Abstract
Background
The LKB1 tumor suppressor gene is commonly inactivated in non-small cell lung carcinomas (NSCLC), a major form of lung cancer. Targeted therapies for LKB1-inactivated lung cancer are currently unavailable. Identification of critical signaling components downstream of LKB1 inactivation has the potential to uncover rational therapeutic targets. Here we investigated the role of INSL4, a member of the insulin/IGF/relaxin superfamily, in LKB1-inactivated NSCLCs.
Methods
INSL4 expression was analyzed using global transcriptome profiling, quantitative reverse transcription PCR, western blotting, enzyme-linked immunosorbent assay, and RNA in situ hybridization in human NSCLC cell lines and tumor specimens. INSL4 gene expression and clinical data from The Cancer Genome Atlas lung adenocarcinomas (n = 515) were analyzed using log-rank and Fisher exact tests. INSL4 functions were studied using short hairpin RNA (shRNA) knockdown, overexpression, transcriptome profiling, cell growth, and survival assays in vitro and in vivo. All statistical tests were two-sided.
Results
INSL4 was identified as a novel downstream target of LKB1 deficiency and its expression was induced through aberrant CRTC-CREB activation. INSL4 was highly induced in LKB1-deficient NSCLC cells (up to 543-fold) and 9 of 41 primary tumors, although undetectable in all normal tissues except the placenta. Lung adenocarcinomas from The Cancer Genome Atlas with high and low INSL4 expression (with the top 10th percentile as cutoff) showed statistically significant differences for advanced tumor stage (P < .001), lymph node metastasis (P = .001), and tumor size (P = .01). The INSL4-high group showed worse survival than the INSL4-low group (P < .001). Sustained INSL4 expression was required for the growth and viability of LKB1-inactivated NSCLC cells in vitro and in a mouse xenograft model (n = 5 mice per group). Expression profiling revealed INSL4 as a critical regulator of cell cycle, growth, and survival.
Conclusions
LKB1 deficiency induces an autocrine INSL4 signaling that critically supports the growth and survival of lung cancer cells. Therefore, aberrant INSL4 signaling is a promising therapeutic target for LKB1-deficient lung cancers.
Lung cancer is the leading cause of cancer mortality globally (1,2). Non-small cell lung carcinomas (NSCLC) represent approximately 85% of all lung cancer cases with lung adenocarcinoma as the major histological subtype. Targeted therapeutics that specifically block cancer gene drivers show improved efficacy and reduced toxicity over the traditional cytotoxic therapeutics (3–5). However, targeted therapies are currently available only for a small population of lung cancer patients carrying specific genetic alterations including EGFR mutations or ALK rearrangements. Mechanism-based therapies for many other genetic abnormalities are needed to improve the survival of NSCLC patients.
The LKB1 (STK11) tumor suppressor gene is frequently altered in NSCLCs. LKB1 serine/threonine kinase is a master regulator of the AMPK family kinases influencing a variety of cellular processes such as cell polarity, proliferation, and metabolism (6,7). Peutz-Jeghers syndrome caused by germline LKB1 mutations is associated with an enhanced cancer risk (8,9). LKB1 somatic alterations have been observed in multiple human cancers, most commonly in NSCLCs (10–12) and cervical cancers (13). Approximately 15%–30% of NSCLC cases have aberrant LKB1 function, which ranks as the third most frequently altered gene after TP53 and KRAS. LKB1 functional loss can result from its intragenic mutations, deletions, epigenetic silencing, or altered expression/activity of the components within the LKB1/AMPK pathway (14–17). LKB1 deficiency confers lung cancer with a distinct biology, promotes cancer metastasis, and modulates tumor microenvironment and drug responses (18–20); therefore, LKB1-inactivated tumors will need specific managements. However, no targeted therapies are currently available for LKB1-inactivated lung cancers, although several potential therapeutic strategies have been proposed (18,20–22). Clinical advancement requires comprehensive molecular studies of the critical mediators for LKB1 loss in tumorigenesis, which will uncover effective biomarkers and therapeutic targets for development.
In this study, we performed global transcriptome profiling analysis to understand the critical signaling events downstream of LKB1 inactivation. We then focused on one potential downstream LKB1 target, the insulinlike 4 (INSL4) gene. INSL4 is a member of the insulin/IGF/relaxin superfamily (23,24) that is restrictively expressed in the placenta (25). INSL4 upregulation was previously identified in a breast cancer cell subclone with increased invasiveness through in vitro selection (26). Conditioned medium with secreted INSL4 enhanced the transendothelial invasiveness of breast cancer cells in vitro (27). However, the mechanisms underlying INSL4 upregulation and its functional relevance in human cancers remain poorly characterized. Therefore, we subsequently carried out expression, functional, and mechanistic studies to investigate the aberrant INSL4 signaling in LKB1-inactivated lung cancers as well as its potential clinical and translational significance.
Methods
Cell Culture and Assays
The culture conditions for human bronchial epithelial cell line BEAS-2B and 15 human NSCLC cancer cell lines, the detailed procedures for viral transduction, quantitative reverse transcription PCR (qRT-PCR), western blotting, enzyme-linked immunosorbent assay (ELISA), chromatin immunoprecipitation assay, reporter assay, cell proliferation, apoptosis, and cell cycle assays as well as plasmid and chemical information were described in the Supplementary Methods (available online). The primer sequences were provided in Supplementary Table 1 (available online).
RNA In Situ Hybridization (RNA ISH)
RNA ISH was carried out on formalin-fixed paraffin-embedded (FFPE) xenografts and human lung adenocarcinoma microarrays (#BCS04017a, US Biomax, Inc, Rockville, MD) using an RNAscope 2.5 HD Reagent Kit (Advanced Cell Diagnostics [ACD], Newark, CA) and custom Hs-INSL4, Hs-LINC00473, and Hs-PPIB probes.
Microarray Analyses
RNAs were isolated from A549 cells transduced with lentiviruses expressing INSL4-shRNA or scrambled shRNA control in two biological replicates. Microarray experiments were conducted using GeneChip Human Transcriptome Array 2.0 (Affymetrix) and the data (GSE107246) were deposited in the Gene Expression Omnibus repository. The Ingenuity Pathway Analysis was performed as previously described (28).
Xenograft Tumor Studies
Mouse study was approved by the Institutional Animal Care and Use Committee at the University of Florida. Luciferase-expressing cells transduced with scrambled shRNA control or INSL4-shRNA lentiviruses were subcutaneously injected in 12-week-old NOD/SCID mice (Jackson Laboratory, Bar Harbor, ME). Five mice per group were used; control and treatment groups were matched by sex and age. The measurement of tumor growth and tumor immunohistochemistry and TUNEL assays are described in Supplementary Methods (available online).
Analysis of Gene Expression and Clinical Features
Lung adenocarcinoma (LUAD) RNAseq data (version: 2016-08-16, 515 primary tumor samples) and clinical information (version: 2016-04-27) from The Cancer Genome Atlas (TCGA) were used for the analysis. This RNAseq dataset included the gene-level transcription estimates, as in normalized RSEM (RNA-Seq by the expectation-maximization method) counts. The clinical data matrix included clinicopathological features such as overall survival and tumor stage, as well as cancer gene mutational data. Only 503 patient samples had both the RNAseq data and overall survival information, and 11 patients were removed as they had overall survival of less than one month. Thus, a total of 492 patients were used for the analysis for the potential association of INSL4 expression and tumor stages and survival. Patients were divided into INSL4-high and -low groups using the top 10th or top 25th percentile of INSL4 expression as cutoffs.
Statistical Analyses
Statistical analyses of experimental data were carried out using Prism Graphpad 6.0 or R package. The two-sided t test was used to analyze data from qRT-PCR, cell proliferation, apoptosis, and xenograft assays and data were presented as mean and standard deviation (±SD). The Fisher exact test was used to determine the statistical significance of association between gene expression and clinical features. The log-rank test was used to determine the P value in the survival difference between INSL4-high and -low groups in Kaplan-Meier survival analysis. All statistical tests were two-sided and P values were considered statistically significant when not greater than .05.
Results
INSL4 Expression in LKB1-Inactivated and -Intact NSCLC Cells
A global transcriptome profiling of LKB1-null NSCLC A549 cells transduced with retroviruses containing vector control (Ctl), wild-type LKB1 (LKB1-wt), or a kinase-dead LKB1 mutant (LKB1-K78I) was performed to detect LKB1-regulated genes (29). Using the criteria of fold change (≥|2.0|) and P value (≤.05), we detected 197 downregulated and 47 upregulated protein-coding genes after reintroduction of LKB1-wt (Figure 1A;Supplementary Table 2, available online). We also detected 41 downregulated and 35 upregulated genes after LKB1-K78I expression (Figure 1B;Supplementary Table 3, available online). Moreover, there were 588 upregulated and 1406 downregulated genes in LKB1-null NSCLC lines (A549, H460) in comparison with LKB1-wt lines (H322, H3123) (Figure 1C;Supplementary Table 4, available online). By combining these data, eight genes showed repressed expression by LKB1-wt, but not LKB1-K78I mutant, and displayed elevated levels in LKB1-null NSCLC cells (Figure 1D;Supplementary Figure 1, available online). Within this gene list, CPS1 is a known LKB1 target (29–31) and the remaining genes are novel. One of the novel genes, INSL4, exhibited the greatest increase in expression (543-fold) in LKB1-null vs LKB1-expressing cell lines.
Figure 1.
INSL4 expression in LKB1-inactivated and -intact non-small cell lung carcinoma (NSCLC) cells. A, B) Differentially expressed genes in LKB1-null NSCLC A549 cells after expression of wild-type LKB1 (LKB1-wt) (A) or a kinase-dead K78I mutant (LKB1-K78I) (B) are shown in volcano plots. C) Differentially expressed genes in two LKB1-null cell lines (A549 and H460) vs two LKB1-wt cell lines (H322 and H3123) are shown. D) A list of genes was identified with downregulated expression by exogenous LKB1-wt, but not the LKB1-K78I mutant, and elevated expression in LKB1-null vs LKB1-wt NSCLC cells. E) Western blotting detected LKB1 expression status in immortalized lung epithelial cell line BEAS-2B, LKB1-null, and LKB1-wt human NSCLC lines. F) INSL4 expression in LKB1-mutant group as compared with BEAS-2B and LKB1-wt NSCLCs by qRT-PCR. G) INSL4 expression levels in LKB1-mutant NSCLC cell lines in comparison with LKB1-wt cell lines from the Cancer Cell Line Encyclopedia database. The two-sided t test was used to calculate the P values. Ctl = control.
INSL4 belongs to the insulin/IGF/relaxin superfamily (23,24) and showed placenta-specific expression with undetectable levels in 26 adult tissues examined (32) (Supplementary Figure 2A, available online). We then assessed INSL4 expression in a panel of NSCLC cell lines and an immortalized normal human lung epithelial cell line (BEAS-2B) by qRT-PCR assays. LKB1 protein expression status of these cell lines was confirmed by western blotting (Figure 1E). We observed extremely low INSL4 expression in BEAS-2B cells and all seven LKB1-wt NSCLC lines, but highly elevated levels in five out of seven LKB1-null NSCLC lines (A427, A549, H460, H2122, and H2126) (Figure 1F). Two LKB1-null lines (H23 and H157) expressed undetectable INSL4, which likely resulted from epigenetic silencing, as the treatment of the DNA methyltransferase inhibitor (5-Aza-2’-deoxycytidine) and/or histone deacetylase inhibitor (TSA) increased INSL4 expression (Supplementary Figure 3, available online). However, secondary effects with these compounds cannot be excluded. To evaluate the extent of INSL4 expression and its potential correlation with LKB1 mutation status, we examined the Cancer Cell Line Encyclopedia cell lines with available expression data and mutational data (Supplementary Table 5, available online) and found statistically significant INSL4 upregulation in LKB1-mutated NSCLC lines (n = 32) as compared with LKB1-wt lines (n = 59) (P < .001) (Figure 1G). Therefore, INSL4 is an LKB1-regulated gene and has elevated expression in the majority of LKB1-inactivated NSCLC cell lines. Epigenetic silencing may contribute to the failure of INSL4 induction in a small subset of LKB1-null cell lines.
Analysis of INSL4 Expression, Clinical Features, and Cancer Gene Mutations in the TCGA Lung Adenocarcinomas
To gain insights into the functional relevance of INSL4 in human cancers, we surveyed its expression in public datasets. INSL4 displayed outlier expression mainly in a subset of NSCLCs and cervical cancer, two cancer types reported to have high LKB1 alterations, in cross-cancer expression analyses from TCGA (https://cancergenome.nih.gov/) and the Gene Expression across Normal and Tumor tissue databases (33) (Figure 2A;Supplementary Figure 2B, available online). We further examined potential differences between INSL4-high and -low tumors for the clinical features using the TCGA lung adenocarcinoma (TCGA-LUAD) dataset (12). INSL4 expression was statistically significantly higher in lung tumor samples than in normal tissues (P = .008) (Figure 2B). When those patients with both INSL4 expression and survival data available (n = 492) were separated at the upper 10th percentile or the upper quantile into INSL4-high (red) and INSL4-low (blue) groups, INSL4-high and -low groups showed a statistically significantly difference in patient survival by log-rank test (P < .001 and .009 for the respective 10th and 25th percentile cutoffs) (Figure 2C;Supplementary Figure 4A, available online). Analysis of a different lung adenocarcinoma patient cohort (n = 720) (34) also showed that patients with high INSL4 expression (upper quartile) had worse overall survival and shorter time to first progression (Supplementary Figure 4, B and C, available online). The INSL4-high and -low group, separated at the top 10th percentile of INSL4 expression, showed statistically significant differences for advanced tumor stage (P < .001), lymph node metastasis (P = .001), and tumor size (T2-4) (P = .01), but not distant metastasis based on Fisher exact test analysis (Table 1). Furthermore, these two groups showed a statistically significant difference in LKB1 mutations (P = .01), but not other cancer genes such as KRAS, EGFR, BRAF, MET, PIK3CA (Table 2). Also, there was a negative correlation of INSL4 and LKB1 messenger RNA levels (Pearson r = −0.46 and P < .001).
Figure 2.
Analysis of INSL4 expression and its associations with patient survival in non-small cell lung carcinomas (NSCLCs). A) Pan-cancer analysis of INSL4 expression in the The Cancer Genome Atlas (TCGA) dataset based on the RNA-seq studies from cBioPortal. B) INSL4 expression levels in matched normal tissues (N) (n = 58) and tumors (T) (n = 58) and unpaired tumor (UT) (n = 457) in TCGA-LUAD samples. The y-axis represented normalized RSEM read counts. Note that samples with normalized RSEM value less than 1 were not shown in the graph. C) Survival analysis of the TCGA-LUAD data for the association of INSL4 expression and patient survival. The top 10th percentile was defined as the INSL4-high group and the remaining as INSL4-low groups based on the expression values. The two survival curves were compared statistically using the log-rank test. D) RNA in situ hybridization (RNA ISH) analysis of INSL4 and LINC00473 transcripts in LKB1-null A549 xenograft sections. Housekeeping gene PPIB signal indicated good sample RNA quality. E) INSL4 and INC00473 RNA ISH staining in a representative lung adenocarcinoma case. Scale bars: 400 µm (left panels), 20 µm (right panels). ACC = adrenocortical carcinoma; AML = acute myeloid leukemia; Ca = carcinoma; DLBC = diffuse large B-cell lymphoma; HNCC = head & neck carcinoma; LUAD = lung adenocarcinoma; LSCC = lung squamous cell carcinoma; NSCLC = non-small cell lung carcinoma; PCPG = pheochromocytoma and paraganglioma; uterine CS = uterine carcinosarcoma; ccRCC = clear cell renal cell carcinoma; chRCC = chromophobe renal cell carcinoma; pRCC = papillary renal cell carcinoma.
Table 1.
Analysis of INSL4 expression and clinical features in TCGA lung adenocarcinomas
| Characteristics | Total cases* | INSL4-low cases† | INSL4-high cases‡ | P § |
|---|---|---|---|---|
| Clinical stage | ||||
| Early stage (≤I) | 264 | 250 | 14 | <.001 |
| Advanced stage (>I) | 220 | 186 | 34 | |
| Early stage (≤IIa) | 313 | 295 | 18 | <.001 |
| Advanced stage (>IIa) | 171 | 141 | 30 | |
| Early stage (≤IIB) | 380 | 352 | 28 | .001 |
| Advanced stage (>IIB) | 104 | 84 | 20 | |
| Distant metastases | ||||
| M0 | 326 | 290 | 36 | .50 |
| M1 | 24 | 20 | 4 | |
| Lymph node metastasis | ||||
| N0 | 318 | 298 | 20 | .001 |
| N1–3 | 163 | 136 | 27 | |
| Tumor size | ||||
| T1 | 164 | 156 | 8 | .01 |
| T2–4 | 325 | 286 | 39 |
The total number of lung cancer cases with available clinical data were used.
The number of lung cancer cases with INSL4 expression within the bottom 90th percentile was defined as INSL4-low group.
The number of lung cancer cases with INSL4 expression within the top 10th percentile was defined as INSL4-high group.
P values were calculated by two-sided Fisher exact test. The italic values indicated P values less than .05.
Table 2.
Analysis of the association of INSL4 expression and cancer gene mutations in TCGA lung adenocarcinomas
| Gene | Mutation | Total cases* | INSL4-low cases† | INSL4-high cases‡ | P§ |
|---|---|---|---|---|---|
| LKB1 | No | 180 | 168 | 12 | .01 |
| Yes | 36 | 28 | 8 | ||
| EGFR | No | 186 | 166 | 20 | .08 |
| Yes | 30 | 30 | 0 | ||
| BRAF | No | 197 | 177 | 20 | .23 |
| Yes | 19 | 19 | 0 | ||
| KRAS | No | 153 | 141 | 12 | .30 |
| Yes | 63 | 55 | 8 | ||
| PIK3CA | No | 202 | 182 | 20 | .37 |
| Yes | 14 | 14 | 0 | ||
| CTNNB1 | No | 207 | 188 | 19 | .59 |
| Yes | 9 | 8 | 1 | ||
| ERBB4 | No | 196 | 177 | 19 | .70 |
| Yes | 20 | 19 | 1 | ||
| MET | No | 201 | 182 | 19 | 1.00 |
| Yes | 14 | 13 | 1 | ||
| AKT1 | No | 214 | 194 | 20 | 1.00 |
| Yes | 2 | 2 | 0 | ||
| MAP2K1 | No | 213 | 193 | 20 | 1.00 |
| Yes | 3 | 3 | 0 | ||
| NRAS | No | 215 | 195 | 20 | 1.00 |
| Yes | 1 | 1 | 0 | ||
| HRAS | No | 215 | 195 | 20 | 1.00 |
| Yes | 1 | 1 | 0 | ||
| ERBB2 | No | 210 | 190 | 20 | 1.00 |
| Yes | 6 | 6 | 0 |
The total number of lung cancer cases with available mutational data were used.
The number of lung cancers with INSL4 expression within the bottom 90th percentile was defined as INSL4-low group.
The number of lung cancer cases with INSL4 expression within the top 10th percentile was defined as INSL4-high group.
P values were calculated by two-sided Fisher exact test.
As several commercial INSL4 antibodies were not specific in our immunohistochemistry and western blotting experiments, we detected INSL4 RNA transcripts in tumor specimens using RNAscope RNA ISH. The lncRNA-LINC00473 ISH was simultaneously performed on serial sections to assess the potential correlation of INSL4 expression and LKB1 inactivation, as LINC00473 expression was the most consistently upregulated in LKB1-inactivated lung cancers (29). RNA ISH for the housekeeping gene PPIB was performed for assessing sample RNA quality. We first observed diffused cytoplasmic signals for INSL4 RNA and nuclear dot signals for LINC00473 in FFPE-A549 xenograft tumor sections (Figure 2D) but no signals in FFPE-H322 cell pellet sections (Supplementary Figure 5A, available online). Using serial sections of a tumor microarray, which contained 48 cases of human lung adenocarcinoma with duplicate cores per case, we found 41 PPIB-positive lung adenocarcinoma cases. A total of 9 out of 41 PPIB-positive cases (22.0%) showed coexpression of INSL4 and LINC00473 with the respective diffused cytoplasmic and nuclear dotted straining, whereas the remaining cases were negative for both INSL4 and LINC00473. Representative images are shown in Figure 2E and Supplementary Figure 5, B–D (available online). These data support that INSL4 expression is likely associated with LKB1 inactivation in lung adenocarcinoma and that it is a potential regulator of lung cancer progression.
Analysis of Upstream LKB1-CRTC-CREB Signaling in Regulating INSL4 Expression
LKB1 loss renders salt-inducible kinases inactive and increases dephosphorylated cAMP-regulated transcriptional coactivators (CRTCs) that subsequently enter the nucleus and activate the cAMP-responsive element-binding protein (CREB) transcriptional program (35,36). Because the INSL4 gene promoter contains two putative half-cAMP-responsive elements (CRE), LKB1 may regulate INSL4 expression through the CRTC-CREB pathway. To test this hypothesis, we first examined the effects of LKB1 overexpression and depletion on INSL4 expression. Expression of LKB1-wt statistically significantly repressed INSL4 expression in LKB1-null A549 cells (Vector = 1.01 [0.14]; LKB1-wt = 0.36 [0.08]; P = .03), whereas LKB1-K78I had no statistically significant effect (Figure 3A). Conversely, LKB1 depletion via two shRNAs (shLKB1-2 and -5) in LKB1-wt H322 cells statistically significantly enhanced INSL4 expression (shCtl = 1.00 [0.11]; shLKB1-2 = 5.44 [0.36]; shLKB1-5 = 4.31 [1.01]; P = .004 for shCtl vs shLKB1-2; P = .04 for shCtl vs shLKB1-5) (Figure 3B). Therefore, the LKB1 level modulates INSL4 expression in NSCLC cells. Next, we depleted CREB expression or expressed a dominant-negative CRTC (dnCRTC) that blocked CRTC-CREB binding and found that both approaches decreased INSL4 expression (shCtl = 1.00 [0.04]; shCREB-B9 = 0.12 [0.03]; shCREB-G9 = 0.45 [0.01]; P = .002 for shCtl vs shCREB-B9 and P = .003 for shCtl vs shCREB-G9) (GFP = 1.000 [0.003]; dnCRTC = 0.017 [0.002]; P < .001 for GFP vs dnCRTC) (Figure 3, C and D). We further observed that the activity of an INSL4 promoter-driven luciferase reporter (−758 to +81 containing 2 half-CRE) was statistically significantly repressed by LKB1-wt (Vector = 1.00 [0.14]; LKB1-wt = 0.22 [0.03]; P = .02) or the dominant-negative mutant A-CREB that interferes with CREB’s DNA binding (Vector = 1.00 [0.16]; A-CREB = 0.42 [0.03]; P = .04) (Figure 3, E–G). Moreover, CRTC1, CRTC2, and CREB were statistically significantly enriched in the proximal INSL4 promoter region by chromatin immunoprecipitation (IgG = 1.01 [0.18]; CRTC1 = 11.62 [0.46]; CRTC2 = 15.37 [1.18]; CREB = 13.88 [0.08]; P < .001 for both IgG vs CRTC1 or CREB and P = .003 for IgG vs CRTC) (P < .001) (Figure 3H). Taken together, INSL4 expression is induced by CRTC-CREB transcriptional activation in NSCLC cells with LKB1 deficiency.
Figure 3.
Analysis of LKB1-CRTC-CREB signaling axis in regulating INSL4 expression. A) INSL4 expression in LKB1-null NSCLC A549 cell line transduced with vector, LKB1-wt, or the LKB1-K78I mutant by qRT-PCR (n = 3). LKB1 protein expression was confirmed by western blotting. B) INSL4 expression in LKB1-wt NSCLC cell line H322 transduced with control or LKB1 shRNA lentiviruses qRT-PCR analysis (n = 3). C) INSL4 expression in A549 cells transduced with control or CREB shRNAs by qRT-PCR (n = 3). D) INSL4 expression in A549 cells transduced with control or dominant-negative CRTC retroviruses by qRT-PCR. E) Schematic diagram of an INSL4 promoter-driven luciferase reporter. F) The INSL4 promoter reporter activity in LKB1-null A549 cells expressing LKB1 or the K78I mutant in (n = 3). G) The INSL4 promoter reporter activity in LKB1-null A549 cells expressing A-CREB or vector (n = 3). H) Chromatin immunoprecipitation assay assays for the enrichment of CREB and CRTC1/2 on the INSL4 promoter region in A549 cells (n = 3). Data are mean ± SD. Two-sided t test was used to calculate the P values.
Effects of Modulating INSL4 Expression on NSCLC Cell Growth and Viability In Vitro and In Vivo
To assess the role of INSL4 in lung cancer, we determined the impact of INSL4 knockdown on lung cancer cell behaviors. A549 cells transduced with lentiviral pLKO.1-based INSL4 shRNAs (shINSL4-1 and shINSL4-4) showed reduced INSL4 transcripts and secreted proteins as compared with cells with scrambled shRNA (shCtl) control by qRT-PCR and ELISA assays, respectively (Figure 4, A and B). INSL4-depleted and control cells were then assayed for cell viability and apoptosis. INSL4 knockdown decreased the viable cell number and increased apoptotic cell population (Figure 4, C and D). Moreover, INSL4 depletion altered cell cycle distributions as evidenced by an increased cell population in the G0/G1 phase and a decreased population in the S phase (Figure 4E). Similar effects were also observed in other LKB1-mutant, INSL4-expressing NSCLC cells (H2122, H1568, H1437), but not in LKB1-wt, INSL4-low cells (H522) (Supplementary Figure 6, available online). Moreover, lentiviral-transduced INSL4 expression in LKB1-wt, INSL4-nonexpressing H522 cells caused a moderate increase in cell proliferation (Supplementary Figure 7, available online). These data indicate that INSL4 positively regulates cell growth and viability.
Figure 4.
Effects of knockdown of INSL4 expression on the growth and apoptosis of LKB1-null non-small cell lung carcinoma cells in vitro and in vivo. A, B) Luciferase-expressing A549 cells were infected with lentiviruses containing INSL4 shRNAs (shINSL4) or the scrambled shRNA control (shCtl), respectively. At 96 h posttransduction, INSL4 transcript levels were quantified by qRT–PCR (A) and secreted INSL4 levels in culture media were measured by enzyme-linked immunosorbent assay (n = 3). C–E) Transduced cells at 96 h post-transduction were also seeded at 2 × 105 per well in six-well plates and cultured for additional 96 h. Viable cells were counted using Trypan blue assay (C), apoptotic cells measured by Annexin V/PI staining (D), and cell cycles determined by PI staining (E) (n = 3). F–L) Luciferase-expressing cells after transduced with shINSL4 or shCtl for 96 h were injected subcutaneously to the dorsal flanks of NOD/SCID mice. Tumor volumes at different days after tumor cell injection was shown (n = 5) (F). Representative bioluminescent images of mice injected with shCtl or shINSL4 A549-luc cells (G) and the weights of excised tumors (H, I) at the endpoints were presented. Immunostaining of Ki-67 (J) and p-Akt (K) and TUNEL staining (L) were performed on the sections from A549-control and A549-shINSL4 xenograft tumors. Two-sided t test was used to calculate the P values.
We next investigated the consequence of INSL4 knockdown on the growth of lung cancer cells in vivo. We transduced luciferase-labeled A549 cells with shINSL4 and shCtl for 72 hours, injected the same number of transduced cells subcutaneously to NOD.SCID mice, and monitored the growth of xenograft tumors. INSL4 depletion inhibited the growth of xenograft tumors, as shown by tumor volumes and weights (Figure 4, F–I). We observed a reduced number of Ki-67-positive cells, reduced p-Akt staining, and an increased number of cells positive for apoptotic DNA fragmentation in INSL4-depleted tumors compared with the control tumors based on immunohistochemical staining and TUNEL assays (Figure 4, J–L). Therefore, the in vitro and xenograft data support that LKB1-deficient lung cancer cells depend on sustained INSL4 expression for the growth and survival.
Analysis of INSL4-Regulated Gene Expression Program
To elucidate how INSL4 promotes cell growth and survival in lung cancer, we examined INSL4-regulated gene expression program through a transcriptome analysis. The INSL4-depleted and control A549 cells were subjected to gene expression profiling using Affymetrix GeneChip Human Transcriptome Array 2.0. Differentially expressed genes were defined using the criteria of fold change (≥|1.5|) and false discovery rate (<0.05) and the heatmap and volcano plots are shown (Figure 5, A and B). We identified 134 downregulated and 21 upregulated genes after INSL4 depletion (Supplementary Table 6, available online). The qRT-PCR assay validated expression changes for a subset of genes important in cell growth and survival regulation (Figure 5C). Ingenuity Pathway Analysis revealed that “cellular growth and proliferation,” “cellular movement,” and “cell death and survival” categories were statistically significantly enriched in the INSL4-regulated candidate gene list (P < .001) (Figure 5D). Also, PI3K and MAPK signaling were identified as upstream regulators of INSL4-regulated genes (Figure 5E), which was further corroborated by the western blotting results showing reduced phosphorylation levels of AKT, Erk1/2, and P70-S6K1 in INSL4-depleted A549 and H2122 cells (Figure 5F). Moreover, exogenous INSL4 overexpression in LKB1-wt, INSL4-low H522 cells showed enhanced p-Akt levels without obvious changes in p-Erk1/2 and p-S6K under serum-free culture conditions (Supplementary Figure 7, available online), likely reflecting cell-context signaling differences. These data therefore demonstrate that INSL4 promotes cell growth and survival through activating PI3k-Akt and/or MAPK signaling.
Figure 5.
Gene expression profiling of INSL4-depleted and control LKB1-null A549 cells. A) A heatmap shows differential gene expression patterns in INSL4-depleted (shINSL4) vs control (shCtl) A549 cells. B) A volcano plot shows differential gene expression between INSL4-depleted and control A549 cells. The green dots correspond to genes with fold change ≤1.5 and false discovery rate (FDR) < 0.05 and the red dots indicate genes with fold change ≥1.5 and FDR < 0.05. C) Validation of several INSL4-regulated genes identified from the expression array analysis. The qRT-PCR assays were performed on the control and two INSL4-depleted A549 cells. D) Functional classification of INSL4-regulated genes was analyzed through the Ingenuity Pathway Analysis tool. E) Gene network analysis. F) Effect of INSL4 depletion on the phosphorylated levels of Akt, Erk1/2, and S6K1 in LKB1-null cell lines, A549 and H2122 by western blotting analysis.
Discussion
Although pharmacological agents that target gain-of-function oncogenic mutations have been developed for cancer treatments, direct targeting of loss-of-function mutations of tumor suppressor genes remains challenging. The LKB1 tumor suppressor gene is frequently altered in NSCLCs. Abundant data indicate that LKB1-mutant cancers have distinct biological features that require specific antitumor strategies. However, there is no targeted therapy available for LKB1-inactivated lung cancer. The elucidation of signaling pathways downstream of aberrant LKB1 inactivation will reveal opportunities for rational therapeutic approaches. In this study, we identified LKB1-regulated genes through gene expression profiling with the goal of uncovering potential biomarkers and rational therapeutic targets for LKB1-inactivated lung cancer. We discovered INSL4 as a novel target downstream of LKB1 deficiency. Our findings support a model in which the loss of function of LKB1 induces CRTC dephosphorylation and subsequent nuclear entry and coactivation of the CREB transcription factor to promote INSL4 expression. INSL4 acts on unknown cognate receptor(s) and activates PI3k/Akt and/or MAPK pathways in an autocrine manner, sustaining cancer cell growth and viability (Figure 6). Therefore, INSL4 signaling is a potential “Achilles heel” for LKB1-inactivated NSCLCs and thus targeting INSL4 signaling presents a novel and effective antitumor strategy.
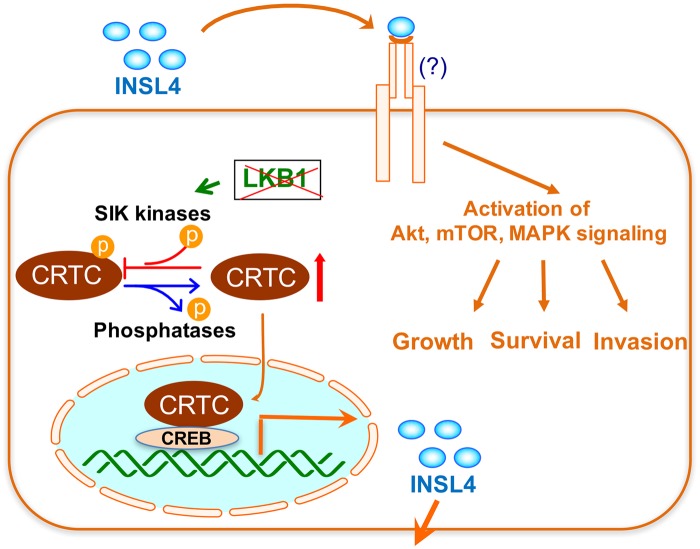
Figure 6.
A model shows that LKB1 deficiency induces an autocrine INSL4 signaling that critically supports lung cancer cell growth and viability. LKB1 inactivation causes CRTC dephosphorylation and nuclear entry, leading to coactivation of the CREB transcription factor in promoting INSL4 transcription. The secreted INSL4 binds to unknown receptor(s) and activates signaling events such as Akt, mTOR, and/or MAPK signaling, which sustain cancer cell growth and survival and likely invasive properties.
INSL4 belongs to the insulin superfamily that is composed of insulin, insulinlike growth factors, and relaxin genes with 44% homology to relaxins and 15% homology to insulin (24,37,38). Intriguingly, INSL4 is the only insulinlike growth factor gene that is specifically expressed in primates. INSL4 was restrictedly expressed in the early placental cytotrophoblasts and syncytiotrophoblasts (25). We showed that INSL4 expression is controlled by a LKB1-CRTC-CREB signaling axis, providing a mechanistic explanation for drastically elevated INSL4 expression in LKB1-null lung cancer. Although high INSL4 expression was found in the majority of LKB1-null NSCLC cells and highly associated with the LKB1 mutations, a minority of LKB1-null NSCLC cells had no INSL4 induction. The treatment with DNA methyltransferase inhibitor and/or histone deacetylase inhibitor enhanced INSL4 expression, suggesting an additional layer of epigenetic regulation. These data were consistent with the finding that the INSL4 gene promoter was hypomethylated in more than 50% of medullary thyroid tumors, which likely caused aberrant INSL4 upregulation (39). Further study is needed to elucidate the transcriptional and epigenetic regulation of INSL4 expression in normal and cancerous cells.
Although INSL4 was cloned more than two decades ago, its biological functions and mechanisms remain poorly understood. INSL4 upregulation was associated with the invasive property of breast cancer cells (26,27). However, the pan-cancer analysis showed general low INSL4 expression in breast cancers, thus the role of INSL4 in human breast cancer was unclear. In this study, we revealed critical pro-growth and pro-survival functions for INSL4 in lung cancer, supported by several lines of evidence: 1) INSL4 positively regulated cell proliferation and viability; 2) changes in INSL4 levels affected PI3K-Akt and/or MAPK signaling; and 3) INSL4 knockdown perturbed expression of genes that critically regulate cell growth and survival. Importantly, high INSL4 expression was correlated with advanced cancer and poor patient survival, strongly implicating its positive regulatory role in lung cancer progression. Intriguingly, INSL4 is a primate-specific gene and is absent in the mouse genome, and it is unclear whether INSL4 contributes to unique attributes of human lung cancers.
The INSL4 gene encodes a 139-aa peptide containing a signal peptide, B-chain, C-peptide and A-chain, which undergo posttranslational cleavages likely resulting in either a two-chain or a single-chain product (26). INSL4 structure and the identity of its cognate receptor(s) are currently unknown. Future studies are needed to define INSL4 structure and to identify its receptor(s) for a comprehensive understanding of INSL4-induced signaling. Because of the dependency of LKB1-inactivated lung cancer cells on INSL4 expression and undetectable INSL4 expression in normal adult tissues, INSL4 signaling represents an attractive and safe therapeutic target. Strategies such as antibodies that interfere with INSL4 and its receptor interaction and agents that reduce INSL4 expression levels or its downstream signaling will have the potential of inducing antitumor responses.
The limitations of this study are the lack of specific INSL4 antibodies and functional INSL4 ligands as well as unknown INSL4 receptors. The development of INSL4 reagents and the identification of INSL4 receptors are needed for comprehensive mechanistic insights into the roles and mechanisms for INSL4-induced signaling in lung cancer development and progression.
Collectively, this study uncovered a novel autocrine INSL4 signaling that is essential for the growth and viability of LKB1-deficient lung cancer cells. Our study has important clinical relevance as INSL4 is a potential indicator for aggressiveness and a promising therapeutic target for lung cancer–carrying LKB1-inactivated alterations.
Funding
This work was supported by the National Institutes of Health (R21CA187730 and R01DE023641 to LW) and UF Health Cancer Center. FJK was supported by the Gatorade Trust Fund through the University of Florida Department of Medicine.
Notes
Affiliations of authors: Department of Molecular Genetics and Microbiology (RY, ZC, XZ, WN, LW), UF Health Cancer Center (RY, SWL, ZC, XZ, JL, FJK, LW), Department of Medicine (SWL, FJK), UF Genetics Institute (WN, LW), Department of Pathology, Immunology and Laboratory Medicine (DAF), and Department of Biochemistry and Molecular Biology, College of Medicine (JL), University of Florida, Gainesville, FL.
The study funder had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
The authors declare that no conflicts of interest exist.
Supplementary Material
References
- 1. Torre LA, Siegel RL, Jemal A.. Lung cancer statistics. Adv Exp Med Biol. 2016;893:1–19. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A.. Cancer statistics. CA Cancer J Clin. 2017;671:7–30. [DOI] [PubMed] [Google Scholar]
- 3. Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;3045676:1497–1500. [DOI] [PubMed] [Google Scholar]
- 4. Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;1413:4275–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pao W, Chmielecki J.. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat Rev Cancer. 2010;1011:760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alessi DR, Sakamoto K, Bayascas JR.. LKB1-dependent signaling pathways. Annu Rev Biochem. 2006;75:137–163. [DOI] [PubMed] [Google Scholar]
- 7. Shackelford DB, Shaw RJ.. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;98:563–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giardiello FM, Welsh SB, Hamilton SR, et al. Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987;31624:1511–1514. [DOI] [PubMed] [Google Scholar]
- 9. Hemminki A, Markie D, Tomlinson I, et al. A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998;3916663:184–187. [DOI] [PubMed] [Google Scholar]
- 10. Matsumoto S, Iwakawa R, Takahashi K, et al. Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene. 2007;2640:5911–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ding L, Getz G, Wheeler DA, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;4557216:1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Collisson EA, Campbell JD, Brooks AN, et al. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;5117511:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wingo SN, Gallardo TD, Akbay EA, et al. Somatic LKB1 mutations promote cervical cancer progression. PLoS One. 2009;44:e5137.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boudeau J, Baas AF, Deak M, et al. MO25alpha/beta interact with STRADalpha/beta enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;2219:5102–5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esteller M, Avizienyte E, Corn PG, et al. Epigenetic inactivation of LKB1 in primary tumors associated with the Peutz-Jeghers syndrome. Oncogene. 2000;191:164–168. [DOI] [PubMed] [Google Scholar]
- 16. Imielinski M, Berger AH, Hammerman PS, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;1506:1107–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng B, Jeong JH, Asara JM, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;332:237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shackelford DB, Abt E, Gerken L, et al. LKB1 inactivation dictates therapeutic response of non-small cell lung cancer to the metabolism drug phenformin. Cancer Cell. 2013;232:143–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carretero J, Shimamura T, Rikova K, et al. Integrative genomic and proteomic analyses identify targets for Lkb1-deficient metastatic lung tumors. Cancer Cell. 2010;176:547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Z, Cheng K, Walton Z, et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature. 2012;4837391:613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu Y, Marks K, Cowley GS, et al. Metabolic and functional genomic studies identify deoxythymidylate kinase as a target in LKB1-mutant lung cancer. Cancer Discov. 2013;38:870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cao C, Gao R, Zhang M, et al. Role of LKB1-CRTC1 on glycosylated COX-2 and response to COX-2 inhibition in lung cancer. J Natl Cancer Inst. 2015;1071:358.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lu C, Lam HN, Menon RK.. New members of the insulin family: regulators of metabolism, growth and now … reproduction. Pediatr Res. 2005;57(5 Pt 2):70R–73R. [DOI] [PubMed] [Google Scholar]
- 24. Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. 2012;123:159–169. [DOI] [PubMed] [Google Scholar]
- 25. Laurent A, Rouillac C, Delezoide AL, et al. Insulin-like 4 (INSL4) gene expression in human embryonic and trophoblastic tissues. Mol Reprod Dev. 1998;512:123–129. [DOI] [PubMed] [Google Scholar]
- 26. Brandt B, Roetger A, Bidart JM, et al. Early placenta insulin-like growth factor (pro-EPIL) is overexpressed and secreted by c-erbB-2-positive cells with high invasion potential. Cancer Res. 2002;624:1020–1024. [PubMed] [Google Scholar]
- 27. Brandt B, Kemming D, Packeisen J, et al. Expression of early placenta insulin-like growth factor in breast cancer cells provides an autocrine loop that predominantly enhances invasiveness and motility. Endocr Relat Cancer. 2005;124:823–837. [DOI] [PubMed] [Google Scholar]
- 28. Chen J, Li JL, Chen Z, et al. Gene expression profiling analysis of CRTC1-MAML2 fusion oncogene-induced transcriptional program in human mucoepidermoid carcinoma cells. BMC Cancer. 2015;151:803.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Z, Li JL, Lin S, et al. cAMP/CREB-regulated LINC00473 marks LKB1-inactivated lung cancer and mediates tumor growth. J Clin Invest. 2016;1266:2267–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim J, Hu Z, Cai L, et al. CPS1 maintains pyrimidine pools and DNA synthesis in KRAS/LKB1-mutant lung cancer cells. Nature. 2017;5467656:168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Celiktas M, Tanaka I, Tripathi SC, et al. Role of CPS1 in cell growth, metabolism and prognosis in LKB1-inactivated lung adenocarcinoma. J Natl Cancer Inst. 2017;1093:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fagerberg L, Hallstrom BM, Oksvold P, et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2014;132:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shin G, Kang TW, Yang S, et al. GENT: Gene expression database of normal and tumor tissues. Cancer Inform. 2011;10:149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Győrffy B, Surowiak P, Budczies J, et al. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013;812:e82241.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gu Y, Lin S, Li JL, et al. Altered LKB1/CREB-regulated transcription co-activator (CRTC) signaling axis promotes esophageal cancer cell migration and invasion. Oncogene. 2012;314:469–479. [DOI] [PubMed] [Google Scholar]
- 36. Komiya T, Coxon A, Park Y, et al. Enhanced activity of the CREB co-activator Crtc1 in LKB1 null lung cancer. Oncogene. 2010;2911:1672–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chassin D, Laurent A, Janneau JL, et al. Cloning of a new member of the insulin gene superfamily (INSL4) expressed in human placenta. Genomics. 1995;292:465–470. [DOI] [PubMed] [Google Scholar]
- 38. Bieche I, Laurent A, Laurendeau I, et al. Placenta-specific INSL4 expression is mediated by a human endogenous retrovirus element. Biol Reprod. 2003;684:1422–1429. [DOI] [PubMed] [Google Scholar]
- 39. Rodríguez-Rodero S, Fernández AF, Fernández-Morera JL, et al. DNA methylation signatures identify biologically distinct thyroid cancer subtypes. J Clin Endocrinol Metab. 2013;987:2811–2821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.