Abstract
Purpose
To report a case of adult-onset Coats’ disease that had worsening of macular edema and progressive macular traction following cryotherapy and repeated intravitreal bevacizumab injections due to formation of a secondary epiretinal membrane which only improved following pars plana vitrectomy and membrane peeling.
Observations
A 35-year-old male presented with diminution of vision in his left eye and was found to have localized telangiectatic retinal vessels and aneurysmal dilatations with massive exudation and cystoid macular edema. He was diagnosed as adult-onset Coats’ disease and treated with cryotherapy and a concomitant intravitreal injection of 2.5 mg bevacizumab followed by 3 monthly intravitreal injections of 2.5 mg bevacizumab and a single injection of 4 mg triamcinolone acetonide. Partial obliteration of the telangiectatic vessels and aneurysmal dilatations with improvement in surrounding lipid and fluid exudate was achieved, however, this was associated with progressive worsening of macular edema and macular traction due to formation of an epiretinal membrane which only improved following vitrectomy and membrane peeling. Effect of therapy at each stage was evaluated using visual acuity testing, fundus examination, fundus fluorescein angiography, and optical coherence tomography.
Conclusion and Importance
A secondary epiretinal membrane can develop following treatment of adult-onset Coats’ disease and cause traction especially when combining cryotherapy with bevacizumab injections. Vitrectomy in such cases with membrane peeling may result in improvement of anatomical and functional outcomes.
Keywords: Adult-onset coats' disease, Bevacizumab, Cryotherapy, NDP gene, Secondary epiretinal membrane, Vitrectomy
1. Introduction
Coats’ disease is a condition characterized by the development of abnormal telangiectatic retinal vessels associated with aneurysmal dilatations and ischemia which result in massive intra and subretinal exudation of fluid and lipid.1 It is usually unilateral and more common in males. It can occur in children or adults and is suspected to be caused by a somatic mutation in the NDP gene.2
Vascular endothelial growth factor (VEGF) levels have been found to be markedly elevated in patients with Coats’ disease and is thought to play an important role in the pathogenesis of the disease.3 Therapy depends on the disease stage and consists of ablation of the telangiectatic vessels using laser photocoagulation or cryotherapy, and injection of anti-VEGF agents or steroids to treat the exudation and macular edema.4 Vitrectomy is reserved for advanced cases or cases associated with an epiretinal membrane (ERM).4,5
We describe a patient with adult-onset Coats’ disease who had progressive worsening of his macular edema and macular traction following treatment with cryotherapy and intravitreal injections of bevacizumab, despite significant obliteration of the telangiectatic vessels and aneurysmal dilatations, due to formation of an epiretinal membrane that responded favorably to vitrectomy with membrane peeling.
2. Case report
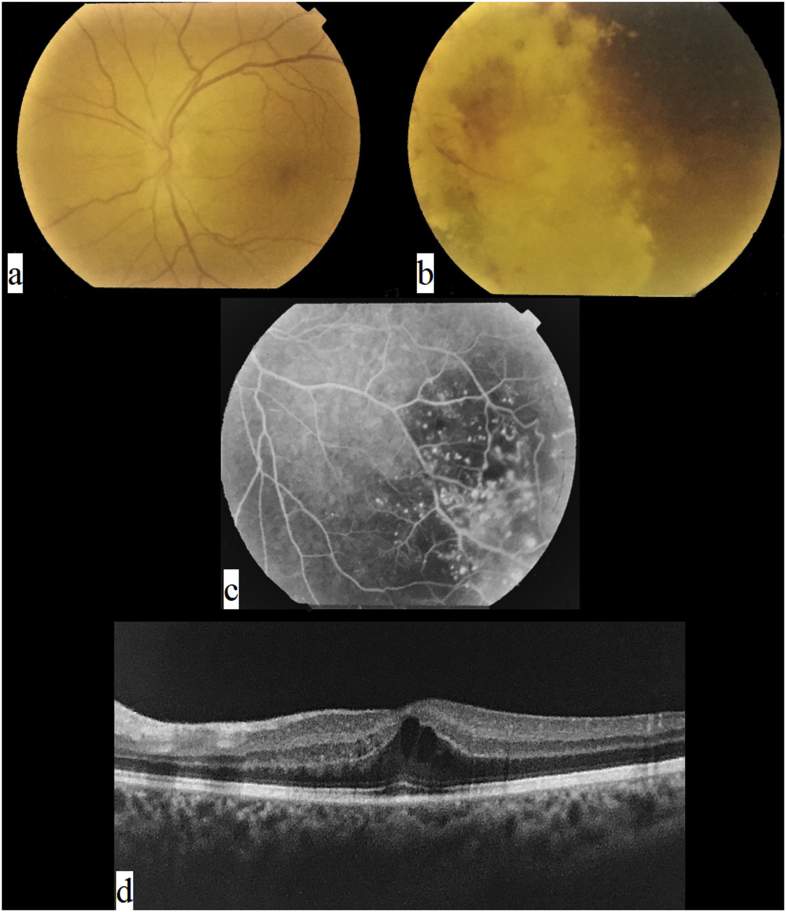
A 35-year-old male presented with gradually progressive diminution of vision in his left eye for 3 months. The patient had no past ocular or medical history. Examination revealed a corrected distance visual acuity (CDVA) of 20/60 in the left eye and 20/20 in the right. Intraocular pressure was 15 mmHg in both eyes. Anterior segment exam was normal in both eyes. Posterior segment examination of the left eye revealed cystoid macular edema (CME) (Fig. 1a) and localized telangiectatic retinal vessels with aneurysmal dilatations in the midperipheral inferotemporal quadrant with marked yellowish exudation and subretinal fluid (Fig. 1b). Posterior segment exam of the right eye was normal. Fundus fluorescein angiography (FFA) was performed and revealed left CME and telangiectatic vessels in the midperipheral inferotemporal quadrant with multiple aneurysmal dilatations and areas of capillary drop out (Fig. 1c). Optical coherence tomography (OCT) of the left macular area allowed quantitative and qualitative assessment of the CME and revealed no ERM or traction (Fig. 1d). This picture was consistent with a diagnosis of Stage 3A1 adult-onset Coats’ disease.
Fig. 1.
Baseline imaging of the patient. a. Posterior pole photograph showing cystoid macular edema, with no lipid exudate or traction affecting foveal area. b. Presence of lipid exudates and subretinal fluid in the inferotemporal midperiphery with telangiectatic vessels and aneurysmal dilations. c. Fluorescein angiography showing telangiectatic vessels and aneurysmal dilations with leakage in the inferotemporal midperiphery associated with areas of ischemia. d. Optical coherence tomography scan of the macula showing cystoid macular edema.
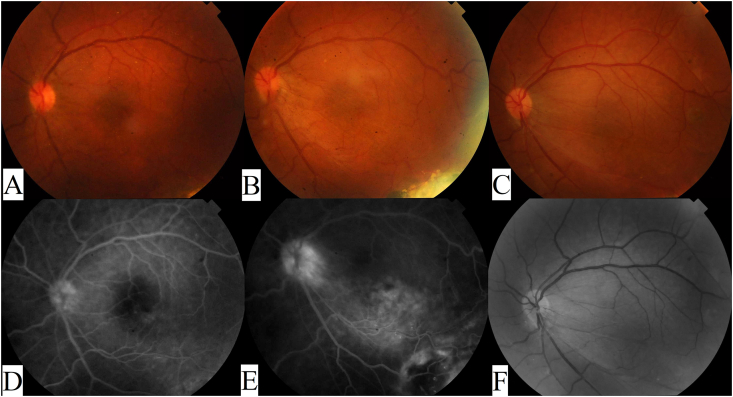
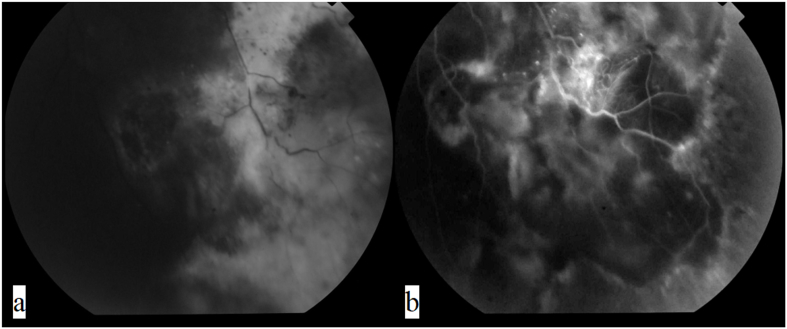
After discussing treatment options with the patient, the decision taken was to perform cryotherapy, using a double freeze-thaw technique, to treat the aneurysmal dilatations and ischemic areas since it was thought that the marked exudation and subretinal fluid would impede effective laser treatment. Off-label intravitreal bevacizumab 2.5 mg injection was given at the same time to treat the CME. One month after treatment, CDVA worsened to 20/100, fundus exam showed +1 vitreous cells (Fig. 2A) and inferotemporal chorio-retinal scarring (Fig. 3a), FFA showed decreased leakage and partial obliteration of the telangiectatic vessels and aneurysmal dilatations (Fig. 2, Fig. 3b), and OCT showed increased CME (Fig. 4A). A second intravitreal injection of bevacizumab was given, and OCT was done one month after the injection which showed improving CME with mild inner retinal corrugations (Fig. 4B), however, CDVA did not improve. Two more monthly injections of bevacizumab were given with no improvement in CDVA, and progressive worsening of CME and traction was seen clinically and by FFA which showed increased leakage at the macula and optic disc (Fig. 2B and E). Intravitreal injection of 4 mg triamcinolone acetonide was done but was not effective in treating the CME with CDVA dropping to 20/200 and OCT revealing further worsening of retinal traction with subretinal fluid (Fig. 4C and D). Because of worsening CDVA and progressive traction by an ERM, pars plana vitrectomy with peeling of the ERM and internal limiting membrane (ILM) was done (See Video 1 showing vitrectomy with peeling of the epiretinal and internal limiting membrane). Briefly, following core vitrectomy and induction of posterior vitreous detachment using triamcinolone acetonide, the ERM and ILM were stained and peeled up to the scar of the previous cryotherapy followed by trimming of the firmly adherent membrane parts, retinal massage was then performed by using a Tano membrane scraper and by injecting a perfluorocarbon liquid to help restore macular anatomy, then fluid-air exchange was done with post-operative prone positioning of the patient for 2 days. CDVA improved to 20/100 one-month post-operatively, fundus examination and red free photography revealed decreased traction (Fig. 2C and F), and OCT showed resolution of the CME and subretinal fluid with no apparent traction (Fig. 4E). The patient continued to do well and 3 months later his CDVA was 20/80.
Fig. 2.
Fundus photography and fluorescein angiography of the posterior pole. A and D. One-month after cryotherapy and intravitreal bevacizumab there was mild vitritis with no traction or leakage on FFA. B and E. Four-months after treatment with 3 additional monthly bevacizumab injections, macular traction was seen towards the area of chorio-retinal scarring and FFA showed increased leakage at the macula and optic disc. C and F. One-month after vitrectomy with membrane peeling, macular traction improved as seen on color and red-free photographs. . (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 3.
Partial obliteration of telangiectatic vessels and aneurysmal dilations one month after cryotherapy and concomitant intravitreal bevacizumab injection with decreased lipid and fluid exudation is seen on a. Fundus photography and b. Fluorescein angiography.
Fig. 4.
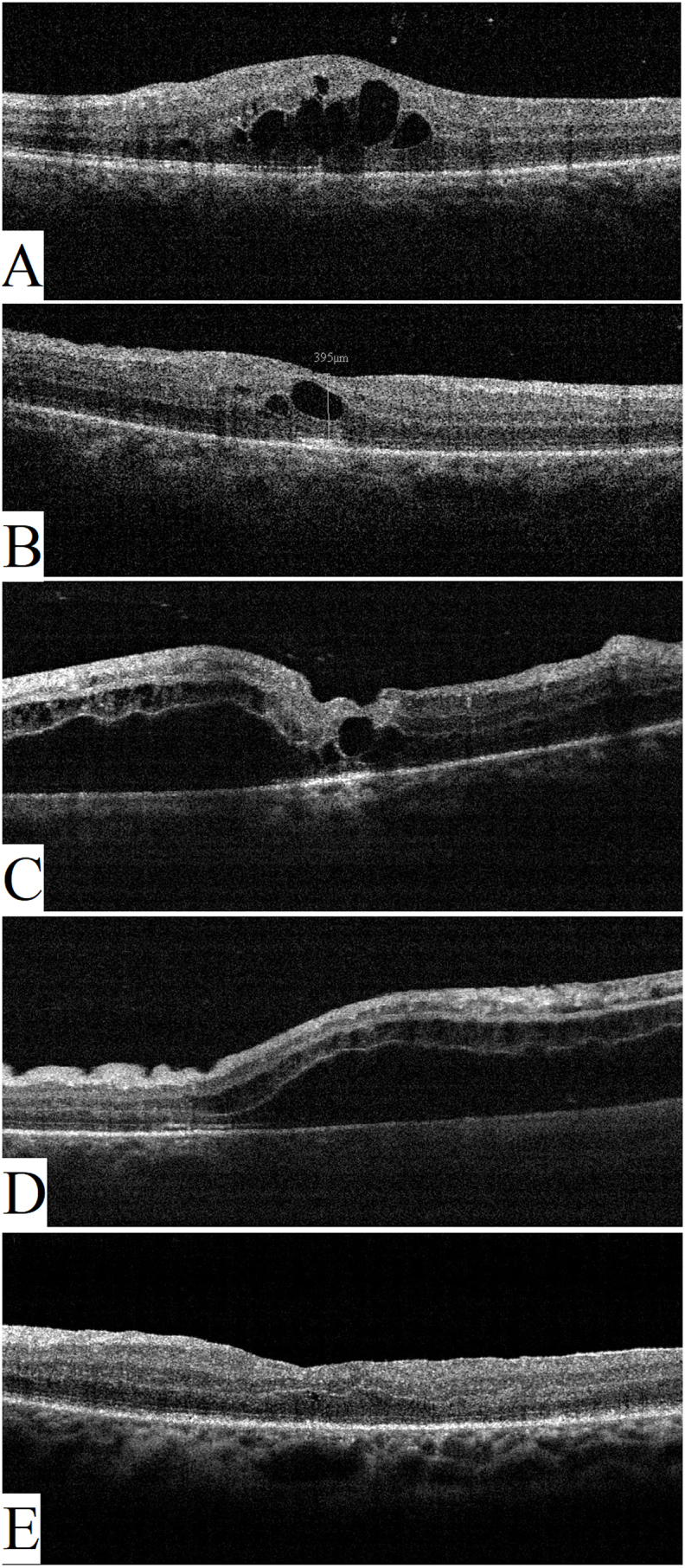
Optical coherence tomography scan of the macular area. A. One-month after cryotherapy and intravitreal bevacizumab there was increased cystoid macular edema and some vitreous cells. B. Following the second bevacizumab injection, cystoid macular edema improved but there was starting macular traction with mild inner retinal corrugations. C and D. Five-months after treatment there was progressive macular traction with subretinal fluid. E. One-month following vitrectomy there is relief of macular traction and resolution of macular edema.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.ajoc.2019.100508.
The following is the supplementary data related to this article:
Video showing 23G vitrectomy with peeling of epiretinal and internal limiting membrane
2
3. Discussion
The cause of development of an epiretinal membrane in our case is unknown. Ramasubramanian and Shields reported similar findings with development of vitreoretinal fibrosis in 50% of their patients treated with cryotherapy and intravitreal bevacizumab injections despite improvement of subretinal fluid and exudate following treatment.6 None of their patients, however, underwent vitrectomy since their visual prognosis was poor.
In a study of 124 eyes with Coats' disease treated by laser photocoagulation or cryotherapy, epiretinal membrane formation occurred in only 2%.1 This discrepancy has lead Ramasubramanian and Shields to advice caution while using anti-VEGF therapy to treat Coats’ disease due to a possible increase in the risk of vitreoretinal fibrosis.6 Other studies, however, reported good results with no complications following treatment with laser photocoagulation and intravitreal bevacizumab.7,8
Cryotherapy has been reported to increase the risk of epiretinal membrane development and retinal traction in the presence of retinal tears. This effect may be the result of dispersion of retinal pigment epithelial cells or break down of the blood-retinal barrier.9 The dispersion of retinal pigment epithelial cells in the absence of retinal tears, however, as in Coats’ disease, should result in subretinal instead of epiretinal fibrosis.
Bevacizumab is a full-length humanized monoclonal antibody that blocks all VEGF-A isoforms and that is used in the treatment of macular edema due to various causes.10,11 Previously, intravitreal bevacizumab has also been reported to increase the risk of vitreoretinal fibrosis and retinal traction in patients with proliferative disease.12 Combining cryotherapy and bevacizumab may therefore have a possible synergistic adverse effect of increasing vitreoretinal fibrosis and traction through different mechanisms.
Vitrectomy for epiretinal membrane associated with Coats' disease has been reported to have good results in a few reports.5,13 In a case of juvenile-onset Coats' disease which developed a secondary ERM following multiple sessions of laser and cryotherapy, a good anatomical and functional result was obtained following vitrectomy with ERM removal.13 To the best of our knowledge, no similar report has been made for a case of adult-onset Coats’ disease treated with cryotherapy and multiple intravitreal bevacizumab injections.
In conclusion, we report a case of Stage 3A1 adult-onset Coats’ disease who had worsening of macular edema and progressive macular traction following cryotherapy and multiple intravitreal bevacizumab injections that improved following vitrectomy with membrane peeling. Caution should be exercised when combining cryotherapy with intravitreal anti-VEGF injections for these cases especially when the visual acuity is good and laser treatment may be a safer option. In cases complicated by an epiretinal membrane and traction, early vitrectomy with membrane peeling is recommended to improve anatomical and functional outcomes.
4. Patient consent
Patient has signed a written consent to publish this information.
Acknowledgement and disclosures
There are no sources of funding or conflict of interest. All authors attest that they meet the current ICMJE criteria for Authorship. There are no acknowledgements.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2019.100508.
Ethical approval
This report was approved by Cairo University research ethics committee and followed the tenets of the Declaration of Helsinki.
Financial support
None.
Conflicts of Interest and Source of Funding
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shields J.A., Shields C.L., Honavar S.G., Demirci H., Cater J. Classification and management of Coats disease. The 2000 Proctor lecture. Am J Ophthalmol. 2001;131:572–583. doi: 10.1016/s0002-9394(01)00896-0. [DOI] [PubMed] [Google Scholar]
- 2.Black G.C., Perveen R., Bonshek R. Coats' disease of the retina (unilateral retinal telangiectasis) caused by somatic mutation in the NDP gene: a role for norrin in retinal angiogenesis. Hum Mol Genet. 1999;8:2031–2035. doi: 10.1093/hmg/8.11.2031. [DOI] [PubMed] [Google Scholar]
- 3.He Y.G., Wang H., Zhao B., Lee J., Bahl D., McClusky J. Elevated vascular endothelial growth factor level in Coats' disease and possible therapeutic role of bevacizumab. Graefes Arch Clin Exp Ophthalmol. 2010;248:1519–1521. doi: 10.1007/s00417-010-1366-1. [DOI] [PubMed] [Google Scholar]
- 4.Grosso A., Pellegrini M., Cereda M.G., Panico C., Staurenghi G., Sigler E.J. Pearls and pitfalls in diagnosis and management of coats disease. Retina. 2015;35:614–623. doi: 10.1097/IAE.0000000000000485. [DOI] [PubMed] [Google Scholar]
- 5.Shukla D., Chakraborty S., Behera U.C., Kim R. Vitrectomy for epimacular membrane secondary to adult-onset coats' disease. Ophthalmic Surg Laser Imag. 2008;39(3):430–431. doi: 10.3928/15428877-20080501-15. [DOI] [PubMed] [Google Scholar]
- 6.Ramasubramanian A., Shields C.L. Bevacizumab for Coats' disease with exudative retinal detachment and risk of vitreoretinal traction. Br J Ophthalmol. 2012;96:356–359. doi: 10.1136/bjophthalmol-2011-300141. [DOI] [PubMed] [Google Scholar]
- 7.Goel N., Kumar V., Seth A., Raina U.K. Role of intravitreal bevacizumab in adult onset Coats' disease. Int Ophthalmol. 2011;31:183–190. doi: 10.1007/s10792-011-9436-x. [DOI] [PubMed] [Google Scholar]
- 8.Park S., Cho H.J., Lee D.W., Kim C.G., Kim J.W. Intravitreal bevacizumab injections combined with laser photocoagulation for adult-onset Coats' disease. Graefes Arch Clin Exp Ophthalmol. 2016;254:1511–1517. doi: 10.1007/s00417-015-3233-6. [DOI] [PubMed] [Google Scholar]
- 9.Glaser B.M., Vidaurri-Leal J., Michels R.G., Campochiaro P.A. Cryotherapy during surgery for giant retinal tears and intravitreal dispersion of viable retinal pigment epithelial cells. Ophthalmology. 1993;100:466–470. doi: 10.1016/s0161-6420(93)31620-9. [DOI] [PubMed] [Google Scholar]
- 10.Elnahry A.G., Hassan F.K., Abdel-Kader A.A. Bevacizumab for the treatment of intraretinal cystic spaces in a patient with gyrate atrophy of the choroid and retina. Ophthalmic Genet. 2018;39(6):759–762. doi: 10.1080/13816810.2018.1536220. [DOI] [PubMed] [Google Scholar]
- 11.Elnahry A.G., Abdel-Kader A.A., Raafat K.A., Elrakhawy K. Evaluation of the effect of repeated intravitreal bevacizumab injections on the macular microvasculature of a diabetic patient using optical coherence tomography angiography. Case Rep Ophthalmol Med. 2019;2019:3936168. doi: 10.1155/2019/3936168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arevalo J.F., Maia M., Flynn H.W., Jr. Tractional retinal detachment following intravitreal bevacizumab (Avastin) in patients with severe proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92(2):213–216. doi: 10.1136/bjo.2007.127142. [DOI] [PubMed] [Google Scholar]
- 13.Yadav N.K., Vasudha K., Gupta K., Shetty K.B. Vitrectomy for epiretinal membrane secondary to treatment for juvenile Coats' disease. Eye. 2013;27:278–280. doi: 10.1038/eye.2012.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video showing 23G vitrectomy with peeling of epiretinal and internal limiting membrane
2