Abstract
Background
Vitamins are among the most frequently used supplements (48% of US adults). However, little is known about contributions of genetic variation to their efficacy and safety. Multiple pathways link catechol-O-methyltransferase (COMT) to the vitamin E supplement, alpha-tocopherol, and cancer.
Methods
Here we determined if COMT exerted pharmacogenetic effects on cancer prevention in two randomized trials of alpha-tocopherol supplementation. Pharmacogenetic effects of common COMT rs4680 (val158met), which encodes a nonsynonymous valine-to-methionine substitution, were examined in the trial plus a 10-year post-trial follow-up (overall) period of The Women’s Genome Health Study (WGHS, N = 23 294), a 10-year alpha-tocopherol and aspirin trial with 10 years post-trial follow-up. Results were validated in a case/control (N = 2396/2235) subset of the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study (ATBC, N = 29 133). The primary outcome was total cancers. Rates of cancer types prevalent in women (colorectal, breast, lung, uterine, and lymphoma/leukemia) were also examined. All statistical tests were two-sided.
Results
Random-effects meta-analysis of rs4680 genotype strata, in WGHS and ATBC overall periods, revealed differential alpha-tocopherol effects compared with placebo: met/met (hazard ratio [HR] = 0.88; 95% confidence interval [CI] = 0.80 to 0.97; P = .01), val/met (HR = 0.99; 95% CI = 0.92 to 1.06; P = .74), and val/val (HR = 1.18; 95% CI = 1.06 to 1.31; P = .002) with a statistically significant COMT by alpha-tocopherol interaction (Pinteraction <.001). Timing of effects differed, with stronger effects in WGHS trial and ATBC post-trial.
Conclusion
Pharmacogenetic analysis of COMT and cancer prevention in two large randomized trials revealed statistically significant COMT by alpha-tocopherol interaction, such that alpha-tocopherol was beneficial among rs4680 met-allele (28.0%), but not val-allele (22.8%) homozygotes. These effects indicate the need for additional studies of genetic variation as a determinant of the benefits and possible harms of over-the-counter supplements, like alpha-tocopherol, used for health promotion.
The potential of pharmacogenomics to guide precision supplement use in chronic disease prevention remains unrealized (1). This is due, in part, to the dearth of studies with the combination of large sample size, randomization to supplement over a prolonged period, and genetic data required to find gene-supplement interaction effects. The Women’s Genome Health Study (WGHS, N = 23 294) (2,3) and Alpha-Tocopherol Beta-Carotene Cancer Prevention Study (ATBC, N = 29 133) (4), with their randomized alpha-tocopherol supplementation, genome-wide association study (GWAS) data, plus ongoing long-term observational follow-up, provide an ideal setting to evaluate alpha-tocopherol pharmacogenetic effects.
Based on its antioxidant properties, alpha-tocopherol (vitamin E) was thought to prevent tumorigenesis by mitigating free-radical damage to biomolecules such as DNA and lipids (5). Although there was considerable epidemiological and experimental evidence that antioxidants like alpha-tocopherol could reduce risk of cancer, randomized controlled trials (RCTs) failed to support a role in cancer prevention (6). This paradox was attributed to lack of efficacy, variability in patient demographics, disease state, or supplement dose and duration. Further, recent studies suggest vitamin E may accelerate tumorigenesis (7). Despite poor evidence of efficacy, vitamins remain some of the most popular supplements and are used by 48% of adults in the United States for disease prevention (8). Individual responses to supplements vary widely; however, the contribution of genetics to this variability is not well understood.
We previously showed that genetic polymorphisms in COMT modified alpha-tocopherol effects on the rate of cardiovascular disease in the WGHS (9). COMT is a phase II enzyme that transfers a methyl group from S-adenosyl methionine to the phenolic group of catecholamines, catechol-containing xenobiotics, and their metabolites (10). COMT is encoded on chromosome 22q11.2, and the increased incidence of malignancy observed in chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome) is thought to be related to the loss of COMT and its role in detoxification of carcinogens (11). Results of COMT association with a wide variety of cancers, including breast (12–18), prostate (19), lung (20), and colorectal (21,22), are inconsistent, suggesting that gene-gene or gene-environment interactions might modulate COMT’s role in carcinogenesis (23). The most extensively studied COMT single nucleotide polymorphism (SNP), rs4680 or val158met, contains a valine (val) variant that is 3–4 times more enzymatically active than the methionine (met) variant (24,25). Differences in enzymatic activity of rs4680 variants are inversely related to endogenous levels of COMT substrates (24,26). A second SNP, rs4818, in partial linkage disequilibrium, shares effects on several clinical outcomes (9,27,28) and encodes a synonymous transversion that contributes to differential mRNA stability (29). Given the link between COMT, cancer, and alpha-tocopherol, we examined how genetic variation in COMT influenced alpha-tocopherol effects on cancer incidence in two seminal alpha-tocopherol clinical trials.
Methods
Study Populations
WHS and WGHS
The Women’s Health Study (WHS) began in 1992 as a large scale, long-term, 2 × 2 factorial design trial of alpha-tocopherol (600 IU Natural Source Alpha-tocopherol Association) and low-dose aspirin (100 mg) in primary prevention of cancer and cardiovascular disease among 39 876 female health professionals aged 45 years and older at enrollment (2). WHS participants were also randomized to beta-carotene (BC), but this was terminated 2.1 years after randomization because of null findings in the Physicians’ Health Study (30). Prerandomization blood samples from 28 345 women were collected and stored for biomarker and genetic analyses. GWAS and exome scans were carried on 23 294 women (31); this primarily self-reported European ancestry subset formed the WGHS (Supplementary Figure 1, available online). Participants were followed observationally since the trial ended in 2004 with annual follow-up questionnaires assessing demographic, lifestyle, medical history, alpha-tocopherol use, and cancer endpoints (32). Follow-up rates after 17.9 years until December 31, 2015 were morbidity at 85% and mortality at 100%. Only confirmed cases of primary cancer, validated through medical record review by an endpoints committee of physicians, were used here. The primary outcome was total invasive cancer (excluding nonmelanoma skin cancer). Secondary endpoints were main site-specific cancers in women: breast, colorectal, lung, and uterine in the WGHS trial plus post-trial (overall) period. Lymphoma/leukemia was also examined.
All subjects provided informed consent before being randomly assigned, and the study was approved by the institutional review board of Brigham and Women’s Hospital and was monitored by an external data and safety monitoring board.
ATBC
COMT association with total cancer was validated in a case-control subset from the ATBC (4). ATBC was an RCT testing daily supplementation with alpha-tocopherol and BC for prevention of lung and other cancers. Details were published previously (33,34). Between 1985 and 1988, 29 133 male cigarette smokers aged 50–69 years from southwestern Finland were randomly assigned using a 2 × 2 factorial design to receive alpha-tocopherol (50 mg/50 IU) or BC (20 mg) daily for 5–8 years (median 6.1 years) through April 30, 1993. Men with preexisting cancer other than nonmelanoma skin cancer were excluded. All subjects provided informed consent, and the study was approved by institutional review boards at both the US National Cancer Institute and the Finnish National Public Health Institute. A total of 14 564 men were allocated to alpha-tocopherol. For this analysis, follow-up was censored 10 years post-trial. Cancer cases were identified through the Finnish Cancer Registry, all cases diagnosed through April 1999, and 40% of all others were confirmed by physician medical record review. Whole blood for DNA extractions was collected in 1992–1993, near the end of the trial; hence, there were fewer genotyped cases during the trial for analysis.
The primary outcome in the ATBC validation analysis was total cancer (excluding nonmelanoma skin cancer), with data drawn from GWAS scans of prostate, colorectal, pancreas, lung, bladder, and kidney cancers as well as glioma and non-Hodgkin’s lymphoma. Cases (N = 2396) were matched with controls (N = 2235) on age (±1 year) and blood collection date. Controls were alive and cancer-free at time of diagnosis of cases. Baseline characteristics of this case-control set were nearly identical to those of the entire ATBC cohort.
Genotyping
WGHS
As previously described (31), genotyping was performed with Human-Hap300 Duo “+” (Illumina, San Diego, CA) using the Infinium II protocol. Self-reported European ancestry was confirmed by a multi-dimensional scaling procedure in PLINK (35). Genotyping was successful for 99.9%-rs4680 and 99.6%-rs4818 of participants.
ATBC
Genotyping (77%) was performed using Illumina 610 K (Illumina, San Diego, CA) and remaining genotyping using Illumina 550 K, Illumina Human 660W-Quad, Illumina OmniExpress, Illumina Human 1M-Duo, and Illumina Custom Infinium genotyping array (OncoArray). All men were of self-reported European ancestry. Imputation was conducted based on 1000 G phase 1, version 3 reference panel.
Statistical Analysis
Cox proportional-hazard models were used to assess COMT rs4680 and rs4818- alpha-tocopherol effects on rates of total invasive cancer assuming a standard additive (on the log scale) genetic model. For estimates of alpha-tocopherol effects, we conducted an intention-to-treat analysis in WGHS and ATBC comparing the alpha-tocopherol treatment arms to arms without alpha-tocopherol (placebo) during the trial, 10-year post-trial, and overall periods. The interaction of COMT SNPs with alpha-tocopherol was tested using the model coefficients of an interaction term corresponding to the product of SNP genotype (0, 1, or 2) and indicator variable(s) for alpha-tocopherol allocation (0 = placebo, 1 = alpha-tocopherol). Alpha-tocopherol effects compared with placebo were evaluated in models stratified by COMT genotype. Cox models were adjusted for age and randomized BC allocation. The WGHS models were also adjusted for aspirin and fully adjusted models included the following risk factors: smoking, body mass index (BMI), family history of cancer, alcohol use, physical activity, use of hormone therapy, and menopausal status. Additional analyses were conducted to adjust for the first 10 principal components of population structure. Sensitivity analyses were conducted on off-trial use of alpha-tocopherol in the post-trial period. ATBC main models were adjusted for cigarettes smoked/day, and years smoked and interaction models included BMI and a BC-SNP interaction term. The analysis was repeated for a second COMT SNP rs4818. Statistically significant interactions were observed between COMT and aspirin in WGHS, and COMT and BC in ATBC. Analyses of COMT-aspirin and COMT-BC interactions will be reported elsewhere. The P values presented here are two-tailed and not adjusted for multiple comparisons. P less than 0.05 was considered statistically significant. Analyses of cancer subtypes were conducted such that cancer of any type led to censoring. For all models, the proportionality assumption was verified using a time-exposure interaction term. Hardy–Weinberg equilibrium was assessed by an exact test (36). Comprehensive Meta-Analysis (Biostat, NJ) was used for random-effects meta-analysis of the WGHS and ATBC gene dosage and genotype strata effects to derive an estimate of COMT effects across men and women.
Results
Baseline Characteristics of WGHS Participants
Baseline characteristics of the WGHS participants enrolled in the trial period (N = 23 294) and those who opted in for the 10-year observational period (N = 20 315) (Supplementary Figure 1, available online) did not differ between alpha-tocopherol and placebo treatment allocation (Supplementary Table 1, available online). Likewise, baseline characteristics of ATBC case-control participants (N = 4631) did not differ by alpha-tocopherol allocation (Supplementary Table 2, available online).
The rs4680 G (val) allele, which encodes the high-activity form of COMT, was the minor allele in both WGHS (minor allele frequency [MAF] = 0.47) and ATBC (MAF = 0.45) and the low-activity A (met) was the major allele (data not shown). For rs4818, the G allele was the minor allele in both WGHS (MAF = 0.39) and ATBC (MAF = 0.32), and the major allele was C. The two SNPs were in moderate linkage disequilibrium in WGHS (r2 = 0.70; D′ = 1.00), and the distributions of both SNPs were in Hardy-Weinberg Equilibrium (P > .05).
COMT Gene-Dosage Effect on Alpha-Tocopherol and Cancer Prevention
During the average 10.1 years of the WGHS trial, there were 936 cancer events in the alpha-tocopherol and 959 in the placebo treatment arms. Among those randomized to alpha-tocopherol during the trial, the COMT rs4680 val-allele was associated with a statistically significant 13% increase in rate of total cancer (HR = 1.13; 95% CI = 1.03 to 1.23; P = .009) (Table 1). In contrast, the val-allele association among women randomly assigned to placebo was lower and statistically nonsignificant (HR = 0.93; 95% CI = 0.85 to 1.02; P = .10). These differential effects were reflected in a statistically significant COMT gene by alpha-tocopherol interaction (Pinteraction = .003), which was not modified by adjustment for cancer risk factors (Pinteraction = .004). Although these COMT effects were attenuated during the 10-year post-trial observational period, the overall (trial plus 10-year post-trial) COMT gene by alpha-tocopherol interaction effect remained statistically significant (Pinteraction = .002).
Table 1.
COMT rs4680 and rs4818 gene dosage (per allele) effects on total cancer* among participants randomized to alpha-tocopherol or placebo in the trial, post-trial, and overall (trial plus 10 years) time periods of the WGHS† and ATBC‡ separately and combined
| Study and period by SNP | Alpha-tocopherol allocation |
Placebo allocation |
COMT-alpha-tocopherol Pinteraction | ||||
|---|---|---|---|---|---|---|---|
| Ev/N or Ca/Co§ | Per allele |
Ev/N or Ca/Co | Per allele |
||||
| HR (95% CI) | P‖,¶ | HR (95% CI) | P ¶ | ||||
| rs4680 | |||||||
| WGHS | |||||||
| Trial | 936/11 665 | 1.13 (1.03 to 1.23) | .009 | 959/11 629 | 0.93 (0.85 to 1.02) | .10 | .003 |
| Post-trial | 893/10 367 | 1.05 (0.95 to 1.15) | .35 | 896/10 328 | 0.96 (0.87 to 1.05) | .38 | .20 |
| Overall | 1829/11 665 | 1.09 (1.02 to 1.16) | .01 | 1855/11 629 | 0.94 (0.88 to 1.01) | .07 | .002 |
| ATBC | |||||||
| Trial | 153/2134 | 1.19 (0.95 to 1.49) | .13 | 156/2188 | 1.17 (0.93 to 1.46) | .18 | .95 |
| Post-trial | 1048/1083 | 1.12 (1.02 to 1.22) | .01 | 1039/1146 | 0.94 (0.86 to 1.03) | .17 | .007 |
| Overall | 1201/1086 | 1.13 (1.04 to 1.22) | .004 | 1195/1149 | 0.97 (0.89 to 1.05) | .40 | .01 |
| Meta-analysis# | — | 1.11 (1.05 to 1.16) | <.001 | — | 0.95 (0.90 to 1.00) | .07 | <.001 |
| rs4818 | |||||||
| WGHS | |||||||
| Trial | 936/11 665 | 1.15 (1.05 to 1.26) | .003 | 959/11 629 | 0.92 (0.84 to 1.01) | .08 | <.001 |
| Post-trial | 893/10 367 | 1.02 (0.93 to 1.13) | .63 | 896/10 328 | 0.95 (0.87 to 1.05) | .33 | .30 |
| Overall | 1829/11 665 | 1.09 (1.02 to 1.16) | .01 | 1855/11 629 | 0.94 (0.88 to 1.00) | .05 | .002 |
| ATBC | |||||||
| Trial | 153/2134 | 1.15 (0.91 to 1.46) | .25 | 156/2188 | 1.22 (0.97 to 1.54) | .09 | .67 |
| Post-trial | 1048/1083 | 1.08 (0.99 to 1.19) | .09 | 1039/1146 | 0.91 (0.83 to 1.00) | .06 | .01 |
| Overall | 1201/1086 | 1.09 (1.00 to 1.18) | .06 | 1195/1149 | 0.95 (0.87 to 1.04) | .25 | .03 |
| Meta-analysis | — | 1.09 (1.04 to 1.15) | <.001 | — | 0.94 (0.90 to 0.99) | .03 | <.001 |
Total cancer was a composite of all invasive cancers. ATBC = The Alpha-Tocopherol and Beta-Carotene Cancer Prevention Study; CI = confidence interval; HR = hazard ratio; SNP = single nucleotide polymorphism; WGHS = Women’s Genome Health Study.
WGHS main effect and interaction models were adjusted for age, aspirin, and BC randomized allocation. No differences were observed with models fully adjusted for cancer risk factors (smoking, body mass index [BMI], family history of cancer, alcohol use, physical activity, and menopausal status). The Cox proportionality assumption was verified by inclusion of time-dependent interaction terms for covariates with survival time in the models.
ATBC main effect models were adjusted for age, cigarettes smoked per day, and years smoked. Interaction models also included AT randomized allocation, BC randomized allocation, BMI and interaction terms for alpha-tocopherol by SNP and BC by SNP. The Cox proportionality assumption was verified by inclusion of time-dependent interaction terms for covariates with survival time in the models.
Ev/N = events/N in WGHS and Ca/Co = cases/controls in ATBC.
Allele key: rs4680 coded allele = val (G), reference = met (A); rs4818 coded allele = G, reference = C.
A Wald χ2 test was used to test whether Cox proportional hazard model coefficients were different from zero. P values were two-sided.
Random-effects meta-analysis of WGHS and ATBC overall time periods, no heterogeneity was observed between the studies, I2 = 0.
With fewer available cases and controls and a shorter ATBC trial (see Methods), the COMT gene-dosage effects during the trial period were statistically nonsignificant. In the post-trial period, there was a statistically significant 12% higher risk of total cancer associated with the rs4680 val-allele among those allocated to alpha-tocopherol (HR = 1.12; 95% CI = 1.02 to 1.22; P = .01) and a statistically nonsignificant 6% lower rate of total cancer in the placebo arm (HR = 0.94; 95% CI = 0.86 to 1.03; P = .17) (Table 1). These differential effects resulted in a statistically significant COMT gene by alpha-tocopherol interaction (Pinteraction = .007). In the overall period, the COMT effect among those allocated to alpha-tocopherol (HR = 1.13; 95% CI = 1.04 to 1.22; P = .004) and COMT gene by alpha-tocopherol interaction (Pinteraction = .01) were also statistically significant.
In random effects meta-analysis of the overall rs4680 val-allele effect in the WGHS and ATBC, there was a statistically significant 11% higher rate of total cancer associated with alpha-tocopherol treatment (HR = 1.11; 95% CI = 1.05 to 1.16; P < .001) and a trend toward lower rates with placebo (HR = 0.95; 95% CI = 0.90 to 1.00; P = .07), resulting in a statistically significant overall COMT gene by alpha-tocopherol interaction (Pinteraction < .001) (Table 1). Results were similar for the other COMT SNP, rs4818.
Alpha-Tocopherol Effects on Total Cancer in COMT Genotype Strata
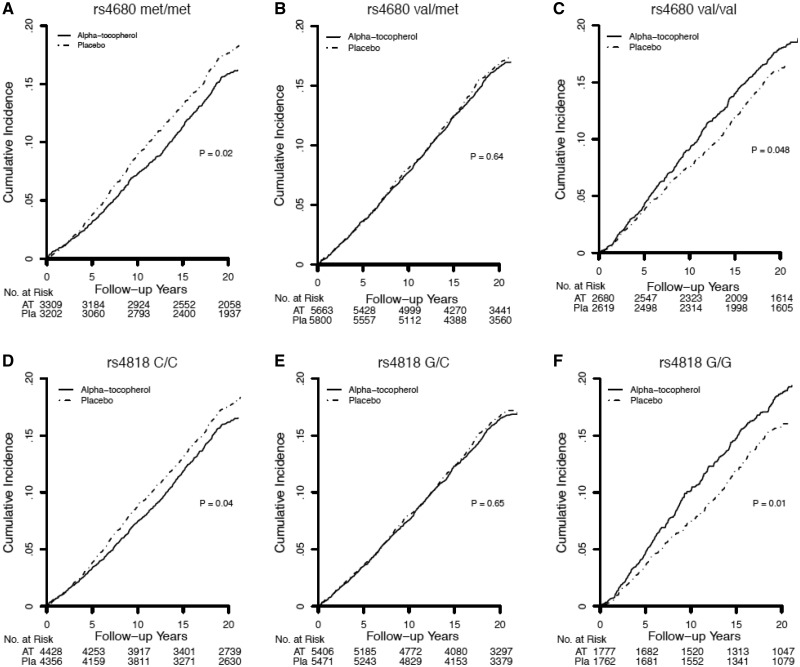
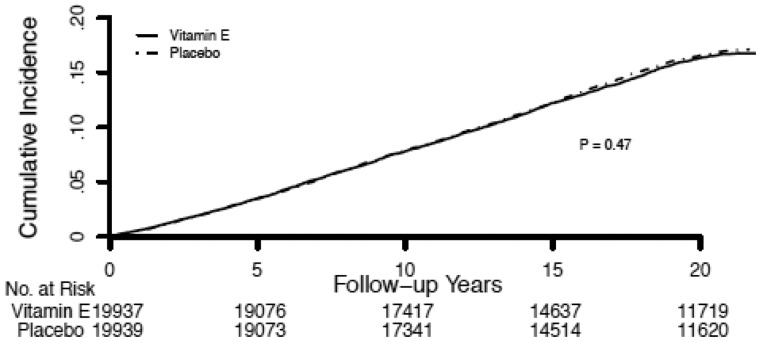
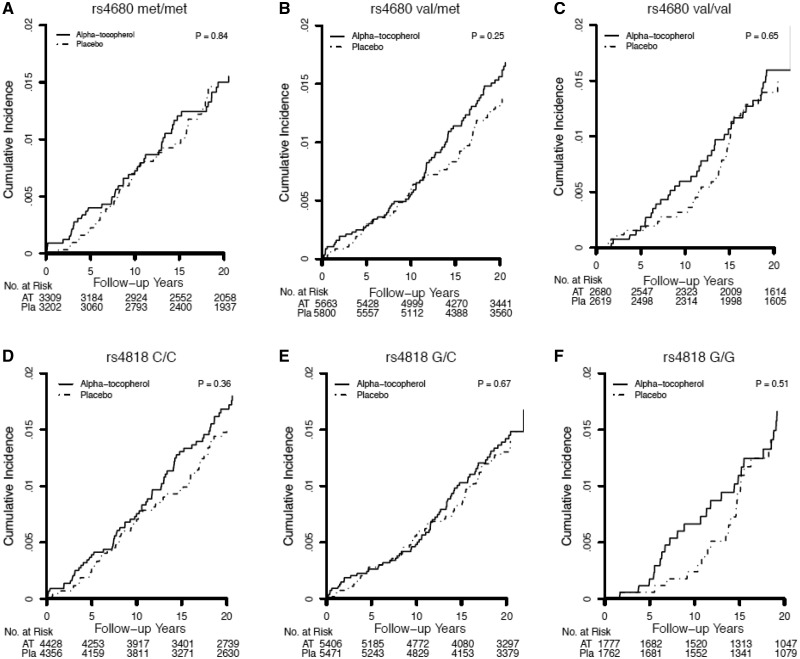
In both the WGHS and ATBC, there were differential effects of randomized alpha-tocopherol by COMT genotype and, consistent with the gene-dosage results, the strength of the effects differed by study period. In the WGHS, genotype-dependent effects were evident during the trial period and attenuated during the post-trial period (Table 2; Figure 1). These effects were not modified when post-trial alpha-tocopherol use or principal components for population structure were included in the models. In the overall period, rates of total cancer were 15% higher for women homozygous for the val-allele randomized to vitamin-E compared with placebo (val/val: HR = 1.15; 95% CI = 1.00 to 1.31; P = .047). Conversely, for women homozygous for the met-allele, rates of total cancer were 14% statistically significantly lower (met/met: HR = 0.86; 95% CI = 0.76 to 0.98; P = .02). There was no effect among women who were heterozygous (val/met: HR = 0.98; 95% CI = 0.89 to 1.07; P = .62). These findings were in marked contrast to the null effect previously reported for alpha-tocopherol without accounting for COMT gene variation during the trial period (2) and the overall period reported here (HR = 0.98; 95% CI = 0.93 to 1.03; P = .48) (Figure 2).
Table 2.
Alpha-tocopherol vs placebo effects on total cancer* in COMT rs4680 and rs4818 genotype strata in the trial, post-trial, and overall (trial plus 10 years) time periods of the WGHS† and ATBC‡ separately and combined
| Study period by SNP and genotype | Alpha-tocopherol | Placebo | Alpha-tocopherol vs placebo |
|
|---|---|---|---|---|
| Ev/N or Ca/Co§ | Ev/N or Ca/Co | HR (95% CI) | P ‖ | |
| rs4680 | ||||
| WGHS | ||||
| Trial | ||||
| met/met | 243/3309 | 286/3202 | 0.82 (0.69 to 0.97) | .02 |
| val/met | 446/5663 | 469/5800 | 0.97 (0.85 to 1.11) | .66 |
| val/val | 246/2680 | 203/2619 | 1.19 (0.99 to 1.44) | .06 |
| Post-trial | ||||
| met/met | 247/2966 | 252/2812 | 0.92 (0.77 to 1.09) | .34 |
| val/met | 433/5042 | 451/5162 | 0.98 (0.86 to 1.12) | .78 |
| val/val | 212/2348 | 193/2347 | 1.10 (0.90 to 1.33) | .36 |
| Overall | ||||
| met/met | 490/3309 | 538/3202 | 0.86 (0.76 to 0.98) | .02 |
| val/met | 879/5663 | 920/5800 | 0.98 (0.89 to 1.07) | .62 |
| val/val | 458/2680 | 396/2619 | 1.15 (1.00 to 1.31) | .047 |
| ATBC | ||||
| Trial | ||||
| met/met | 35/639 | 37/653 | 0.94 (0.59 to 1.49) | .80 |
| val/met | 84/1060 | 84/1093 | 1.01 (0.75 to 1.37) | .94 |
| val/val | 34/435 | 35/442 | 0.98 (0.61 to 1.57) | .92 |
| Post-trial | ||||
| met/met | 301/337 | 325/328 | 0.91 (0.78 to 1.07) | .26 |
| val/met | 500/558 | 508/582 | 1.00 (0.88 to 1.13) | .95 |
| val/val | 247/188 | 206/236 | 1.29 (1.07 to 1.55) | .007 |
| Overall | ||||
| met/met | 336/338 | 362/328 | 0.91 (0.79 to 1.06) | .22 |
| val/met | 584/560 | 592/585 | 1.00 (0.89 to 1.12) | .97 |
| val/val | 281/188 | 241/236 | 1.23 (1.04 to 1.47) | .02 |
| Meta-analysis¶ | ||||
| Overall | ||||
| met/met | — | — | 0.88 (0.80 to 0.97) | .01 |
| val/met | — | — | 0.99 (0.92 to 1.06) | .74 |
| val/val | — | — | 1.18 (1.06 to 1.31) | .002 |
| rs4818 | ||||
| WGHS | ||||
| Trial | ||||
| C/C | 334/4428 | 385/4356 | 0.85 (0.73 to 0.98) | .03 |
| C/G | 417/5406 | 438/5471 | 0.96 (0.84 to 1.10) | .59 |
| G/G | 181/1777 | 134/1762 | 1.36 (1.09 to 1.70) | .007 |
| Post-trial | ||||
| C/C | 338/3964 | 343/3832 | 0.94 (0.81 to 1.10) | .45 |
| C/G | 417/4820 | 426/4886 | 0.99 (0.87 to 1.14) | .91 |
| G/G | 136/1535 | 126/1575 | 1.11 (0.87 to 1.41) | .42 |
| Overall | ||||
| C/C | 672/4428 | 728/4356 | 0.89 (0.80 to 0.99) | .03 |
| C/G | 834/5406 | 864/5471 | 0.98 (0.89 to 1.08) | .65 |
| G/G | 317/1777 | 260/1762 | 1.24 (1.05 to 1.46) | .01 |
| ATBC | ||||
| Trial | ||||
| C/C | 60/1003 | 63/1036 | 0.97 (0.68 to 1.39) | .88 |
| C/G | 79/922 | 73/944 | 1.05 (0.77 to 1.45) | .76 |
| G/G | 14/209 | 20/208 | 0.72 (0.36 to 1.42) | .34 |
| Post-trial | ||||
| C/C | 482/519 | 512/522 | 0.95 (0.84 to 1.08) | .42 |
| C/G | 444/477 | 433/510 | 1.03 (0.90 to 1.18) | .68 |
| G/G | 122/87 | 94/114 | 1.49 (1.14 to 1.95) | .004 |
| Overall | ||||
| C/C | 542/521 | 575/524 | 0.95 (0.85 to 1.07) | .42 |
| C/G | 523/478 | 506/511 | 1.03 (0.91 to 1.16) | .67 |
| G/G | 136/87 | 114/114 | 1.32 (1.03 to 1.69) | .03 |
| Meta-analysis | ||||
| Overall | ||||
| C/C | — | — | 0.92 (0.85 to 0.99) | .03 |
| C/G | — | — | 1.00 (0.89 to 1.08) | .93 |
| G/G | — | — | 1.29 (1.10 to 1.45) | <.001 |
Total cancer was a composite of all invasive cancers. ATBC = Alpha-Tocopherol and Beta-Carotene Cancer Study; CI = confidence interval; HR = hazard ratio; SNP = single nucleotide polymorphism; WGHS = Women’s Genome Health Study.
In WGHS models no differences were observed, with models fully adjusted for cancer risk factors (smoking, body mass index [BMI], family history of cancer, alcohol use, physical activity, and menopausal status) or models adjusted for post-trial alpha-tocopherol use. The Cox proportionality assumption was verified by inclusion of time-dependent interaction terms for covariates with survival time in the models.
ATBC models were adjusted for age, BC allocation, cigarettes smoked per day, and years smoked. The Cox proportionality assumption was verified by inclusion of time-dependent interaction terms for covariates with survival time in the models.
Ev/N = events/N in WGHS and Ca/Co = cases/controls in ATBC.
A Wald χ2 test was used to test whether Cox proportional hazard model coefficients were different from zero. P values were two-sided.
Random-effects meta-analysis of WGHS and ATBC overall time periods, no heterogeneity was observed between the studies, I2 = 0.
Figure 1.
Kaplan-Meier curves comparing cumulative incidence of total cancer (all invasive cancers) by COMT rs4680 (A–C) and rs4818 (D–F) genotype in Women’s Genome Health Study participants randomized to alpha-tocopherol or placebo during the overall (trial plus 10 years) time period. Differences between genotype strata were tested using the log-rank test.
Figure 2.
Kaplan-Meier curves of cumulative incidence of total cancer during the original Women’s Health Study (N = 39 876) overall (trial plus post-trial) period by randomized assignment to alpha-tocopherol or placebo. Differences between drug treatment strata were tested using the log-rank test; P values were two-sided.
COMT genotype strata effects on alpha-tocopherol during the ATBC trial period were statistically nonsignificant. However, differential alpha-tocopherol effects were evident in the post-trial period. The associations were directionally similar to WGHS, with a statistically significant 29% increase in rate of total cancer among val-allele homozygotes (val/val: HR = 1.29; 95% CI = 1.07 to 1.55; P = .007) compared with placebo, no effect among heterozygotes (val/met: HR = 1.00; 95% CI = 0.88 to 1.13; P = .95), and the 9% lower rate among met-allele homozygotes was statistically nonsignificant (met/met: HR = 0.91; 95% CI = 0.78 to 1.07; P = .26) (Table 2). The statistically significant val/val association in the overall period was driven by the post-trial period.
To evaluate the overall effect of alpha-tocopherol compared with placebo in both genders, we conducted a random effects meta-analysis of COMT genotype strata in the overall period in the WGHS and ATBC (Table 2). Among the 22% of individuals homozygous for the val-allele, random assignment to alpha-tocopherol was associated with an 18% higher rate of total cancer (val/val: HR = 1.18; 95% CI = 1.06 to 1.31; P = .002) compared with placebo. In contrast, among the 28% of individuals homozygous for the met-allele, the rate was 12% lower (met/met: HR = 0.88; 95% CI = 0.80 to 0.97; P = .01). There was no association among the remaining 50.0% who were val/met (val/met: HR = 0.99; 95% CI = 0.92 to 1.06; P = .74). Results for rs4818 were similar.
Cancer Types
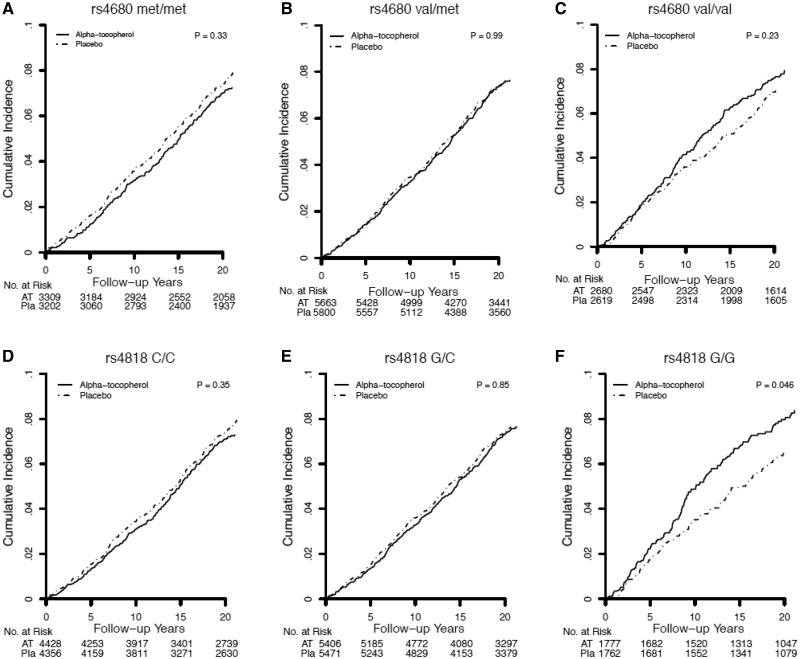
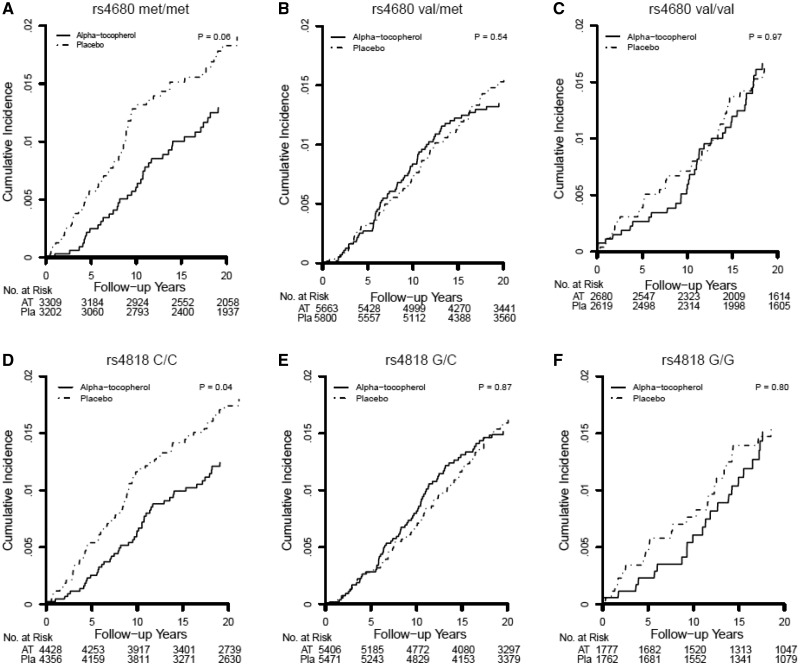
The pattern of alpha-tocopherol effects on rates of individual invasive cancers (Figures 3–6) and lymphoma/leukemia (Supplementary Figure 2, available online) was generally consistent with that observed for total invasive cancer in the WGHS. Among met-allele homozygotes, rates of breast and colorectal cancer were lower with alpha-tocopherol compared with placebo though not statistically significant. For val/met individuals, rates of lung cancer and lymphoma/leukemia were nonsignificantly higher with alpha-tocopherol. Among val/val individuals, rates of breast, lung, uterine, and lymphoma/leukemia but not colorectal cancer were not statistically significantly higher with alpha-tocopherol.
Figure 3.
Kaplan-Meier curves comparing cumulative incidence of first cancer, which is breast cancer, in COMT rs4680 (A–C) and rs4818 (D–F) genotype strata among Women’s Genome Health Study participants randomized to alpha-tocopherol or placebo in the overall period (trial plus 10 years). Differences between genotype strata were tested using the log-rank test; P values were two-sided.
Figure 4.
Kaplan-Meier curves comparing cumulative incidence of first cancer, which is colorectal cancer, in COMT rs4680 (A–C) and rs4818 (D–F) genotype strata among Women’s Genome Health Study participants randomized to alpha-tocopherol or placebo in the overall period (trial plus 10 years). Differences between genotype strata were tested using the log-rank test; P values were two-sided.
Figure 5.
Kaplan-Meier curves comparing cumulative incidence of first cancer, which is lung cancer, in COMT rs4680 (A–C) and rs4818 (D–F) genotype strata among Women’s Genome Health Study participants randomized to alpha-tocopherol or placebo in the overall period (trial plus 10 years). Differences between genotype strata were tested using the log-rank test; P values were two-sided.
Figure 6.
Kaplan-Meier curves comparing cumulative incidence of first cancer, which is uterine cancer, in COMT rs4680 (A–C) and rs4818 (D–F) genotype strata among Women’s Genome Health Study participants randomized to alpha-tocopherol or placebo in the overall period (trial plus 10 years). Differences between genotype strata were tested using the log-rank test; P values were two-sided.
Discussion
COMT statistically significantly modified alpha-tocopherol effects on cancer incidence, in a gene dose-response manner, in two independent RCTs of alpha-tocopherol supplementation with physician-confirmed cancer outcomes and 10 years of observational follow-up. Although originally the two RCTs examined in this study found no overall benefit of alpha-tocopherol for cancer prevention (2,4), among individuals homozygous for high-activity COMT rs4680 val-allele, assignment to alpha-tocopherol was associated with increased cancer risk, had no effect on heterozygotes, and was associated with reduced cancer risk among low-activity met-allele homozygotes. These COMT alpha-tocopherol interactions were consistent across the trials, and hence among men and women, and were also unchanged by multivariable adjustment. These results provide the first replicated evidence that pharmacogenetic effects might contribute to cancer-increasing or -decreasing effects of alpha-tocopherol supplementation.
Despite the many differences between the WGHS and ATBC trial designs including sex, age at entry, smoking status, geographical location, alpha-tocopherol dose, randomization to aspirin and BC, and duration, the long-term COMT main and alpha-tocopherol interaction effects in the trial plus 10-year periods were strikingly similar. Whereas the WGHS women and ATBC men were healthy at outset, the ATBC men were older and exclusively smokers, putting them at higher risk of cancer and suggesting these results may have broad applicability to both healthy and cancer-prone individuals. Alpha-tocopherol dose and timing of use differed in WGHS (alternate day 600 IU) and ATBC (50 IU daily), suggesting that a broad range of doses may be modified by COMT genotype. Hence, COMT genotype is a potential modifier of alpha-tocopherol effectiveness. In the WGHS, the COMT effect was statistically significant in the trial and attenuated in the post-trial period; in ATBC the reverse was true. It is difficult to assess the difference in the trial periods because in ATBC there were fewer available genotyped cases during the trial period (see Methods), and differences in total cancer between alpha-tocopherol and placebo tended to emerge 3–5 years into the trial. Hence, with the shorter ATBC trial, there may have been less time for the alpha-tocopherol effects to emerge. In the post-trial period, whereas the WGHS alpha-tocopherol effects were attenuated, in ATBC they were robust and statistically significant. One possible explanation for this is that, whereas WGHS events included all invasive cancers minus nonmelanoma skin cancers, ATBC only had those cancer types that were genotyped. Another possibility is that this difference could reflect COMT sexual dimorphism related to COMT metabolism of catechol estrogens, which is evident in multiple differential phenotypes among men and women (37).
This study has several limitations. Both the WGHS and ATBC consist of participants of European ancestry. Consequently, the applicability of these findings to other races and ethnicities remains to be determined. Dietary contribution to alpha-tocopherol intake was not assessed, hence this study does not inform dietary effects of alpha-tocopherol. Finally, it is possible that other common variants in linkage disequilibrium with COMT are responsible for these effects.
Although our study provides some indication that COMT- alpha-tocopherol interaction may affect a cross-section of cancer types, larger studies are needed to understand which cancers are affected. Still, our findings of COMT- alpha-tocopherol effect modification offer one explanation for inconsistent findings in the literature on COMT association with multiple cancers (38,39). Although the mechanism underlying COMT- alpha-tocopherol interactions is not known, the association between COMT and metabolites of vitamin C (40,41), an antioxidant with links to alpha-tocopherol (42,43), is striking (P < 10–106). Hence, future studies should examine COMT effects in oxidative stress and potential interactions with vitamin C. Further, studies are warranted to understand the potential for catechol-containing drugs and metabolites or drugs that target catecholamine pathways to perturb COMT function and in so doing modify cancer risk (9,44,45).
Vitamins are taken by 48% of US adults and are used popularly to prevent disease (8). Here, we report the pharmacogenetic effects of COMT and alpha-tocopherol on cancer prevention in two large randomized trials resulting in beneficial effects among rs4680 met-allele, but not among val-allele homozygotes. In an era when interest in supplements is increasing and optimal use is under debate, these novel findings are a strong exemplar of the importance of considering genetic composition in evaluating clinical efficacy and using pharmacogenomics to guide development of precision medicine strategies.
Funding
The WHS is supported by grants CA047988, HL043851, HL080467, HL099355, and UM1 CA182913 from the NIH (Bethesda, MD). This study was also funded by a research supplement from the Office of Dietary Supplements. KTH is supported by NHLBI K01HL130625 and Harvard CATALYST faculty fellowship.
TJK is supported by NCCIH 2K24 AT004095. The ATBC Study is supported by the Intramural Research Program of the US National Cancer Institute, National Institutes of Health and by US Public Health Service contract HHSN261201500005C from the National Cancer Institute, Department of Health and Human Services.
Notes
Affiliations of authors: Divisions of Preventive Medicine (KTH, JEB, MVM, PW, PMR, HDS, NRC, DIC) and Cardiovascular Medicine (EMB), Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA; Division of General Medicine and Primary Care, Beth Israel Deaconess Medical Center, Boston, MA (KJM, TJK); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD (SJW, DA).
The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication. The authors have no conflicts of interest to disclose.
Supplementary Material
References
- 1. Preuss C, Das MK, Pathak YV.. Genomics and natural products: role of bioinformatics and recent patents. Recent Pat Biotechnol. 2014;82:144–151. [DOI] [PubMed] [Google Scholar]
- 2. Lee IM, Cook NR, Gaziano JM, et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;2941:56–65. [DOI] [PubMed] [Google Scholar]
- 3. Ridker PM, Cook NR, Lee IM, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;35213:1293–1304. [DOI] [PubMed] [Google Scholar]
- 4. Alpha-Tocopherol BCCPSG. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;33015:1029–1035. [DOI] [PubMed] [Google Scholar]
- 5. Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: impact on human health. Phcog Rev. 2010;48:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fortmann SP, Burda BU, Senger CA, et al. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;15912:824–834. [DOI] [PubMed] [Google Scholar]
- 7. Sayin VI, Ibrahim MX, Larsson E, et al. Antioxidants accelerate lung cancer progression in mice. Sci Transl Med. 2014;6221:221ra15.. [DOI] [PubMed] [Google Scholar]
- 8. Kantor ED, Rehm CD, Du M, et al. Trends in dietary supplement use among US adults from 1999-2012. JAMA. 2016;31614:1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall KT, Nelson CP, Davis RB, et al. Polymorphisms in catechol-O-methyltransferase modify treatment effects of aspirin on risk of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2014;349:2160–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Axelrod J, Tomchick R.. Enzymatic O-methylation of epinephrine and other catechols. J Biol Chem. 1958;2333:702–705. [PubMed] [Google Scholar]
- 11. Lambert MP, Arulselvan A, Schott A, et al. The 22q11.2 deletion syndrome: cancer predisposition, platelet abnormalities and cytopenias. Am J Med Genet A. 2017; doi:10.1002/ajmg.a.38474. [DOI] [PubMed] [Google Scholar]
- 12. Chenevix-Trench G, Barnes DR, Antoniou AC.. More on co-occurrence of COMT and BRCA1/2 variants in a population. N Engl J Med. 2017;3778:793–795. [DOI] [PubMed] [Google Scholar]
- 13. Movassagh M, Mudvari P, Horvath A.. Co-occurrence of COMT and BRCA1/2 variants in a population. N Engl J Med. 2017;37621:2090–2091. [DOI] [PubMed] [Google Scholar]
- 14. Brauner EV, Loft S, Wellejus A, et al. Adipose tissue PCB levels and CYP1B1 and COMT genotypes in relation to breast cancer risk in postmenopausal Danish women. Int J Environ Health Res. 2014;243:256–268. [DOI] [PubMed] [Google Scholar]
- 15. Day FR, Ruth KS, Thompson DJ, et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;4711:1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji Y, Olson J, Zhang J, et al. Breast cancer risk reduction and membrane-bound catechol O-methyltransferase genetic polymorphisms. Cancer Res. 2008;6814:5997–6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kuo SH, Yang SY, You SL, et al. Polymorphisms of ESR1, UGT1A1, HCN1, MAP3K1 and CYP2B6 are associated with the prognosis of hormone receptor-positive early breast cancer. Oncotarget. 2017;813:20925–20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li K, Li W, Zou H.. Catechol-O-methyltransferase Val158Met polymorphism and breast cancer risk in Asian population. Tumour Biol. 2014;353:2343–2350. [DOI] [PubMed] [Google Scholar]
- 19. Xiao L, Tong M, Jin Y, et al. The l58Val/Met polymorphism of catechol-O-methyl transferase gene and prostate cancer risk: a meta-analysis. Mol Biol Rep. 2013;402:1835–1841. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Hua S, Zhang A, et al. Association between polymorphisms in COMT, PLCH1, and CYP17A1, and non-small-cell lung cancer risk in Chinese nonsmokers. Clin Lung Cancer. 2013;141:45–49. [DOI] [PubMed] [Google Scholar]
- 21. Landi S, Gemignani F, Moreno V, et al. A comprehensive analysis of phase I and phase II metabolism gene polymorphisms and risk of colorectal cancer. Pharmacogenet Genomics. 2005;158:535–546. [DOI] [PubMed] [Google Scholar]
- 22. Passarelli MN, Newcomb PA, Makar KW, et al. No association between germline variation in catechol-O-methyltransferase and colorectal cancer survival in postmenopausal women. Menopause. 2014;214:415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sak K. The Val158Met polymorphism in COMT gene and cancer risk: role of endogenous and exogenous catechols. Drug Metab Rev. 2017;491:56–83. [DOI] [PubMed] [Google Scholar]
- 24. Chen J, Lipska BK, Halim N, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;755:807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lachman HM, Papolos DF, Saito T, et al. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;63:243–250. [DOI] [PubMed] [Google Scholar]
- 26. Dawling S, Roodi N, Mernaugh RL, et al. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: comparison of wild-type and variant COMT isoforms. Cancer Res. 2001;6118:6716–6722. [PubMed] [Google Scholar]
- 27. Białecka M, Kurzawski M, Roszmann A, et al. Association of COMT, MTHFR, and SLC19A1(RFC-1) polymorphisms with homocysteine blood levels and cognitive impairment in Parkinson’s disease. Pharmacogenet Genomics. 2012;2210:716–724. [DOI] [PubMed] [Google Scholar]
- 28. Fijal B, Perlis RH, Heinloth AN, et al. The association of single nucleotide polymorphisms in the catechol-O-methyltransferase gene and pain scores in female patients with major depressive disorder. J Pain. 2010;119:910–915. [DOI] [PubMed] [Google Scholar]
- 29. Nackley AG, Shabalina SA, Tchivileva IE, et al. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;3145807:1930–1933. [DOI] [PubMed] [Google Scholar]
- 30. Lee IM, Cook NR, Manson JE, et al. Beta-carotene supplementation and incidence of cancer and cardiovascular disease: the Women’s Health Study. J Natl Cancer Inst. 1999;9124:2102–2106. [DOI] [PubMed] [Google Scholar]
- 31. Ridker PM, Chasman DI, Zee RY, et al. Rationale, design, and methodology of the Women’s Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin Chem. 2008;542:249–255. [DOI] [PubMed] [Google Scholar]
- 32. Cook NR, Lee IM, Zhang SM, et al. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Ann Intern Med. 2013;1592:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Albanes D, Heinonen OP, Huttunen JK, et al. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995;626:1427S–1430S. [DOI] [PubMed] [Google Scholar]
- 34. The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. The ATBC Cancer Prevention Study Group. Ann Epidemiol. 1994;41:1–10. [DOI] [PubMed] [Google Scholar]
- 35. Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;813:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wigginton JE, Cutler DJ, Abecasis GR.. A note on exact tests of Hardy-Weinberg equilibrium. Am J Hum Genet. 2005;765:887–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harrison PJ, Tunbridge EM.. Catechol-O-methyltransferase (COMT): a gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;3313:3037–3045. [DOI] [PubMed] [Google Scholar]
- 38. Bergman-Jungestrom M, Wingren S.. Catechol-O-methyltransferase (COMT) gene polymorphism and breast cancer risk in young women. Br J Cancer. 2001;856:859–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peterson NB, Trentham-Dietz A, Garcia-Closas M, et al. Association of COMT haplotypes and breast cancer risk in Caucasian women. Anticancer Res. 2010;301:217–220. [PMC free article] [PubMed] [Google Scholar]
- 40. Shin SY, Fauman EB, Petersen AK, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;466:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suhre K, Shin SY, Petersen AK, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;4777362:54–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Packer JE, Slater TF, Willson RL.. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature. 1979;2785706:737–738. [DOI] [PubMed] [Google Scholar]
- 43. Niki E. Interaction of ascorbate and alpha-tocopherol. Ann N Y Acad Sci. 1987;498(1):186–199. [DOI] [PubMed] [Google Scholar]
- 44. Hall KT, Kossowsky J, Oberlander TF, et al. Genetic variation in catechol-O-methyltransferase modifies effects of clonidine treatment in chronic fatigue syndrome. Pharmacogenomics J. 2016;165:454–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schacht JP. COMT val158met moderation of dopaminergic drug effects on cognitive function: a critical review. Pharmacogenomics J. 2016;165:430–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.