Abstract
Background
The antibacterial property of new atraumatic restorative treatment (ART) materials incorporated with Azadirachta indica (Neem) on Streptococcus mutans was carried out.
Materials and methods
The study was carried out by using the agar diffusion method to determine the antibacterial property of ART materials (ART-I and ART-II). The zone of inhibition was tabulated, and the data was statistically analyzed using the student t-test. The minimum inhibitory concentrations (MIC) and the minimum bactericidal concentrations (MBC) of the ethanolic extract of Neem were recorded.
Results
The MIC and MBC of the mixture of the ethanolic extract of Neem was 3.13% and 12.5% respectively. The zone of inhibition of ART-I and ART-II was 11.81 mm and 11.97 mm respectively. Significant differences were observed between these two ART materials (P = 0,08).
Conclusion
Both the new ART materials i.e. ART-I and ART–II have considerable antibacterial activity against S. mutans.
Keywords: Antibacterial activity, ART, Minimum inhibition concentration (MIC), Minimum bactericidal concentration (MBC), Neem extract, Soxhlet method
1. Introduction
Dental caries is a multi-factorial disease characterized by dissolution and destruction of organic and inorganic parts of the tooth through a complex process, and consequently a cavity can be developed. The cavity often needs restorative treatment (filling). As a process of caries development, the microorganisms ferment carbohydrate and produce lactic acid which demineralize the tooth tissue and initiate dental caries, which can result in a cavity on a tooth.1,2
Several treatment procedures have been developed to manage dental caries namely; restoration with restorative materials such as glass ionomer cements, composite resins and amalgam. But these materials are relatively expensive and can be technique sensitive. New ART materials (filling material) have been developed in the laboratory, which can be considered as dental restorative materials, and they are relatively inexpensive. This study investigated new ART materials incorporated with Neem extract as an antibacterial agent, which is different from those existing in the market.3,4
There are microorganisms in oral cavity, and many of them are in biofilms, called dental microbial plaque. The species Streptococcus mutans and Lactobacilli are common bacteria living within dental plaque. Streptococcus mutans (and its several sub-species) is believed to be the most common bacteria associated with the initiation of dental caries.2. Previous studies evaluated different plant extracts for antimicrobial properties against cariogenic bacteria. The herbal agent, Azadirachta indica (Neem) has an extract which expresses an antibacterial property.5,6Azadirachta indica (Neem) belongs to the Melicea plant family. Extracts from different parts of Neem are claimed to have positive health effects. 7, 8, 9, 10
Previous studies reported that, the ethanolic Neem extract exhibited a higher antibacterial property than the aqueous Neem extract.11,12 Thus, in the present study, ethanolic Neem extracts from different parts of Neem tree were prepared and assessed as an antibacterial agent incorporated into new ART materials.
With this background, the objectives of the present study were;
-
1.
To evaluate the Minimum Inhibition Concentration (MIC), and the Minimum Bactericidal Concentration (MBC) of the Neem extracts (extracts of leaves, twigs and barks).
-
2.
To determine the zone of inhibition of 2 ART materials incorporated with Neem extract.
2. Materials and Methods
2.1. Sample (Neem barks, twigs and leaves) collection
The barks, twigs and leaves of Azadirachta indica (Neem) were collected from the garden/herbarium of the Nitte Deemed to be a University, Deralakatte, Mangalore, India. The parts were cleaned and washed under tap water, followed by sterilized distilled water, and then chopped into small pieces to dry continuously for two weeks. This drying was carried out during the dry season. The dried barks, leaves and twigs pieces were pulverized separately into a coarse powder. The powder was then stored at room temperature in a clean, air-tight container for further use.
2.2. Preparation of Neem extract
The extraction from the Neem twigs, barks and leaves were carried-out separately by the Soxhlet method.13,14 The coarse powder of Neem was weighed (150 g) and extracted with 250 ml of 98% ethanol Soxhlet apparatus. Constant heat was provided for recycling of the solvent. The recycling process continued for 23 cycles to yield the completed extract. Later, the extract (150 ml to 170 ml) was transferred into a clean and pre-weighed Borosil beaker. The extraction was passed through a process in order to evaporate the ethanol solvent completely at 37 °C. The semi-solid extract was stored at 4 °C in sterile labeled plastic tubes until further use.
2.3. Determination of MIC and MBC
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) was determined using a micro broth dilution method.4 Pure cultures of Streptococcus mutans were grown in heart infusion broth (BHI Broth, Hi-media India). The optical density of planktonic suspensions of each culture was adjusted to 1.5 x 10 8 CFU/ml (0.5 McFarland standard). One hundred percent Neem stock solutions were prepared by dissolving 0.1 grams of Neem extract in 0.1 ml of dimethyl sulfoxide (DMSO) and ethanol separately. One hundred percent extracts were serially diluted in broth in micro well titre plates. By serial doubling dilution, the concentration of the Neem extract was achieved as 100%, 50%, 25%, 12.5%, 6.25%, 3.125% and 1.56%.
A loop full (10 μl) of Streptococcus mutans, was inoculated into 0.5 McFarland micro litre plates containing 100 μl of various concentrations of Neem extracts in BHI broth.
The microtitre plates were incubated at 37 °C for 18–24 h and thereafter observed for growth or turbidity. A loop full of broth from each microtube was not showing growth; hence they were inoculated into nutrient agar plate. Thereafter, equal volumes of sterile nutrient broth were added into the micro tube cultures and incubated further for 24 h at 37 °C. Then, the microtiter plates and agar plates were examined for growth and turbidity using the unaided eye (CLSI, 2012). The procedure was repeated three times.
The inoculums of the organism without Neem extract were used as the control. The growth of the control was compared to the growth of the organism exposed to the test material. The MIC is the least concentration of antimicrobial agent that prevents microbial growth, as well as the MBC, is the least concentration of antimicrobial agent required to kill microorganisms.15
Determination of the zone of inhibition associated with the ART materials incorporated Neem extract.
A total of 20 samples of ART materials were used, ten samples were ART-I and remaining ten were ART-II.30 These samples were prepared according to the composition discussed in Table 1. Individual ART mixes incorporated with Neem extract were placed in Teflon moulds (an inner diameter 6 mm and a depth of 3.5 mm) and compressed for 20 min untill final set. After setting, all the specimens were removed from the mould and stored in an airtight container for 24 h to allow maturation of the cement.
Table 1.
Compositions of the new ART materials.
| Composition of ART material | ART-I (%) | ART -II (%) | Action |
|---|---|---|---|
| Zinc oxide | 20 | 20 | Base of the Cement |
| Aluminum oxide | 70 | 70 | Base of the cement |
| Hydrogenated rosin | 6 | 6 | Bonding Agent |
| Sodium fluoride (NaF) | 3 | 3 | Fluoride Agent |
| Neem extract | 6 | 11.5 | Antibacterial agent |
| Eugenol | 40 | 40 | Binding Agent |
| Ethoxybenzoic acid (EBA) | 60 | 60 | Bonding Agent |
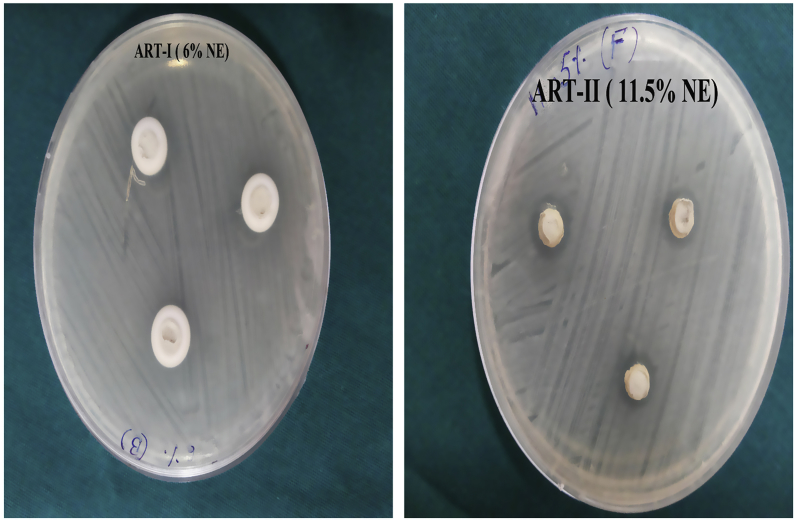
The test microorganisms were sub-cultured on selective media and incubated aerobically at 37 °C for 24 h. A total of three wells were made into a nutrient agar plate using sterile cork borers (6 mm in diameter) and inoculums containing 1 × 105 CFU/ml of bacteria were spread on the solid plate with the bacterial suspensions. The ART-I and ART-II were placed in the respective wells of agar plates. The plates were then incubated at 37 °C for 24 h in an aerobic environment. After overnight incubation, the plates were observed for the zone of inhibition and the diameters of the inhibition zone. These were measured in millimetres at four different points and using a scale and the mean was calculated. The above procedures were repeated in triplicate.
2.4. Statistical analysis
Statistical analysis for the values of zone of inhibition was carried out using SPSS version 20 (IBM Corporation, Armonk, New York, United States). The Student t-test was conducted to assess the level of significance of differences between the mean values.
3. Results
The MIC and MBC values in percentages, of the Neem extracts diluted with two solvents, are discussed in Table 2. Neem twigs extracts without bark showed better MIC values in both the solvents than the other extracts. The MIC and MBC value of the Neem twigs without bark in both solvents were 3.13% and 12.5% respectively.
Table 2.
MIC and MBC values of ethanolic extracts of Neem with different concentrations, diluted by using each of the two solvents.
| Neem extract | Solvent used | MIC | MBC |
|---|---|---|---|
| Barks | Ethanol | 50% | ---- |
| DMSO | 25% | 100% | |
| Leaves | Ethanol | 6.25% | 25% |
| DMSO | 6.25% | 25% | |
| Twigs without barks | Ethanol | 3.125% | 12.5% |
| DMSO | 3.125% | 12.5% | |
| Mixture (equal weights of twigs and leaves) | Ethanol | 6.25% | 12.5% |
| DMSO | 6.25% | 12.5% | |
| Twigs with barks | Ethanol | 50% | 100% |
| DMSO | 6.25% | 12.5% |
The average and standard deviations of the inhibition zones of S. mutans on nutrient agar treated with ART materials are shown in Table 3 and Fig. 2. The mean ± SD (standard deviation) zone of inhibition of the ART-I was 11.81 ± 0.255 mm and that of ART-II was 11.97 ± 0.067 mm. This result showed no significant difference in antibacterial effects between these two different ART materials (P = 0.08).
Table 3.
Zone of inhibition associated with different ART materials incorporated with different amounts (%) of Neem extracts.
| Material | Number of the samples | Zone of inhibition (mm) Mean | SD | SE | p-value |
|---|---|---|---|---|---|
| ART-I | 10 | 11.81 | 0.25582 | 0.08090 | 0.08 |
| ART-II | 10 | 11.97 | 0.06749 | 0.02134 |
Fig. 2.
Zone of inhibition associated with the ART materials with Streptococcus mutans.
4. Discussion
ART is a minimal intervention approach and is recommended by the World Health Organization for an atraumatic tooth restoration technique. ART technique involves removing soft, demineralized tooth tissue using only by hand instruments, followed by restoration with an adhesive dental restorative material. This treatment is now used all over the world which is cost effective and can be carried out by a trained health worker in a primary health care setting.16, 17, 18
New ART materials were developed in the laboratory of the fluoride research unit of the department of Oral Biology & Genomic Studies of the AB Shetty Dental College, Nitte Deemed to be a University, India, in collaboration with the National Institute of Technology Karnataka, Surathkal. The ratio of the composition used was fixed by assessing the physical and mechanical properties such as compressive strength, micro-hardness, adhesiveness and solubility properties as per the ISO standardized assessment procedure of dental materials.19,20
It is impossible to eliminate all the bacteria completely from the tooth cavity in an ART approach. There is a possibility of the development of secondary caries after restoring a tooth cavity. Therefore, it could be important to add an antibacterial agent to ART materials.16 Neem extract was chosen as an antibacterial agent for these new ART materials.
Extraction of the antibacterial agent was done from Neem in the laboratory. The Soxhlet extraction method was chosen for the extraction process. This is considered the standard method to compare the positive outcome of newly developed materials.13,14 The procedure for extraction of plant parts plays an important role in this study. The common factors affecting the extraction processes are matrix properties of the plant part, solvent, temperature and time.9
Ninety eight percent ethanol was used as a solvent for the extraction process in the present study, rather than water as a solvent. This is because the organic solvent itself has antimicrobial activity and a minimum inhibitory concentration unlike the water extraction method.22 The active component present in Neem, is made of phenol groups that dissolve in organic solvent but not in water.9,12,23,24 However one of the previous studies reported that there was no statistical significance between the antimicrobial effects of alcoholic Neem extracts compared to those from water Neem extracts.25
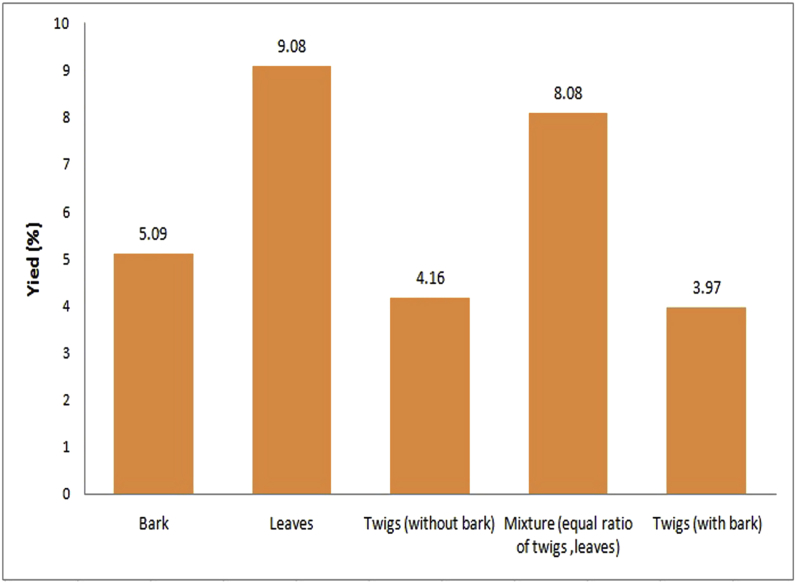
Fig. 1 summarizes the percentages of the yield from different parts of Neem extracted by using the Soxhlet extraction method. The highest percentage yield was obtained from the Neem leaves and whilst the least yield was obtained from the twigs and barks.
Fig. 1.
Schematic representation of the yield of the ethanolic Neem extracts assessed in the present study.
Dimethyl sulfoxide (DMSO) was used as one of the solvents to prepare different concentrations of the Neem extract in the present study. DMSO has a unique capacity to penetrate through living tissues without affecting the activity of the compounds in the Neem extract. The unique capability of the DMSO is due to its relative polar nature, capacity to accept hydrogen bonds and relative compact nature.22
Both DMSO and absolute ethanol were used as solvents in the present study, to prepare the different concentrations of Neem extract. DMSO was observed to provide a better effect. The MIC and MBC values associated with the ethanolic Neem extract was comparable with a previous study.26
The MIC values associated with the Neem twigs without barks were better than the values obtained associated with other parts of Neem extracts (Table 2). The MBC values of the Neem twigs extracts (without barks) and the mixtures extracts (equal amounts of Neem twigs and leaves) showed similar values. However, the yield of the mixture extracts (equal ratio of Neem twigs and leaves) obtained was higher than the Neem twigs extracts (without barks) but the MIC value of the Neem twigs extracts without barks was better than that of mixture extracts (equal ratio of Neem twigs and leaves). Also, previous studies have shown that ethanolic Neem twigs extracts had higher antibacterial properties than Neem leaves extracts.5,26, 27, 28, 29 Therefore, Neem twigs extracts without barks were selected as the antibacterial agent for the new ART materials in the present study.
However, there is a potential disadvantage associated with adding an antibacterial agent to restorative materials related to the physical and chemical properties. The threshold of the maximum amount of this antibacterial agent which can be added to the dental restorative material is 30%.21 Accordingly, 6% and 11.5% of ethanolic Neem extracts were decided to be incorporated into the new ART materials as an antibacterial agent so as not to compromise the physical properties of the restorative material. The new ART material containing 6% Neem extract was named as ART-I and the material containing 11.5% Neem extract was named ART-II.
A large variety of bacteria play a role in caries development. However, S. mutans plays a more prominent role in the initiation of the dental caries process and hence was assessed in this study.21,29 Therefore, the antibacterial property of these new ART materials incorporated with Neem extract was assessed using S. mutans.
In the present study, zones of inhibition associated with the ART-I and ART-II were 11.81 mm and 11.97 mm respectively. On comparing these zones of inhibition, ART-II was associated with a trend towards slightly better antibacterial properties compared to ART-I. However, statistically there was no significant difference in this antibacterial effect (P = 0.08).
A previous study reported that the zones of inhibition of associated with the use of a conventional glass ionomer cement containing an addition of 5% of the marketed antibacterial agent chlorhexidine diacetate/cetrimide were in the range of 12 mm -13 mm against S. mutans.16 Similar values were observed in the present study.
Physical-mechanical properties, chemical property and biological property of new ART material were estimated and summarized.30, 31, 32 Both ART-I and ART-II were found to be non-mutagen against S. typhimurium.30 Solubility of ART-I and ART-II in de-ionized water in the first 24 h was 7.4% and 7.2% respectively.31 Microhardness and compressive strength of ART-I was in between 21.1 VHN and 25.8 VHN and 38.2 MPa and 56 MPa respectively. On the other hand, micro-hardness and compressive strength of ART-II were in between 13.2 VHN and 16.3 VHN as well as 43.3 MPa and 61 Mpa. There was no marginal gap present between the ART-II and dental structure compared to ART-I.32
5. Conclusion
The zones of inhibition in the agar diffusion test showed the antibacterial efficiency of the new ART materials incorporated with the ethanolic Neem extract. Therefore, Neem can be considered as a potential natural source of an anti-bacterial agent – and can be incorporated in an atraumatic restorative material (ART) to be used for the management of dental caries, and it should be further investigated for this potential use.
Funding
No specific grants-in-aid were allotted for this study.
The study was supported within the facilities of the department of Oral Biology & Genomic Studies, AB Shetty Dental College and Department of Microbiology, KS Hegde Medical Academy, Nitte Deemed to be a University, Mangalore, India.
Acknowledgement
Statistical analysis was supported by Professor Krishna Bhat, Department of Statistics of KS Hegde Medical Academy (KSHEMA), Nitte Deemed to be a University, Mangalore, Deralakatte, Mangalore, India.
References
- 1.Klai S., Altenburger M., Spitzmüller B., Anderson A., Hellwig E., Al-Ahmad A. Antimicrobial effects of dental luting glass ionomer cements on Streptococcus mutans. Sci World J. 2014;2014:7. doi: 10.1155/2014/807086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pai M.R., Acharya L.D., Udupa N. The effect of two different dental gels and a mouthwash on plaque and gingival scores: a six‐week clinical study. Int Dent J. 2004;54:219–223. doi: 10.1111/j.1875-595x.2004.tb00283.x. [DOI] [PubMed] [Google Scholar]
- 3.Pai M.R., Acharya L.D., Udupa N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel - a 6-week clinical study. J Ethnopharmacol. 2004;90:99–103. doi: 10.1016/j.jep.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 4.Mistry K.S., Sanghvi Z., Parmar G., Shah S. The antimicrobial activity of Azadirachta indica, Mimusops elengi, Tinospora cardifolia, Ocimum sanctum and 2% chlorhexidine gluconate on common endodontic pathogens: an in vitro study. Eur J Dent. 2014;8:172–177. doi: 10.4103/1305-7456.130591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siswomihardjo W., Badawi S.S., Nishimura M., Hamada T. The difference of antibacterial effect of neem leaves and stick extracts. Int Chin J Dent. 2007;7:27–29. [Google Scholar]
- 6.Mahfuzul Hoque M.D., Bari M.L., Inatsu Y., Juneja V.K., Kawamoto S. Antibacterial activity of guava (Psidium guajava L.) and neem (Azadirachta indica A. Juss.) extracts against foodborne pathogens and spoilage bacteria. Foodb Pathog Dis. 2007;4:481–488. doi: 10.1089/fpd.2007.0040. [DOI] [PubMed] [Google Scholar]
- 7.Subapriya R., Nagini S. Medicinal properties of neem leaves: a review. Curr Med Chem Anti Cancer Agents. 2005;5:149–156. doi: 10.2174/1568011053174828. [DOI] [PubMed] [Google Scholar]
- 8.Lakshmi T., Krishnan V., Rajendran R., Madhusudhanan N. Azadirachta indica: a herbal panacea in dentistry–An update. Pharm Rev. 2015;9:41–44. doi: 10.4103/0973-7847.156337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmad I., Beg A.Z. Antimicrobial and phytochemical studies on 45 Indian medicinal plants against multi-drug resistant human pathogens. J Ethnopharmacol. 2001;74:113–123. doi: 10.1016/s0378-8741(00)00335-4. [DOI] [PubMed] [Google Scholar]
- 10.Atawodi S.E., Atawodi J.C. Azadirachta indica (neem): a plant of multiple biological and pharmacological activities. Phytochem Rev. 2009;8:601–620. [Google Scholar]
- 11.Sewani S., Qureshi M. Antimicrobial activity of neem, clove, curry leaves, cardamom, tulsi stem and tulsi leaves. Int Res J Biol Sci. 2016;5:42–46. [Google Scholar]
- 12.Jain I., Jain P., Bisht D., Sharma A., Srivastava B., Gupta N. Comparative evaluation of antibacterial efficacy of six Indian plant extracts against Streptococcus mutans. J Clin Diagn Res. 2015;9:ZC50–ZC53. doi: 10.7860/JCDR/2015/11526.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azmir J., Zaidul I.S., Rahman M.M. Techniques for extraction of bioactive compounds from plant materials: a review. JFoodEng. 2013;117:426–436. [Google Scholar]
- 14.De Castro M.L., Garcıa-Ayuso L.E. Soxhlet extraction of solid materials: an outdated technique with a promising innovative future. Anal Chim Acta. 1998;369:1–10. [Google Scholar]
- 15.Hombach M., Bloemberg G.V., Böttger E.C. Effects of clinical breakpoint changes in CLSI guidelines 2010/2011 and EUCAST guidelines 2011 on antibiotic susceptibility test reporting of Gram-negative bacilli. J Antimicrob Chemother. 2011;67:622–632. doi: 10.1093/jac/dkr524. [DOI] [PubMed] [Google Scholar]
- 16.TÜzÜner T., KuşgÖz A., Er K., Taşdemir T., Buruk K., Kemer B. Antibacterial activity and physical properties of conventional glass‐ionomer cements containing chlorhexidine diacetate/cetrimide mixtures. J Esthetic Restor Dent. 2011;23:46–55. doi: 10.1111/j.1708-8240.2010.00385.x. [DOI] [PubMed] [Google Scholar]
- 17.Smales R.J., Yip H.K. The atraumatic restorative treatment (ART) approach for primary teeth: review of literature. Pediatr Dent. 2000;22:294–298. [PubMed] [Google Scholar]
- 18.da Mata C., Allen P.F., Cronin M., O'mahony D., McKenna G., Woods N. Cost‐effectiveness of ART restorations in elderly adults: a randomized clinical trial. Community Dent Oral Epidemiol. 2014;42:79–87. doi: 10.1111/cdoe.12066. [DOI] [PubMed] [Google Scholar]
- 19.Tyas M.J. Dental materials science-the maintenance of standard. J Oral Rehabol. 1991;18:105–110. doi: 10.1111/j.1365-2842.1991.tb00037.x. [DOI] [PubMed] [Google Scholar]
- 20.Anusavice Kenneth. twelfth ed. Elsevier Health Sciences; 2013. Dental Cements. Phillips' Science of Dental Materials; pp. 303–339. [Google Scholar]
- 21.Fernandes J.M., Menezes V.A., Albuquerque A.J. Emerging Trends in Oral Health Sciences and Dentistry. InTech; 2015. Improving antimicrobial activity of dental restorative materials; pp. 65–82. [Google Scholar]
- 22.Dzulkarnain S.M., bin Abdul Rahim I. Antimicrobial activity of methanolic Neem extract on wound infection bacteria. Int. Conf. Biol. Chem. Environ. Sci. 2014;4:72–75. [Google Scholar]
- 23.Subramaniam S.K., Siswomihardjo W., Sunarintyas S. The effect of different concentrations of Neem (Azadiractha indica) leaves extract on the inhibition of Streptococcus mutans (In vitro) Dent J (Majalah Kedokteran Gigi) 2005;38:176–179. [Google Scholar]
- 24.Sanguri S., Kapil S., Gopinathan P., Pandey F.K., Bhatnagar T. Comparative screening of antibacterial and antifungal activities of some weeds and medicinal plants leaf extracts: an in-vitro study. Elixir Appl. Bot. 2012;47:8903–8905. [Google Scholar]
- 25.Nayak A., Nayak R.N., Soumya B., Bhat K., Kudalkar M. Evaluation of antibacterial and anticandidial efficacy of aqueous and alcoholic extract of Neem (Azadirachta indica) an in vitro study. Int J Res Ayurveda Pharm. 2011;2:230–235. [Google Scholar]
- 26.Adyanthaya S., Pai V., Jose M. Antimicrobial potential of the extracts of the twigs of Azadirachta indica (Neem): an in vitro study. J. Med. Plants Stud. 2014;2(6):53–57. [Google Scholar]
- 27.Irshad S., Butt M., Younus H. In-vitro antibacterial activity of two medicinal plants Neem (Azadirachta indica) and Peppermint. Int Res J Pharm. 2011;1(01):9–14. [Google Scholar]
- 28.Singh R.K., Khan S.A., Murawat K., Sharma P. Neem the miracle tree-A medicinal and dental update. Asian J Oral Health Allied Sci. 2012;2(2):84–85. [Google Scholar]
- 29.Siswomihardjo W., Badawi S.S., Nishimura M., Hamada T. The difference of antibacterial effect of neem leaves and stick extracts. Int Chin J Dent. 2007;7:27–29. [Google Scholar]
- 30.Divya Kumari P., Shetty A Veena, Khijimatgar Shahnawaz, Chowdhury Avidyuti, Lynch Edward, Chowdhury Chitta R. Mutagenicity potential (affect) of new atraumatic restorative treatment (ART) material incorporated with Azadirachta indica (Neem) against Salmonella typhimurium. J Oral Biol Craniofac Res. 2018 doi: 10.1016/j.jobcr.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumari P.D., Khijmatgar S., Chowdhury A., Grootveld M., Lynch E., Chowdhury C.R. Solubility and water sorption of novel atraumatic restorative treatment materials: a in vitro Study. Indian J. Oral Health Res. 2018;4(1):6. http://www.ijohr.org/text.asp?2018/4/1/6/245675 [Google Scholar]
- 32.Kumari D., Khijmatgar S., Chowdhury A. An evaluation of the physical properties of a new atraumatic restorative treatment material containing Azadirachta indica (neem) J Evol Med Dent Sci. 2018;7(38):5001–5008. https://www.jemds.com/data_pdf/divya-i-sept%2017-Ori-Checked.pdf [Google Scholar]