Abstract
Background
Patients undergoing treatment for cancer are at increased risk of acute kidney injury (AKI). There are few data on AKI incidence and risk factors in the current era of cancer treatment.
Methods
We conducted a population-based study of all patients initiating systemic therapy (chemotherapy or targeted agents) for a new cancer diagnosis in Ontario, Canada (2007–2014). The primary outcome was hospitalization with AKI or acute dialysis. We estimated the cumulative incidence of AKI and fitted Fine and Gray models, adjusting for demographics, cancer characteristics, comorbidities, and coprescriptions. We modeled exposure to systemic therapy (the 90-day period following treatments) as a time-varying covariate. We also assessed temporal trends in annual AKI incidence.
Results
We identified 163 071 patients initiating systemic therapy of whom 10 880 experienced AKI. The rate of AKI was 27 per 1000 person-years, with overall cumulative incidence of 9.3% (95% CI = 9.1% to 9.6%). Malignancies with the highest 5-year AKI incidence were myeloma (26.0%, 95% CI = 24.4% to 27.7%), bladder (19.0%, 95% CI = 17.6% to 20.5%), and leukemia (15.4%, 95% CI = 14.3% to 16.5%). Advanced cancer stage, chronic kidney disease, and diabetes were associated with increased risk of AKI (adjusted hazard ratios [aHR] = 1.41, 95% CI = 1.28 to 1.54; 1.80, 95% CI = 1.67 to 1.93; and 1.43, 95% CI = 1.37 to 1.50, respectively). In patients aged 66 years or older with universal drug benefits, diuretic, and angiotensin-converting enzyme inhibitor/angiotensin receptor blocker coprescription was associated with higher AKI risk (aHR = 1.20, 95% CI = 1.14 to 1.28; 1.30, 95% CI = 1.23 to 1.38). AKI risk was further accentuated during the 90-day period following systemic therapy (aHR = 2.34, 95% CI = 2.24 to 2.45). The annual incidence of AKI increased from 18 to 52 per 1000 person-years between 2007 and 2014.
Conclusion
Cancer-related AKI is common and associated with advanced stage, chronic kidney disease, diabetes, and concomitant receipt of diuretics or angiotensin-converting enzyme inhibitors/angiotensin receptor blockers. Risk is heightened in the 90 days after systemic therapy. Preventive strategies are needed to address the increasing burden of AKI in this population.
Cancer patients receiving treatment are known to have elevated risk for acute kidney injury (AKI) (1–4). Chemotherapy-associated nephrotoxicity, hypercalcemia, tumor lysis syndrome, paraneoplastic glomerulonephritis, and obstructive nephropathy are among the multiple causes of AKI inherent to patients with cancer (5–7). These patients are also subject to increased risks of noncancer-specific causes of AKI, such as volume depletion, nephrotoxic medications (eg, nonsteroidal anti-inflammatory drugs, diuretics, renin-angiotensin system blockade) and contrast-induced nephropathy. AKI is of particular concern in this population, as a reduction in kidney function may delay or even preclude appropriate cancer therapies. Single-center studies have estimated that, short-term (ie, <6 months) mortality ranges from 51% to 87% after an episode of severe AKI for which dialysis was administered (AKI-D) (8–12).
Despite myriad risks to kidney function and its important prognostic implications, there is a paucity of data characterizing the burden of AKI in cancer patients, particularly in the contemporary era of cancer treatment. A 2006 study estimated that 12% of admissions to a comprehensive cancer center were complicated by AKI (13). A Danish study using data from 1999 to 2006 estimated that the 1-year incidence of more severe forms of AKI (eg, RIFLE criteria categories of “injury” or “failure,” which are more likely to require hospitalization or dialysis) (14) was 13% (15).
However, considerable advances in the treatment of many cancer types have been made in the last decade, including in multiple myeloma (16,17) and kidney cancers (including kidney-preserving approaches, such as partial nephrectomy) (18,19). Also, targeted and immunotherapies have changed both outcomes in many cancers, as well as the potential adverse kidney sequelae (20–23). As such, a reassessment of AKI incidence across various cancer types in the current era of cancer treatment is warranted.
We conducted a population-based cohort study of patients undergoing systemic treatment for cancer in Ontario, Canada. Our objectives were to assess the incidence of clinically relevant AKI (including hospitalizations for AKI or receipt of dialysis) and to identify patient-level risk factors. We also evaluated temporal trends in AKI and AKI-D incidence in this high-risk population.
Methods
Study Design and Setting
We designed a population-based study of all adult patients initiating systemic therapy for an incident cancer diagnosis in Ontario, Canada between April 1, 2007 and March 31, 2014. Ontario is Canada’s most populous province with 13 million residents who receive single-payer publically funded healthcare under the Ontario Health Insurance Plan.
This study was conducted using data from the Institute of Clinical Evaluative Sciences with a prespecified protocol, and was approved by the Research Ethics Board at Sunnybrook Health Sciences Centre (Supplementary Table 1, available online).
Population and Data Sources
We used Ontario-wide administrative datasets to identify patients, determine baseline characteristics, and ascertain outcomes. These datasets were linked using unique encoded identifiers and analyzed at the Institute of Clinical Evaluative Sciences. We included all adult patients (>18 years of age) who initiated systemic therapy for an incident cancer diagnosis during the study period. We did not impose restriction on the time from cancer diagnosis to initiation of systemic treatment and therefore allowed for the inclusion of individuals who may have received (their first) systemic therapy due to disease progression or recurrence. We excluded patients with more than one cancer diagnosis in the five years before starting therapy because we could not definitively ascribe their therapy to a specific cancer diagnosis. We also excluded patients with a history of end-stage renal disease (ESRD), defined as receipt of dialysis in the one year before to the start of systemic cancer therapy or kidney transplant (after 1981).
Patients with cancer were identified using the Ontario Cancer Registry. This registry contains data on all incident cancers in Ontario (except nonmelanoma skin cancers) since 1964, and has been estimated to be more than 95% complete (24). Cancer diagnoses were coded according to International Classification of Diseases for Oncology, Third Edition (ICD-O-3) codes. Classification of 29 cancer diagnoses was done as per the ICD-O-3 definitions used by the 2016 Ontario Cancer Statistics report (Supplementary Table 2, available online) (25). The Registered Persons Database was used to obtain vital status, age, sex, and other demographic information.
The initiation of systemic treatment was determined using evidence from one or more of four administrative data sources that record the receipt of cancer therapies (Supplementary Methods, available online). The earliest date of any entry within these four datasets was used to identify the start of systemic treatment and this served as the index date for the time-to-event analyses. Data from these sources was also used to determine the receipt of subsequent courses of systemic therapy during the follow-up period.
Comorbidities and receipt of hematopoietic stem cell transplant (HSCT) were ascertained using the Canadian Institute for Health Information Discharge Abstract Database (CIHI DAD) using International Classification of Diseases, Tenth Revision (ICD-10) codes as well as physician billing codes under the Ontario Health Insurance Plan in the three years before systemic therapy initiation (26,27).
Outcomes
The primary outcome was defined as time to first hospitalization with AKI or receipt of acute dialysis. We identified hospitalization with AKI using the ICD-10 “N17” within the CIHI DAD. This diagnostic code for AKI has a positive predictive value of more than 90% and has been shown to reflect more severe AKI, with an associated median (interquartile range [IQR]) serum creatinine increase of 1.11 (0.49 to 2.26) mg/dL from baseline (28,29). Acute dialysis was ascertained from dialysis billing claims (Supplementary Table 3, available online) (30). Dialysis codes, which are associated with physician reimbursement and are less likely to be inaccurate, have been used in previous studies of AKI incidence (30,31). A secondary outcome restricted to AKI-D was assessed on the basis of these codes as well.
Statistical Analyses
We calculated the 1- and 5-year cumulative incidences of AKI and AKI-D for all cancers, as well as individual cancer types, and reported events per 1000 patient-years. We used multivariable Fine and Gray models for the risk of AKI and AKI-D. Model covariates included age, sex, cancer type (breast cancer as the referent because it was the most common malignancy in our cohort), cancer stage (stage I as the referent), year of systemic therapy start, and the presence of one or more comorbid conditions (including myocardial infarction, coronary artery disease, heart failure, hypertension, diabetes, cancer, chronic liver disease, peripheral vascular disease, cerebrovascular disease, dementia, chronic kidney disease, chronic lung disease, gastrointestinal bleeding, and HIV). We accounted for the competing risks of death and ESRD by estimating subdistribution hazard ratios as per the method of Fine and Gray (32). We considered a two-sided P value less than .05 as statistically significant. We performed all analyses using SAS version 9.4 (SAS Institute, Cary, NC).
Secondary Analyses
We conducted secondary analyses to further characterize AKI risk in this population, including: 1) the effect of recent systemic therapy (ie, AKI risk in the 90-day period after systemic treatments); 2) the effect of coprescribed medications; 3) temporal trends in AKI incidence; and 4) AKI risk associated with specific therapies in high-risk cancers. Descriptions of these analyses are included in the Supplementary Methods (available online).
Results
Baseline Characteristics
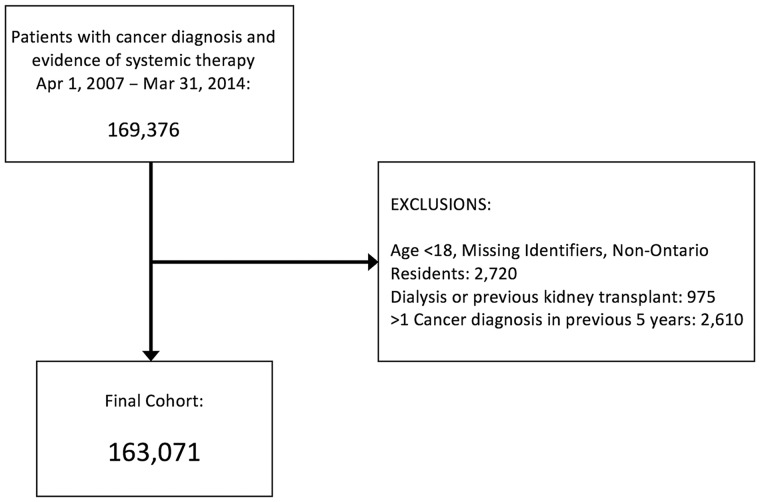
We identified 163 071 individuals initiating systemic therapy for an incident cancer diagnosis between 2007 and 2014 (Figure 1). Median (IQR) follow-up was 1.85 (0.77 to 3.83) years. Baseline characteristics are shown in Table 1. Mean age was 61.9 (SD = 13.3) years and 57.1% were female. The cancer stage at diagnosis was available for 71.4% of the cohort, with 19.3% of patients having stage IV cancers. The most common cancers were breast (23.4%), colorectal (13.2%), lung (12.7%), non-Hodgkin lymphoma (6.3%), and prostate (4.7%). Noncancer comorbidities were frequent, including hypertension (41.2%), diabetes mellitus (20.0%), and coronary artery disease (14.3%). Preexisting chronic kidney disease (CKD) was recorded in 4.0%.
Figure 1.
Study flow diagram for cohort of patients initiating systemic cancer therapy in Ontario (2007–2014). AKI = acute kidney injury; ESRD = end-stage renal disease.
Table 1.
Baseline characteristics for patients initiating systemic cancer therapy in Ontario between 2007 and 2014 (N = 163 071)*
| Baseline Characteristics | No. of patients | % |
|---|---|---|
| Mean age at index date, y (SD) | 61.89 | 13.29 |
| Sex, female | 93 034 | 57.1 |
| Index year | ||
| 2007 | 9062 | 5.6 |
| 2008 | 17 816 | 10.9 |
| 2009 | 19 716 | 12.1 |
| 2010 | 22 146 | 13.6 |
| 2011 | 23 883 | 14.6 |
| 2012 | 25 690 | 15.8 |
| 2013 | 25 944 | 15.9 |
| 2014 | 16 842 | 10.3 |
| 2015 | 1972 | 1.2 |
| Income quintile | ||
| 1 (low) | 29 277 | 18.0 |
| 2 | 32 533 | 20.0 |
| 3 (mid) | 31 967 | 19.6 |
| 4 | 34 337 | 21.1 |
| 5 (high) | 34 398 | 21.1 |
| Residence in a rural region | 23 967 | 14.7 |
| Residence in long-term care facility (among patients age >66 y) | 421 | 0.3 |
| Cancer characteristics | ||
| Stage at diagnosis | ||
| Missing | 46 591 | 28.6 |
| I | 19 867 | 12.2 |
| II | 31 751 | 19.5 |
| III | 33 451 | 20.5 |
| IV | 31 411 | 19.3 |
| Initial systemic therapy (5 most common) | ||
| CCO Regimen 1 (CHOP-Rituximab) | 43 238 | 7.5 |
| CCO Regimen 2 (carboplatin-paclitaxel) | 45 097 | 7.8 |
| CCO Regimen 3 (FOLFOX) | 23 385 | 4.0 |
| CCO Regimen 4 (FEC 100) | 19 281 | 3.3 |
| CCO Regimen 5 (cisplatin/gemcitabine-cisplatin) | 26 175 | 4.5 |
| NDFP Drug 1 (paclitaxel) | 13 588 | 14.8 |
| NDFP Drug 2 (epirubicin) | 11 821 | 12.9 |
| NDFP Drug 3 (rituximab) | 11 455 | 12.5 |
| NDFP Drug 4 (oxaliplatin) | 11 394 | 12.4 |
| NDFP Drug 5 (gemcitabine) | 9521 | 10.4 |
| Comorbidities | ||
| Charlson score | ||
| Mean (SD) | 1.83 | 2.45 |
| 0 | 86 309 | 52.9 |
| 1 | 3729 | 2.3 |
| 2 | 31 456 | 19.3 |
| ≥3 | 41 577 | 25.5 |
| ADG score | ||
| Mean (SD) | 9.24 | 3.39 |
| Acute myocardial infarction | 3508 | 2.2 |
| Congestive heart failure | 9601 | 5.9 |
| Cerebrovascular disease | 7540 | 4.6 |
| Diabetes mellitus, type 1 and 2 | 32 641 | 20.0 |
| Chronic liver disease | 2562 | 1.6 |
| Peripheral vascular disease | 3717 | 2.3 |
| Previous acute kidney injury | 4918 | 3.0 |
| Cardiac arrhythmia | 15 896 | 9.7 |
| Ischemic heart disease | 23 256 | 14.3 |
| Chronic lung disease | 17 193 | 10.5 |
| HIV/AIDS | 454 | 0.3 |
| Hypertension | 67 120 | 41.2 |
| Upper GI hemorrhage | 1301 | 0.8 |
| Lower GI hemorrhage | 1623 | 1.0 |
| Chronic kidney disease | 6570 | 4.0 |
| Health-care use (in the year preceding initiation of systemic cancer therapy) | ||
| Nephrology consultation | 5584 | 3.4 |
| No. of hospitalizations (mean, SD) | 0.21 | 0.62 |
| No. of ER visits | 0.75 | 1.71 |
| Coprescription within 120 days of index date | ||
| No. of individuals age >66 y on index date | 68 481 | 42.0 |
| Angiotensin-converting enzyme inhibitor | 21 220 | 13.0 |
| Angiotensin-receptor blocker | 13 897 | 8.5 |
| NSAIDs | 10 394 | 6.4 |
| Diuretics | 19 914 | 12.2 |
| Beta-blockers | 19 658 | 12.1 |
| DHP calcium channel blockers | 14 592 | 8.9 |
| Non-DHP calcium channel blockers | 3998 | 2.5 |
| Statins | 31 127 | 19.1 |
*ADG = Aggregated Diagnosis Groups;: CCO = Cancer Care Ontario dataset; CHOP = cyclophosphamide-hydroxyldaunorubicin (doxorubicin)-oncovin (vincristine)-prednisone; DHP = dihydropyridine; FEC 100 = fluorouracil-epirubicin-cyclophosphamide; FOLFOX = folinic acid (leucovorin)-fluorouracil-oxaliplatin; NDFP = New Drug Funding Plan dataset; NSAIDs = non-steroidal anti-inflammatory drugs; SD = standard deviation.
AKI Incidence Across Cancer Types
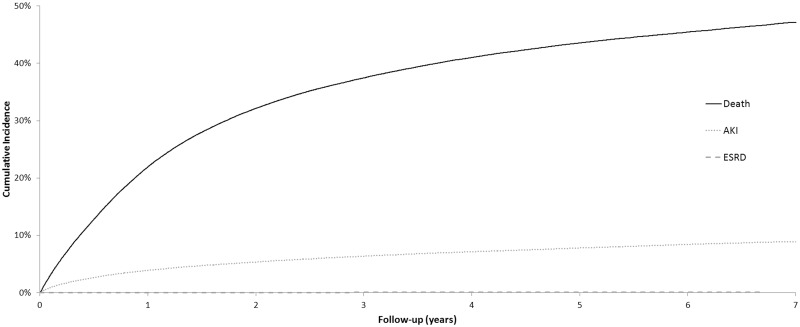
A total of 10 880 patients experienced an AKI-associated hospitalization or received acute dialysis over 403 538 patient-years of follow-up (Table 2). The rate of AKI was 27 per 1000 person-years (PY). The overall cumulative incidence of AKI (over 8 years) was 9.3% (95% CI = 9.1% to 9.6%) for patients initiating systemic therapy for any cancer. The overall cumulative incidence of AKI-D was 0.9% (95% CI = 0.8% to 1.0%). Median (IQR) time from initiation of systemic therapy to AKI was 276 (87–704) days. Median (IQR) time from the most recent systemic therapy exposure to AKI was 33 (9–177) days. Cumulative incidence curves for death, AKI, and ESRD are shown in Figure 2.
Table 2.
Incidence of acute kidney injury (AKI) and acute kidney injury requiring dialysis (AKI-D) by primary cancer type, 2007–2014, ordered by 5-year cumulative incidence
| Cancer type by primary site | N | No. of patients (%) | Total person-years | Event rate per 1000 person-years | 1-yr Cumulative incidence, % (95% CI) | 5-yr Cumulative incidence, % (95% CI) |
|---|---|---|---|---|---|---|
| Incidence of AKI | ||||||
| Total cohort | 163 071 | 10 880 (6.7) | 403 538 | 27.0 | 3.9 (3.8 to 4.0) | 7.8 (7.7 to 8.0) |
| Myeloma | 4244 | 893 (21.0) | 9833 | 90.8 | 10.1 (9.2 to 11.1) | 26.0 (24.4 to 27.7) |
| Bladder | 3811 | 611 (16.0) | 7925 | 77.1 | 10.7 (9.7 to 11.7) | 19.0 (17.6 to 20.5) |
| Leukemia | 5766 | 740 (12.8) | 12 730 | 58.1 | 7.8 (7.2 to 8.6) | 15.4 (14.3 to 16.5) |
| Kidney | 2021 | 223 (11.0) | 3388 | 65.8 | 6.4 (5.4 to 7.6) | 13.9 (12.1 to 15.9) |
| Peritoneal | 311 | 30 (9.6) | 446 | 67.3 | 6.6 (4.1 to 9.7) | 13.8 (8.4 to 20.6) |
| Liver | 1143 | 108 (9.4) | 1326 | 81.4 | 7.4 (5.9 to 9.0) | 11.7 (9.5 to 14.2) |
| Biliary | 1217 | 124 (10.2) | 1657 | 74.9 | 7.0 (5.6 to 8.5) | 11.6 (9.6 to 13.7) |
| Prostate | 7626 | 586 (7.7) | 16 939 | 34.6 | 4.5 (4.0 to 5.0) | 10.3 (9.4 to 11.2) |
| Cervix | 1714 | 139 (8.1) | 5359 | 25.9 | 4.4 (3.5 to 5.4) | 9.3 (7.8 to 11.0) |
| Anal | 1031 | 83 (8.1) | 3430 | 24.2 | 3.9 (2.8 to 5.2) | 9.1 (7.2 to 11.3) |
| Colorectal | 21 614 | 1752 (8.1) | 61 941 | 28.3 | 4.4 (4.2 to 4.7) | 9.1 (8.7 to 9.5) |
| Non-Hodgkin lymphoma | 10 238 | 836 (8.2) | 30 507 | 27.4 | 4.7 (4.3 to 5.1) | 9.1 (8.5 to 9.7) |
| Uterus | 3239 | 237 (7.3) | 7532 | 31.5 | 4.4 (3.7 to 5.1) | 8.8 (7.7 to 10.0) |
| Stomach | 3919 | 293 (7.5) | 6916 | 42.4 | 5.4 (4.7 to 6.2) | 8.3 (7.4 to 9.3) |
| Ovary | 4772 | 297 (6.2) | 12 372 | 24.0 | 2.9 (2.5 to 3.4) | 7.0 (6.3 to 7.9) |
| Pancreas | 4292 | 263 (6.1) | 4259 | 61.7 | 4.4 (3.8 to 5.0) | 6.8 (6.0 to 7.7) |
| Esophagus | 2262 | 132 (5.8) | 3351 | 39.4 | 4.3 (3.6 to 5.2) | 6.4 (5.3 to 7.6) |
| Oral cavity | 4183 | 239 (5.7) | 11 409 | 20.9 | 3.9 (3.3 to 4.5) | 6.3 (5.5 to 7.1) |
| Larynx | 599 | 28 (4.7) | 1402 | 20.0 | 2.5 (1.5 to 4.1) | 6.0 (3.9 to 8.5) |
| Lung | 20 804 | 1057 (5.1) | 28 126 | 37.6 | 3.6 (3.4 to 3.9) | 5.6 (5.3 to 6.0) |
| Thyroid | 961 | 27 (2.8) | 2492 | 10.8 | 1.7 (1.0 to 2.7) | 4.7 (2.9 to 7.2) |
| Melanoma | 2742 | 100 (3.6) | 6115 | 16.4 | 2.4 (1.8 to 3.0) | 4.6 (3.7 to 5.6) |
| Hodgkin lymphoma | 2199 | 83 (3.8) | 8056 | 10.3 | 2.1 (1.6 to 2.8) | 4.3 (3.4 to 5.3) |
| Breast | 38 217 | 902 (2.4) | 127 883 | 7.1 | 0.9 (0.8 to 1.0) | 3.1 (2.9 to 3.3) |
| Bones and joints* | 242 | 20 (8.3) | 597 | 33.5 | 7.5 (4.6 to 11.3) | – |
| Brain* | 2976 | 58 (1.9) | 5041 | 11.5 | 1.3 (1.0 to 1.8) | – |
| Testicular* | 981 | 39 (4.0) | 3542 | 11.0 | 3.1 (2.1 to 4.3) | – |
| Adrenal† | 77 | 12 (15.6) | 133 | 90.5 | 8.3 (3.3 to 16.2) | 38.5 (4.2 to 75.1) |
| Other‡ | 9870 | 968 (9.8) | 18 832 | 51.4 | 6.2 (5.7 to 6.7) | 12.5 (11.7 to 13.3) |
| Incidence of AKI-D | ||||||
| Total cohort | 163 071 | 1042 (0.6) | 411 900 | 2.5 | 0.4 (0.3 to 0.5) | 0.8 (0.7 to 1.0) |
| Myeloma | 4244 | 142 (0.09) | 10 500 | 13.5 | 1.5 (1.2 to 1.9) | 4.1 (3.4 to 4.9) |
| Leukemia | 5766 | 127 (0.08) | 13 298 | 9.6 | 1.4 (1.1 to 1.7) | 2.6 (2.2 to 3.1) |
| Bladder | 3811 | 59 (0.04) | 8352 | 7.1 | 0.9 (0.7 to 1.3) | 2.0 (1.5 to 2.7) |
| Kidney | 2021 | 25 (0.02) | 3543 | 7.1 | 0.7 (0.4 to 1.2) | 1.7 (1.1 to 2.7) |
| Esophagus | 2262 | 17 (0.01) | 3417 | 5.0 | 0.7 (0.4 to 1.1) | 1.1 (0.5 to 2.2) |
| Non-Hodgkin lymphoma | 10 238 | 89 (0.05) | 31 212 | 2.9 | 0.6 (0.4 to 0.7) | 1.1 (0.8 to 1.4) |
| Cervix | 1714 | 11 (0.01) | 5463 | 2.0 | 0.4 (0.2 to 0.8) | 1.0 (0.4 to 2.2) |
| Prostate | 7626 | 50 (0.03) | 17 304 | 2.9 | 0.4 (0.3 to 0.6) | 1.0 (0.7 to 1.3) |
| Colorectal | 21 614 | 149 (0.09) | 63 941 | 2.3 | 0.4 (0.3 to 0.5) | 0.8 (0.7 to 0.9) |
| Hodgkin lymphoma | 2199 | 12 (0.01) | 8119 | 1.5 | 0.3 (0.1 to 0.7) | 0.8 (0.4 to 1.5) |
| Breast | 38 217 | 103 (0.06) | 128 683 | 0.8 | 0.1 (0.1 to 0.2) | 0.3 (0.3 to 0.4) |
| Ovary | 4772 | 9 (0.01) | 12 579 | 0.7 | 0.1 (0.0 to 0.2) | 0.3 (0.1 to 0.5) |
| Pancreas | 4292 | 9 (0.01) | 4334 | 2.1 | 0.2 (0.1 to 0.4) | 0.3 (0.1 to 0.7) |
| Lung* | 20 804 | 47 (0.03) | 28 679 | 1.6 | 0.2 (0.1 to 0.2) | – |
| Melanoma* | 2742 | 10 (0.01) | 6170 | 1.6 | 0.3 (0.2 to 0.6) | – |
| Oral cavity* | 4183 | 15 (0.01) | 11 729 | 1.3 | 0.3 (0.2 to 0.5) | – |
| Stomach* | 3919 | 21 (0.01) | 7080 | 3.0 | 0.5 (0.3 to 0.8) | – |
| Testicular* | 981 | 6 (0.00) | 3604 | 1.7 | 0.5 (0.2 to 1.2) | – |
| Uterus* | 3239 | 12 (0.01) | 7645 | 1.6 | 0.2 (0.1 to 0.5) | – |
| Other‡ | 9870 | 106 (0.07) | 19 443 | 5.5 | 0.6 (0.5 to 0.8) | 1.4 (1.2 to 1.8) |
Insufficient events to calculate a 5-year cumulative incidence estimate. CI = confidence interval.
Diagnoses with fewer than 100 patients.
Malignancies not categorized in the other diagnoses listed.
Figure 2.
Cumulative incidence curves for acute kidney injury (AKI), death, and end-stage renal disease (ESRD) for patients initiating systemic cancer in Ontario between 2007 and 2014. Cumulative incidence estimates obtained from multivariable Fine and Gray regression models (N = 163 071).
AKI and AKI-D event rates, as well as 1- and 5-year cumulative incidence estimates, for each of the 29 cancer diagnoses are shown in Table 2. Cancers with the highest 5-year cumulative incidence of AKI included multiple myeloma (26.0%, 95% CI = 24.4% to 27.7%), bladder cancer (19.0%, 95% CI = 17.6% to 20.5%), leukemia (15.4%, 95% CI = 14.3% to 16.5%), renal (13.9%, 95% CI = 12.1% to 15.9%), and liver cancer (11.7%, 95% CI = 9.5% to 14.2%). AKI-D was comparatively infrequent, with multiple myeloma patients most frequently experiencing AKI-D (4.1%, 95% CI = 3.4% to 4.9%), followed by patients with leukemia (2.6%, 95% CI = 2.2% to 3.1%).
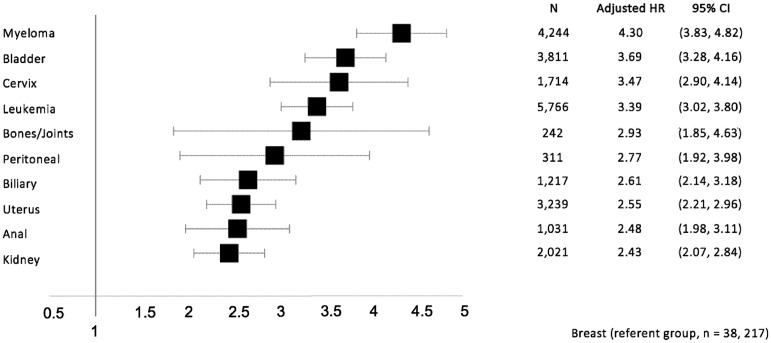
After adjustment for potential confounders, the highest hazard ratios for AKI (relative to breast cancer) were observed in multiple myeloma, bladder cancer, and cervical cancer with adjusted hazard ratios (aHR) (95% CI) of 4.30 (3.83 to 4.82), 3.69 (3.28 to 4.16), and 3.47 (2.90 to 4.14), respectively (Figure 3).
Figure 3.
Forest plot of adjusted hazard ratios for the 10 cancer diagnoses most strongly associated with acute kidney injury in multivariable regression. Effect estimates from multivariable Fine and Gray regression models with breast cancer as the referent category (N = 163 071). HR = hazard ratio; CI = confidence interval. AKI = acute kidney injury; AKI-D = acute kidney injury requiring dialysis.
Patient-Level Risk Factors for AKI and Effect of Recent Systemic Therapy
Increasing age and male sex were modestly associated with increased AKI risk (Table 3). More advanced cancer stage at the time of diagnosis was also associated with increased AKI risk (HR = 1.41, 95% CI = 1.28 to 1.54).
Table 3.
Adjusted hazard ratios (aHR) for the association between patient-level risk factors and acute kidney injury
| Covariates | aHR* (95% CI) | P † |
|---|---|---|
| Age (per decade) | 1.10 (1.08 to 1.12) | <.001 |
| Male vs female | 1.26 (1.20 to 1.32) | <.001 |
| Year of cohort entry (by year) | 1.01 (1.00 to 1.01) | .31 |
| Cancer stage | ||
| Stage I | 1.00 (Ref) | |
| Stage II | 1.09 (1.00 to 1.19) | .07 |
| Stage III | 1.25 (1.15 to 1.37) | <.001 |
| Stage IV | 1.41 (1.28 to 1.54) | <.001 |
| Missing | 1.37 (1.26 to 1.50) | <.001 |
| Comorbidities | ||
| Chronic kidney disease | 1.80 (1.67 to 1.93) | <.001 |
| Previous AKI | 1.69 (1.56 to 1.83) | <.001 |
| Diabetes mellitus | 1.43 (1.37 to 1.50) | <.001 |
| Congestive heart failure | 1.36 (1.27 to 1.45) | <.001 |
| HIV/AIDS | 1.36 (1.00 to 1.84) | .05 |
| Chronic liver disease | 1.30 (1.14 to 1.47) | <.001 |
| Hypertension | 1.28 (1.23 to 1.34) | <.001 |
| Peripheral vascular disease | 1.22 (1.11 to 1.34) | <.001 |
| Arrhythmia | 1.09 (1.03 to 1.16) | .002 |
| Ischemic heart disease | 1.06 (1.01 to 1.12) | .02 |
| Previous acute myocardial infarction | 1.05 (0.95 to 1.16) | .34 |
| COPD | 1.05 (0.99 to 1.11) | .15 |
| Cerebrovascular disease | 0.98 (0.91, 1.06) | .65 |
| Gastrointestinal bleeding | 0.97 (0.84 to 1.13) | .72 |
| Charlson score | ||
| 0 | 1.00 (Ref) | |
| 1 | 1.07 (0.96 to 1.20) | .21 |
| 2 | 0.97 (0.92 to 1.02) | .27 |
| ≥3 | 0.99 (0.94 to 1.05) | .80 |
Hazard ratios reflect fully adjusted model, including baseline demographic, cancer type/stage, and comorbidity covariates. AKI = acute kidney injury; CI = confidence interval; COPD = chronic obstructive pulmonary disease
P values were obtained from two-sided Wald χ2 test.
Comorbidities most strongly associated with AKI included CKD, diabetes mellitus, and congestive heart failure (aHR = 1.80, 95% CI = 1.67 to 1.93; 1.43, 95% CI = 1.37 to 1.50; and 1.36, 95% CI = 1.27 to 1.45, respectively). A previous history of AKI was also statistically significantly associated with subsequent risk (aHR = 1.69, 95% CI = 1.56 to 1.83) (Table 3).
The 90-day period after systemic therapy exposure was associated with a heightened (cause-specific) aHR (95% CI) for AKI of 2.34 (2.24 to 2.45) vs time periods more distant (ie, >90 days) from systemic therapy exposure (Table 4). The cause-specific aHR (95% CI) for AKI-D following recent systemic therapy was similarly elevated at 2.03 (1.75 to 2.35) in the time-varying covariate model.
Table 4.
Adjusted hazard ratios (aHR) for acute kidney injury associated with systemic therapy exposure (within 90 days) and coprescriptions
| Covariate | aHR* (95% CI) | P † |
|---|---|---|
| Recent systemic therapy exposure‡ | 2.34 (2.24 to 2.45) | <.001 |
| Coprescription§ | ||
| ACEi or ARB | 1.30 (1.23 to 1.38) | <.001 |
| Diuretic | 1.20 (1.14 to 1.28) | <.001 |
| Beta-blocker | 1.10 (1.04 to 1.17) | .002 |
| Calcium channel blocker | 1.18 (1.07 to 1.30) | .001 |
| Statin | 1.02 (0.96 to 1.07) | .61 |
*Adjusted for all (time-fixed) covariates used in primary model. ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin-receptor blockers; CI = confidence interval.
P values were obtained from two-sided Wald χ2 test.
90-day period following each treatment.
Within 120 days of systemic therapy start (patients aged ≥66 years, n = 68 481).
Effect of Coprescription at Systemic Therapy Initiation and Temporal Trends in AKI Incidence
There were 68 481 individuals (42.0%) who were more than 65 years of age at the time of systemic therapy initiation (Table 1). Of these, angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers were prescribed in 51.3%, beta-blockers in 28.7%, calcium channel blockers in 27.1%, diuretics in 29.1%, NSAIDs in 15.2%, and statins in 45.4%.
Prescription of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers at the time of systemic therapy was most strongly associated with AKI risk (aHR = 1.30, 95% CI = 1.23 to 1.38), followed by diuretic prescription (aHR = 1.20, 95% CI = 1.14 to 1.28) (Table 4). Beta-blocker and calcium channel blocker prescriptions were also modestly associated with AKI risk, although statins were not.
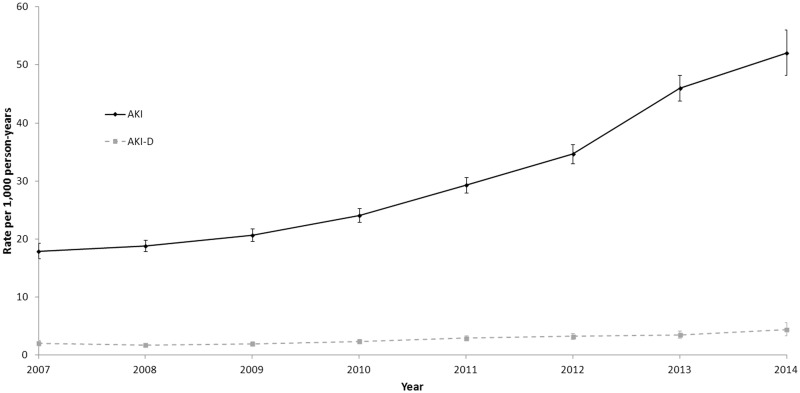
The annual incidence of AKI increased nearly threefold over the study period (from 18 to 52 events per 1000 patient-years, P value for trend <.001) and the AKI-D rate more than doubled (from 2.1 to 4.4 events per 1000 patient-years) (Figure 4).
Figure 4.
Trends in annual incidence of acute kidney injury (AKI) by year of systemic therapy initiation, 2007–2014. Error bars represent 95% confidence intervals. Annual number of AKI events per 1000 patient-years report (with AKI events attributed to the year in which patients initiated systemic therapy). Cochran-Armitage test used for P-value trend (P < .001).
AKI Risk Associated With Therapies in High-Risk Cancers (Post Hoc Analysis)
Adjusted hazard ratios for AKI risk associated with bladder cancer, multiple myeloma, and leukemia therapies are shown in Table 5. Cisplatin- vs carboplatin-based regimens did not differ with respect to AKI risk; however, regimens without platinum-based agents were less likely to associate with AKI (aHR = 0.76, 95% CI = 0.59 to 0.99).
Table 5.
Association of specific therapies on acute kidney injury risk in bladder cancer, multiple myeloma, and leukemia
| Cancer type and therapy | n | aHR* (95% CI) | P † |
|---|---|---|---|
| Bladder cancer | 2141 | ||
| Cisplatin-based regimens (eg, single-agent cisplatin, cisplatin-gemcitabine, MVAC) | 1210 | 1.00 (referent) | |
| Carboplatin-based regimen (eg, single-agent carboplatin, carboplatin-gemcitabine) | 400 | 0.79 (0.60 to 1.05) | .10 |
| Other (eg, single-agent gemcitabine, mitomycin C, 5-FU- mitomycin C) | 531 | 0.76 (0.59 to 0.99) | .04 |
| Multiple myeloma | 4244 | ||
| Bortezomib | 1471 | 0.83 (0.70 to 0.99) | .04 |
| Cyclophosphamide | 739 | 0.77 (0.62 to 0.96) | .02 |
| IMiD (eg, lenalidomide, thalidomide) | 32 | 0.30 (0.10 to 0.95) | .04 |
| Melphalan | 564 | 0.93 (0.75 to 1.16) | .52 |
| Vincristine, doxorubicin, and dexamethasone | 57 | 0.61 (0.32 to 1.16) | .13 |
| Bisphosphonate | 935 | 0.80 (0.67 to 0.95) | .01 |
| HSCT treatment (time-varying covariate) | 1276 | ||
| 30-day period post-HCT (vs pre-/no HSCT, or >1-year post-HSCT) | 1.25 (0.68 to 2.28) | .47 | |
| 31–90-day period post-HSCT | 0.41 (0.19 to 0.87) | .02 | |
| 91-day to 1-year period post-HSCT | 0.64 (0.46 to 0.88) | .006 | |
| Leukemia | 2561 | ||
| Acute leukemia regimen (eg, “7 + 3,” azacitadine, cytarabine, daunorubicin mitoxantrone) | 1298 | 2.79 (2.16 to 3.59) | <.001 |
| Chronic leukemia regimen [eg, bendamustine, chlorambucil, FC, FC-R, TKI) | 1263 | 1.00 (referent) | |
| HSCT treatment (time-varying covariate) | 445 | ||
| 30-day period post-HSCT (vs pre-/no HSCT, or >1-year post-HSCT) | 3.61 (1.86 to 7.02) | <.001 | |
| 31–90-day period post-HSCT | 5.25 (3.43 to 8.01) | <.001 | |
| 91-day to 1-year period post-HSCT | 2.60 (1.84 to 3.69) | <.001 |
Adjusted for all (time-fixed) covariates used in primary model (including demographics, comorbidities, etc.). “7 + 3” = cytarabine-daunorubicin; 5-FU = 5-fluorouracil; aHR = adjusted hazard ratio; FC = fludarabine-cyclophosphamide; FC-R = fludarabine-cycIophosphamide-rituximab; HSCT = hematopoietic stem cell transplant; IMiD = immunomodulatory drugs; MVAC = methotrexate, vinblastine, doxorubicin, and cisplatin; TKI = tyrosine kinase inhibitor (dasatinib, imatinib, nilotinib).
P values obtained from two-sided Wald χ2 test.
In multiple myeloma, bortezomib, cyclophosphamide, immunomodulatory, and bisphosphonate therapies were associated with reduced risk of AKI. The 30-day period following HSCT was associated with increased AKI risk; however, the 31–90-day and 91-day-to-1-year periods after HSCT were associated with reduced risk.
In leukemia, regimens associated with acute leukemia treatment were associated with an increased risk of AKI. HSCT was associated with an increase in AKI risk (in all postexposure time periods).
Discussion
Our results demonstrate the considerable burden of AKI among patients who initiate systemic therapy for cancer in the current era of cancer treatment. Nearly one in 10 patients initiating systemic cancer therapy will experience a hospitalization or receive acute dialysis for AKI. The 5-year cumulative incidence for AKI reached as high as 15% to 26% for the top three high-risk malignancies. This magnitude of AKI risk may be underappreciated by both clinicians and patients commencing systemic cancer treatment, given the paucity of existing data (13). Moreover, despite the advances in cancer therapy, our results remain largely congruent with estimates observed in the 1999 to 2006 data from a Danish cohort study (in which the 5-year cumulative incidence of the RIFLE “injury” and “failure” categories was 14.6% and 7.6%, respectively) (15). However, unlike patients in the Danish study, our cohort was restricted to those who received systemic treatment and were likely more susceptible to AKI.
Patients initiating treatment for multiple myeloma, bladder cancer, and leukemia had the highest incidence of AKI. The high risk of AKI associated with multiple myeloma is recognized and attributable to the many mechanisms by which kidney injury may occur in paraprotein disease, including cast nephropathy, hypercalcemia, and glomerulopathies (eg, immunoglobulin deposition diseases and amyloidosis) (33–35). Novel treatments including bortezomib-based regimens have been purported to decrease mortality (36–38) and ESRD (16); however our data suggests the risk of hospitalization and acute dialysis for AKI remains high. When we assessed the risk of AKI across myeloma treatments, receipt of bortezomib was associated with decreased risk. We also observed decreased AKI risk associated with cyclophosphamide and immunomodulatory drug therapies. This is consistent with a recent observational study reporting improved kidney outcomes in 83 patients receiving bortezomib “triplet” therapies (ie, bortezomib and dexamethasone plus cyclophosphamide or thalidomide) versus bortezomib-dexamethasone (“doublet”) therapy (39). Bisphosphonate use was also associated with reduced AKI risk. This suggests that the benefit of mitigating hypercalcemia-related AKI may outweigh the well-described, but rare, phenomenon of bisphosphonate-related nephrotoxicity (40).
The excess AKI risk observed in bladder and cervical cancers is likely reflective of obstructive (postrenal) AKI in these malignancies (3,41), as well as exposure to potentially nephrotoxic platinum-based chemotherapies (42). This risk is of particular concern as AKI in the setting of bladder cancer has been linked with substantially increased risks of de novo CKD and death (43). When we assessed specific therapies in bladder cancer, cisplatin- and carboplatin-based therapies did not differ with respect to AKI risk, despite putatively lower nephrotoxicity with the latter (44). This finding supports results of a small phase 2 study that did not demonstrate a difference in kidney toxicity in patients receiving gemcitabine-cisplatin vs gemcitabine-carboplatin for bladder cancer (45). It is likely, however, that patients receiving carboplatin in our cohort had reduced baseline kidney function and, as such, were predisposed to AKI due to underlying CKD.
The increased risk of AKI seen in leukemias and other hematologic cancers in our study confirms the findings of smaller cohorts (9,46), and may be attributable to the risks of sepsis, volume depletion, and tumor lysis syndrome (which are more likely to occur in the setting of acute vs chronic leukemia treatments).
HSCT was associated with a statistically significant increase in AKI risk in leukemia and may be attributable to the recognized kidney risks of acute tubular necrosis, hepatic sinusoidal obstructive syndrome, and thrombotic microangiopathy (47). In myeloma, however, HSCT was associated with AKI risk only in the first 30 days post-HSCT and was associated with decreased risk in later time periods post-HSCT, suggesting improved disease control associated with HSCT results in less myeloma-related kidney injury after the initial period in which periprocedural kidney complications may occur. This finding supports data from smaller cohorts suggesting that HSCT is associated with more favorable kidney prognosis and may be considered in some patients with kidney dysfunction (48,49).
The comparative risks of AKI across cancer types in our study differ from the findings of the Danish study (15), in which renal cancers were associated with the highest risk. This difference may reflect more recent trends toward less invasive and kidney-sparing treatment options for renal cancers, such as partial nephrectomy, which has been shown to reduce the incidence of AKI vs radical nephrectomies (50,51).
Our findings highlight the substantial burden of comorbidity in “real-world” patients initiating systemic therapies for cancer and demonstrate that those with a history of CKD, diabetes, and/or congestive heart failure are at substantially increased risk for AKI. These conditions have been shown to potentiate the risks of systemic therapy-associated nephrotoxicities (52,53), as well as increase the likelihood of prerenal states, and polypharmacy (4,54). As such, close monitoring of blood pressure and volume status in patients with congestive heart failure, CKD, and hypertension during systemic therapy is warranted. Similarly, among patients with diabetes, hypoglycemic agents may require adjustment based on current glycemic control and kidney function (55).
In our cohort, a sizable proportion of older patients were filling prescriptions for medications such as diuretics, angiotensin-converting enzyme inhibitors, or angiotensin-receptor blockers when starting systemic treatment. These agents were associated with an increased AKI risk of 20%–30%. This may be because an elevated risk of hemodynamic/prerenal insults to the kidney when patients are hypovolemic as a result of reduced oral intake or gastrointestinal side effects of cancer therapy. Holding or discontinuing these medications at the time of systemic therapy initiation, particularly when emetogenic anticancer therapies are administered, may represent a risk reduction strategy for selected patients. Current oncology clinical practice guidelines do not comment on modifying antihypertensives during systemic therapy. Routine dose modification or temporary cessation of antihypertensives and other potentially nephrotoxic drugs during systemic cancer therapy warrants investigation as a method to mitigate adverse events (56,57), including AKI.
Most AKI events in this population occurred in close proximity to cancer treatment itself (median 33 days from last treatment), rather than after treatment discontinuation or end-of-life care. The more than twofold increased hazard of AKI during the 90 days following systemic therapy may represent a window for heightened clinical and biochemical (eg, serum creatinine) surveillance.
The incidence of AKI increased statistically significantly over the study period. Although this may reflect increased recognition and administrative coding of AKI hospitalizations, the rates of AKI-D also increased substantially during this period. This may reflect trends toward increasing age and comorbidities among patients initiating cancer treatment (58–60). Despite the advent of novel therapies, the burgeoning population of elderly cancer patients with AKI-predisposing comorbidities may continue to present challenges to oncology and nephrology care providers in the coming years.
Our study has several strengths. We evaluated all adult patients undergoing systemic cancer treatment in a diverse universal health-care system. We employed clinically relevant and validated AKI outcomes, accounting for the competing risk of death. Our data permitted assessment of multiple comorbidities. We also assessed AKI risk conferred by common coprescriptions in a large subcohort of older patients with available medication data.
Our study also has important limitations to consider. As we did not have serum creatinine data and were limited to AKI hospitalizations and acute dialysis events, less severe AKI episodes were likely not captured in our analysis; this may have resulted in an underestimation of overall AKI incidence. It is likely, however, that the AKI events we captured represented clinically relevant episodes of kidney injury. Our analysis of patient-level risk factors for AKI may have also been susceptible to confounding by indication. The observed increased risk associated with angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, diuretics, and other antihypertensives may have been confounded by the increased risk conferred by conditions for which these drugs are indicated (ie, diabetes, congestive heart failure, and CKD). Similarly, our analysis of risk related to specific therapies (ie, use of cisplatin vs carboplatin, or receipt of HSCT) may have been affected by confounding by indication, as risk factors for AKI (and general prognostic markers) may influence the receipt of these treatments. Also, missing covariate data, particularly on cancer staging at diagnosis, may have biased our effect estimates. Finally, our analysis was limited by the inability to ascribe etiologies to the AKI events observed. Distinguishing events related to therapy (including prerenal insults and nephrotoxicity) vs direct effects of disease would be of benefit in devising cancer- and therapy-specific AKI risk reduction strategies.
In conclusion, patients undergoing systemic treatment for cancers are at high risk of hospitalization and acute dialysis for AKI, with nearly one in 10 patients experiencing an episode of kidney injury. Patients with multiple myeloma, bladder cancer, cervical cancer, and leukemia are at highest risk. Comorbidities and coprescriptions influenced this risk, which was highest in the peritreatment period. Further efforts should focus on the development of robust prediction tools for AKI as well as viable strategies for AKI prevention in patients undergoing cancer therapy.
Funding
This study was supported by the Institute for Clinical Evaluative Sciences (ICES) Western site. ICES is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Core funding for ICES Western is provided by the Academic Medical Organization of Southwestern Ontario (AMOSO), the Schulich School of Medicine and Dentistry (SSMD), Western University, and the Lawson Health Research Institute (LHRI). The research was conducted by members of the ICES Kidney, Dialysis and Transplantation team, at the ICES Western facility, who are supported by a grant from the Canadian Institutes of Health Research (CIHR).
Notes
Affiliations of authors: Department of Medicine (AK), Division of Nephrology, University of Toronto, Toronto, ON, Canada (AK, CTC, SJK, RW); Institute for Clinical Evaluative Sciences, London, ON, Canada (EM, CMB, RS, DMN, AXG, SJK); Department of Medical Oncology, Princess Margaret Cancer Centre, Toronto, ON, Canada (EA, HM); Department of Oncology, Queen's University, Kingston, ON, Canada (CMB); Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada (RS); Division of Nephrology, Queen's University, Kingston, ON, Canada (SAS); Division of Nephrology, Western University, London, ON, Canada (AXG).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Parts of this material are based on data and/or information compiled and provided by CIHI. The opinions, results, and conclusions are those of the authors and are independent from the funding and data sources. No endorsement by ICES, AMOSO, SSMD, LHRI, CIHI, CIHR, or the MOHLTC is intended or should be inferred.
Supplementary Material
References
- 1. Cohen EP, Krzesinski JM, Launay-Vacher V, et al. Onco-nephrology: core curriculum 2015. Am J Kidney Dis. 2015;665:869–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Janus N, Launay-Vacher V, Byloos E, et al. Cancer and renal insufficiency results of the BIRMA study. Br J Cancer. 2010;10312:1815–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lam AQ, Humphreys BD.. Onco-nephrology: AKI in the cancer patient. Clin J Am Soc Nephrol. 2012;710:1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosner MH, Perazella MA.. Acute kidney injury in patients with cancer. N Engl J Med. 2017;37618:1770–1781. [DOI] [PubMed] [Google Scholar]
- 5. Darmon M, Ciroldi M, Thiery G, Schlemmer B, Azoulay E.. Clinical review: specific aspects of acute renal failure in cancer patients. Crit Care. 2006;102:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lameire NH, Flombaum CD, Moreau D, et al. Acute renal failure in cancer patients. Ann Med. 2005;371:13–25. [DOI] [PubMed] [Google Scholar]
- 7. Lahoti A, Humphreys BD. AKI associated with malignancies. In: Onconephrology Curriculum [online curriculum]. American Society of Nephrology; 2016. https://www.asn-online.org/education/distancelearning/curricula/onco/Chapter3.pdf
- 8. Darmon M, Thiery G, Ciroldi M, et al. Should dialysis be offered to cancer patients with acute kidney injury? Intensive Care Med. 2007;335:765–772. [DOI] [PubMed] [Google Scholar]
- 9. Darmon M, Vincent F, Canet E, et al. Acute kidney injury in critically ill patients with haematological malignancies: results of a multicentre cohort study from the Groupe de Recherche en Réanimation Respiratoire en Onco-Hématologie. Nephrol Dial Transplant. 2015;3012:2006–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lahoti A, Kantarjian H, Salahudeen AK, et al. Predictors and outcome of acute kidney injury in patients with acute myelogenous leukemia or high-risk myelodysplastic syndrome. Cancer. 2010;11617:4063–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Libório AB, Abreu KL, Silva GB Jr, et al. Predicting hospital mortality in critically ill cancer patients according to acute kidney injury severity. Oncology. 2011;80(3–4):160–166. [DOI] [PubMed] [Google Scholar]
- 12. Soares M, Salluh JI, Carvalho MS, et al. Prognosis of critically ill patients with cancer and acute renal dysfunction. J Clin Oncol. 2006;2424:4003–4010. [DOI] [PubMed] [Google Scholar]
- 13. Salahudeen AK, Doshi SM, Pawar T, et al. Incidence rate, clinical correlates, and outcomes of AKI in patients admitted to a comprehensive cancer center. Clin J Am Soc Nephrol. 2013;83:347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bellomo R, Ronco C, Kellum JA, et al. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;84:R204–R212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Christiansen CF, Johansen MB, Langeberg WJ, et al. Incidence of acute kidney injury in cancer patients: a Danish population-based cohort study. Eur J Intern Med. 2011;224:399–406. [DOI] [PubMed] [Google Scholar]
- 16. Reule S, Sexton DJ, Solid CA, et al. ESRD due to multiple myeloma in the United States, 2001-2010. J Am Soc Nephrol. 2016;275:1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sonneveld P, De Wit E, Moreau P.. How have evolutions in strategies for the treatment of relapsed/refractory multiple myeloma translated into improved outcomes for patients? Crit Rev Oncol Hematol. 2017;112:153–170. [DOI] [PubMed] [Google Scholar]
- 18. Calvo E, Schmidinger M, Heng DY, et al. Improvement in survival end points of patients with metastatic renal cell carcinoma through sequential targeted therapy. Cancer Treat Rev. 2016;50:109–117. [DOI] [PubMed] [Google Scholar]
- 19. Posadas EM, Limvorasak S, Figlin RA.. Targeted therapies for renal cell carcinoma. Nat Rev Nephrol. 2017;138:496–511. [DOI] [PubMed] [Google Scholar]
- 20. Abbas A, Mirza MM, Ganti AK, et al. Renal toxicities of targeted therapies. Target Oncol. 2015;104:487–499. [DOI] [PubMed] [Google Scholar]
- 21. Bedke J, Gauler T, Grunwald V, et al. Systemic therapy in metastatic renal cell carcinoma. World J Urol. 2017;352:179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Launay-Vacher V, Aapro M, De Castro G Jr, et al. Renal effects of molecular targeted therapies in oncology: a review by the Cancer and the Kidney International Network (C-KIN). Ann Oncol. 2015;268:1677–1684. [DOI] [PubMed] [Google Scholar]
- 23. Lefebvre J, Glezerman IG.. Kidney toxicities associated with novel cancer therapies. Adv Chronic Kidney Dis. 2017;244:233–240. [DOI] [PubMed] [Google Scholar]
- 24. McLaughlin JR, Kreiger N, Marrett LD, et al. Cancer incidence registration and trends in Ontario. Eur J Cancer. 1991;2711:1520–1524. [DOI] [PubMed] [Google Scholar]
- 25. Cancer Care Ontario. Ontario Cancer Statistics. Toronto, Ontario: Cancer Care Ontario; 2016. [Google Scholar]
- 26. The Johns Hopkins ACG® Case-Mix System Version 10.0 Release Notes [computer program]. PC (DOS/WIN/NT) and Unix Version 10.0 - December 2011. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health; 2011.
- 27. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;405:373–383. [DOI] [PubMed] [Google Scholar]
- 28. Hwang YJ, Shariff SZ, Gandhi S, et al. Validity of the International Classification of Diseases, Tenth Revision code for acute kidney injury in elderly patients at presentation to the emergency department and at hospital admission. BMJ Open. 2012;26:e001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grams ME, Waikar SS, MacMahon B, et al. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;94:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quinn RR, Laupacis A, Austin PC, et al. Using administrative datasets to study outcomes in dialysis patients: a validation study. Med Care. 2010;488:745–750. [DOI] [PubMed] [Google Scholar]
- 31. Wald R, McArthur E, Adhikari NK, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis. 2015;656:870–877. [DOI] [PubMed] [Google Scholar]
- 32. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94446:496–509. [Google Scholar]
- 33. Eleutherakis-Papaiakovou V, Bamias A, Gika D, et al. Renal failure in multiple myeloma: Incidence, correlations, and prognostic significance. Leuk Lymphoma. 2007;482:337–341. [DOI] [PubMed] [Google Scholar]
- 34. Finkel KW, Cohen EP, Shirali A, et al. Paraprotein-related kidney disease: evaluation and treatment of myeloma cast nephropathy. Clin J Am Soc Nephrol. 2016;1112:2273–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Leung N, Nasr SH.. Myeloma-related kidney disease. Adv Chronic Kidney Dis. 2014;211:36–47. [DOI] [PubMed] [Google Scholar]
- 36. Dimopoulos MA, Delimpasi S, Katodritou E, et al. Significant improvement in the survival of patients with multiple myeloma presenting with severe renal impairment after the introduction of novel agents. Ann Oncol. 2014;251:195–200. [DOI] [PubMed] [Google Scholar]
- 37. Scott K, Hayden PJ, Will A, et al. Bortezomib for the treatment of multiple myeloma. Cochrane Database Syst Rev. 2016;4:CD010816.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Uttervall K, Duru AD, Lund J, et al. The use of novel drugs can effectively improve response, delay relapse and enhance overall survival in multiple myeloma patients with renal impairment. PLoS One. 2014;97:e101819.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dimopoulos MA, Roussou M, Gavriatopoulou M, et al. Bortezomib-based triplets are associated with a high probability of dialysis independence and rapid renal recovery in newly diagnosed myeloma patients with severe renal failure or those requiring dialysis. Am J Hematol. 2016;915:499–502. [DOI] [PubMed] [Google Scholar]
- 40. Pozzi S, Raje N.. The role of bisphosphonates in multiple myeloma: mechanisms, side effects, and the future. Oncologist. 2011;165:651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Campbell GA, Hu D, Okusa MD.. Acute kidney injury in the cancer patient. Adv Chronic Kidney Dis. 2014;211:64–71. [DOI] [PubMed] [Google Scholar]
- 42. Latcha S, Jaimes EA, Patil S, et al. Long-term renal outcomes after cisplatin treatment. Clin J Am Soc Nephrol. 2016;117:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kwon T, Jeong IG, Lee C, et al. Acute kidney injury after radical cystectomy for bladder cancer is associated with chronic kidney disease and mortality. Ann Surg Oncol. 2016;232:686–693. [DOI] [PubMed] [Google Scholar]
- 44. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;302:191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dogliotti L, Carteni G, Siena S, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: Results of a randomized phase 2 trial. Eur Urol. 2007;521:134–141. [DOI] [PubMed] [Google Scholar]
- 46. Luciano RL, Brewster UC.. Kidney involvement in leukemia and lymphoma. Adv Chronic Kidney Dis. 2014;211:27–35. [DOI] [PubMed] [Google Scholar]
- 47. Sawinski D. The kidney effects of hematopoietic stem cell transplantation. Adv Chronic Kidney Dis. 2014;211:96–105. [DOI] [PubMed] [Google Scholar]
- 48. Glavey SV, Gertz MA, Dispenzieri A, et al. Long-term outcome of patients with multiple [corrected] myeloma-related advanced renal failure following auto-SCT. Bone Marrow Transplant. 2013;4812:1543–1547. [DOI] [PubMed] [Google Scholar]
- 49. Parikh GC, Amjad AI, Saliba RM, et al. Autologous hematopoietic stem cell transplantation may reverse renal failure in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;157:812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim CS, Bae EH, Ma SK, et al. Impact of partial nephrectomy on kidney function in patients with renal cell carcinoma. BMC Nephrol. 2014;15:181.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Schmid M, Krishna N, Ravi P, et al. Trends of acute kidney injury after radical or partial nephrectomy for renal cell carcinoma. Urol Oncol. 2016;347:293.e1–293.e10. [DOI] [PubMed] [Google Scholar]
- 52. Izzedine H, Perazella MA.. Anticancer drug-induced acute kidney injury. Kidney Int Rep. 2017;24:504–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Máthé C, Bohács A, Duffek L, et al. Cisplatin nephrotoxicity aggravated by cardiovascular disease and diabetes in lung cancer patients. Eur Respir J. 2011;374:888–894. [DOI] [PubMed] [Google Scholar]
- 54. Perazella MA. Onco-nephrology: renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol. 2012;710:1713–1721. [DOI] [PubMed] [Google Scholar]
- 55. Singer M. Management of comorbid diabetes and cancer. Oncology (Williston Park). 2007;21(8 suppl):26–37. [PubMed] [Google Scholar]
- 56. Kutner JS, Blatchford PJ, Taylor DH Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness. JAMA Intern Med. 2015;1755:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. LeBlanc TW, McNeil MJ, Kamal AH, et al. Polypharmacy in patients with advanced cancer and the role of medication discontinuation. Lancet Oncol. 2015;167:e333–e341. [DOI] [PubMed] [Google Scholar]
- 58. Aarts MJ, Aerts JG, van den Borne BE, et al. Comorbidity in patients with small-cell lung cancer: trends and prognostic impact. Clin Lung Cancer. 2015;164:282–291. [DOI] [PubMed] [Google Scholar]
- 59. Hurria A, Levit LA, Dale W, et al. Improving the evidence base for treating older adults with cancer: American Society of Clinical Oncology Statement. J Clin Oncol. 2015;3332:3826–3833. [DOI] [PubMed] [Google Scholar]
- 60. Jorgensen TL, Hallas J, Friis S, et al. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. Br J Cancer. 2012;1067:1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.