Highlights
-
•
Icariin protects against rotenone-induced neuronal cell injury in vitro and in vivo.
-
•
Icariin improves mitochondrial respiration in neuronal cells damaged by rotenone
-
•
Icariin modulates phosphorylation of mTOR.
-
•
Icariin attenuates accumulation of p62 caused by rotenone.
-
•
Upregulation of autophagic flux is involved in the protective action of icariin.
Abbreviations: BCA, bicinchoninic acid; DA, dopamine; DMEM, Dulbecco's modified Eagle's medium; HRP, horseradish peroxidase; ICA, icariin; LDH, lactate dehydrogenase; mTOR, mammalian target of rapamycin; OCR, oxygen consumption rate; PE, phosphatidylethano-lamine; PD, Parkinson`s disease; ROT, rotenone; SN, substantia nigra
Keywords: Icariin, Rotenone, Neurotoxicity, Mitochondrial function, Autophagy
Abstract
Rotenone (ROT) is an environmental neurotoxin which has been demonstrated to cause characteristic loss of dopamine (DA) neurons in Parkinson’s disease (PD). Icariin (ICA) is a flavonoid glucoside isolated from Herba Epimedii that has been shown to display neuroprotective functions. The present study evaluated protective effects of ICA on ROT-induced neurotoxicity and determined the modulation of ICA on the regulation of autophagy in vivo and in vitro. Rats were treated with ROT (1.0 mg/kg/day) with a co-administration of ICA (15 or 30 mg/kg/day) for 5 weeks. Immunohistochemical analysis showed a significant loss in DA neurons in the substantia nigra (SN) of rats treated with ROT, accompanied by an increase in the accumulation of α-synuclein and a compromised mitochondrial respiration. However, co-administration of ICA potently ameliorated the ROT-induced neuronal cell injury and improved mitochondrial function and decreased the accumulation of α-synuclein. ROT treatment resulted in a decrease in the protein expression of LC3-II and Beclin-1, and an increase in the protein level of P62, and upregulated the activation of mammalian target of rapamycin (mTOR), whereas ICA significantly reversed these aberrant changes caused by ROT. Furthermore, the neuroprotective effect of ICA was further verified in PC12 cells. Cells treated with ROT displayed an increased cytotoxicity and a decreased oxygen consumption which were rescued by the presence of ICA. Furthermore, ROT decreased the protein expression level of LC3-II, enhanced Beclin-1 expression, and activated phosphorylation of mTOR, whereas ICA markedly reversed this dysregulation of autophagy caused by ROT in the PC12 cells. Collectively, these results suggest that ICA mediated activation of autophagic flux confers a neuroprotective action on ROT-induced neurotoxicity.
1. Introduction
Environmental toxicants such as pesticides and heavy metals are commonly recognized to be associated with the development of Parkinson’s disease (PD) [1,2]. ROT is a pesticide that is neurotoxic. Epidemiological investigation has established a positive link between human exposure to ROT and the occurrence of PD [3]. Extensive studies on ROT toxicity have revealed the molecular mode of action of ROT including inhibition of mitochondrial complex I, oxidative stress, and induction of inflammation, all of which may be critically involved in the pathological progression of neurodegeneration [[4], [5], [6]]. Although ROT has been shown to recapitulate many key features of PD in various experimental models, the molecular mechanism for rotenone-induced PD remains not fully elucidated [7,8]. The exploration of etiology and pathogenesis of PD is of great importance as there is no cure to PD, and current treatments available can only delay the pathological progress of PD [9].
An increasing number of studies demonstrate that the dysregulation of cellular autophagy is involved in the molecular signaling of progressive neuronal cell death occurring in various neurodegenerative disorders including PD [10,11]. A wide range of environmental neurotoxicants including pesticides such as ROT and paraquat and heavy metals have been shown to adversely affect the regulation of autophagy [[12], [13], [14], [15]]. However, it has been recognized that toxicants may largely differ in their effects on the autophagy signaling, eventually leading to either excessive autophagy activation or insufficient capacity of removal of damaged or unwanted organelles or proteins, interfering with physiological functions of neurons. On the other hand, pharmacological modulation of autophagy emerges to be an active area in the studies of therapeutic protection of neuronal degeneration and other neuronal injuries [[16], [17], [18]]. With regards to ROT, it has been shown that ROT inhibits autophagic flux, however, the underlying mechanism has yet to be further studied. More importantly, exploring an approach to pharmacologically activate autophagic flux to protect against ROT-neurotoxicity may unravel a promising clue to the prevention and treatment of the currently uncurable disease PD.
ICA is a flavonoid glucoside extracted from Epimedium that displays various pharmacological functions. Previous studies have demonstrated that ICA exhibits neuroprotective capacity in mice model of Alzheimer’s disease [[19], [20], [21]]. ICA has also been shown to be protective in PD models and neurotoxicity induced by a wide variety of neurotoxicants such as 6-OHDA, LPS and MPTP [[22], [23], [24]]. More recently we demonstrated that ICA also exerts a promoting effect on the proliferation of neural stem cells of rat hippocampus [25]. However, the full beneficial efficacy of ICA in PD has been less studied. Therefore, whether ICA has a neuroprotection over ROT-toxicity through activation of autophagy is worthy of full investigation.
In this study, we aimed to evaluate the effects of ICA on neurotoxicity in the ROT-induced rat PD model, and to determine the role of autophagy in the mode of action of ICA on the DA neuronal injury caused by ROT. Utilizing PC12 cells, a rat pheochromocytoma cell line, we further investigated whether the activation of autophagy was involved in the effect of ICA on ROT-induced cytotoxicity in vitro.
2. Materials and methods
2.1. Chemicals and reagents
Rotenone was purchased from Sigma-Aldrich (St Louis, MO, USA). Icariin was purchased from Nanjing Zelang Biological Technology Co., Ltd (Nanjing, China). Dulbecco's modified Eagle's medium (DMEM), horse serum, and fetal bovine serum (FBS) were supplied by Hyclone (Logan, UT). Antibodies against α-synuclein, Beclin-1, SQSTTM1/P62, mTOR, and Phospho-mTOR were purchased from Cell Signaling Technology (Boston, MA, USA). Antibody for LC3-I/II was purchased from Abcam (Cambridge, UK), and antibody for β-actin was purchased from Beyotime (Shanghai, China). LDH kit was purchased from Jiancheng Bioengineering Institute (Nanjing, China).
2.2. Animals and treatment
100 male Sprague Dawley (SD) rats (weighting 220–250 g) were randomly divided into five groups (20 in each group): control, ICA (30 mg/kg/day), ROT (1 mg/kg/day), ROT (1 mg/kg/day)+ ICA (15 mg/kg/day), and ROT (1 mg/kg/day) + ICA (30 mg/kg/day). Considering the sex-specific differences in redox homeostasis and other biological function in the brain, only male animals were included in this study [26]. ICA was dissolved in ddH2O, and ROT was dissolved in DMSO [27]. ROT was given by subcutaneous injection once per day at a dose of 1 mg/kg, and ICA was administrated by oral gavage. The ICA and ROT concentrations were selected according to previous studies [22,28,29]. The control group was subjected to an equivalent volume of vehicles. The animals were treated for 5 weeks [29].
All animal experiments were strictly carried out in accordance with NIH guidelines for the Care and Use of Laboratory Animals (ISBN:13:978-0-309-15400-0, revised in 2011), and all animal experiments were performed in accordance with Chinese Guidelines of Animal Care and Welfare, and the present study was approved by the Animal Care and Use Committee of Zunyi Medical University (approval code 20142-016).
2.3. Preparation of brain mitochondria
Animals were sacrificed at 24 h following the last administration of the chemicals. Rat brain tissue was quickly removed and homogenized on ice in 20 volumes of a homogenizing buffer consisting of 0.25 M sucrose, 10 mM Tris-HCI, 1 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride, PH 7.4. Homogenates were centrifuged at 900 g for 10 min at 4 ℃, and the supernatant was collected and then centrifuged at 9000 g for 10 min at 4℃. The resultant precipitates were mitochondrial fraction that was resuspended in respiratory buffer and stored on ice for the following experiments. Determination of mitochondrial protein concentration was conducted by bicinchoninic acid (BCA) kit (Beyotime, Beijing).
2.4. Measurement of oxygen consumption
The oxygen consumption rate (OCR) of isolated mitochondria was determined by high-resolution respirometry (Oxygraph-2 K, Oroboros Instruments, Austria). Briefly, the isolated brain mitochondria were loaded in the oxygraph chambers at a concentration of 0.1 mg/mL in respiration buffer. After equilibration, mitochondrial respiration was started by energizing with 5 mM succinate. State 3 respiration was initiated by adding 2 mM ADP into the chambers. Mitochondrial respiration was recorded continuously, the DatLab software (Oroboros Instruments) was used for data acquisition and calculation of OCR. The cellular respiration of PC12 cells was evaluated by measuring oxygen consumption with the high-resolution respirometry. Briefly, after 24 h incubation with the indicated chemicals, cells were digested with trypsin and then resuspended in serum-free DMEM medium. The cell suspension was adjusted to a density of 1 × 106 cells/ml, and a total of 2 ml of cell suspension was loaded into the closed chamber with a magnetic stirring. Oxygen flux (pmol O2/s/106 cells) was then recorded continuously using DatLab software.
2.5. Immunohistochemical analysis
Under anesthesia the animals were transcardially perfused with PBS followed by 4% paraformldehyde (PFA)/PBS. After the rat brain was removed, it was transferred to 4% PFA and postfixed for 48 h. The brain tissue was then transferred into 30% sucrose to dehydrate for 48 h. Following the dehydration, the brain tissue was cut with a horizontal sliding microtome into 35 μm transverse free-floating sections. For immunostaining TH, the brain slices were collected in a 24-well plate with PBS and stored at 4℃. The brain tissue sections were incubated with 0.3% Triton for 15 min to breach cell membrane, then subjected to 3% H2O2 for 15 min, and blocked in goat serum for 30 min, and incubated with a primary antibody to TH (1:300, Abcam) overnight at 4℃. After the completion of the incubation with the primary antibody, the brain slices were washed with PBS, then were incubated with a biotinylated secondary antibody for 1 h in 37 °C. After air-drying, the brain slices were mounted onto the slides and sealed with gel resin. The brain sections were visualized using a DAB detection kit (Solarbio, Beijing, China). Digital images of SN TH-positive neurons were obtained by an Olympus microscope (Olympus, Tokyo, Japan).
2.6. Cell culture and treatment
PC12 cell lines were purchased from American Type Culture Collection (Rockville, MD, USA), and were cultured in DMEM/high glucose medium supplemented with 10% fetal bovine serum (MRC, China), 5% horse serum (Hyclone, USA), 100 U/ml penicillin and 100 μg/ml streptomycin. For measurements of cytotoxicity and mitochondrial respiration, and detection of protein expressions, PC12 cells were seeded on 6-well plate. The cells were pre-treated with 2 or 4 μM ICA for 2 h followed by exposure to 1.0 μM ROT for 24 h. The supernatant and the cell pellets were then collected for corresponding experiments.
2.7. Western blot analysis
The protein expressions in SN tissues or PC12 cells were analyzed by Western blot analysis. The brain was removed under anesthesia. Each brain was dissected on a cold glass plate to separate the SN tissues. The SN tissues or PC12 cells pellets were homogenized in RIPA lysis buffer. The lysates were incubated on ice for 30 min, then centrifuged at 12,000 g for 15 min. The supernatant was then collected for the analysis of protein expressions. Determination of protein concentrations was carried out by BCA kit (Beyotime, Beijing, China). The protein was separated on 10% Bis-Tris NuPAGE gel and transferred to PVDF membrane. The PVDF membrane was blocked with 4% BSA (Sigma) for 2 h, and then reacted with primary antibodies at 4℃ for overnight. The primary antibodies included those for α-synuclein (1:1000, Abcam), LC3-I/II (1:1000, Abcam), Beclin-1 (1:1000, Cell signaling), SQSTTM1/P6
2 (1:1000, Cell signaling), mTOR (1:1000, Cell signaling), Phospho-mTOR (1:1000, Cell signaling), and β-actin (1:2000, Beyotime). After washing, the membranes were then incubated in horseradish peroxidase (HRP)-conjugated secondary antibodies (1:2000) for 2 h. The membrane-bound secondary antibody was detected with ECL Western blot detection kit. The band intensities were quantified using Quantity One 1-D analysis software v4.52 (BioRad).
2.8. LDH assay
The lactate dehydrogenase (LDH) activity was determined using a commercial LDH assay kit per manufacturer’s instruction (Beyotime, Beijing). Briefly. After treatments of PC12 cells, supernatants were collected and centrifuged at 400 g for 5 min for the measurement of activity of LDH. The reaction was initiated by mixing 0.2 ml of cell-free supernatant with 50 μl LDH work buffer (Beyotime, Beijing). The mixture was incubated in room temperature for 30 min, and the OD value indicating the highly colored and soluble formazan was then measured at 490 nm spectrophotometrically.
2.9. Statistical analysis
Data were analyzed using statistical package for social sciences (SPSS) version 19.0. Statistical evaluation of the difference between three or more groups of individual data was analyzed by one-way analysis of variance (ANOVA) and post hoc multiple comparisons using Tukey's test. A value of p < 0.05 was considered as statistical significance. Data were expressed as mean ± SEM (standard error of mean) of three or more independent experiments.
3. Results
3.1. Protective effect of ICA on ROT-induced loss of DA cells
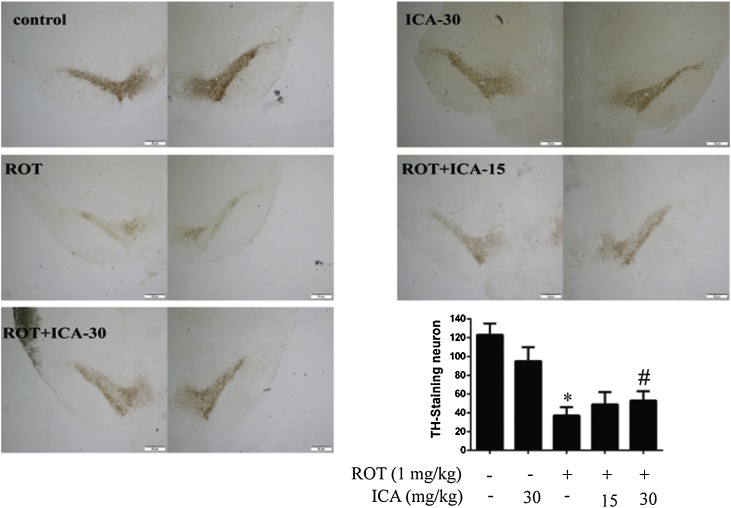
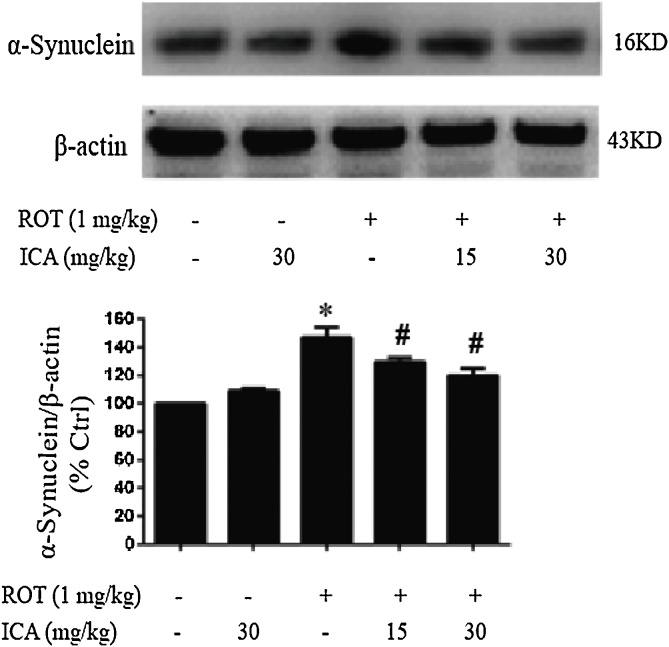
It has been well demonstrated that neuronal toxicant ROT causes progressive loss of DA neurons and the Lewy body formation in the nigral-striatal system [30]. To determine the in vivo protective effects of ICA on DA cells, rats were administered ROT (1 mg/kg/day) by subcutaneous injection with co-treatment of ICA (15 or 30 mg/kg/day) for 5 weeks. Following the last administration of the chemicals, the damage to DA neurons in the SN was examined by immunohistochemistry staining with anti-TH antibody. As shown in Fig. 1A, a significant reduction in DA neurons was observed in the ROT-treated rats, about 40% of that in the control. However, the loss of DA neurons was markedly ameliorated when co-treated with ICA (Fig. 1). To further examine the in vivo protection of ICA, next we examined the expression level of α-synuclein in the SN. The aberrant accumulation of α-synuclein causes deleterious impact on neurons and is a pathological hallmark of PD [31]. Western blot analysis showed the treatment of rats with ROT caused an increase in the protein level of α-synuclein in the SN, about 47% increase relative to the control, whereas the increased accumulation of α-synuclein was noticeably reduced when co-treated with ICA (Fig. 2). These results indicated clearly a beneficial effect of ICA on ROT-induced neurotoxicity.
Fig. 1.
Protective effect of ICA on ROT-induced loss of DA cells. The animals were administrated with ROT and ICA for 5 weeks. ICA (15 and 30 mg/kg) was given by oral gavage, and ROT was administered by subcutaneous injection once per day at the dose of 1 mg/kg. Twenty-four hours following the last treatment, rats were sacrificed, and the brains were collected. Brain tissues were processed as described in the Material and methods, and brain sections were immunostained with an anti-TH antibody. (A) and (B), Immunostaining of DA cells and quantification of TH-positive cells in SN, respectively. The results were the mean ± SEM from three rats (n = 3). *, p < 0.05 as compared with control; #, p < 0.05 as compared with ROT group.
Fig. 2.
Effect of ICA on ROT-induced of protein expression levels of α-synuclein in SN. After treatment, the SN of rats were collected, then the protein expression level of α-synuclein was determined by Western blot. The results were the mean ± SEM from three rats (n = 3). *, p < 0.05 as compared with control; #, p < 0.05 as compared with ROT group.
3.2. Protective effect of icariin on brain mitochondrial function
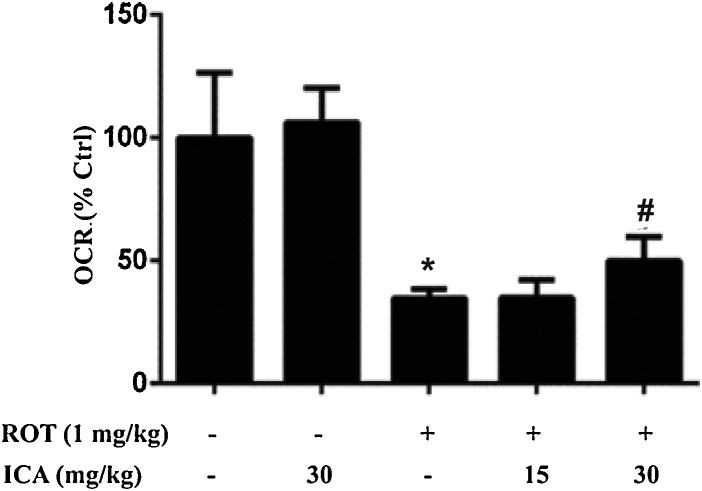
The mitochondrial function was evaluated by measuring oxygen consumption of isolated mitochondria from brain tissues by Oxygraph-2k high-resolution respirometry. As shown in Fig. 3, treatment of ROT markedly inhibited mitochondrial respiration as compared with the control group (p < 0.05), whereas icariin significantly improved the respiratory function of brain mitochondria as compared with the ROT group (p < 0.05).
Fig. 3.
Protective effect of ICA on brain mitochondrial respiration. Mitochondria were isolated from the whole brain tissues as described in the Materials and methods. Mitochondrial respiration flux was detected by high-resolution respirometry. The results were the mean ± SEM from three rats (n = 3). *, p < 0.05 as compared with control; #, p < 0.05 as compared with ROT group.
3.3. ICA activates autophagic flux in vivo
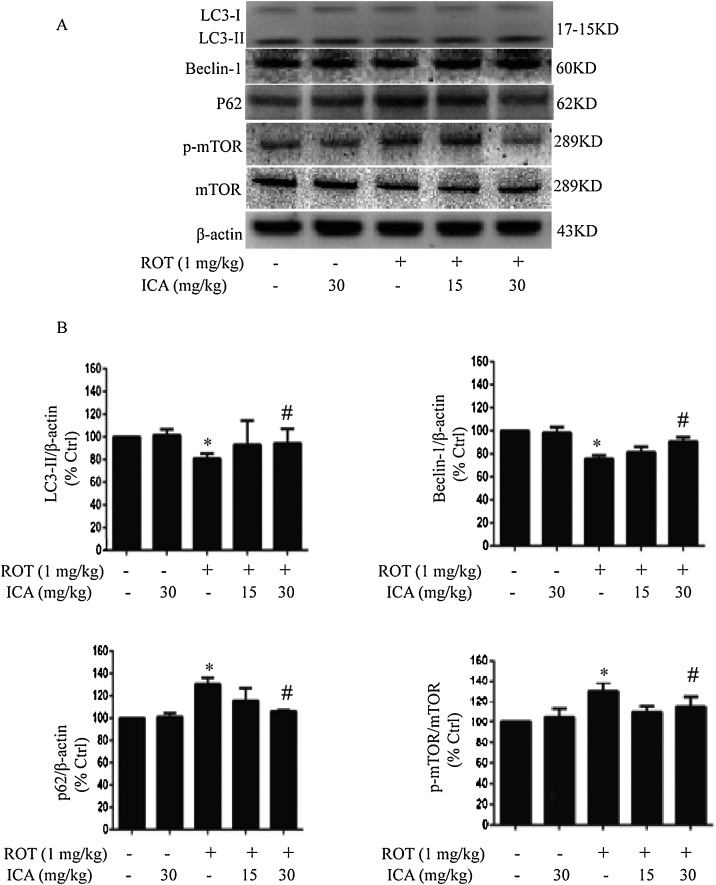
To determine whether modulation of autophagic flux is involved in the mechanism of ICA-mediated protection on ROT neurotoxicity, we examined the protein expression levels of LC3-II, Beclin-1, and P62, in the SN tissue by Western blot analysis. As shown in Fig. 4, treatment of ROT significantly decreased the expression of LC3-II, indicating an inhibitory effect of ROT on autophagy in the SN. However, the decrease in the protein expression level of LC3-II was reversed when rats were co-treated with ICA of 30 mg/kg (p < 0.05 vs ROT group). The activation effect of ICA was further dissected by examining the expression level of the autophagy protein Beclin-1. Western blot analysis found that ROT suppressed the protein level of Beclin-1, whereas ICA enhanced significantly the Beclin-1 level in the SN of rats when treated with ROT combined with ICA (Fig. 4). In addition, we examined autophagic flux by measuring the protein expression of autophagy substrate p62. It was found that treatment of ROT caused a higher accumulation of p62 compared to the control, whereas ICA was able to significantly decrease the p62 level (Fig. 4), indicating a promoting effect of ICA on autophagic flux.
Fig. 4.
Effects of ROT and ICA on the expression levels of autophagic protein LC3-II, Beclin-1, P62, mTOR and p-mTOR in SN. After treatment, the SN of rats were collected, then the protein expression levels of LC3-II, Beclin-1, P62, mTOR and p-mTOR were determined by Western blot. The results were the mean ± SEM from three rats (n = 3). *, p < 0.05 as compared with control; #, p < 0.05 as compared with ROT group.
To further explore the protective mechanism of ICA over ROT-induced neurotoxicity, we next determined the expression level of mTOR, an evolutionarily conserved serine/threonine protein kinase. As an upstream regulatory pathway for autophagy, studies have shown that activation of mTOR leads to the blockage of autophagy [32,33]. Both mTOR and phosphor-mTOR (p-mTOR) were detected by Western blot analysis. The results revealed that ROT treatment resulted in a significant increase in the expression of p-mTOR (p < 0,05 vs. the control), indicating that the mTOR pathway was activated. However, the expression of p-mTOR protein decreased significantly when ICA was co-administered. These data suggested a potential role of ICA in the activation of autophagy, which may account for its neuroprotective effects in vivo.
3.4. Protective effects of ICA on ROT-induced neurotoxicity in PC12 cells
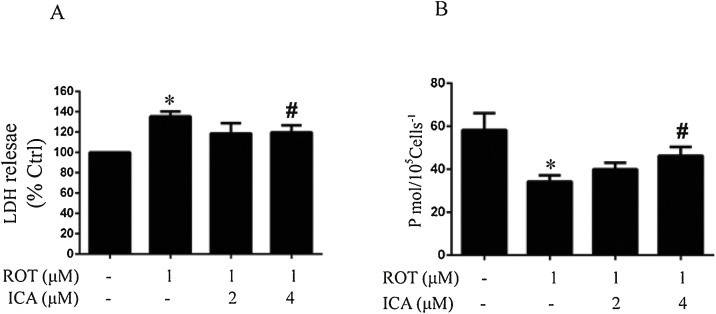
The protective effects of ICA on neurotoxicity was further examined in PC12 cells. The PC12 cell line has been widely used in the studies of neurotoxicity and characterization of various neuroprotective agents [34]. As shown in Fig. 5A, cells treated with ROT displayed a robust cytotoxicity as measured by an increase in the release of LDH. This increased LDH release appeared to be significantly negated in the presence of 4 μM ICA, demonstrating the protective capacity of ICA on the ROT-induced cytotoxicity in vitro. We further evaluated the cellular respiration. As shown in Fig. 5B, the oxygen consumption rate in the cells treated with ROT dropped to about 50% as compared to the control, while the co-treatment of ICA potently improved the cellular respiration (p < 0.05 as compared to ROT alone).
Fig. 5.
Protective effects of ICA on ROT-induced neurotoxicity in PC12 cells. PC12 cells were treated with ICA for 2 h, then exposed to ROT for 24 h. LDH release was measured by the LDH assay kit and cellular respiration was detected by high-resolution respirometry. The results were the mean ± SEM from three independent experiments (n = 3). *, p < 0.05 as compared with control; #, p < 0.05 as compared with ROT group.
3.5. Effects of ICA on the expression of proteins LC3-II, P62, mTOR and p-mTOR in PC12 cells
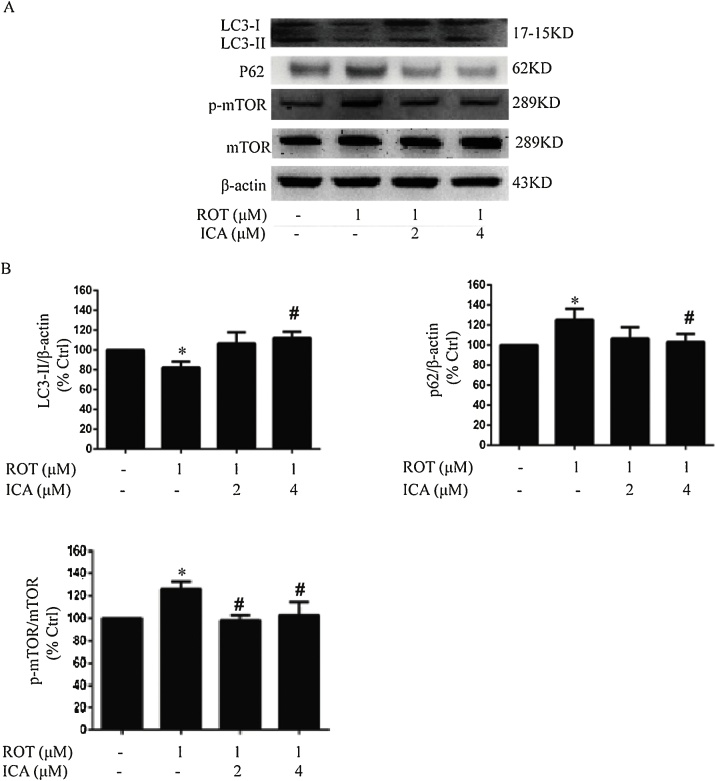
We next determined whether ICA modulates the regulation of autophagic flux in vitro. The protein expression levels of LC3-II, P62 as well as mTOR and p-mTOR were detected by Western blot analysis. As shown in Fig. 6, a lower protein expression in LC3-II along with a higher accumulation of P62 was found in the cells treated with ROT, while ICA significantly reversed the changes in the protein expression of LC3-II and P62, indicating the role of ICA in the activation of autophagy flux in vitro. Furthermore, we examined the activation of mTOR. Consistent with the in vivo findings, ROT treatment upregulated the phosphorylation of mTOR, while ICA significantly suppressed the expression level of p-mTOR (p < 0.05). These results further corroborated the modulation of ICA on the regulation of autophagy flux in the context of ROT neurotoxicity.
Fig. 6.
Effects of ROT and ICA on LC3-II, P62, mTOR and p-mTOR protein expression in PC12 cells. PC12 cells were treated with ICA for 2 h, then exposed to ROT for 24 h. The protein expression levels of LC3-II, P62, mTOR and p-mTOR were determined by Western blot. The results were the mean ± SEM from three independent experiments (n = 3). *, p < 0.05 as compared with control; #, p < 0.05 as compared with ROT group.
4. Discussion
Pesticides are increasingly recognized to cause a wide range of toxicities in various organs [35,36]. Particularly environmental exposure to neurotoxicant ROT is associated with an increased risk in the development of PD [3]. In vivo studies have shown that ROT causes progressive degeneration of DA neurons and thus is widely used in PD animal models. In this study, we described the protective efficacy of ICA on the cell loss of DA neurons in rats treated with ROT. Analysis of autophagy related proteins suggests that activation of autophagy flux may account at least partially for the protective mode of action of ICA against ROT induced loss of DA neurons.
Deregulation of autophagy has been implicated in the pathogenesis of neurodegenerative disorders and a wide range of environmental chemical-elicited neurotoxicity [10,14]. It has been shown that ROT impairs autophagy, although the mechanism behind it, however, has not been fully established yet. In agreement with the previous report showing accumulation of autophagy substrate P62 in SH-SY5Y cells [12], in the present study we demonstrated that treatment of ROT resulted in an elevated P62 level in the SN tissue of ROT-intoxicated rats. Moreover, we found that ROT also affected the regulation of mTOR, which negatively regulates the activation of autophagy [37,38]. Our results showed that ROT treatment caused elevation in phosphorylation of mTOR in SN tissues, suggesting an inhibitory effect of ROT on autophagy. However, we demonstrated that when co-administered, ICA not only decreased P62 accumulation but also inhibited phosphorylation of mTOR, showing that ICA plays a role in the promotion of autophagic flux in the context of ROT induced neurotoxicity. We attributed this ICA-mediated activation of autophagic flux to its neuroprotective capacity over ROT induced neuronal cell loss. In this regard, further studies on the regulation of phosphorylation of mTOR may reveal more mechanistic insights. The PI3K/AKT/mTOR pathway is an intracellular signaling pathway that has been widely implicated into the mechanism of a variety of pharmacological agents [39]. Whether ICA could regulate the phosphorylation of mTOR through the PI3K/AKT/mTOR pathway to exert neuroprotective effects has yet to be investigated.
Activation of autophagy may exert cytoprotective effects through multiple intracellular events including elimination of malfunctional mitochondria and other damaged proteins. It has been well established that impairment in mitochondrial function represents an early intracellular event that is critical to lead to the death of neuronal cells in PD [40]. Mitochondrial dysfunction results in not only bioenergetic collapse that is directly detrimental to neuronal cells, but also excess production of reactive oxygen species leading to oxidative stress status, which in turn damages mitochondrial molecules and cellular function. Accordingly, a timely clearance of dysfunctional mitochondria is thus protective against cytotoxicity caused by environmental chemicals. Extensive studies have shown that mitochondria appear to be the target of many environmental toxicants, ROT for instance, which is a specific inhibitor of mitochondrial complex I. Inhibition of mitochondrial complex I may suppress the respiration and increase cellular oxidative stress. Thus, activation of autophagy by ICA may be beneficial to mitochondrial function and cellular homeostasis. Indeed, our results showed that mitochondria isolated from ROT treated rat brain displayed a significantly lower respiration, whereas co-treatment with ICA significantly improved mitochondrial function. Although we did not dissect the exact molecular mechanism by which activation of autophagy confers protection over ROT neurotoxicity, suppression of ROS generation might be at least one of the accountable events. However, this does not rule out other possibilities. ICA, as a flavonoid compound, has been demonstrated to be of antioxidant capacity. In this regard, the antioxidant function exerted by ICA might be a combination of activation of autophagy and a direct scavenging action of ICA. It is also likely that ICA mediated inhibition of cellular oxidative stress may directly protect cells against ROT neurotoxicity, and thus prevent a possible modulation of rotenone on the regulation of autophagy. Further studies of interaction between ICA and ROT-mediated mitochondrial ROS generation may reveal more mechanistic insights on the mode of action of ICA. In addition, recent studies have shown that ICA participates in the regulation of ion metabolism that may also be implicated in the neuroprotective mechanism of ICA [41,42]. Therefore, more studies on ICA mediated mechanism of autophagy are warranted. Nevertheless, it is worthy of emphasis: (1) ROT causes DA neuron loss in the rat model of ROT administration paralleling with dysregulation of autophagy; (2) ICA protects ROT induced neurotoxicity accompanied by an increased autophagic flux. Interestingly, in the PC12 cell model, the cytoprotective function of ICA over ROT toxicity was verified. Consistent with the findings in vivo, we showed that ICA protected ROT induced cell killing, and meanwhile activated autophagic flux. These results supported a critical role of activation of autophagy in the mode of action of ICA.
Another finding in this study was that ICA abolished the accumulation of α-synuclein caused by ROT treatment. The α-synuclein is a component of LBs (Lewy bodies) which is a hallmark of PD. Although a causative role of α-synuclein in the pathogenesis of PD remains to be fully understood, the accumulation of α-synuclein is toxic to the cell. A great number of studies support that α-synuclein causes damage to mitochondria and plays a pivotal role in the pathogenesis of neurodegenerative disorders [43,44]. However, opposing results also exist in the literature showing [45]. The relationship between α-synuclein accumulation and mitochondrial impairment appears to be not conclusively known. Nevertheless, we found that the α-synuclein accumulation was significantly reduced when the animals were co-treated with ICA, and the decrease in α-synuclein is likely protective to neuronal cell survival under the attack of ROT.
Despite great progress in the understanding the pathogenesis, the etiology and molecular mechanism of PD continue to be elusive. Intervention and treatment of PD still remain a big challenge. Identification of ICA in the activation of autophagy in the context of ROT induced neurotoxicity thus offers not only a promising therapeutic agent but also mechanistic clues to further elucidation of the molecular basis of pathogenesis of PD.
5. Conclusions
ROT administration induced a loss of DA neurons accompanied by a decrease in brain mitochondrial function in a rat model of ROT intoxication for 5 weeks. Co-administration of ICA significantly attenuated neurotoxicity and improved mitochondrial function in rats treated with ROT. ICA treatment decreased phosphorylation of mTOR and the P62 protein level in the SN of rats treated with ROT, suggesting a role of activation of autophagy in the protective mode of action that ICA exerts on the ROT-elicited neuronal cell injury. The ICA mediated effect of neuroprotective capacity and promotion of autophagy was further verified in PC12 cell mode, which showed a significant decrease in cell injury as compared to the cells treated with ROT alone, and an elevated level of autophagy flux. Further studies of molecular signaling in the ICA-mediated regulation of autophagy may be a promising direction to fully exploit the neuroprotective efficacy of ICA in PD.
Acknowledgements
This work was supported by grants from the National Natural Science Fund of China (No. 81460548) and Tutorial Studio Foundation of Education Department of Guizhou Province (No. 99050).
References
- 1.Dick F.D., Palma G.D., Ahmadi A., Scott N.W., Prescott G.J., Bennett J., Semple S., Dick S., Counsell C., Mozzoni P., Haites N., Wettinger S.B., Mutti A., Otelea M., Seaton A., Söderkvist P., Felice A., Geoparkinson Study Group Environmental risk factors for Parkinson’s disease and parkinsonism: the Geoparkinson study. Occup. Environ. Med. 2007;64(10):673–680. doi: 10.1136/oem.2006.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neal S.L., Zheng W. Manganese toxicity upon overexposure: a decade in review. Curr. Environ. Health Rep. 2015;2(3):315–328. doi: 10.1007/s40572-015-0056-x. https://doi.org/10.1007/s40572-015-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanner C.M., Kamel F., Ross G.W., Hoppin J.A., Goldman S.M., Korell M., Marras C., Bhudhikanok G.S., Kasten M., Chade A.R., Comyns K., Richards M.B., Meng C., Priestley B., Fernandez H.H., Cambi F., Umbach D.M., Blair A., Sandler D.P., Langston J.W. Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 2011;119(6):866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi W.S., Kim H.W., Xia Z.G. JNK inhibition of VMAT2 contributes to rotenone-induced oxidative stress and dopamine neuron death. Toxicology. 2015;328:75–81. doi: 10.1016/j.tox.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keane P.C., Kurzawa M., Blain P.G., Morris C.M. Mitochondrial dysfunction in Parkinson’s disease. Parkinsons Dis. 2011;14(17):1261–1266. doi: 10.4061/2011/716871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Main B.S., Zhang M., Brody K.M., Kirby F.J., Crack P.J., Taylor J.M. Type-I interferons mediate the neuroinflammatory response and neurotoxicity induced by rotenone. J. Neurochem. 2017;141:75–85. doi: 10.1111/jnc.13940. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt W.J., Alam M. Controversies on new animal models of Parkinson’s disease pro and con: the rotenone model of Parkinson’s disease. J. Neural Transm. Suppl. 2006;70:273–276. [PubMed] [Google Scholar]
- 8.Johnson M.E., Bobrovskaya L. An update on the rotenone models of Parkinson’s disease: their ability to reproduce the features of clinical disease and model gene-environment interactions. Neurotoxicology. 2015;46:101–116. doi: 10.1016/j.neuro.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Jamebozorgi K., Taghizadeh E., Rostami D., Pormasoumi H., Barreto G.E., Hayat S.M.G., Sahebkar A. Cellular and molecular aspects of parkinson treatment: future therapeutic perspectives. Mol. Neurobiol. 2018 doi: 10.1007/s12035-018-1419-8. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Vicente M. Autophagy in neurodegenerative diseases: from pathogenic dysfunction to therapeutic modulation. Semin. Cell Dev. Biol. 2015;40:115–126. doi: 10.1016/j.semcdb.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Koentjoro B., Park J.S., Sue C.M. Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson’s disease. Sci. Rep. 2017;7(1):44373. doi: 10.1038/srep44373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chatterjee S., Sarkar S., Bhattacharya S. Toxic metals and autophagy. Chem. Res. Toxicol. 2014;27(11):1887–1900. doi: 10.1021/tx500264s. [DOI] [PubMed] [Google Scholar]
- 13.Mader B.J., Pivtoraiko V.N., Flippo H.M., Klocke B.J., Roth K.A., Mangieri L.R., Shacka J.J. Rotenone inhibits autophagic flux prior to inducing cell death. ACS Chem. Neurosci. 2012;3(12):1063–1072. doi: 10.1021/cn300145z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou Q., Zhang H., Wu Q., Shi J., Zhou S. Pharmacological manipulations of autophagy modulate paraquat-induced cytotoxicity in PC12 cells. Int. J. Biochem. Mol. Biol. 2017;8(2):13–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou Q., Fu X., Wang X., Wu Q., Lu Y., Shi J., Klaunig J.E., Zhou S. Autophagy plays a protective role in Mn-induced toxicity in PC12 cells. Toxicology. 2018;394:45–53. doi: 10.1016/j.tox.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Singh A.K., Kashyap M.P., Tripathi V.K., Singh S., Garg G., Rizvi S.I. Neuroprotection through rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB signaling against amyloid-β-Induced oxidative stress, synaptic/neurotransmission dysfunction, and neurodegeneration in adult rats. Mol. Neurobiol. 2017;54:5815–5828. doi: 10.1007/s12035-016-0129-3. [DOI] [PubMed] [Google Scholar]
- 17.Thellung S., Scoti B., Corsaro A., Villa V., Nizzari M., Gagliani M.C., Porcile C., Russo C., Pagano A., Tacchetti C., Cortese K., Florio T. Pharmacological activation of autophagy favors the clearing of intracellular aggregates of misfolded prion protein peptide to prevent neuronal death. Cell Death Dis. 2018;9:166. doi: 10.1038/s41419-017-0252-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vakifahmetoglu-Norberg H., Hong G., Xia H.G., Yuan J. Pharmacologic agents targeting autophagy. J. Clin. Invest. 2015;125:5–13. doi: 10.1172/JCI73937. https:doi.org/ 10.1172/JCI73937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li F., Gong Q.H., Wu Q., Lu Y.F., Shi J.S. Icariin isolated from epimedium brevicornum maxim attenuates learning and memory deficits induced by d-galactose in rats. Pharmacol. Biochem. Behav. 2010;96(3):301–305. doi: 10.1016/j.pbb.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Nie J., Luo Y., Huang X.N., Gong Q.H., Wu Q., Shi J.S. Icariin inhibits beta-amyloid peptide segment25-35 induced expression of beta-secretase in rat hippocampus. Eur. J. Pharmacol. 2010;626(2-3):213–218. doi: 10.1016/j.ejphar.2009.09.039. https://doi.org/10.1016/j.ej-phar.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Jin F., Gong Q.H., Xu Y.S., Wang L.N., Jin H., Li F., Li L.S., Ma Y.M., Shi J.S. Icariin, a phosphodiesterase-5 inhibitor, improves learning and memory in APP/PS1 transgenic mice by stimulation of NO/cGMP signaling. Int. J. Neuropsychopharmacol. 2014;17:871–881. doi: 10.1017/S1461145713001533. [DOI] [PubMed] [Google Scholar]
- 22.Wang G.Q., Li D.D., Huang C., Lu D.S., Zhang C., Zhou S.Y., Liu J., Zhang F. Icariin reduces dopaminergic neuronal loss and microglia-mediated inflammation in vivo and in vitro. Front. Mol. Neurosci. 2018;10:441. doi: 10.3389/fnmol.2017.00441. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Zeng K.W., Fu H., Liu G.X., Wang X.M. Icariin attenuates lipopolysaccharide-induced microglial activation and resultant death of neurons by inhibiting TAK1/IKK/NF-kappaB and JNK/p38 MAPK pathways. Int. Immunopharmacol. 2010;10:668–678. doi: 10.1016/j.intimp.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 24.Chen W.F., Wu L., Wong M.S. Neuroprotective properties of icariin in MPTP-induced mouse model of Parkinson’s disease: involvement of PI3K/Akt and MEK/ERK signaling pathways. Phytomedicine. 2017;25:93–99. doi: 10.1016/j.phymed.2016.12.017. https://doi.org/10. 1016/j.phymed.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 25.Fu X., Li S., Zhou S., Wu Q., Jin F., Shi J. Stimulatory effect of icariin on the proliferation of neural stem cells from rat hippocampus. BMC Complement. Altern. Med. 2018;18:34. doi: 10.1186/s12906-018-2095-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruszkiewicz J.A., Miranda-Vizuete A., Tinkov A.A., Skalnaya M.G., Skalny A.V., Tsatsakis A., Aschner M. Sex-specific differences in redox homeostasis in brain norm and disease. J. Mol. Neurosci. 2019 doi: 10.1007/s12031-018-1241-9. [DOI] [PubMed] [Google Scholar]
- 27.Thiffault C., Langston J.W., Monte D.A. Increased striatal dopamine turnover following acute administration of rotenone to mice. Brain Res. 2000;885(2):283–288. doi: 10.1016/s0006-8993(00)02960-7. [DOI] [PubMed] [Google Scholar]
- 28.Liu J., Mattheos N., Su C., Deng C., Luo N., Wang Z., Tang H. The effects of icariin on wound healing of extraction sites with administration of zoledronic and dexamethasone: a rat model study. J. Oral Pathol. Med. 2017;47:198–205. doi: 10.1111/jop.12659. [DOI] [PubMed] [Google Scholar]
- 29.Abdelsalam R.M., Safar M.M. Neuroprotective effects of vildagliptin in rat rotenone Parkinson’s disease model: role of RAGE-NFκB and Nrf2-antioxidant signaling pathways. J. Neurochem. 2015;133:700–707. doi: 10.1111/jnc.13087. [DOI] [PubMed] [Google Scholar]
- 30.Alam M., Schmidt W.J. Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav. Brain Res. 2002;136(1):317–324. doi: 10.1016/s0166-4328(02)00180-8. [DOI] [PubMed] [Google Scholar]
- 31.Goedert M., Spillantini M.G., Tredici K.D. 100 years of Lewy pathology. Nat. Rev. Neurol. 2012;9(1):13–24. doi: 10.1038/nrneurol.2012.242. [DOI] [PubMed] [Google Scholar]
- 32.Lipton J.O., Sahin M. The neurology of mTOR. Neuron. 2014;84:275–291. doi: 10.1016/j.neuron.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim Y.C., Guan K.L. mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 2015;125:25–32. doi: 10.1172/JCI73939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura K. α-synuclein and mitochondria: partners in crime? Neurotherapeutics. 2013;10(3):391–399. doi: 10.1007/s13311-013-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rizzati V., Briand O., Guillou H., Gamet-Payrastre L. Effects of pestici-de mixtures in human and animal models: an update of the recent literature. Chem. Biol. Interact. 2016;254:231–246. doi: 10.1016/j.cbi.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Georgiadis N., Tsarouhas K., Tsitsimpikou C., Vardavas A., Rezaee R., Germa-nakis I., Tsatsakis A., Stagos D., Kouretas D. Pesticides and cardiotoxi-city. where do we stand? Toxicol. Appl. Pharmacol. 2018;353:1–14. doi: 10.1016/j.taap.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Díaz-Troya S., Pérez-Pérez M.E., Florencio F.J., Crespo J.L. The role of TOR in autophagy regulation from yeast to plants and mammals. Autophagy. 2008;4:851–865. doi: 10.4161/auto.6555. [DOI] [PubMed] [Google Scholar]
- 38.Jung C.H., Ro S.H., Cao J., Otto N.M., Kim D.H. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim J.Y., Lee J.Y., Byun B.J., Kim S.H. Fisetin targets phosphatidylino-sitol-3-kinase and induces apoptosis of human B lymphoma Raji cells. Toxicol. Rep. 2015;2:984–989. doi: 10.1016/j.toxrep.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muñoz Y., Carrasco C.M., Campos J.D., Aguirre P., Núñez M.T. Parkin-son’s disease: the mitochondria-iron link. Parkinsons Dis. 2016:1–21. doi: 10.1155/2016/7049108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernández B., Ferrer I., Gil F., Hilfifiker S. Biomonitorization of iron accumulation in the substantia nigra from Lewy body disease patients. Toxicol. Rep. 2017;4:188–193. doi: 10.1016/j.toxrep.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M., Liu J., Guo W.L., Liu X., Liu S.J., Yin H.J. Icariin regulates systemic iron metabolism by increasing hepatic hepcidin expression through Stat3 and Smad1/5/8 signaling. Int. J. Mol. Med. 2016;37:1379–1388. doi: 10.3892/ijmm.2016.2545. [DOI] [PubMed] [Google Scholar]
- 43.Benskey M.J., Perez R.G., Manfredsson F.P. The contribution of alpha s-ynuclein to neuronal survival and function - Implications for Parkinson’s disease. J. Neurochem. 2016;137(3):331–359. doi: 10.1111/jnc.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vicario M., Cieri D., Brini M., Calì T. The Close Encounter Between Al- pha-Synuclein and Mitochondria. Front. Neurosci. 2018;12(388) doi: 10.3389/fnins.2018.00388. https://doi.org/10.338- 9/Fnins.eCollection2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McFarland N.R., Fan Z., Xu K., Schwarzschild M.A., Feany M.B., Hyman B.T., McLean P.J. Alpha-synuclein S129 phosphorylation mutants do not alter nigrostriatal toxicity in a rat model of Parkinson disease. J. Neuropathol. Exp. Neurol. 2009;68(5):515–524. doi: 10.1097/NEN.0b013e3181a24b53. https://doi.org/10.1097/NEN.0b013e3181a24b53. [DOI] [PMC free article] [PubMed] [Google Scholar]