins in the CD203c basophil activation test. Blood cells from 7 patients with cat allergy (Table 1, nos. 4, 7, 13, 14, 17, 18, and 23) were exposed to PBS (unstim.), anti-IgE, and serial dilutions (10-0.01 μg/mL) of rFel d 1, PreS-2xP1, PreS-2xP5, or PreS-P1-P5 (x-axes). CD203c expression on basophils was determined by means of FACS analysis. CD203c upregulation is displayed as the stimulation index (y-axes).
PreS fusion proteins induce Fel d 1–specific IgG responses in rabbits and mice
Rabbits were immunized with PreS-2xP1, PreS-2xP5, and PreS-P1-P5 by using complete Freund adjuvant. Table E2 (available in this article’s Online Repository at www.jacionline.org) shows that each of the fusion proteins induced robust Fel d 1–specific IgG antibodies in rabbits up to a serum dilution of 1:160,000, with a titer comparable with that of the complete rFel d 1 allergen.
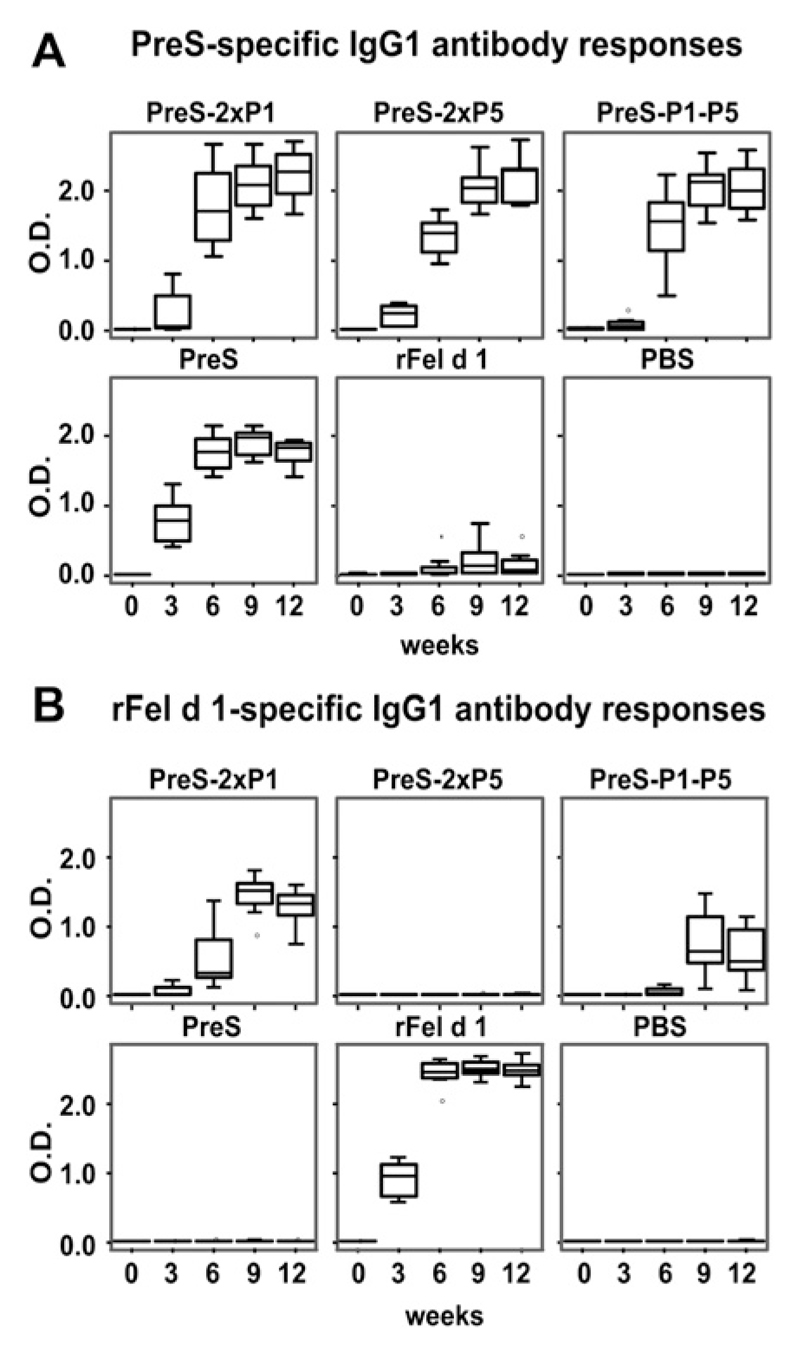
Next, groups of 8 mice were immunized with the PreS fusion proteins, PreS alone, or rFel d 1 adsorbed to aluminum hydroxide. PreS-specific IgG1 started to develop already 3 weeks after the first immunization and continued to increase at weeks 6 and 9 in mice that had received PreS alone or PreS fusion proteins but not in mice that had been immunized with rFel d 1 or aluminum hydroxide alone (Fig 4, A). Likewise, Fel d 1–specific IgG1 could be detected after 3 weeks in rFel d 1–immunized mice and after 6 weeks in mice that had received PreS-2xP1 and PreS-P1-P5 (Fig 4, B). No Fel d 1–specific IgG1 antibody response was observed in mice immunized with PreS-2xP5. Fel d 1–specific antibody responses were stronger in mice that had received a PreS fusion protein containing 2 copies of P1 (ie, PreS-2xP1) than in mice immunized with a PreS fusion protein with only a single P1 copy (ie, PreS-P1-P5; Fig 4, B). Similar results regarding PreS- and Fel d 1–specific responses were observed for IgA, IgG2a, and IgG2b antibodies, but the titers were much lower than for IgG1 (data not shown).
Fig 4.
PreS- (A) and rFel d 1–specifc (B) IgG1 responses in mice. Groups of 8 mice each were immunized subcutaneously with the antigen shown at the top of the boxes (PreS-2xP1, PreS-2xP5, PreS-P1-P5, PreS, rFel d 1, and PBS). Serum samples were taken 1 day before each immunization in 3-week intervals (0-12 weeks; x-axes). IgG1 reactivities (OD values, y-axes) were determined by means of ELISA and are displayed as box plots, where 50% of the values are within the boxes and nonoutliers are between the bars. The lines within the boxes indicate the median values.
Immunizations with PreS fusion proteins do not induce allergic sensitization to Fel d 1
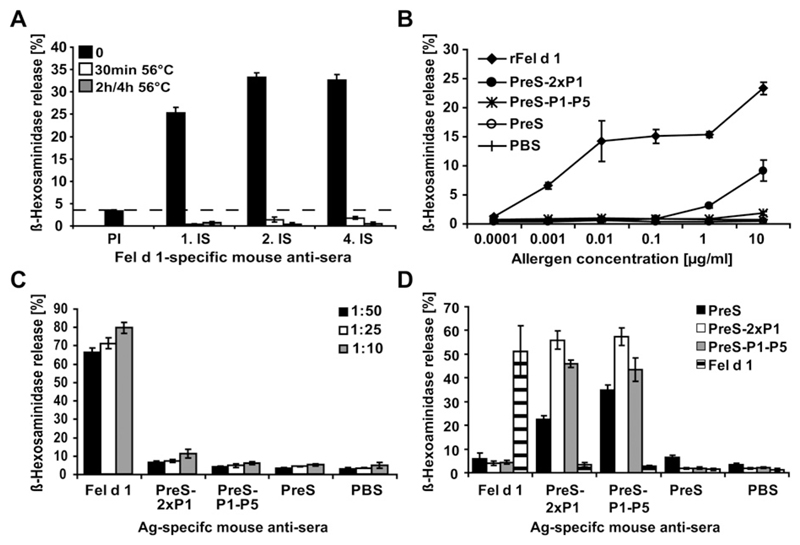
RBL cells expressing the high-affinity receptor for murine IgE were incubated with sera from immunized mice to investigate whether immunization with the PreS fusion proteins induces an allergenic immune response to rFel d 1 (Fig 5). Only non–heat-inactivated sera from mice immunized with rFel d 1 induced a strong Fel d 1–specific degranulation of basophils, whereas incubation at 56°C for 30 minutes was sufficient to abolish degranulation, indicating that the reaction is caused by IgE antibodies (Fig 5, A). A titration of Fel d 1 shows that immunization with the fusion proteins induced more than 1000-fold less Fel d 1–specific reaginic IgE antibodies, even when tested at different serum IgE concentrations (1:50, 1:25, and 1:10; Fig 5, B and C). A strong degranulation with Fel d 1 was only observed when RBL cells were sensitized with sera from mice immunized with rFel d 1 but not from mice immunized with fusion proteins (Fig 5, D).
Fig 5.
Immunization with PreS fusion proteins does not induce allergic sensitization to Fel d 1. A, Sera from mice obtained before (PI) and 3 (1. IS), 6 (2. IS), and 12 (4. IS) weeks after immunization with rFel d 1 (x-axis) were heated at 56°C for different times (30 minutes, 2 hours, or 4 hours) or left untreated (0). These sera were loaded onto RBL and exposed to rFel d 1. B, RBL cells were sensitized with mouse antigen (Ag)–specific sera and stimulated with increasing concentrations of rFel d 1 (x-axis). C, RBL cells were sensitized with different dilutions (1:50, 1:25, and 1:10) of allergen-specific murine sera (x-axis) and stimulated with rFel d 1. D, Sensitized RBL cells (x-axis) were stimulated with PreS-2xP1, PreS-P1-P5, and rFel d 1. In each figure β-hexosaminidase releases are displayed as a percentage of total β-hexosaminidase release on the y-axes.
Antibodies induced by immunization with PreS fusion proteins inhibit IgE binding of patients with cat allergy to Fel d 1 and CD203c upregulation on basophils
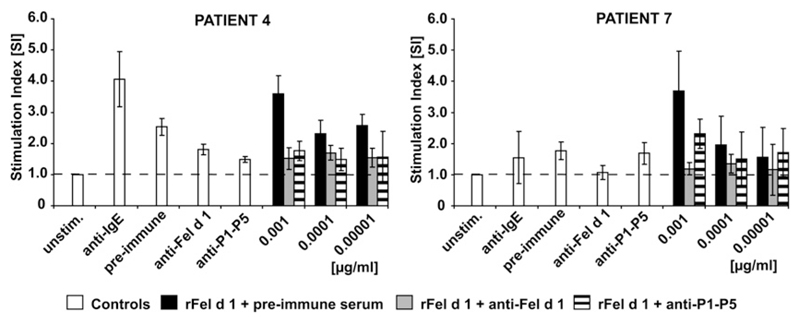
The inhibition of IgE binding of patients with cat allergy to rFel d 1 by rabbit IgG antibodies raised against the PreS fusion proteins and rFel d 1 was determined by means of ELISA competition experiments (Table II). A strong inhibition of allergic patients’ IgE reactivity to Fel d 1 was achieved with anti–PreS-P1-P5 antibodies (68% mean inhibition). This inhibition was as good as that achieved with anti–Fel d 1–specific antibodies (66% mean inhibition). The mean inhibition of IgE binding to Fel d 1 achieved with antibodies specific for PreS-2xP1 and PreS-2xP5 was 53% and 49%, respectively, and was thus lower than that obtained with antibodies specific for the PreS fusion protein containing both Fel d 1 peptides (ie, P1 and P5). The blocking activity of IgG antibodies induced by means of immunization with PreS-P1-P5 was also tested in a functional assay (ie, regarding their ability to inhibit allergen-induced basophil activation). As exemplified in Fig 6, preincubation of rFel d 1 with the anti–PreS-P1-P5 antibodies inhibited allergen-induced CD203c upregulation on basophils almost to the same extent as antibodies obtained by means of immunization with the complete rFel d 1 allergen (Fig 6).
Table II. Percentages of inhibition of IgE binding of sera from patients with cat allergy to Fel d 1 by rabbit IgG antibodies.
| Patient no. | Anti–rFel d 1 | Anti–PreS-2xP1 | Anti–PreS-2xP5 | Anti–PreS-P1-P5 |
|---|---|---|---|---|
| 1 | 56 | 59 | 48 | 70 |
| 2 | 81 | 85 | 79 | 84 |
| 3 | 50 | 25 | 13 | 53 |
| 4 | 69 | 33 | 52 | 70 |
| 5 | 62 | 41 | 38 | 65 |
| 6 | 55 | 53 | 50 | 72 |
| 7 | 69 | 24 | 28 | 60 |
| 8 | 69 | 52 | 52 | 70 |
| 9 | 71 | 70 | 59 | 76 |
| 10 | 75 | 61 | 55 | 75 |
| 11 | 82 | 70 | 56 | 78 |
| 12 | 57 | 30 | 55 | 65 |
| 13 | 73 | 55 | 60 | 74 |
| 14 | 67 | 70 | 57 | 73 |
| 15 | 74 | 75 | 63 | 79 |
| 16 | 56 | 39 | 39 | 64 |
| 17 | 75 | 47 | 53 | 76 |
| 18 | 66 | 51 | 43 | 67 |
| 19 | 65 | 65 | 57 | 72 |
| 20 | 58 | 48 | 52 | 58 |
| 21 | 70 | 74 | 72 | 79 |
| 22 | 66 | 55 | 47 | 63 |
| 23 | 42 | 0 | 0 | 10 |
| Means | 66 | 53 | 49 | 68 |
The percentages of inhibition of sera from patients with cat allergy (n = 23) to rFel d 1 by rabbit anti–rFel d 1, anti–PreS-2xP1, anti–PreS-2xP5, and anti–PreS-P1-P5 are shown. Mean inhibitions are displayed in boldface in the bottom line.
Fig 6.
Inhibition of rFel d 1–induced CD203c upregulation on basophils from patients with cat allergy by rabbit IgG antibodies. Increasing concentrations of rFel d 1 (0.00001-0.001 μg/mL) were preincubated with rabbit anti–rFel d 1 or anti–PreS-P1-P5 IgG antibodies or the corresponding preimmune serum. Basophils from 2 patients with cat allergy (Table I, nos. 4 and 7) were then exposed to PBS (unstim.), anti-IgE, and the preincubated mixtures of rFel d 1 (x-axes). Upregulation of CD203c expression on basophils determined by means of FACS analysis is displayed as the stimulation index (y-axes).
Fel d 1–specific IgG responses can be boosted by means of reimmunization with PreS fusion protein or peptide alone
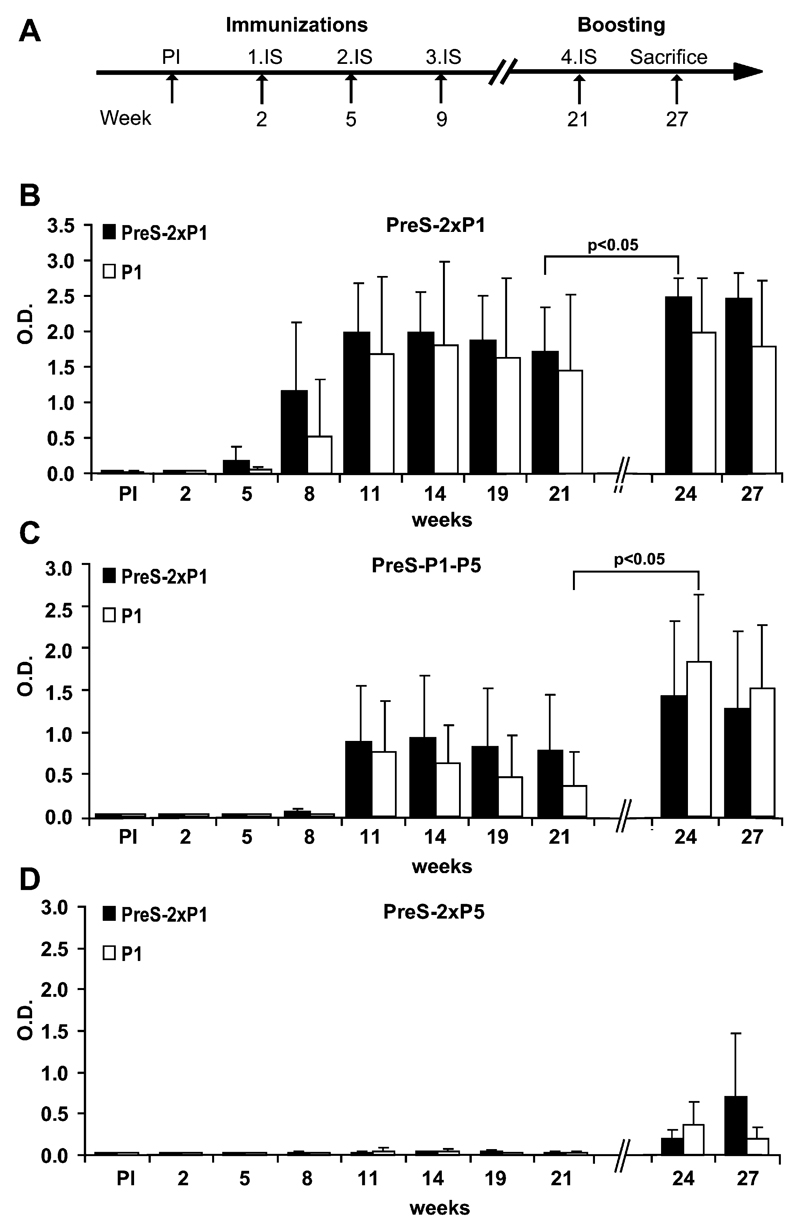
In a next set of immunization experiments, we studied whether and how Fel d 1–specific IgG responses induced in mice by means of immunization with PreS fusion proteins can be boosted. For this purpose, groups of 8 mice each were immunized 3 times (weeks 2, 5, and 9) with aluminum hydroxide–adsorbed PreS-2xP1, PreS-2xP5, or PreS-P1-P5 after priming with PreS on day 0 (see Fig E2, A, in this article’s Online Repository at www.jacionline.org). As in the first experiments, high titers of Fel d 1–specific antibodies were found in mice immunized with PreS-2xP1 and PreS-P1-P5 but not in mice immunized with PreS-2xP5 (see Fig E2, B-D). After 3 immunizations, the Fel d 1–specific IgG antibody levels peaked at week 14 and showed only a very moderate decrease, remaining still high at week 21. At week 21, each group of mice was divided into 2 groups, of which one was reimmunized with aluminum hydroxide–adsorbed peptide P1 alone or PreS-2xP1. Increases in Fel d 1–specific IgG1 levels were observed in mice boosted with the isolated peptide and the fusion protein. Statistically significant increases in Fel d 1–specific IgG1 responses were observed when PreS-2xP1–immunized mice were boosted with PreS-2xP1 and in PreS-P1-P5–immunized mice after boosting with the isolated P1 peptide (P < .05). Interestingly, the booster effect appeared strongest in PreS-P1-P5–preimmunized mice that had received the free peptide (P1). Even in PreS-2xP5–preimmunized mice who had not mounted detectable Fel d 1–specific IgG responses at week 21, a Fel d 1–specific response could be induced with PreS-2xP1 and P1.
Majority of the T-cell response in PreS fusion protein–immunized mice is specific for PreS
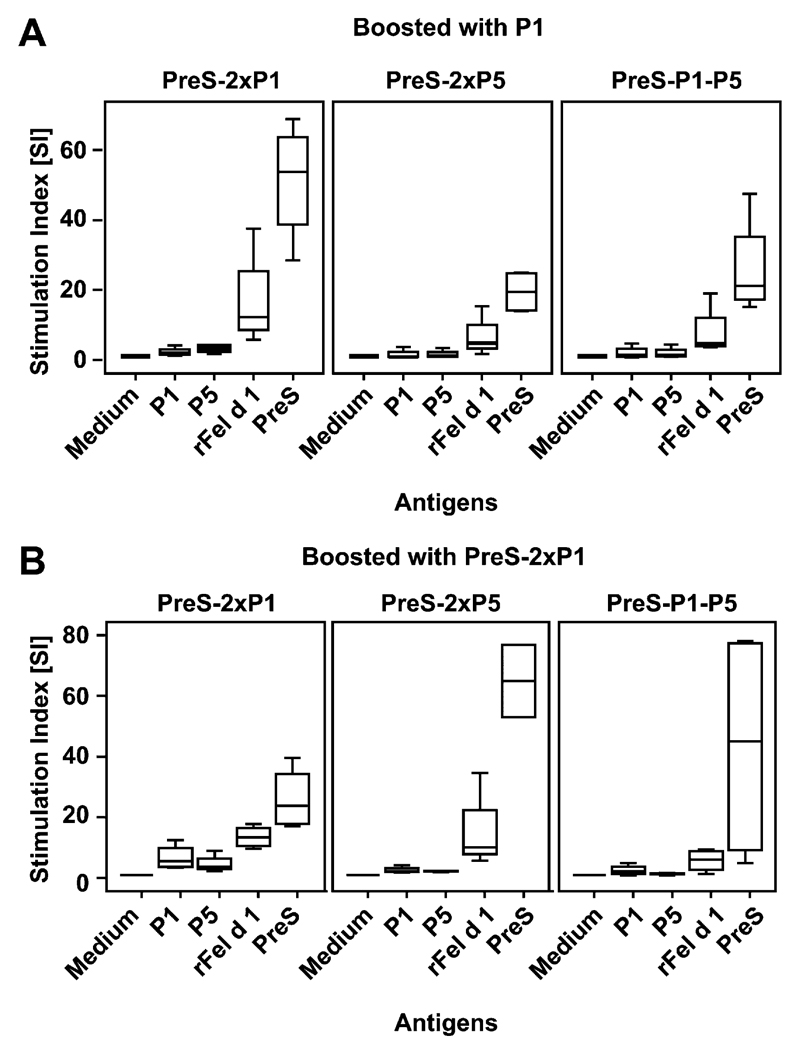
Spleen cells obtained at week 27 (see Fig E3 in this article’s Online Repository at www.jacionline.org) were stimulated with P1, P5, rFel d 1, or PreS to determine the specificity of T-cell responses in mice that had been immunized with PreS fusion proteins and were then boosted with P1 or PreS-2xP1 (see Fig E3, A and B, x-axes). In each of the immunized mouse groups the majority of T-cell response was directed against PreS, whereas only a low Fel d 1–specific T-cell response was observed.
Discussion
SIT performed as injection treatment is a clinically effective therapy for cat allergy but often induces severe side effects.11 Furthermore, currently used allergen extracts are often of poor quality, which might limit the success of treatment.33 The majority of patients with cat allergy are sensitized to Fel d 1, a 35-kd tetrameric glycoprotein that is composed of 2 covalently linked heterodimers, each comprising 2 polypeptides, Fel d 1 chain 1 and chain 2, that are derived from 2 independent genes.34–36 rFel d 1 resembling the allergenic activity of natural Fel d 1 and of cat dander extract has been produced in E coli.25
Here we report the expression, purification, and characterization of 3 fusion proteins for vaccination against cat allergy, which should allow overcoming of problems related to both IgE-mediated and T cell–mediated side effects and poor vaccine quality. They consist of the PreS domain of HBV as a carrier containing 2 nonallergenic peptides derived from chains 1 and 2 of Fel d 1. Hepatitis B PreS is part of hepatitis B vaccines, which have been safely applied in human subjects.37 We demonstrate that the fusion proteins can be easily produced as defined proteins in large quantities by means of expression in E coli and, when compared with Fel d 1, lack any detectable IgE-related allergenic activity. Even in the fusion protein containing both Fel d 1–derived peptides (P1 and P5), more than 60% of the primary Fel d 1 sequence has been removed. It might thus be expected that the fusion proteins exhibit reduced side effects because of activation of Fel d 1–specific T cells. Despite the reduction of Fel d 1 sequence elements in the constructs, we found that immunization of mice and rabbits with the recombinant fusion proteins induced Fel d 1–specific IgG antibodies that, as tested for the rabbit antibodies, inhibited allergic patients’ IgE reactivity to a similar extent as IgG antibodies induced by vaccination with complete Fel d 1. This result can be explained according to the hapten-carrier principle described by Siskind et al,38 who demonstrated that robust antibody responses can be induced against peptides using T-cell help from a covalently bound unrelated carrier molecule.20 In fact, our murine immunization experiments demonstrate that the majority of T-cell help for the production of Fel d 1–specific IgG antibodies was derived from the PreS carrier molecule. An IgG response against Fel d 1 could only be obtained with PreS-bound P5 but not with P5 alone, indicating the importance of the carrier.
By using this principle, similar results have been recently obtained for a grass pollen allergy vaccine based on a peptide derived from the major timothy grass pollen allergen Phl p 1 fused to rhinovirus VP1 protein.39 Because the IgG antibodies induced by means of immunization with the fusion proteins inhibited IgE binding of patients with cat allergy to Fel d 1, it is expected that such protective antibodies will also develop in vaccinated patients. These IgG antibodies might then contribute to clinical improvement by blocking allergen-induced mast cell degranulation, inhibiting IgE-facilitated antigen presentation to T cells, and reducing boosts of IgE memory responses.40 Another potentially very important finding was that immunization with the recombinant fusion proteins did not induce allergic sensitization to Fel d 1. It might thus be expected that the fusion proteins will not increase allergic sensitization to Fel d 1 during SIT and perhaps be useful for prophylactic vaccination. We observed that immunization with PreS fusion proteins induced murine IgE responses against the immunogens and PreS, but these IgE responses are likely not of clinical relevance because PreS has been already used to safely to vaccinate newborns.41
We think that the recombinant fusion proteins might have several advantages over previous strategies for cat immunotherapy. In contrast to the mix of Fel d 1 T cell epitope–containing peptides that have been extensively studied in clinical trials,3,13,14,16–18 the fusion proteins induce robust Fel d 1–specific IgG antibodies, which should block immediate allergic inflammation. Furthermore, the T cell–mediated side effects should be lower than observed for the T cell peptides because of the elimination of a large portion of the Fel d 1 sequence. A chimeric fusion protein consisting of Fel d 1 and human Fcγ has been shown to block allergen-induced mast cell activation but seems to be less suitable for vaccination because, on repeated administration, it might induce autoantibodies against human Fcγ, and the intact Fel d 1 might give rise to allergic side effects.42
Recently, a vaccine based on Fel d 1 coupled to bacteriophage Qβ–derived virus-like particles has been described.43 This approach induces Fel d 1–specific IgG antibodies, but the proteins tend to aggregate, and the chemical coupling process might be difficult to standardize for vaccine production. Intact Fel d 1 has been coupled to the virus-like particles and the vaccine might induce both immediate and T cell–mediated side effects.
Hypoallergenic recombinant derivatives made for Fel d 1 exhibited a reduced allergenic activity, but the reduction of the allergenic activity and IgE reactivity of these earlier reported derivatives42 was much lower than that of the fusion proteins described by us. We observed a more than 1000-fold reduction in allergenic activity of the fusion proteins compared with that of wild-type rFel d 1 so that allergic patients should theoretically tolerate an up to 1000-fold higher dose of the fusion proteins compared with Fel d 1 in the course of SIT. Furthermore, recombinant hypoallergenic Fel d 1 derivatives contained the majority of Fel d 1–specific T-cell epitopes and thus might give rise to T cell–mediated side effects.44
In summary, the recombinant fusion proteins described here should represent extremely well-defined and safe vaccines for the treatment of cat allergy. Based on this approach, it might be possible to develop broadly applicable and safe forms of immunotherapy for cat allergy and eventually also prophylactic vaccines against cat allergy.
Methods
Lymphoproliferative responses in PBMCs from patients with cat allergy
PBMCs were isolated from patients with cat allergy by means of Ficoll (Amersham Bioscience, Uppsala, Sweden) density gradient centrifugation. Cells were then resuspended in AIM V medium (Life Technologies, Grand Island, NY) to a final concentration of 2 × 105 cells/well and stimulated with increasing doses of rFel d 1–derived peptide (1-5; 0.0625-1 μg/well) or medium alone in triplicates. After 6 days, proliferative responses were measured by means of tritiated thymidine incorporation and are displayed as stimulation indices.E1
IgE inhibition experiments
Sera from patients with cat allergy (n = 22) were diluted 1:10 in PBS containing 0.5% wt/vol BSA and 0.05% vol/vol Tween 20 and were preincubated with rFel d 1, PreS, PreS-2xP1, PreS-2xP5, and PreS-P1-P5 (10 μg/mL) and, for control purposes, with buffer alone overnight at 4°C.E2 Sera were then loaded onto ELISA plate–coated rFel d 1, and bound human IgE was detected with HRP-coupled goat anti-human IgE antibodies diluted 1:2,500 (KPL). The OD values corresponding to bound antibodies were measured at 405 and 490 nm. An OD of 0.06, representing the 2-fold value of the ODs found for nonallergic subjects, served as a cutoff value for specific IgE reactivity and was subtracted from each of the OD values. The percentage of inhibition of IgE binding was calculated as follows:
where ODA and ODB represent the OD values after preincubation with the antigen or the buffer (0.5% wt/vol BSA), respectively.
Immunization of rabbits and measurement of Fel d 1–specific IgG antibodies
Rabbits were immunized 3 times in monthly intervals with 200 μg of antigen (rFel d 1, PreS-2xP1, PreS-2xP5, and PreS-P1-P5) emulsified once in complete Freund adjuvant and twice in Freund incomplete adjuvant (Charles River). Plates were coated with rFel d 1 (5 μg/mL), rabbit sera were diluted up to 1:160,000, and bound rabbit IgG was detected with 1:2,000 diluted donkey anti-rabbit HRP-coupled IgG antibodies (Amersham Bioscience) to determine antigen-specific antibodies in sera from immunized rabbits. The OD values corresponding to the levels of antigen-specific antibodies were measured at 405 and 490 nm in an ELISA reader (Dynatech).
Immunization of mice and measurement of PreS and Fel d 1 immune responses
Six-week-old female BALB/c mice were purchased from Charles River. The animals were maintained in the animal care unit of the Institute of Pathophysiology (Medical University of Vienna), according to the local guidelines for animal care. In a first set of experiments, groups of 8 mice each were immunized subcutaneously twice with 10 μg and once with 20 μg of the purified antigens (P1, P5, P1+P5, PreS, PreS-2xP1, PreS-2xP5, PreS-P1-P5, and rFel d 1) adsorbed to aluminum hydroxide (SERVA Electrophoresis, Heidelberg, Germany) in 3-week intervals and bled from the tail veins 1 day before each immunization.
In a second set of experiments, groups of 8 mice were preimmunized subcutaneously with aluminum hydroxide–adsorbed carrier protein (10 μg of PreS; ie, priming). After 2 weeks, each group received twice 10 μg and once 20 μg (3-week intervals, aluminum hydroxide adsorbed) of PreS-2xP1, PreS-2xP5, or PreS-P1-P5 by means of subcutaneous injection. At week 21, the groups of mice were split into 2 groups, of which one group received a booster injection with 20 μg of alum-adsorbed peptide (P1) and the other received one 20-μg injection of alum-adsorbed PreS-2xP1. One day before each immunization, mice were bled, and the sera were stored at −20°C until use.
ELISA plates (Nunc, Langenselbold, Germany) were coated with 5 μg/mL of the above antigens and incubated with murine sera diluted 1:100 for IgA and IgM and 1:1000 for IgG1, IgG2a, and IgG2b to determine the PreS- and rFel d 1–specific antibody responses.E3,E4 Bound murine antibodies were detected with monoclonal rat anti-mouse IgA, IgM, IgG1, IgG2a, and IgG2b antibodies (BD PharMingen, San Diego, Calif) diluted 1:1000, followed by detection with HRP-conjugated goat anti-rat IgG antibodies (Amersham Bioscience) diluted 1:2000. The OD values corresponding to the levels of antigen-specific antibodies were measured at 405 and 490 nm in an ELISA reader (Dynatech).
T-cell proliferation assay
Lymphoproliferative responses in boosted mice were measured in spleen cell cultures at week 27 stimulated with equimolar amounts of P1 (0.26 μg/well), P5 (0.22 μg/well), rFel d 1 (2 μg/well), PreS (2 μg/well), concanavalin A (0.5 μg/well, positive control), or medium alone, as previously described.E5
Statistical analysis
Differences in antibody levels induced by means of immunization of mice were assessed by using Mann-Whitney U tests. The SPSS statistical software system (SPSS, Inc, Chicago, Ill) was used for calculations. A P value of less than .05 was considered significant.
Extended Data
Fig E1.
Lymphoproliferative responses of PBMCs from 4 patients with cat allergy to the rFel d 1–derived peptides (1-5). Mean stimulation indices are shown for 5 antigen concentrations: 1 μg/well, 0.5 μg/well, 0.25 μg/well, 0.125 μg/well, and 0.0625 μg/well. Stimulation indices (SIs) of greater than 2.0 are displayed in gray.
Fig E2.
Boosting of Fel d 1–specific IgG responses in vaccinated mice with isolated peptides. A, Groups of 8 mice each were preimmunized with PreS, followed by 3 immunizations either with PreS-2xP1 (B), PreS-P1-P5 (C), or PreS-2xP5 (D). At week 21, when Fel d 1–specific IgG1 responses decreased, mice were randomized into 2 groups of 4 mice each and boosted either with peptide 1 (P1; white bars) alone or with the PreS-2xP1 fusion protein (black bars). Bars represent Fel d 1–specific IgG1 antibody levels (y-axes; OD values).
Fig E3.
Proliferative responses of splenocytes. Displayed are proliferative responses (y-axes; stimulation indices) of splenocytes from immunized mice (top of the boxes, immunogens) that had been boosted with P1 (A) or PreS-2xP1 (B) and were stimulated with medium alone, P1, P5, rFel d 1, or PreS (x-axes). Mean values of triplicates are shown as box plots, where 50% of the values are within the boxes and nonoutliers are between the bars. The lines within the boxes indicate the median values of the groups.
Table E1. Inhibition of IgE binding to rFel d 1 after preabsorption of patients’ sera with rFel d 1 or recombinant fusion proteins.
| Fel d 1 | PreS-2xP1 | PreS-2xP5 | PreS-P1-P5 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| BSA OD |
|||||||||
| Patient no. | OD | Percent | OD | Percent | OD | Percent | OD | Percent | |
| 1 | 0.05 | 90 | 0.48 | 4 | 0.53 | 0 | 0.49 | 2 | 0.50 |
| 2 | 0.01 | 99 | 0.40 | 13 | 0.43 | 7 | 0.41 | 10 | 0.46 |
| 3 | 0.05 | 94 | 0.68 | 15 | 0.76 | 5 | 0.70 | 13 | 0.80 |
| 4 | 0.04 | 97 | 1.09 | 18 | 1.20 | 10 | 1.08 | 19 | 1.33 |
| 5 | 0.30 | 88 | 2.14 | 14 | 2.34 | 6 | 2.20 | 11 | 2.48 |
| 6 | 0.00 | 100 | 0.30 | 9 | 0.32 | 4 | 0.30 | 8 | 0.33 |
| 7 | 0.13 | 93 | 1.67 | 7 | 1.74 | 4 | 1.65 | 8 | 1.80 |
| 8 | 0.10 | 95 | 1.96 | 11 | 2.09 | 5 | 2.02 | 9 | 2.21 |
| 9 | 0.03 | 94 | 0.45 | 13 | 0.50 | 3 | 0.44 | 15 | 0.52 |
| 10 | 0.00 | 100 | 0.42 | 14 | 0.45 | 8 | 0.44 | 11 | 0.49 |
| 11 | 0.04 | 81 | 0.18 | 5 | 0.18 | 4 | 0.17 | 11 | 0.19 |
| 12 | 0.03 | 95 | 0.47 | 11 | 0.40 | 24 | 0.38 | 29 | 0.53 |
| 13 | 0.10 | 92 | 1.05 | 15 | 1.15 | 6 | 1.08 | 12 | 1.23 |
| 14 | 0.00 | 100 | 0.20 | 13 | 0.23 | 0 | 0.20 | 12 | 0.23 |
| 15 | 0.00 | 100 | 0.09 | 18 | 0.10 | 7 | 0.09 | 18 | 0.11 |
| 16 | 0.00 | 100 | 0.18 | 28 | 0.22 | 10 | 0.20 | 21 | 0.25 |
| 17 | 0.10 | 93 | 1.25 | 17 | 1.41 | 6 | 1.37 | 9 | 1.50 |
| 18 | 0.12 | 91 | 1.17 | 13 | 1.20 | 10 | 1.15 | 14 | 1.34 |
| 20 | 0.19 | 80 | 0.82 | 12 | 0.86 | 8 | 0.82 | 12 | 0.93 |
| 21 | 0.08 | 60 | 0.16 | 20 | 0.18 | 8 | 0.17 | 14 | 0.20 |
| 22 | 0.00 | 100 | 0.53 | 13 | 0.56 | 8 | 0.55 | 11 | 0.61 |
| 23 | 0.28 | 88 | 2.41 | 0 | 2.49 | 0 | 2.40 | 0 | 2.32 |
| Means | 0.07 | 92 | 0.82 | 13 | 0.88 | 7 | 0.83 | 12 | 0.93 |
Displayed are the OD values corresponding to the levels of bound IgE antibodies of sera from patients with cat allergy (n = 22) to rFel d 1 after preincubation with rFel d 1 and PreS-2xP1, PreS-2xP5, and PreS-P1-P5. The cutoff value is an OD of 0.06. The percentages of inhibition of IgE binding are shown. Mean OD values and inhibitions for each antigen are displayed in boldface.
Table E2. Fel d 1–specific titers in anti-sera from rFel d 1–, PreS-2xP1–, PreS-2xP5–, and PreS-P1-P5–immunized rabbits.
| Serum dilution | Anti–rFel d 1 | Anti–PreS-2xP1 | Anti–PreS-2xP5 | Anti–PreS-P1-P5 |
|---|---|---|---|---|
| 1:5,000 | 3.68 | 3.69 | 3.71 | 3.65 |
| 1:10,000 | 3.21 | 2.62 | 2.12 | 2.97 |
| 1:20,000 | 2.68 | 1.96 | 1.39 | 2.11 |
| 1:40,000 | 2.01 | 1.28 | 0.91 | 1.59 |
| 1:80,000 | 1.03 | 0.67 | 0.48 | 1.04 |
| 1:160,000 | 0.47 | 0.29 | 0.23 | 0.47 |
Displayed are OD values corresponding to Fel d 1–specific IgG antibody levels for different dilutions of rabbit anti-sera.
Clinical implications.
Fusion proteins consisting of nonallergenic Fel d 1–derived peptides fused to hepatitis B PreS should represent safe vaccines for cat allergy.
Acknowledgments
Supported by grants DK-W1212-B13, L214-B13, F1815, and 1820 of the Austrian Science Fund (FWF); by research grants from Biomay, Vienna, Austria, and the Christian Doppler Research Association, Austria; and by the Swedish Research Council and Swedish Asthma and Allergy Association’s Research Foundation.
Abbreviations used
- HBV
Hepatitis B virus
- HRP
Horseradish peroxidase
- RBL
Rat basophil leukemia
- SI
Stimulation index
- SIT
Allergen-specific immunotherapy
Footnotes
Disclosure of potential conflict of interest: M. van Hage has received research support from the Swedish Research Council and the Swedish Asthma and Allergy Association’s Research Foundation. R. Valenta has received research support from Biomay, Phadia, and the Austrian Science Fund (FWF) and has consulted for Biomay and Phadia. The rest of the authors have declared that they have no conflict of interest.
References
- 1.Perzanowski MS, Ronmark E, Nold B, Lundback B, Platts-Mills TA. Relevance of allergens from cats and dogs to asthma in the northernmost province of Sweden: schools as a major site of exposure. J Allergy Clin Immunol. 1999;103:1018–24. doi: 10.1016/s0091-6749(99)70173-9. [DOI] [PubMed] [Google Scholar]
- 2.Roost HP, Kunzli N, Schindler C, Jarvis D, Chinn S, Perruchoud AP, et al. Role of current and childhood exposure to cat and atopic sensitization. European Community Respiratory Health Survey. J Allergy Clin Immunol. 1999;104:941–7. doi: 10.1016/s0091-6749(99)70072-2. [DOI] [PubMed] [Google Scholar]
- 3.Gronlund H, Saarne T, Gafvelin G, van Hage M. The major cat allergen, Fel d 1, in diagnosis and therapy. Int Arch Allergy Immunol. 2010;151:265–74. doi: 10.1159/000250435. [DOI] [PubMed] [Google Scholar]
- 4.Durham SR, Walker SM, Varga EM, Jacobson MR, O’Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999;341:468–75. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 5.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006;6:761–71. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 6.Taylor WW, Ohman JL, Jr, Lowell FC. Immunotherapy in cat-induced asthma. Double-blind trial with evaluation of bronchial responses to cat allergen and histamine. J Allergy Clin Immunol. 1978;61:283–7. doi: 10.1016/0091-6749(78)90048-9. [DOI] [PubMed] [Google Scholar]
- 7.Van Metre TE, Jr, Marsh DG, Adkinson NF, Jr, Kagey-Sobotka A, Khattignavong A, Norman PS, Jr, et al. Immunotherapy decreases skin sensitivity to cat extract. J Allergy Clin Immunol. 1989;83:888–99. doi: 10.1016/0091-6749(89)90102-4. [DOI] [PubMed] [Google Scholar]
- 8.Hedlin G, Graff-Lonnevig V, Heilborn H, Lilja G, Norrlind K, Pegelow K, et al. Immunotherapy with cat- and dog-dander extracts. V. Effects of 3 years of treatment. J Allergy Clin Immunol. 1991;87:955–64. doi: 10.1016/0091-6749(91)90417-m. [DOI] [PubMed] [Google Scholar]
- 9.Varney VA, Edwards J, Tabbah K, Brewster H, Mavroleon G, Frew AJ. Clinical efficacy of specific immunotherapy to cat dander: a double-blind placebo-controlled trial. Clin Exp Allergy. 1997;27:860–7. [PubMed] [Google Scholar]
- 10.Nanda A, O’Connor M, Anand M, Dreskin SC, Zhang L, Hines B, et al. Dose dependence and time course of the immunologic response to administration of standardized cat allergen extract. J Allergy Clin Immunol. 2004;114:1339–44. doi: 10.1016/j.jaci.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 11.Mellerup MT, Hahn GW, Poulsen LK, Malling H. Safety of allergen-specific immunotherapy. Relation between dosage regimen, allergen extract, disease and systemic side-effects during induction treatment. Clin Exp Allergy. 2000;30:1423–9. doi: 10.1046/j.1365-2222.2000.00910.x. [DOI] [PubMed] [Google Scholar]
- 12.Counsell CM, Bond JF, Ohman JL, Jr, Greenstein JL, Garman RD. Definition of the human T-cell epitopes of Fel d 1, the major allergen of the domestic cat. J Allergy Clin Immunol. 1996;98:884–94. doi: 10.1016/s0091-6749(96)80004-2. [DOI] [PubMed] [Google Scholar]
- 13.Norman PS, Ohman JL, Jr, Long AA, Creticos PS, Gefter MA, Shaked Z, et al. Treatment of cat allergy with T-cell reactive peptides. Am J Respir Crit Care Med. 1996;154:1623–8. doi: 10.1164/ajrccm.154.6.8970345. [DOI] [PubMed] [Google Scholar]
- 14.Simons FE, Imada M, Li Y, Watson WT, HayGlass KT. Fel d 1 peptides: effect on skin tests and cytokine synthesis in cat-allergic human subjects. Int Immunol. 1996;8:1937–45. doi: 10.1093/intimm/8.12.1937. [DOI] [PubMed] [Google Scholar]
- 15.Haselden BM, Kay AB, Larche M. Immunoglobulin E-independent major histocompatibility complex-restricted T cell peptide epitope-induced late asthmatic reactions. J Exp Med. 1999;189:1885–94. doi: 10.1084/jem.189.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oldfield WL, Kay AB, Larche M. Allergen-derived T cell peptide-induced late asthmatic reactions precede the induction of antigen-specific hyporesponsiveness in atopic allergic asthmatic subjects. J Immunol. 2001;167:1734–9. doi: 10.4049/jimmunol.167.3.1734. [DOI] [PubMed] [Google Scholar]
- 17.Oldfield WL, Larche M, Kay AB. Effect of T-cell peptides derived from Fel d 1 on allergic reactions and cytokine production in patients sensitive to cats: a randomised controlled trial. Lancet. 2002;360:47–53. doi: 10.1016/s0140-6736(02)09332-7. [DOI] [PubMed] [Google Scholar]
- 18.Alexander C, Tarzi M, Larche M, Kay AB. The effect of Fel d 1-derived T-cell peptides on upper and lower airway outcome measurements in cat-allergic subjects. Allergy. 2005;60:1269–74. doi: 10.1111/j.1398-9995.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- 19.Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125:383–9. doi: 10.1016/j.jaci.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 20.Focke M, Swoboda I, Marth K, Valenta R. Developments in allergen-specific immunotherapy: from allergen extracts to allergy vaccines bypassing allergen-specific immunoglobulin E and T cell reactivity. Clin Exp Allergy. 2010;40:385–97. doi: 10.1111/j.1365-2222.2009.03443.x. [DOI] [PubMed] [Google Scholar]
- 21.Heermann KH, Goldmann U, Schwartz W, Seyffarth T, Baumgarten H, Gerlich WH. Large surface proteins of hepatitis B virus containing the pre-s sequence. J Virol. 1984;52:396–402. doi: 10.1128/jvi.52.2.396-402.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neurath AR, Kent SB. The pre-S region of hepadnavirus envelope proteins. Adv Virus Res. 1988;34:65–142. doi: 10.1016/s0065-3527(08)60516-3. [DOI] [PubMed] [Google Scholar]
- 23.Milich DR. T- and B-cell recognition of hepatitis B viral antigens. Immunol Today. 1988;9:380–6. doi: 10.1016/0167-5699(88)91239-X. [DOI] [PubMed] [Google Scholar]
- 24.Gronlund H, Adedoyin J, Reininger R, Varga EM, Zach M, Fredriksson M, et al. Higher immunoglobulin E antibody levels to recombinant Fel d 1 in cat-allergic children with asthma compared with rhinoconjunctivitis. Clin Exp Allergy. 2008;38:1275–81. doi: 10.1111/j.1365-2222.2008.03003.x. [DOI] [PubMed] [Google Scholar]
- 25.Gronlund H, Bergman T, Sandstrom K, Alvelius G, Reininger R, Verdino P, et al. Formation of disulfide bonds and homodimers of the major cat allergen Fel d 1 equivalent to the natural allergen by expression in Escherichia coli. J Biol Chem. 2003;278:40144–51. doi: 10.1074/jbc.M301416200. [DOI] [PubMed] [Google Scholar]
- 26.Focke M, Mahler V, Ball T, Sperr WR, Majlesi Y, Valent P, et al. Nonanaphylactic synthetic peptides derived from B cell epitopes of the major grass pollen allergen, Phl p 1, for allergy vaccination. FASEB J. 2001;15:2042–4. doi: 10.1096/fj.01-0016fje. [DOI] [PubMed] [Google Scholar]
- 27.Vrtala S, Susani M, Sperr WR, Valent P, Laffer S, Dolecek C, et al. Immunologic characterization of purified recombinant timothy grass pollen (Phleum pratense) allergens (Phl p 1, Phl p2, Phl p 5) J Allergy Clin Immunol. 1996;97:781–7. doi: 10.1016/s0091-6749(96)80156-4. [DOI] [PubMed] [Google Scholar]
- 28.Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, et al. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J Allergy Clin Immunol. 2002;110:102–9. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- 29.Vrtala S, Ball T, Spitzauer S, Pandjaitan B, Suphioglu C, Knox B, et al. Immunization with purified natural and recombinant allergens induces mouse IgG1 antibodies that recognize similar epitopes as human IgE and inhibit the human IgE-allergen interaction and allergen-induced basophil degranulation. J Immunol. 1998;160:6137–44. [PubMed] [Google Scholar]
- 30.Focke M, Linhart B, Hartl A, Wiedermann U, Sperr WR, Valent P, et al. Non-anaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin Exp Allergy. 2004;34:1525–33. doi: 10.1111/j.1365-2222.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- 31.Campana R, Vrtala S, Maderegger B, Jertschin P, Stegfellner G, Swoboda I, et al. Hypoallergenic derivatives of the major birch pollen allergen Bet v 1 obtained by rational sequence reassembly. J Allergy Clin Immunol. 2010;126:1024–31. doi: 10.1016/j.jaci.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 32.Linhart B, Bigenzahn S, Hartl A, Lupinek C, Thalhamer J, Valenta R, et al. Costimulation blockade inhibits allergic sensitization but does not affect established allergy in a murine model of grass pollen allergy. J Immunol. 2007;178:3924–31. doi: 10.4049/jimmunol.178.6.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trivedi B, Valerio C, Slater JE. Endotoxin content of standardized allergen vaccines. J Allergy Clin Immunol. 2003;111:777–83. doi: 10.1067/mai.2003.1338. [DOI] [PubMed] [Google Scholar]
- 34.Ohman JL, Jr, Lowell FC, Bloch KJ. Allergens of mammalian origin. III. Properties of a major feline allergen. J Immunol. 1974;113:1668–77. [PubMed] [Google Scholar]
- 35.Morgenstern JP, Griffith IJ, Brauer AW, Rogers BL, Bond JF, Chapman MD, et al. Amino acid sequence of Fel dI, the major allergen of the domestic cat: protein sequence analysis and cDNA cloning. Proc Natl Acad Sci U S A. 1991;88:9690–4. doi: 10.1073/pnas.88.21.9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaiser L, Gronlund H, Sandalova T, Ljunggren HG, van Hage-Hamsten M, Achour A, et al. The crystal structure of the major cat allergen Fel d 1, a member of the secretoglobin family. J Biol Chem. 2003;278:37730–5. doi: 10.1074/jbc.M304740200. [DOI] [PubMed] [Google Scholar]
- 37.Schumann A, Fiedler M, Dahmen U, Grosse-Wilde H, Roggendorf M, Lindemann M. Cellular and humoral immune response to a third generation hepatitis B vaccine. J Viral Hepat. 2007;14:592–8. doi: 10.1111/j.1365-2893.2007.00848.x. [DOI] [PubMed] [Google Scholar]
- 38.Siskind GW, Paul WE, Benacerraf B. Studies on the effect of the carrier molecule on antihapten antibody synthesis. I. Effect of carrier on the nature of the antibody synthesized. J Exp Med. 1966;123:673–88. doi: 10.1084/jem.123.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edlmayr J, Niespodziana K, Linhart B, Focke-Tejkl M, Westritschnig K, Scheiblhofer S, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J Immunol. 2009;182:6298–306. doi: 10.4049/jimmunol.0713622. [DOI] [PubMed] [Google Scholar]
- 40.Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, et al. From allergen genes to allergy vaccines. Annu Rev Immunol. 2010;28:211–41. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- 41.Sylvan SP, Madalinski K, Hellstrom UB. Anti-preS responses influence the anti-HBs response in newborns after vaccination with the third generation Sci-B-Vac vaccine. Vaccine. 2009;28:446–51. doi: 10.1016/j.vaccine.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 42.Zhu D, Kepley CL, Zhang K, Terada T, Yamada T, Saxon A. A chimeric human-cat fusion protein blocks cat-induced allergy. Nat Med. 2005;11:446–9. doi: 10.1038/nm1219. [DOI] [PubMed] [Google Scholar]
- 43.Schmitz N, Dietmeier K, Bauer M, Maudrich M, Utzinger S, Muntwiler S, et al. Displaying Fel d1 on virus-like particles prevents reactogenicity despite greatly enhanced immunogenicity: a novel therapy for cat allergy. J Exp Med. 2009;206:1941–55. doi: 10.1084/jem.20090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saarne T, Kaiser L, Gronlund H, Rasool O, Gafvelin G, van Hage-Hamsten M. Rational design of hypoallergens applied to the major cat allergen Fel d 1. Clin Exp Allergy. 2005;35:657–63. doi: 10.1111/j.1365-2222.2005.02234.x. [DOI] [PubMed] [Google Scholar]
- E1.Jahn-Schmid B, Kelemen P, Himly M, Bohle B, Fischer G, Ferreira F, et al. The T cell response to Art v 1, the major mugwort pollen allergen, is dominated by one epitope. J Immunol. 2002;169:6005–11. doi: 10.4049/jimmunol.169.10.6005. [DOI] [PubMed] [Google Scholar]
- E2.Mittermann I, Zidarn M, Silar M, Markovic-Housley Z, Aberer W, Korosec P, et al. Recombinant allergen-based IgE testing to distinguish bee and wasp allergy. J Allergy Clin Immunol. 2010;125:1300–7. doi: 10.1016/j.jaci.2010.03.017. [DOI] [PubMed] [Google Scholar]
- E3.Vrtala S, Ball T, Spitzauer S, Pandjaitan B, Suphioglu C, Knox B, et al. Immunization with purified natural and recombinant allergens induces mouse IgG1 antibodies that recognize similar epitopes as human IgE and inhibit the human IgE-allergen interaction and allergen-induced basophil degranulation. J Immunol. 1998;160:6137–44. [PubMed] [Google Scholar]
- E4.Focke M, Linhart B, Hartl A, Wiedermann U, Sperr WR, Valent P, et al. Non-anaphylactic surface-exposed peptides of the major birch pollen allergen, Bet v 1, for preventive vaccination. Clin Exp Allergy. 2004;34:1525–33. doi: 10.1111/j.1365-2222.2004.02081.x. [DOI] [PubMed] [Google Scholar]
- E5.Linhart B, Bigenzahn S, Hartl A, Lupinek C, Thalhamer J, Valenta R, et al. Costimulation blockade inhibits allergic sensitization but does not affect established allergy in a murine model of grass pollen allergy. J Immunol. 2007;178:3924–31. doi: 10.4049/jimmunol.178.6.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]