Graphical abstract
Keywords: Perfluorinated alkyl acids, Perfluorinated chemicals, Rats, Toxicokinetics, PFBS, PFHxS, PFOS
Highlights
-
•
In rats, the half-life of perfluoroalkyl sulfonic acids decreased with shorter chain lengths.
-
•
Sex differences in kinetics were found for PFBS and PFHxS but not PFOS.
-
•
Perfluoroalkyl sulfonic acids were highly present in the liver but not the brain.
Abstract
Perfluorinated alkyl substances (PFAS) are persistent contaminants that have been detected in the environment and in humans. With the PFAS chemical class, there are perfluorinated alkyl acids, many of which have been associated with certain toxicities. Because toxicity testing cannot feasibly be conducted for each individual PFAS, the National Toxicology Program (NTP) designed studies to compare toxicities across different subclasses of PFAS and across PFAS of different chain lengths to better understand the structure-toxicity relationship. Pharmacokinetic studies were conducted in parallel to these toxicity studies to facilitate comparisons across PFAS and to provide context for human relevance. Here, the toxicokinetic parameters of perfluorobutane sulfonate (PFBS), perfluorohexane-1-sulphonic acid (PFHxS), or perfluorooctane sulfonate (PFOS) after a single intravenous or gavage administration in male and female Hsd:Sprague-Dawley rats are reported. Concentrations of these PFAS were measured in the liver, kidney, and brain. Plasma half-life increased with longer chain length after gavage administration: PFBS- males averaged 3.3 h, females 1.3 h; PFHxS- males averaged 16.3 days, females 2.1 days; PFOS- males and females averaged ˜ 20 days. There were dose-dependent changes in clearance and systemic exposure for all administered chemicals and the direction of change was different in PFOS compared to the others. Liver:plasma ratios of PFOS were the highest followed by PFHxS and PFBS, while brain:plasma ratios were low in all three sulfonates. Sex differences in plasma half-life and tissue distribution were observed for PFBS and PFHxS, but not PFOS. These data provide a direct comparison of the kinetics of three different perfluoroalkyl sulfonic acids and allow for the contextualization of toxicity data in rats for human risk assessment of this chemical class.
1. Introduction
Poly- and perfluorinated alkyl substances (PFAS) are used in a variety of products to impart repellency of water, oil, and/or grease. They contain chains of carbons bonded to fluorines and some contain other functional groups, such as carboxylate or sulfonate moieties. Due to their chemical properties, PFAS are resistant to degradation and persist in the environment and in organisms [1]. Additionally, rodent and human studies have identified a variety of adverse effects associated with PFAS exposure, including liver, endocrine, developmental, and immune toxicity, among others [[2], [3], [4]]. The presence of PFAS in the environment and the estimated long half-life of PFAS in human sera have led to the removal or reduced use of PFAS like perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS). Replacement PFAS, many of which have shorter carbon chains, have been developed [5]. Some studies show that these replacement chemicals are less toxic for some endpoints (Mahpatra et al., 2017) but others indicate that shorter-chained PFAS still have biological effects which may be toxic [6,7].
Part of the decrease in toxicity observed with the shorter-chain PFAS has been attributed to differences in toxicokinetics. Toxicokinetic properties of PFAS often vary with chain length, where shorter, straight-chain PFAS are eliminated faster than the longer chain varieties (Ohmari, 2003; [8]). Kinetic properties can also vary greatly depending on the sex and species/strain in which the study was conducted. PFOA displays a large sex difference in rats [9,10], with a half-life on the order of hours in female rats but days in male rats. Rodents often display a faster elimination for some long-chain PFAS than humans, typically within hours, whereas in humans the corresponding half-life may extend on the order of years [11]. The high variability of PFAS toxicokinetics makes it challenging to interpret animal toxicity data and extrapolate toxicity to humans in the absence of toxicokinetic data. In addition, any comparison of toxicities across chemicals, e.g. long chain vs short chain, would also necessitate an understanding of the absorption, distribution, and elimination of each PFAS in the same animal strain, species and sex.
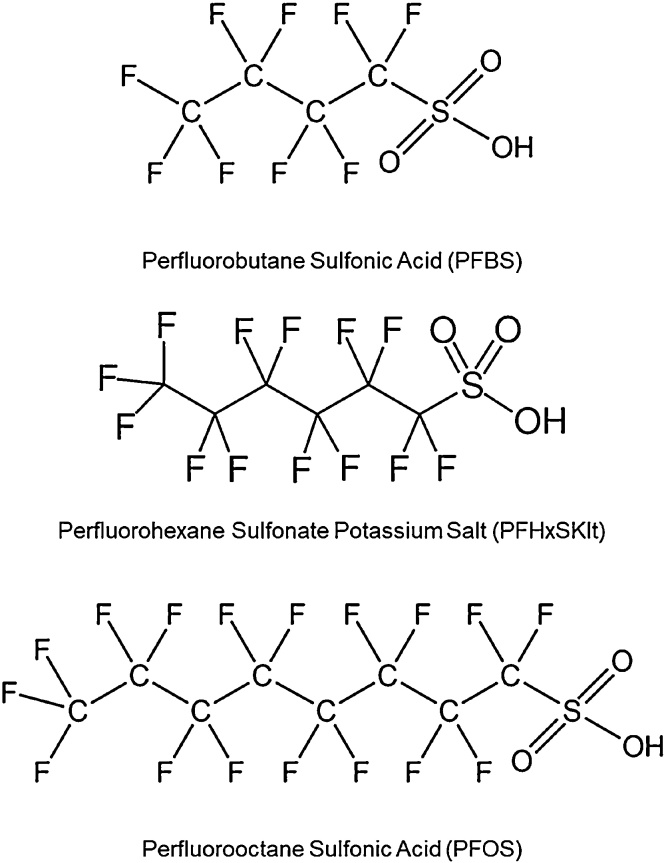
The increasing use of replacement PFAS creates a need for comparative toxicity studies, particularly between different subclasses and chain lengths, to help predict toxicity of new PFAS. To contribute to this effort, the National Toxicology Program (NTP) conducted subchronic toxicity studies of seven perfluorinated alkyl acids, a subgroup of PFAS, in male and female Hsd:Sprague-Dawley rats; three sulfonates and four carboxylates with varying chain lengths (reported in NTP Toxicity Reports 96 and 97). Toxicokinetic studies were completed in the same strain to complement these toxicity studies, providing information on internal dose to enable translation of the toxicities observed. Here, we report the plasma kinetic parameters and tissue concentrations of the three perfluoroalkyl sulfonic acids: perfluorobutane sulfonate (C4HF9O3S; PFBS), perfluorohexane-1-sulphonic acid (C6HF13O3S; PFHxS), and perfluorooctane sulfonate (C8HF17O3S; PFOS) (Fig. 1), in male and female Hsd:Sprague-Dawley rats.
Fig. 1.
Sulfonate perfluorinated alkyl acids evaluated: perfluorobutane sulfonate (PFBS), perfluorohexane-1-sulphonic acid (PFHxS), or perfluorooctane sulfonate (PFOS).
2. Materials and methods
2.1. Chemicals
Potassium perfluorobutane sulfonate (KPFBS; CAS# 29420-49-3; Lot# 20070201; Ivy Fine Chemicals), perfluorohexane-1-sulphonic acid potassium salt (PFHxSKlt; CAS# 3871-99-6; Lot# 230002; Interchim), and perfluorooctane sulfonate (PFOS; CAS# 1763-23-1; Lot# T20 G; Matrix Scientific), were purchased commercially. Chemical identity was confirmed by infrared spectroscopy, 13C and 19F nuclear magnetic resonance (NMR) spectroscopy. Purity (KPFBS = 96%; PFHxSKslt = 99.9%; PFOS = 96.7%) was determined by a combination of ion chromatography, and gas chromatography (GC) with flame ionization or electron capture detection (ECD).
2.2. Animals
Male and female Hsd:Sprague Dawley rats, approximately eight weeks of age, were purchased from Harlan Laboratories, Inc. (now Envigo, Inc., Indianapolis, IN) and delivered to Battelle (Columbus, OH). Prior to shipment, jugular catheters were implanted in rats for intravenous (IV) administration. Irradiated NTP-2000 feed (Zeigler Brothers, Inc., Gardners, PA) was provided ad libitum. Animals were maintained on a 12:12 light cycle. Prior to dosing, rats were randomized using a partitioning algorithm program (Xybion PATH/TOX SYSTEM, Xybion Medical Systems Corporation, Version 4.2.2) to ensure that mean body weights of rats in each group were similar. Additional animals were included as replacements in the event that dosing was believed to be incomplete or an insufficient volume of blood was collected.
2.3. Dose formulation and administration
Doses for each chemical are given in Table 1. All chemicals were formulated in 2% Tween 80 (pH 6–8) in deionized water. Since the primary objective of the study was to evaluate the kinetics of the chemicals and not necessarily toxicity, no vehicle control group was included. All formulations were analyzed by high performance liquid chromatography using validated analytical methods (r ≥ 0.99; precision ≤ 5%; accuracy, ≤ ±10%). All gavage formulations were within 10% of the target concentration. A single bolus IV (4 mL/kg) or gavage dose (three dose levels; 5 mL/kg) was administered based on the body weight on the day of dosing. For PFOS, repeat doses, 2 mg/kg/d for five days, were administered to compare kinetics to the single administration. The chemicals PFBS and PFHxS were purchased as salts but the measurement in blood and tissues was as the original chemical. Thus, when discussing the dose, the abbreviation for the ion form is used whereas in the results, the abbreviated chemical name is used (e.g. KPFBS vs. PFBS).
Table 1.
The route of administration and doses administered for the three sulfonates.
| Chemical Administered |
Route | Dose (mg/kg) |
Dose (mmol/kg) |
Time points of blood collection |
|---|---|---|---|---|
| KPFBS | IV | 4 | 0.0118 | 0.083, 0.25, 0.5, 0.75, 1, 1.5, 3, 6, 12, 24, 48 hrs (M & F) |
| Gavage | 4 | 0.0118 | 0.083, 0.25, 0.5, 0.75, 1, 1.5, 3, 6, 9, 12, 24, 48 hrs (M & F) | |
| 20 | 0.0591 | |||
| 100 | 0.2957 | |||
| PFHxSK | IV | 4 | 0.0091 | 0.083, 0.25, 0.5, 1, 3, 6, 12, 24, 192, 528, 864, 1200 hrs (M) 0.083, 0.25, 0.5, 1, 3, 6, 12, 24, 48, 96, 192, 288, 528 hrs (F) |
| Gavage | 4 | 0.0091 | 0.25, 1, 3, 6, 12, 24, 192, 528, 864, 1200 hrs (M) 0.25, 1, 3, 6, 12, 24, 48, 96, 192, 288, 528 hrs (F) |
|
| 16 | 0.0365 | |||
| 32 | 0.0730 | |||
| PFOS | IV | 2 | 0.004 | 0.083, 1, 6, 12, 24, 192, 528, 864, 1200 hrs, 11, 15, 20 wks (M & F) |
| Gavage | 2 | 0.004 | 0.083, 1, 6, 12, 24, 192, 528, 864, 1200 hrs, 11, 15, 20 wks (M & F) | |
| 2 (x 5)a | 0.020b | |||
| 20 | 0.040 |
a Dose administered for 5 days.
b Total dose administered.
2.4. Sample collection
Blood was collected at 10–13 time points (n = 3/time point) determined based on the preliminary studies and literature. Each animal was bled twice at most. Actual times for blood collection were recorded at each time point; target blood collection times are shown in Table 1. Rats were anesthetized with 70% CO2 (30% O2) and blood was collected via the retro-orbital sinus (˜ 0.7 mL) into glass tubes containing ethylenediaminetetraacetic acid, gently inverted, and placed on wet ice until separated into plasma. Plasma was separated by centrifugation at 1750 x g for 10 min at 4 °C and stored at -20 °C. In specific gavage dose groups (20 mg/kg for KPFBS; 16 mg/kg for PFHxSKlt; 2 mg/kg, 20 mg/kg, and 2 mg/kg x 5 for PFOS), the liver, kidney, and brain were collected from 3 animals per timepoint to measure chemical concentrations. Due to anticipated differences in kinetics, each chemical was evaluated at a different range of time points for tissue collection: PFBS- 0.5, 1, 3, 6, 12 h; PFHxS- 3, 6, 12, 24, 192, 538, 1200 h; PFOS- 6, 24, 864, 1704, 3216 h.
2.5. Sample preparation and analysis
An analytical method using protein precipitation followed by liquid chromatography (LC) tandem mass spectrometry (MS/MS) was used to quantitate PFBS, PFHxS, and PFOS in Hsd:Sprague Dawley rat plasma, liver, kidney, and brain. The validation included an assessment of linearity (r), inter- and intraday accuracy (estimated as standard error, RE), and inter- and intraday precision (estimated as relative standard deviation, RSD), absolute recovery, extract and matrix stability, reinjection reproducibility, and limit of detection (LOD). Analytical method validation parameters are given in Supplemental Table S1-S4.
Stock solutions of chemical analyte were prepared in methanol and further diluted in either methanol (plasma PFBS) or acetonitrile then in American Society for Testing and Materials (ASTM) Type I water to generate concentrations of standards in the working range. Stock solutions of the internal standard (IS) was prepared in methanol and diluted in either acetonitrile (plasma PFBS) or ASTM Type I water followed by dilution in 1% formic acid in acetonitrile (plasma PFBS), 10 mM ammonium acetate, pH 5.0 (plasma PFHxS) or in ASTM Type 1 water to generate working IS solutions. Matrix calibration curves were prepared in duplicate by adding the standard solutions to blank rat plasma/tissue. Quality control (QC) samples were prepared in blank rat plasma/tissue using a procedure similar to that for the matrix standards, using an independent stock solution. Matrix blanks were prepared the same as matrix standards except the addition of the analyte.
Tissues (100–300 mg) were homogenized in a volume of ASTM Type 1 water ten times the volume of the tissue. For plasma, 100 μL of IS solution (PFHxS: 200 ng/mL; PFOS: 500 ng/mL), was added to 100 μL of plasma and mixed thoroughly. For PFBS, 400 μL of IS (50 ng/mL) was added. For tissues, 200 μL of IS (100 ng/mL) was added to 100 μL of tissue homogenate. Then, 0.05 N potassium hydroxide in 1:1 methanol:water was added to the homogenates and rotated for 12 h for digestion. Samples were neutralized with 0.1 N hydrochloric acid, vortexed and centrifuged. The supernatant was analyzed by LC–MS/MS. Matrix calibration standards, QC samples and matrix blanks were also prepared in the same manner as study samples and run with each batch of samples.
The LC–MS/MS system used was an Agilent 1100 and 1200 (Santa Clara, CA) coupled to a Sciex API 3000, 4000 Q Trap, or 5000 mass spectrometer (Toronto, Canada). Chromatography was performed using a Luna C18(2)-HST column (C18, 100 x 3.0 mm, 2.5 μm for PFBS; Phenomenex, Torrance, CA). For the analysis of PFBS, mobile phase (0.1% formic acid in acetonitrile: 1 mM aqueous ammonium acetate, 85:15 (v/v)) was run at an isocratic gradient at a flow rate of 500 μL/minute. Turbo Ionspray™ (Sciex) ionization sources were operated in the negative ion mode.
A quadratic regression with 1/x weighing was used to relate LC–MS/MS peak area response ratio of analyte to IS with the concentration of calibration standards. The concentration in samples was calculated using the response ratio, the regression equation, initial sample volume, and when applicable dilution. The concentration in plasma was expressed as ng/mL and in tissues as ng/g. The limit of quantitation (LOQ) in plasma was 25 ng/mL and 5 ng/g of organ tissue. All concentrations above LOQ were reported. Data from study samples were considered valid if: the matrix calibration curve was linear (r ≥ 0.99); at least 75% of matrix standards were within 15% of nominal (except at the LOQ where it was 20%); at least 67% of the QC samples were within 15% of nominal values.
2.6. Toxicokinetic (TK) modeling
All concentrations above LOQ were used in TK analysis. Semi-log plots of the plasma and tissue (liver, kidney, and brain) concentration versus time data sets were prepared by sex, by dose, and by route of administration. WinNonlin (Version 5.0.1, Pharsight Corporation, Mountain View, CA) was used for TK analysis. Individual animal concentration-time values were used to find a model that best fit the data set. One- and/or two-compartment models with and without weighting were tested based on the appearance of the plasma concentration vs. time plot. The model and the weighting factor that resulted in the best goodness of fit were selected as the final model. The primary and secondary parameters, including a measure of their variability, were estimated using the WinNonlin software.
Cmax is the maximal plasma concentration, with the time of maximum concentration as Tmax. Assuming first-order kinetics, half-lives for the initial (α T1/2) and terminal (β T1/2) elimination phases were calculated as 0.693/α and 0.693/β, respectively, where α and β are the bi-exponential disposition rate constants. Half-lives for the absorption (k01 half-life) and elimination (k10 half-life) were calculated as 0.693/k01 and 0.693/k10, respectively. Apparent volume of central (V1) and peripheral (V2) distribution and apparent clearance (CL) were calculated using standard equations [12]. Bioavailability was calculated using the area under the curve (AUC) and the equation:
| F% = [AUC(gavage))/(Dose(gavage)]/ [AUC(IV))/(Dose(IV)] *100 |
To facilitate comparisons, plasma concentrations and kinetic parameters were converted to a molar basis using the molecular weight of PFBS (300.1 g/mol), PFHxS (400.1 g/mol), and PFOS (500.1 g/mol). The dose administered was converted to a mmol/kg value using the molecular weight of the KPFBS (338.2 g/mol), PFHxSK (438.2 g/mol), and PFOS (500.1 g/mol) (Table 1).
3. Results
There were no animals found dead or moribund due to toxicity and no treatment-related clinical signs observed following an IV or gavage administration at any dose level for the three PFAS.
3.1. PFBS
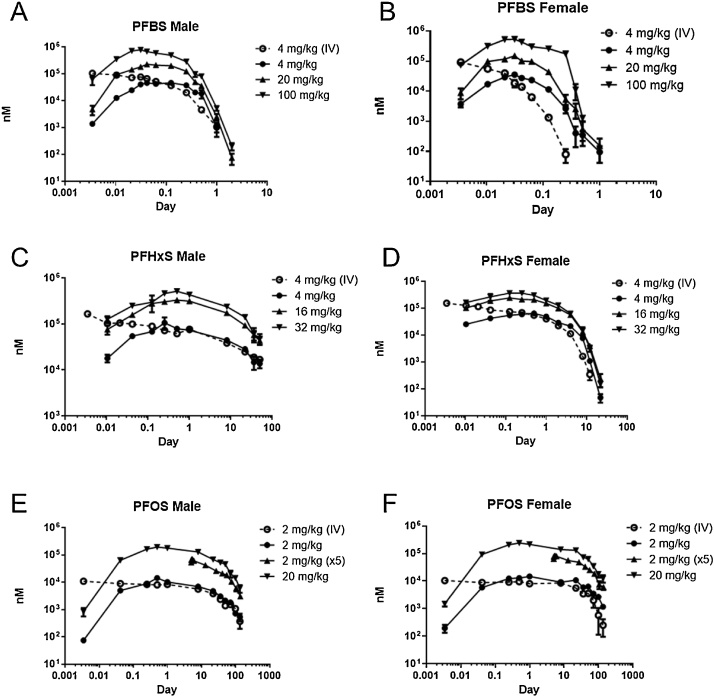
Plasma concentrations of PFBS over time are shown in Fig. 2A and B. A two-compartment model was the best fit for male rats (IV and gavage) and female rats (IV) while a one-compartment model was a better fit for the female gavage exposure data. Toxicokinetic parameters for PFBS are shown in Table 2. After IV administration, the Cmax in males was 0.118 mM. The overall plasma elimination (k10) half-life was around 2 h. In females, the Cmax was similar to that in males. The k10 half-life was 6-fold faster in females compared to males, 0.36 h. The AUC was approximately 7-fold lower and clearance was 7-fold faster in females.
Fig. 2.
Plasma concentrations (nM; mean and SEM) of PFBS, PFHxS, or PFOS in male and female Hsd:Sprague-Dawley rats after a single administration of chemical. Three gavage and one iv dose were administered.
Table 2.
Summary of pharmacokinetic properties (mean ± SEM) in plasma after a single IV or gavage dose (three dose levels) of KPFBS in male and female Hsd:Sprague Dawley Rats.
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | 4a (IV) | 4a | 20a | 100a | 4a (IV) | 4b | 20b | 100b |
| Cmaxc (mM) | 0.118 ± 0.017 | 0.053 ± 0.008 | 0.250 ± 0.026 | 0.750 ± 0.070 | 0.104 ± 0.016 | 0.028 ± 0.001 | 0.129 ± 0.006 | 0.422 ± 0.035 |
| Tmaxc (hr) | n/a | 2.37 ± 0.56 | 2.18 ± 0.24 | 1.42 ± 0.18 | n/a | 0.99 ± 0.13 | 0.71 ± 0.16 | 1.42 ± 0.27 |
| k10 T1/2 (hr) | 2.26 ± 0.33 | 4.37 ± 18.1 | 2.73 ± 0.84 | 2.86 ± 0.39 | 0.36 ± 0.03 | 1.50 ± 0.10 | 1.23 ± 0.12 | 1.11 ± 0.10 |
| α T1/2 (hr) | 0.53 ± 0.25 | 1.37 ± 31.5 | 2.37 ± 1.07 | 2.60 ± 0.61 | 0.28 ± 0.03 | n/a | n/a | n/a |
| β T1/2 (hr) | 4.22 ± 0.28 | 4.89 ± 1.67 | 5.36 ± 1.24 | 5.25 ± 1.19 | 0.95 ± 0.10 | n/a | n/a | n/a |
| AUCc (μM*hr) | 0.387 ± 0.023 | 0.513 ± 0.050 | 1.776 ± 0.150 | 4.399 ± 0.332 | 0.053 ± 0.004 | 0.088 ± 0.011 | 0.364 ± 0.078 | 1.289 ± 0.165 |
| AUCc / Dose (μM *hr/mg/kg) | 0.097 ± 0.006 | 0.128 ± 0.012 | 0.089 ± 0.007 | 0.044 ± 0.003 | 0.013 ± 0.001 | 0.022 ± 0.003 | 0.018 ± 0.004 | 0.013 ± 0.002 |
| CL (mL/hr/kg) | 34.5 ± 2.0 | 26.0 ± 2.5 | 37.6 ± 3.1 | 75.5 ± 5.8 | 252 ± 18 | 152 ± 20 | 183 ± 39 | 259 ± 33 |
| V1 (mL/kg)d | 113 ± 16 | 164 ± 677 | 148 ± 52 | 311 ± 55 | 123 ± 12 | 328 ± 57 | 326 ± 95 | 415 ± 83 |
| V2 (mL/kg)e | 74.8 ± 18.8 | 13.3 ± 544 | 19.0 ± 17.7 | 23.9 ± 19.5 | 42 ± 7 | n/a | n/a | n/a |
| Ff (%) | n/a | 133 | 92 | 46 | n/a | 166 | 137 | 97 |
n/a: not applicable.
CL=clearance.
F=bioavailability.
T1/2=half-life.
a Two-compartment model.
b One-compartment model.
c Predicted from model.
dVolume of distribution for the central compartment.
eVolume of distribution for the peripheral compartment.
f Estimated by dividing dose-normalized IV AUC by dose-normalized Gavage AUC.
In males administered KPFBS by gavage, Cmax was reached by 1.4 to 2.4 h. The k10 half-life ranged from 2.7 to 4.4 h. Cmax and AUC increased with dose in male rats, but these increases did not appear to be dose-proportional. The dose-adjusted AUC decreased roughly 3-fold from the 4 to 100 mg/kg dose, while clearance increased 3-fold. In female rats, Cmax was lower and reached earlier than in males; Tmax was under 1.5 h for all dose groups. The average k10 half-life was between 1.1 and 1.5 h in female rats, which was approximately 2 to 3-fold faster than males. The AUC was 3 to 6-fold lower in females compared to males given the same dose; clearance was around 3 to 6-fold higher in females. As observed in males, the dose-adjusted AUC decreased while clearance increased with increasing doses. The overall volume of distribution (V1+V2) was similar for both males (167–335 mL/kg) and females (165–415 mL/kg). Bioavailability was generally higher in females than males and decreased in both sexes with increasing dose.
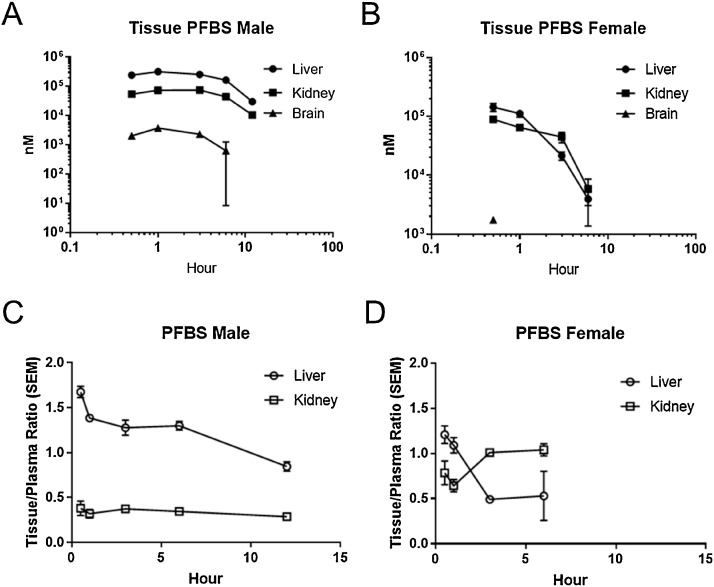
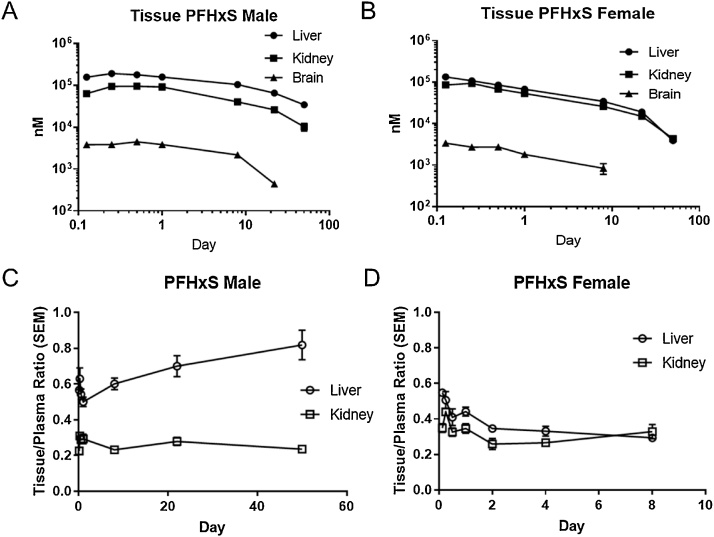
Liver, kidney, and brain tissues were collected at selected time points in the 20 mg/kg group of male and female rats up to 12 h post-dose. Liver and kidney concentrations of PFBS were below the detection limit at the last measured time point in females. Brain concentrations were detected in all but the last time point in males and only at the first time point in females. Overall, concentrations of PFBS were higher in the liver than in the kidney in both sexes. PFBS concentrations in all tissues decreased slightly over time, with females having a faster decrease than males (Fig. 3A, B). In males, liver:plasma ratios were generally above 1, dropping below 1.0 at 12 h, while kidney:plasma ratios remained around 0.29 to 0.38 (Fig. 3C). In female rats, the liver:plasma ratios were lower and kidney:plasma ratios were higher than those in males (Fig. 3D). Brain:plasma ratios ranged from 0.01 to 0.02 in males and was 0.02 in females at 0.5 h (data not shown).
Fig. 3.
Tissue concentrations (nM; mean and SEM) and tissue:plasma ratios (mean and SEM) in male (A, C) and female (B, D) Hsd:Sprague-Dawley rats after a single gavage administration of KPFBS (20 mg/kg).
3.2. PFHxS
Plasma concentrations of PFHxS over time are shown in Fig. 2C and D. A two-compartment model was used to describe the data after an IV administration while a one-compartment model was used for gavage administration (Fig. 2C, D). Toxicokinetic parameters for PFHxS are shown in Table 3. After IV administration, male rats had a Cmax of 0.11 mM and a k10 half-life of 13 days. Female rats had a similar Cmax but the k10 half- life was ˜18-fold shorter, 0.7 days. AUC was 14-fold lower and clearance approximately 14-fold higher in female rats compared to males.
Table 3.
Summary of pharmacokinetic properties (mean ± SEM) after a single IV or gavage dose (three dose levels) of PFHxSK in male and female Hsd:Sprague Dawley Rats.
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | 4 (IV)a | 4b | 16 b | 32 b | 4 (IV) a | 4 b | 16 b | 32 b |
| Cmaxc (mM) | 0.11 ± 0.01 | 0.08 ± 0.01 | 0.29 ± 0.02 | 0.41 ± 0.04 | 0.15 ± 0.03 | 0.06 ± 0.01 | 0.21 ± 0.02 | 0.30 ± 0.02 |
| Tmaxc (hr) | n/a | 6.90 ± 1.26 | 5.89 ± 0.82 | 5.28 ± 0.99 | n/a | 2.81 ± 0.38 | 2.24 ± 0.48 | 1.87 ± 0.43 |
| k10 T1/2 (days) | 13.0 ± 1.5 | 17.6 ± 1.8 | 16.5 ± 1.1 | 14.8 ± 1.2 | 0.70 ± 0.08 | 2.33 ± 0.07 | 2.19 ± 0.06 | 1.98 ± 0.05 |
| α T1/2 (hr) | 13.0 ± 3.8 | n/a | n/a | n/a | 0.70 ± 0.31 | n/a | n/a | n/a |
| β T1/2 (days) | 33.8 ± 6.4 | n/a | n/a | n/a | 1.56 ± 0.04 | n/a | n/a | n/a |
| AUCc (μM*hr) | 50.74 ± 4.97 | 49.74 ± 4.00 | 167.0 ± 9.5 | 212.7 ± 15.0 | 3.65 ± 0.17 | 5.20 ± 0.22 | 16.30 ± 1.02 | 20.84 ± 1.30 |
| AUCc / Dose (μM *hr/mg/kg) | 12.7 ± 1.4 | 12.4 ± 1.0 | 10.4 ± 0.6 | 6.65 ± 0.47 | 0.912 ± 0.044 | 1.30 ± 0.06 | 1.02 ± 0.06 | 0.651 ± 0.041 |
| CL (mL/hr/kg) | 0.197 ± 0.019 | 0.201 ± 0.016 | 0.239 ± 0.014 | 0.376 ± 0.027 | 2.73 ± 0.13 | 1.92 ± 0.09 | 2.46 ± 0.15 | 3.84 ± 0.24 |
| V1d (mL/kg) | 88.4 ± 5.1 | 123 ± 11 | 137 ± 9 | 192 ± 17 | 66.3 ± 7.6 | 155 ± 9 | 186 ± 14 | 264 ± 20 |
| V2e (mL/kg) | 136 ± 27 | n/a | n/a | n/a | 77.6 ± 10.8 | n/a | n/a | n/a |
| Ff (%) | n/a | 98 | 82 | 52 | n/a | 142 | 112 | 71 |
n/a: not applicable.
CL=clearance.
F=bioavailability.
T1/2=half-life.
aTwo-compartment model.
bOne-compartment model.
c Predicted from model.
dVolume of distribution for the central compartment.
eVolume of distribution for the peripheral compartment.
f Estimated by dividing dose-adjusted IV AUC by dose-adjusted Gavage AUC.
After gavage administration, the Cmax in males was reached within 7 h. The k10 half-life was around 2 weeks. The dose-adjusted AUC decreased with increasing dose and clearance remained approximately the same. Females tended to have slightly lower Cmax at the same administered dose and earlier Tmax. k10 half-life was ˜7.5-fold shorter than in males. The AUC was 10-fold lower and clearance 10-fold faster in females compared to males. The overall volume of distribution (V1+V2) was similar for both males (123–224 mL/kg) and females (155–264 mL/kg). Bioavailability decreased approximately 2-fold with increasing dose in both males and females.
Following a single gavage administration of 16 mg/kg PFHxSK in male and female rats, PFHxS was detected in the liver and kidney at all timepoints (Fig. 4A). In the brain, PFHxS was below detection limit at the last time point in males and at the last two time points in females. Concentrations of PFHxS were highest in the liver and around 1 to 3-fold less in the kidney. In the brain, PFHxS concentrations were ˜40-fold lower than liver concentrations. In females, tissue concentrations of PFHxS were similar to concentrations in males but decreased faster over time (Fig. 4B). Liver:plasma ratios were less than one at all time points (range of 0.50 to 0.82) and increased over time (Fig. 4C). In females, liver:plasma ratios ranged from 0.29 to 0.55 and had minimal changes over time (Fig. 4D). Kidney:plasma ratios were between 0.23 and 0.31 in males and 0.26 and 0.44 in females with minimal change over time. Brain:plasma ratios of PFHxS were low in both sexes, ranging from 0.01-0.02 (data not shown).
Fig. 4.
Tissue concentrations (nM; mean and SEM) and tissue:plasma ratios (mean and SEM) in male (A, C) and female (B, D) Hsd:Sprague-Dawley rats after a single gavage administration of PFHxSK (16 mg/kg).
3.3. PFOS
Plasma concentrations of PFOS over time are shown in Fig. 2E and F. A two-compartment model was used to describe the plasma concentration data after IV and gavage exposure (single or repeat dosing of PFOS). Toxicokinetic parameters are shown in Table 4. After IV administration in males, the Cmax was 0.01 mM and the k10 half-life was 22 days. There were no sex differences observed.
Table 4.
Summary of pharmacokinetic properties (mean ± SEM) after a single IV, single gavage dose (2 and 10 mg/kg), or repeated dose (2 mg/kg/day for five days) of PFOS in male and female Hsd:Sprague Dawley Rats.
| Male |
Female |
|||||||
|---|---|---|---|---|---|---|---|---|
| Dose (mg/kg) | 2 (IV) | 2 | 2 (x5)a | 20 | 2 (IV) | 2 | 2 (x5)a | 20 |
| Cmaxb (mM) | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.11 ± 0.01 | 0.21 ± 0.03 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.14 ± 0.02 | 0.27 ± 0.03 |
| Tmaxb (hr) | n/a | 14.3 ± 2.7 | 0.94 ± 0.15 | 16.4 ± 2.78 | n/a | 12.2 ± 5.2 | 0.92 ± 0.16 | 13.7 ± 3.3 |
| k10 T1/2 (days) | 22.0 ± 2.1 | 19.9 ± 3.8 | 19.0 ± 3.2 | 14.5 ± 2.1 | 23.0 ± 3.7 | 28.4 ± 11.0 | 21.1 ± 4.3 | 18.0 ± 3.1 |
| α T1/2 (days) | 4.6 ± 2.7 | 3.1 ± 2.4 | 0.3 ± 0.1 | 4.0 ± 2.9 | 0.3 ± 0.3 | 0.8 ± 2.1 | 0.3 ± 0.2 | 2.2 ± 3.0 |
| β T1/2 (days) | 39.7 ± 4.4 | 40.5 ± 5.5 | 33.4 ± 4.2 | 35.8 ± 4.2 | 32.8 ± 3.7 | 40.7 ± 3.5 | 40.0 ± 2.5 | 36.0 ± 4.0 |
| AUCb (μM*hr) | 7.32 ± 0.42 | 9.86 ± 0.74 | 58.18 ± 3.00 | 149.76 ± 10.60 | 10.72 ± 0.78 | 17.74 ± 1.02 | 89.18 ± 5.00 | 213.94 ± 16.00 |
| AUCb / Dose (μM *hr/mg/kg) | 3.66 ± 0.21 | 4.93 ± 0.37 | 5.82 ± 0.30 | 7.49 ± 0.53 | 5.36 ± 0.39 | 8.87 ± 0.51 | 8.92 ± 0.50 | 10.67 ± 0.80 |
| CL (mL/hr/kg) | 0.546 ± 0.031 | 0.406 ± 0.031 | 0.068 ± 0.004 | 0.267 ± 0.019 | 0.373 ± 0.027 | 0.226 ± 0.013 | 0.045 ± 0.003 | 0.186 ± 0.013 |
| V1c (mL/kg) | 417 ± 31 | 280 ± 48 | 176 ± 27 | 34.6 ± 4.8 | 297 ± 43 | 222 ± 84 | 136 ± 25 | 27.9 ± 4.7 |
| V2d (mL/kg) | 264 ± 71 | 244 ± 81 | 123 ± 42 | 43.9 ± 7.7 | 124 ± 62 | 93.4 ± 93.0 | 86.3 ± 37.3 | 27.5 ± 6.5 |
| Fe (%) | n/a | 135 | 159 | 205 | n/a | 165 | 166 | 200 |
n/a: not applicable.
F = Bioavailability.
T1/2=half-life.
a Dose administered over five days.
b Predicted from model (two-compartment).
cVolume of distribution for the central compartment.
dVolume of distribution for the peripheral compartment.
eEstimated by dividing dose-adjusted IV AUC by dose-adjusted Gavage AUC.
After gavage administration of 2 mg/kg, the Cmax in male rats was similar to the Cmax reached with IV administration and the Tmax was 14 h (Table 4). The Cmax reached in the 20 mg/kg dose group was proportional, 0.21 mM. Additionally, the Cmax in animals following repeat dosing (10 mg/kg total dose) was about half of the Cmax seen with a single dose of 20 mg/kg. The plasma k10 half-life ranged from 14 to 19 days for all doses. The AUC increased proportionally with dose, indicated by only slight increases in the dose-adjusted AUC. Clearance in animals dosed with 20 mg/kg was lower than clearance in 2 mg/kg animals. Clearance following repeat dosing was 4 to 6-fold lower than the clearance observed with single administration doses. In female rats, the Cmax, Tmax, and k10-half-life were similar to those in male rats. The AUC was on average 1.5-fold higher and clearance 1.5-fold lower in females compared to males. Bioavailability slightly increased in both males and females with increasing dose. The overall volume of distribution (V1+V2) was similar at the 2 and 20 mg/kg dose for both males (524 and 78.5 mL/kg, respectively) and females (315 and 55.1 mL/kg, respectively). With repeat dosing, the overall volume of distribution was 299 mL/kg in males and 222.3 mL/kg in females.
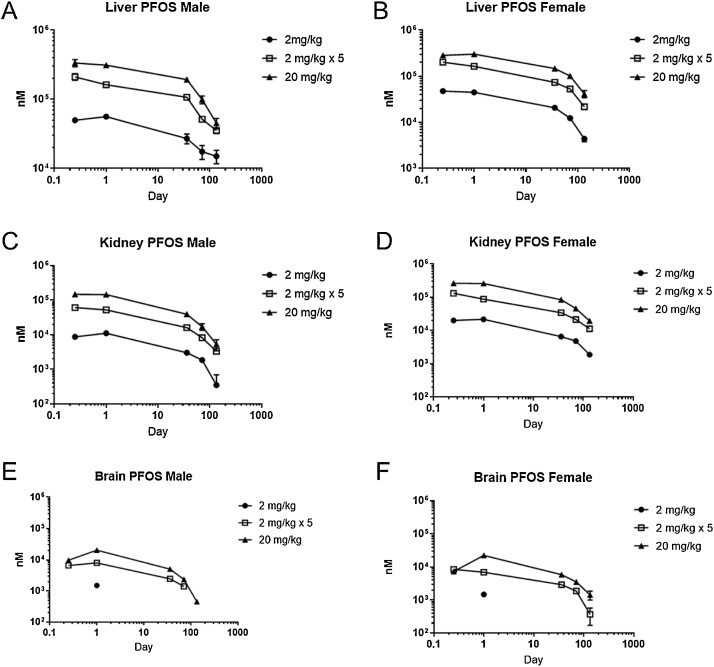
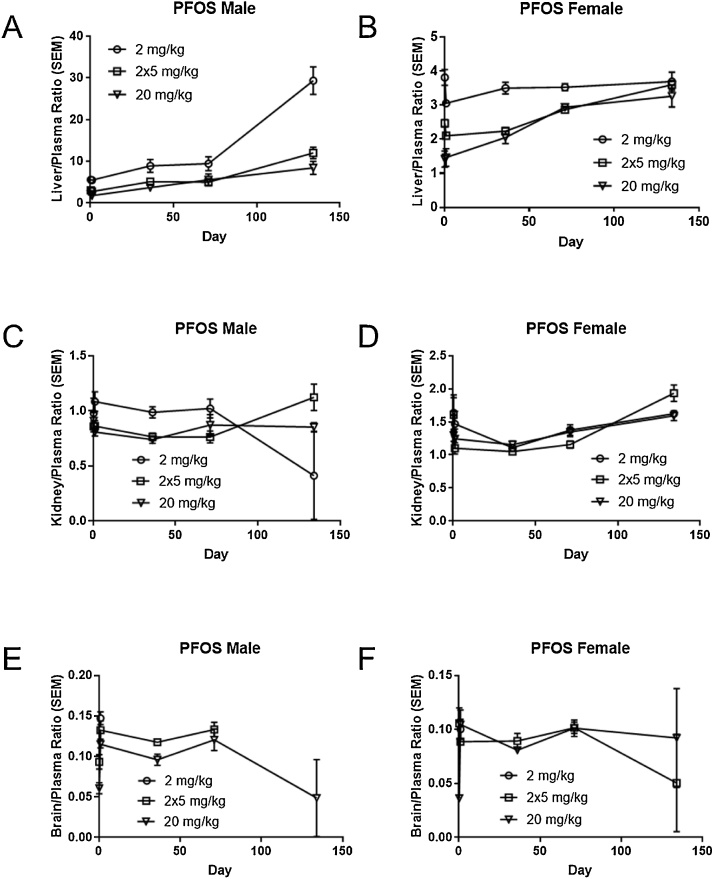
Tissue concentrations of PFOS were dose-dependent and decreased gradually over time (Fig. 5). Following a single administration of either 2 mg/kg or 20 mg/kg PFOS in rats, PFOS was detected in all liver and kidney samples, with liver having the highest concentrations of PFOS. In the brain, PFOS was detected at all timepoints in the 20 mg/kg dose group. PFOS was not detected in the brain at the last time point in male rats with repeat dosing or in the 2 mg/kg animals, except at the 24 h timepoint. In males, the liver:plasma ratio was greater than one at all time points and increased over time for all doses, most dramatically in the 2 mg/kg dose group (Fig. 6A). In female rats, liver:plasma ratio remained between 3 and 4 in the 2 mg/kg dose group over time (Fig. 6B). In the female repeat dose and 20 mg/kg group, liver:plasma ratios were initially lower (1 to 2.5) and increased to similar ratios as seen in the 2 mg/kg group by the last time point. PFOS kidney:plasma ratio was close to one in males and between 1 and 2 in female rats; these ratios remained steady over time (Fig. 6C, D). Brain:plasma ratios ranged from 0.06 to 0.12 in males and 0.04 to 0.10 in females in the 20 mg/kg group, with no apparent change over time (Fig. 6E, F). Repeat gavage dosing of 2 mg/kg over five days led to brain:plasma ratios of 0.09-0.13 in both sexes.
Fig. 5.
Liver and kidney concentrations (nM; mean and SEM) in male and female Hsd:Sprague-Dawley rats after a single gavage administration of PFOS (2 or 20 mg/kg) or multiple administrations of PFOS (2 mg/kg/day for five days).
Fig. 6.
Tissue/plasma ratio (mean and SEM) for male and female Hsd:Sprague Dawley SD rats after a single gavage administration of PFOS (2 or 20 mg/kg) or multiple administrations of PFOS (2 mg/kg/day for five days).
4. Discussion
Understanding the kinetics of PFAS in animals, which can vary due to multiple factors including carbon chain length as well as the sex and strain of the animal, is important for evaluating the human relevance of PFAS toxicity studies and for comparing across subclasses of PFAS. Thus, to inform the interpretation of the NTP’s toxicity data, the NTP evaluated the toxicokinetics of seven PFAS chemicals in male and female Hsd:Sprague-Dawley rats. Here, we report toxicokinetic parameters and tissue concentrations for three of these chemicals: PFBS, PFHxS, and PFOS. Since humans are exposed to PFAS primarily through drinking water, the oral gavage experiments are more pertinent; the IV experiments were used to determine bioavailability.
Following gavage administration, absorption of all three PFAS occurred within 24 h, along with distribution into tissues. PFBS, PHFxS, and PFOS were detected in the liver, kidney, and brain with the most accumulation in the liver, followed by the kidney, and low concentrations in the brain. These findings are consistent with previous reports on tissue distribution of these PFAS [[13], [14], [15]]. The liver:plasma ratios of all three PFAS were much higher than ratios in kidney or brain, which is consistent with the liver being a primary target organ of PFAS accumulation and toxicity [3,16]. With the exception of PFOS in the liver, the tissue:plasma ratios of the PFAS were around one or less. Additionally, the volume of distribution for all PFAS in this study was below the aqueous volume of total body water in rats (668 mL/kg). Taken together, these results support the previous literature indicating that PFAS remain in the plasma, likely due to their high binding affinity to serum albumin [17,18]. No major changes in tissue accumulation of PFOS or in systemic exposure were seen with repeat dosing. Tissue concentrations of PFOS from rats following repeat dosing (5 doses of 2 mg/kg/day) were between concentrations from the 2 and 20 mg/kg dose groups and the tissue:plasma ratios were similar to the other doses.
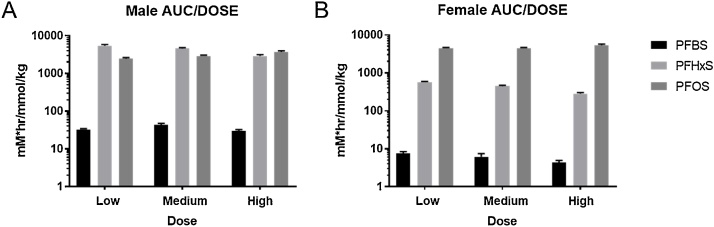
Due to consistencies in study design, we were able to compare kinetics across three PFAS with different chain lengths. Our results showed that PFOS had the highest Tmax out of the three. The dose-adjusted AUC was much lower in males exposed to PFBS compared to males exposed to PFHxS or PFOS (Fig. 7A). AUC also increased with chain length in females, where PFBS had the lowest and PFOS had the highest AUC (Fig. 7B). Shorter chain length PFAS had a shorter half-life than longer-chain analytes, corroborating findings from other studies (Ohmari et al., 2003; [8]). Since blood levels of PFAS were not measured, tissue:plasma ratios were used to compare tissue accumulation. The plasma:blood partitioning for the three sulfonate PFAS studied here have not been determined in rodents. In humans, PFOS, PFHxS and other anionic PFAS show preferential binding in plasma with plasma:blood ratios of around 2:1 [19,20]. Plasma:blood partitioning for PFBS has not been reported in humans. However, PFBS does bind to human serum albumin [18]. Thus, using the assumption that the three PFAS evaluated in this study have plasma:blood ratios of 2:1 in both male and female rats, tissue:plasma ratios reported in this study underestimate tissue:blood ratios and comparisons across chemicals and sexes can be made. In our study, the tissue:plasma ratios of PFOS tended to be higher than PFBS or PFHxS. In summary, shorter chain lengths were associated with faster absorption, less tissue distribution, and higher elimination, leading to less systemic exposure.
Fig. 7.
Dose-adjusted area under the curve (AUC) of plasma concentrations vs. time of PFBS, PFHxS, or PFOS in male (A) and female (B) Hsd:Sprague-Dawley rats after a single gavage administration of chemical.
Differences in elimination and systemic exposure due to chain length of PFAS have been reported in other studies and may be due to a variety of mechanisms. Shorter chain PFAS have increased solubility which could contribute to faster elimination and differences in tissue absorption (Danish EPA 2015). Additionally, longer chain carboxylate PFAS were associated with increased induction of efflux transporter proteins, ABCB1 and ABCC1, in hepatocytes (Rusiecka and Składanowski, 2008), potentially influencing their elimination. Since humans are typically exposed to numerous PFAS at a time, changes in transporter proteins induced by long-chain PFAS may modify the transport kinetics of other PFAS. Furthermore, chain length may influence the binding of PFAS to proteins. Short-chain PFAS have less affinity for serum proteins than the long-chain chemicals and carboxylate PFAS have stronger affinities than the sulfonate ones [17,18]. Because PFAS can also bind to proteins within tissues such as liver fatty acid-binding protein [21], differences in the affinity for these proteins could also lead to differences in tissue retention and elimination. However, given species differences in protein expression and activity, the association between chain length and kinetics observed in rats may not be maintained in humans. For instance, the half-life of PFHxS in humans is considerably longer than that of PFOA and PFOS [11,22,23].
There was some evidence of saturation or induction of elimination with administration of these PFAS. Changes in dose-adjusted AUC with increasing dose have also been observed in Charles River Sprague-Dawley rats exposed to PFOS [15]. Since these PFAS are not known to be metabolized further in the body [3], stimulation of elimination or saturation of binding proteins/transporters may explain the changes in dose-adjusted AUC. High-affinity resorption processes in the kidney may play a primary role in PFAS kinetics, leading to sex differences and longer half-lives for longer chain chemicals, but also are saturable such that at higher doses the dose-adjusted systemic exposure decreases [24]. In addition to renal resorption, perfluorinated alkyl acids, including the sulfonate PFAS, are thought to undergo hepatic transport and enterohepatic circulation (Johnson 1984; [25,3]). PFBS, PFHxS, and PFOS were found to be transported by both rat and human bile-acid transporting protein Na+/taurocholate cotransporting polypeptide (NTCP) [26]. The high bioavailability (>100%) observed at some doses may also be due to enterohepatic circulation, occurring with gavage but not IV administration.
Sex differences in toxicokinetics were observed with PFHxS, to a lesser extent with PFBS, but not with PFOS. These findings are generally consistent with the available literature of these PFSAs. In this study, PFBS, the shortest of the PFAS studied here, had a plasma k10 half-life on the order of ˜3-4 h in males and 1–2 hours in females after gavage administration. Sex differences in PFBS kinetics have been reported but the literature is inconsistent [27,28]. In this study, the male half-life of PFHxS was a little more than two weeks, while females displayed a half-life of around two days. Similarly, a previous report in Sprague-Dawley rats given 4 mg/kg PFHxS orally found females to have a half-life of around 2 days and males, 27 days [29]. For both PFBS and PFHxS, AUC and dose-adjusted AUC in plasma were lower and clearance was higher in females compared to males. The lower Cmax and higher clearance of PFBS in females has been reported in monkeys and rats [28] but was not found in exposures of PFHxS to CD-1 mice or monkeys [14]. In this study, female rats tended to have lower liver, kidney, and brain concentrations of PFBS and PFHxS and faster elimination of these chemicals from the tissues than their male counterparts. This sex difference has been reported for PFHxS [14], but PFBS tissue distribution has been previously studied only in male rodents [13].
The longest chain PFSA, PFOS, had a half-life in the range of 33–40 days and did not display a sex difference in half-life after IV or gavage administration. Similarly, other studies have reported that the plasma half-life of PFOS was not different between sexes [29,30]. One report did find a sex difference in half-life: Charles River Sprague Dawley rats followed for 10 weeks after an oral dose of 2 or 15 mg/kg showed a 2-fold increase in half-life in females compared to males, 62 vs 38 days [15]. AUC was slightly higher in females than males in our study, which was the opposite of PFBS and PFHxS. and consistent with the Chang et al., 2012 report, but not Kim et al., 2016. Sex differences in liver, kidney, and brain concentrations of PFOS were not apparent in our study, which is consistent with values reported in another study [15].
The sex-dependence of elimination and tissue absorption reported in rats has been attributed to differential expression of renal organic anion transporters that facilitate resorption [31,32]. Binding of PFAS to sex-specific proteins such as liver and kidney-form α2μ-globulin may also contribute to inefficient clearance in males [33]. While many of these mechanistic studies focus on PFOA and other perfluorinated carboxylates, sulfonate PFAS may also have these interactions, given their similar structure.
The doses used in these studies greatly exceed the levels that humans are exposed to in the environment. Using body surface area normalization [34], the human equivalent doses of doses in this study range from 0.32 to 16 mg/kg/day. In comparison, concentrations of perfluorinated sulfonic acids that have been detected in drinking water around the world range from 0.24 to 27 ng/L [[35], [36], [37]], though concentrations in the 1700–8000 ng/L range have also been observed [22]. The amounts in drinking water do not come close to the exposures used in this animal study. Internal dose is also helpful in comparing animal with human exposures. Human plasma concentrations of PFHxS and PFOS have been found in the range of 0.1 to 70.7 ng/ml. PFBS in plasma was below detection limit in multiple studies ([38,39]; when detectable, concentrations of PFBS tend to be very low (e.g. 0.06 and 0.46 ng/ml) (Jansen et al., 2019; [37]). Plasma concentrations in animals from this study are multiple orders of magnitude higher than observed human plasma concentrations. Despite the high dose and internal exposure in animals compared to human exposures, this study was designed to evaluate the kinetics of the PFAS, which cannot be done in a controlled and systematic manner in humans, and not necessarily to evaluate risk and toxicity. These animal studies thus provide an idea of the trends in elimination of PFAS that could be expected to occur in humans.
The kinetics of PFOS and PFOA have been studied extensively, but less is known about the toxicokinetics of short-chain PFAS, such as PFBS and PFHxS. Our study corroborated much of the currently available literature on PFOS toxicokinetics and expanded the knowledge base on PFBS and PFHxS. Furthermore, our study provided a direct comparison of kinetics across three different chain length PFAS. In Sprague-Dawley rats in our study, shorter chain PFAS had faster plasma and tissue elimination and lower systemic exposure compared to longer-chained chemicals. Sex differences were found in the kinetic parameters and tissue distribution of PFBS and PFHxS, but not of PFOS. Because these toxicokinetic studies were conducted in the same strain as studies done with carboxylate PFAS and studies assessing PFAS toxicity at the NTP, these data will facilitate future discussions comparing the kinetics and toxicities of perfluoroalkyl carboxylic and sulfonic acids as well as how the toxicity observed in rodents translates to human risk.
Acknowledgements
The authors would like to thank Stephen Ferguson and Brad Collins for their critical review of the manuscript and S. P. Hong and J. R. Pirone for their assistance in data preparation and analysis. This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, Intramural Research project ZIA ES103316-04 and work was performed for the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, U.S. Department of Health and Human Services, under contracts N01-ES-55551, HHSN273201000016C.
References
- 1.Buck R.C. Toxicology data for alternative “short-chain” fluorinated substances. In: DeWitt J.C., editor. Toxicological Effects of Perfluoroalkyl and Polyfluoroalkyl Substances. Humana Press; London: 2014. pp. 451–477. [Google Scholar]
- 2.Lau C., Butenhoff J.L., Rogers J.M. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol. Appl. Pharmacol. 2004;198:231–241. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 3.Lau C., Anitole K., Hodes C., Lai D., Pfahles-Hutchens A., Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol. Sci. 2007;99:366–394. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 4.White S.S., Calafat A.M., Kuklenyik Z., Villanueva L., Zehr R.D., Helfant L., Strynar M.J., Lindstrom A.B., Thibodeaux J.R., Wood C. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol. Sci. 2007;96:133–144. doi: 10.1093/toxsci/kfl177. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z., Cousins I.T., Scheringer M., Hungerbuhler K. Fluorinated alternatives to long-chain perfluoroalkyl carboxylic acids (PFCAs), perfluoroalkane sulfonic acids (PFSAs) and their potential precursors. Environ. Int. 2013;60:242–248. doi: 10.1016/j.envint.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Kjeldsen L.S., Bonefeld-Jorgensen E.C. Perfluorinated compounds affect the function of sex hormone receptors. Environ. Sci. Pollut. Res. Int. 2013;20:8031–8044. doi: 10.1007/s11356-013-1753-3. [DOI] [PubMed] [Google Scholar]
- 7.Gorrochategui E., Perez-Albaladejo E., Casas J., Lacorte S., Porte C. Perfluorinated chemicals: differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol. Appl. Pharmacol. 2014;277:124–130. doi: 10.1016/j.taap.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 8.Kudo N., Suzuki E., Katakura M., Ohmori K., Noshiro R., Kawashima Y. Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chem. Biol. Interact. 2001;134:203–216. doi: 10.1016/s0009-2797(01)00155-7. [DOI] [PubMed] [Google Scholar]
- 9.Hanhijarvi H., Ophaug R.H., Singer L. The sex-related difference in perfluorooctanoate excretion in the rat, 2019 difference in perfluorooctanoate excretion in the rat. Proc. Soc. Exp. Biol. Med. 1982;171:50–55. doi: 10.3181/00379727-171-41476. [DOI] [PubMed] [Google Scholar]
- 10.Heuvel J.V., Kuslikis B., Van Refelghem M., Peterson R. Tissue distribution, metabolism and elimination of perfluorooctanoic acid. J. Biochem. Toxicol. 1991;6:83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- 11.Olsen G.W., Burris J.M., Ehresman D.J., Froehlich J.W., Seacat A.M., Butenhoff J.L., Zobel L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007;115:1298–1305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrielsson J., Weiner D. third edition. Swedish Pharmaceutical Press; Stockholm, Sweden: 2000. Pharmacokinetic and Pharmacodynamic Data Analysis: Concepts and Applications. [Google Scholar]
- 13.Bogdanska J., Sundstrom M., Bergstrom U., Borg D., Abedi-Valugerdi M., Bergman A., DePierre J., Nobel S. Tissue distribution of 35S-labelled perfluorobutanesulfonic acid in adult mice following dietary exposure for 1-5 days. Chemosphere. 2014;98:28–36. doi: 10.1016/j.chemosphere.2013.09.062. [DOI] [PubMed] [Google Scholar]
- 14.Sundstrom M., Chang S.C., Noker P.E., Gorman G.S., Hart J.A., Ehresman D.J., Bergman A., Butenhoff J.L. Comparative pharmacokinetics of perfluorohexanesulfonate (PFHxS) in rats, mice, and monkeys. Reprod. Toxicol. 2012;33:441–451. doi: 10.1016/j.reprotox.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Chang S.C., Noker P.E., Gorman G.S., Gibson S.J., Hart J.A., Ehresman D.J., Butenhoff J.L. Comparative pharmacokinetics of perfluorooctanesulfonate (PFOS) in rats, mice, and monkeys. Reprod. Toxicol. 2012;33:428–440. doi: 10.1016/j.reprotox.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 16.The Danish Environmental Protection Agency . 2015. Short-chain Polyfluoroalkyl Substances (PFAS) [Internet]. Copenhagen, Denmark.https://www2.mst.dk/Udgiv/publications/2015/05/978-87-93352-15-5.pdf Accessed Nov 2018. Available at. [Google Scholar]
- 17.Jones P.D., Hu W., De Coen W., Newsted J.L., Giesy J.P. Binding of perfluorinated fatty acids to serum proteins. Environ. Toxicol. Chem. 2003;22:2639–2649. doi: 10.1897/02-553. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y.M., Guo L.H. Fluorescence study on site-specific binding of perfluoroalkyl acids to human serum albumin. Arch. Toxicol. 2009;83:255–261. doi: 10.1007/s00204-008-0359-x. [DOI] [PubMed] [Google Scholar]
- 19.Hanssen L., Dudarev A.A., Huber S., Odland J.O., Nieboer E., Sandanger T.M. Partition of perfluoroalkyl substances (PFASs) in whole blood and plasma, assessed in maternal and umbilical cord samples from inhabitants of arctic Russia and Uzbekistan. Sci. Total Environ. 2013;447:430–437. doi: 10.1016/j.scitotenv.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Ehresman D.J., Froehlich J.W., Olsen G.W., Chang S.C., Butenhoff J.L. Comparison of human whole blood, plasma, and serum matrices for the determination of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), and other fluorochemicals. Environ. Res. 2007;103:176–184. doi: 10.1016/j.envres.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Luebker D.J., Hansen K.J., Bass N.M., Butenhoff J.L., Seacat A.M. Interactions of fluorochemicals with rat liver fatty acid-binding protein. Toxicol. 2002;176:175–185. doi: 10.1016/s0300-483x(02)00081-1. [DOI] [PubMed] [Google Scholar]
- 22.Li Y., Fletcher T., Mucs D., Scott K., Lindh C.H., Tallving P., Jakobsson K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 2018;75:46–51. doi: 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worley R.R., Moore S.M., Tierney B.C., Ye X., Calafat A.M., Campbell S., Woudneh M.B., Fisher J. Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ. Int. 2017;106:135–143. doi: 10.1016/j.envint.2017.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersen M.E., Clewell H.J., 3rd, Tan Y.M., Butenhoff J.L., Olsen G.W. Pharmacokinetic modeling of saturable, renal resorption of perfluoroalkylacids in monkeys--probing the determinants of long plasma half-lives. Toxicol. 2006;227:156–164. doi: 10.1016/j.tox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Genuis S.J., Birkholz D., Ralitsch M., Thibault N. Human detoxification of perfluorinated compounds. Public Health. 2010;124:367–375. doi: 10.1016/j.puhe.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W., Zitzow J.D., Ehresman D.J., Chang S.C., Butenhoff J.L., Forster J., Hagenbuch B. Na+/Taurocholate cotransporting polypeptide and apical sodium-dependent bile acid transporter are involved in the disposition of perfluoroalkyl sulfonates in humans and rats. Toxicol. Sci. 2015;146:363–373. doi: 10.1093/toxsci/kfv102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olsen G.W., Chang S.C., Noker P.E., Gorman G.S., Ehresman D.J., Lieder P.H., Butenhoff J.L. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicol. 2009;256:65–74. doi: 10.1016/j.tox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Chengelis C.P., Kirkpatrick J.B., Myers N.R., Shinohara M., Stetson P.L., Sved D.W. Comparison of the toxicokinetic behavior of perfluorohexanoic acid (PFHxA) and nonafluorobutane-1-sulfonic acid (PFBS) in cynomolgus monkeys and rats. Reprod. Toxicol. 2009;27:400–406. doi: 10.1016/j.reprotox.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.J., Heo S.H., Lee D.S., Hwang I.G., Lee Y.B., Cho H.Y. Gender differences in pharmacokinetics and tissue distribution of 3 perfluoroalkyl and polyfluoroalkyl substances in rats. Food Chem. Toxicol. 2016;97:243–255. doi: 10.1016/j.fct.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 30.Loccisano A.E., Campbell J.L., Jr., Butenhoff J.L., Andersen M.E., Clewell H.J., 3rd Comparison and evaluation of pharmacokinetics of PFOA and PFOS in the adult rat using a physiologically based pharmacokinetic model. Reprod. Toxicol. 2012;33:452–467. doi: 10.1016/j.reprotox.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Kudo N. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem. Biol. Interact. 2002;139:301–316. doi: 10.1016/s0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 32.Ohmori K., Kudo N., Katayama K., Kawashima Y. Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain length. Toxicol. 2003;184:135–140. doi: 10.1016/s0300-483x(02)00573-5. [DOI] [PubMed] [Google Scholar]
- 33.Han X., Hinderliter P.M., Snow T.A., Jepson G.W. Binding of perfluorooctanoic acid to rat liver-form and kidney-form alpha2u-globulins. Drug Chem. Toxicol. 2004;27:341–360. doi: 10.1081/dct-200039725. [DOI] [PubMed] [Google Scholar]
- 34.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 35.Zhang S., Kang Q., Peng H., Ding M., Zhao F., Zhou Y., Dong Z., Zhang H., Yang M., Tao S. Relationship between perfluorooctanoate and perfluorooctane sulfonate blood concentrations in the general population and routine drinking water exposure. Environ. Int. 2019;126:54–60. doi: 10.1016/j.envint.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 36.Kabore H.A., Vo Duy S., Munoz G., Meite L., Desrosiers M., Liu J., Sory T.K., Sauve S. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci. Total Environ. 2018;616-617:1089–1100. doi: 10.1016/j.scitotenv.2017.10.210. [DOI] [PubMed] [Google Scholar]
- 37.Hölzer J., Midasch O., Rauchfuss K., Kraft M., Reupert R., Angerer J., Kleeschulte P., Marschall N., Wilhelm M. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Int. Environ. Health Perspect. 2008:651–657. doi: 10.1289/ehp.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olsen G.W., Lange C.C., Ellefson M.E., Mair D.C., Church T.R., Goldberg C.L., Herron R.M., Medhdizadehkashi Z., Nobiletti J.B., Rios J.A. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000-2010. Environ. Sci. Technol. 2012;46:6330–6338. doi: 10.1021/es300604p. [DOI] [PubMed] [Google Scholar]
- 39.Manzano-Salgado C.B., Casas M., Lopez-Espinosa M.J., Ballester F., Basterrechea M., Grimalt J.O., Jimenez A.M., Kraus T., Schettgen T., Sunyer J. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ. Res. 2015;142:471–478. doi: 10.1016/j.envres.2015.07.020. [DOI] [PubMed] [Google Scholar]