Abstract
We describe the first known blood fluke from a marine mammal, the dugong, Dugong dugon (Sirenia: Dugongidae), which represents a new species of aporocotylid, Cardicola dhangali n. sp. (Digenea: Aporocotylidae). Eggs presumed to be of blood flukes have been previously reported from dugongs. This exciting discovery raises questions regarding evolution and host-switching in the Aporocotylidae, which prior to this study were only known to infect actinopterygian and chondrichthyan fishes. The new species has male and female genital pores opening on the right side of the body, with the male genital pore opening posterior to the entire reproductive system and the testis is extra-caecal. The uterus is highly convoluted, and the ovary is irregularly lobate. These features, together with the size and number of the tegumental spines per row, easily distinguish the new species from the most similar congeners Cardicola aurata Holzer et al., 2008, Cardicola chaetodontis Yamaguti, 1970, Cardicola currani Bullard and Overstreet, 2004, Cardicola forsteri Cribb et al., 2000, C. jiingurru Yong et al., 2016, and Cardicola palmeri Bullard and Overstreet, 2004, all of which infect actinopterygian fishes. Given that Cardicola is the most diverse and least host-specific of the marine aporoctoylid genera, it seems credible that a successful host-switch has occurred from an actinopterygian to D. dugon. Further sampling of sirenians and other marine mammals is warranted to gain a more comprehensive understanding of the evolutionary biology and biodiversity of the blood flukes (superfamily Schistosomatoidea Stiles and Hassall, 1898), but presents a substantial challenge with respect to their conservation status and large size.
Keywords: Aporocotylidae, Schistosomidae, Spirorchiidae, Blood fluke, Cardicola, Dugong
Graphical abstract
Highlights
-
•
a new species of aporocotylid, Cardicola dhangali n. sp. was discovered in the heart of a dugong.
-
•
The new species is the first known blood fluke from a marine mammal.
-
•
Infection of dugong with an aporocotylid blood fluke may represent a host-switch event from a fish.
1. Introduction
Blood flukes (Platyhelminthes: Digenea: Schistosomatoidea) are aquatic parasites that infect the cardiovascular system of their definitive host. Historically, blood flukes have been assigned to three families corresponding to the vertebrate definitive host lineages they infect; the Schistosomatidae, which infect birds and terrestrial mammals, the Spirorchiidae, which infect reptiles and the Aporocotylidae, which infect fishes. The schistosomatids are dioecious while the aporocotylids and paraphyletic spirorchiids (Snyder, 2004; Orélis-Ribeiro et al., 2014) are hermaphrodites (with the exception of the dioecious “spirorchiid” infecting crocodiles, Griphobilharzia amoena Platt et al., 1991; see Brant and Loker, 2005; Platt et al., 1991). Blood flukes exhibit a two-host life cycle with asexual reproduction occurring in an invertebrate host and direct penetration of the skin of the definitive host. Schistosomes and most spirorchiids utilize gastropods as intermediate hosts (e.g. Brant et al., 2006; Cribb et al., 2017a; see de Buron et al., 2018 for evidence of spirorchiids using a polychaete intermediate host), while aporocotylids of actinopterygians exploit freshwater gastropods (e.g. Evans and Heckmann, 1973; Schell, 1974; Kirk and Lewis, 1993) and marine terebellid polychaetes (e.g. Køie, 1982; Cribb et al., 2011; Sugihara et al., 2014; Shirakashi et al., 2012). Recently, Cribb et al. (2017b) provided compelling evidence that bivalves serve as distinct intermediate hosts for aporocotylids of chondrichthyan fishes. The rate of proposal of new genera and species of Aporocotylidae suggests that a potentially high diversity remains to be discovered in this family (Cribb and Bray, 2011).
To date, blood flukes have not been recorded from marine mammals. The first indication that marine mammals may be susceptible was reported by Marsh et al. (1984) who documented trematode parasite eggs in ovary sections of the dugong, Dugong dugon (Müller, 1776; Sirenia: Dugongidae). These eggs exhibited ‘distinct black paired spots’ which is consistent with the description of dark bodies within developing Cardicola miracidia (McVay et al., 2011). Later, one of the authors (DB) recovered a single aporocotylid blood fluke of suitable quality for description from a stranded dugong in Townsville, Queensland. Subsequent efforts by the authors to recover additional specimens were unsuccessful due to a combination of the rarity of opportunities to necropsy fresh dugongs and the rapid decomposition of the host and soft-bodied parasites in tropical environments (Eros et al., 2007). Indeed, opportunities to conduct dugong necropsies are few because they are protected in Australia under the Environment Protection and Biodiversity Act (1999).
Herein we describe the new species of aporocotylid discovered by DB from Dugong dugon. This is timely given current global research momentum in documenting species richness within the Aporocotylidae. This novel species represents an important discovery that warrants description despite the absence of molecular data. Future material and molecular analyses could permit new inferences into the potential for evolutionary expansion of the Aporocotylidae into marine mammals.
2. Materials and methods
A single male dugong, Dugong dugon (273 cm total length; tail width 90 cm; collectors' ID MM313) originating from the Strand, Townsville, Queensland (19°15′00.0″S 146°49′59.9″E), in post-mortem condition D2 (“carcass in good condition, fresh/edible”; Eros et al., 2007) was dissected by DB on the 8th September 1992. A single aporocotylid was recovered from heart washings. The specimen was not apparently alive but was in good condition. It was fixed beneath a coverslip, with a small dab of Vaseline® jelly under each corner (to prevent excess flattening) while 10% formalin was drawn under the coverslip using absorbent paper, prior to being stained with Gower's carmine, and mounted in Canada balsam on a glass slide under a cover slip. A fragment of a second specimen was noticed in heart washings but was lost. Examination of the heart, a common site for spirorchiid and aporocotylid blood flukes, had been prompted by the report in Marsh et al. (1984) of probable blood-fluke eggs in the ovaries of several dugongs. Animal ethics statement: note that the animal was dead and found washed up on a beach and therefore did not require specific animal ethics approval.
The parasite specimen was observed using an Olympus BX53 compound light microscope fitted with direct interference contrast optics, or a Leica DM LS2 compound light microscope fitted with phase-contrast optics. Drawings were made using a drawing tube connected to the Leica DM LS2. All measurements are given in microns. Measurements were taken using Olympus LabSens® image analysis software. Where specifically indicated, measurements of curved structures following the curvature were taken using the polyline function of the software. In the interest of accessibility to the scientific community, images through the focal plane of this rare specimen were prepared and made available as supplementary video files: Hutson, K. (2019). Z-stack videos files for: First record of a ‘fish’ blood fluke (Digenea: Aporocotylidae) from a marine mammal: Cardicola dhangali n. sp. James Cook University. (dataset). https://doi.org/10.25903/5c527ca5c8b6d Digital Object Identifier (DOI):10.25903/5c527ca5c8b6d.
The following specimens were viewed for comparative purposes: Cardicola brasiliensis Knoff and Amato, 1992 (University of Nebraska State Museum 31717, 31718 paratypes; two slides), Cardicola whitteni Manter, 1954 (KSH personal collection, voucher, one slide), Paradeontacylix sanguinicoloides McIntosh, 1934 (South Australian Museum Australian Helminth Collection [SAMA AHC] vouchers AHC 28909, 28910; two slides), Paradeontacylix sp. (SAMA AHC vouchers AHC 28911, 28912; two slides).
3. Results
Family Aporocotylidae Odhner, 1912
Genus Cardicola Short, 1953
Cardicola dhangali n. sp.
3.1. Description (Fig. 1, Fig. 2, Fig. 3)
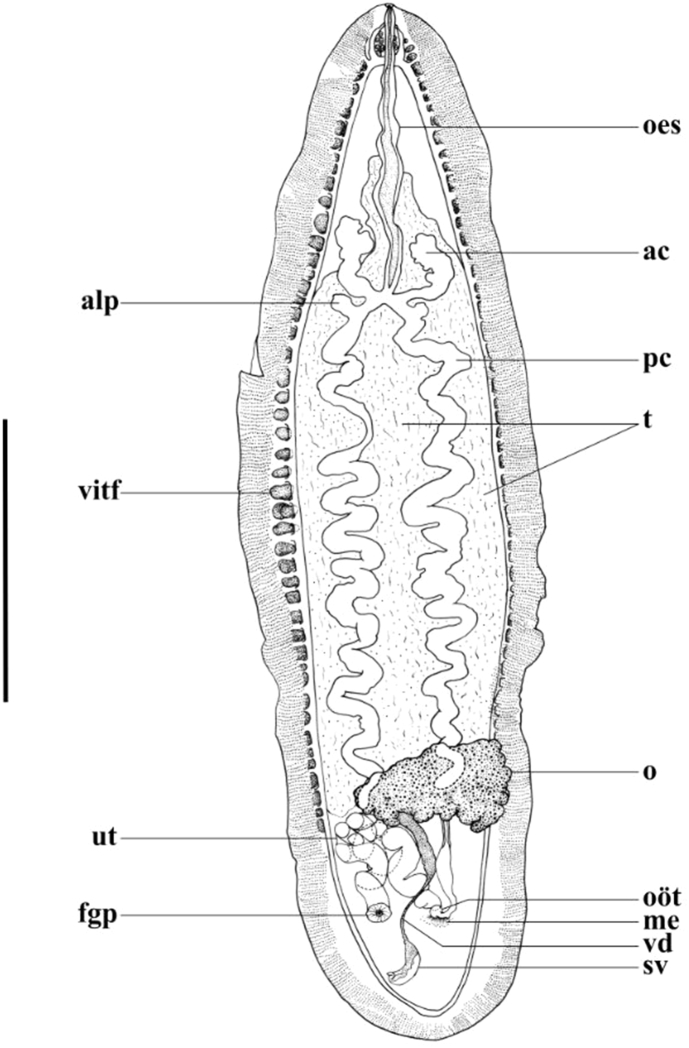
Fig. 1.
Cardicola dhangali n. sp. whole mount ventral view. Abbreviations: ac, anterior caecal branch; alp, antero-lateral projection of posterior caecal branch; fgp, female genital pore; me, Mehlis' glands; o, ovary; oes, oesophalgus, oöt, oötype; pc, posterior caecal branch; sv, seminal vesicle; t, testicular field; ut, uterus; vd, vas deferens; vitf, vitelline follicle. Scale bar = 1000 μm.
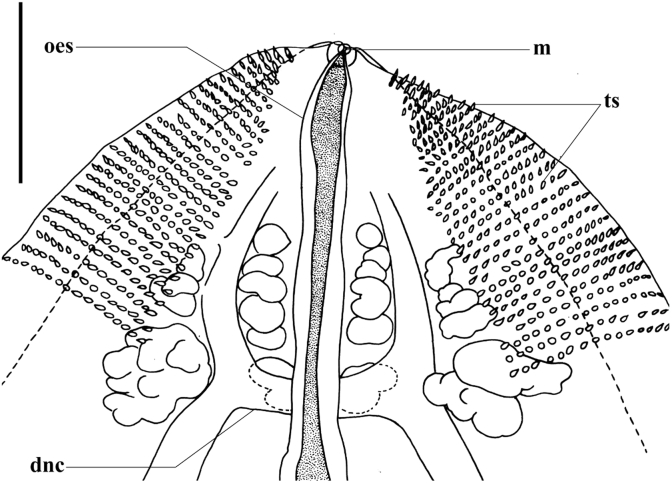
Fig. 2.
Cardicola dhangali n. sp. anterior region detail. Abbreviations: dnc, dorsal nerve commissure; m, mouth; ts, tegumental spines. Other abbreviations as for Fig. 1. Scale bar = 100 μm.
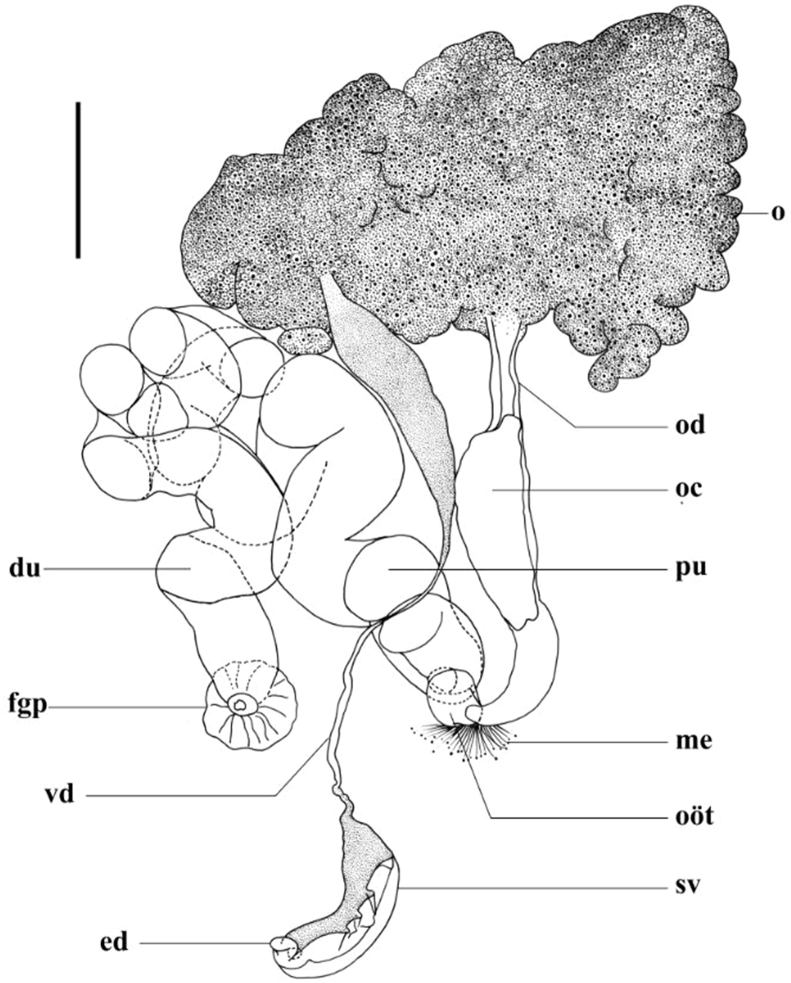
Fig. 3.
Cardicola dhangali n. sp. reproductive system (excluding testis). Abbreviations: du; distal portion of uterus; ed, ejaculatory duct; oc, oviductal chamber; od, oviduct; pu, proximal portion of uterus. Other abbreviations as for Fig. 1. Scale bar = 150 μm.
[Based on single whole-mounted holotype specimen] Body lanceolate (Fig. 1), 3690 in total length; 1105 wide at widest point; 3.3 times longer than wide. Between 2 (anterior-most), and 20 lanceolate tegumental spines (Fig. 2) 7–8 long arranged marginally in ventro-lateral rows initially 7–8 apart anteriorly, increasing to 11–14 apart at body mid region, decreasing to 9–10 posteriorly. Tegumental bristles or papillae not observed. Dorsal nerve commissure 18 thick, 186 from anterior extremity. No discernible oral sucker present. Mouth small, aperture 7 in diameter, immediately subterminal, ventral, slightly raised from body surface and weakly domed (Fig. 2). Oesophagus 1071 long, occupying ∼29% of body length (Fig. 1). Caecum roughly H-shaped (Fig. 1). Anterior caecal branches slightly curved, terminally lobate (Fig. 1). Right anterior branch 359 long, left anterior branch 379 long, following curvature. Anterior caecal branches 796 and 828 from anterior body extremity, respectively. Posterior caecal branches sigmoidally convoluted (Fig. 1), each initially forming short antero-laterally directed protuberance post-bifurcation (Fig. 1). Right protuberance 59 long, left protuberance 55 long. Right posterior caecal branch 3055 long, left caecal branch 2757 long (following curvature), terminating non-confluently dorsal to ovary (Fig. 1). Caecal extent occupies ∼58% of body length.
Testis (Figs. 1), 2388 long, 674 wide, not easily discernible, extracaecal, extending anteriorly, medially beyond anterior caecal branches, but not reaching dorsal nerve commissure; extending posteriorly to level of ovary and immediately anterior to uterus. Vas deferens dorsal to ovary, arising medially from posterior portion of testis, initially broad, narrowing distally and turning right, ventrally over proximal uterine fold (Fig. 1), then posteriorly to meet curved seminal vesicle on right side of body, posterior to remainder of reproductive system (Fig. 1, Fig. 3). Seminal vesicle curved to right, 194 long (following curvature), 50 wide. Short, terminally folded ejaculatory duct present (Fig. 1, Fig. 3). Male genital pore difficult to discern, opens dorsally on right of body, posterior to remainder of reproductive system in immediate vicinity of short ejaculatory duct.
Ovary distinct, irregularly lobate, 327 long, 504 wide, positioned medially and left in posterior body portion (Fig. 1, Fig. 3). Oviduct exits ovary posteriorly, left of body mid-line, travelling posteriorly before opening into empty oviductal chamber 464 long, 147 wide. Duct leading from oviductal chamber curves right to enter oötype (Fig. 1, Fig. 3). Mehlis’ gland present (Fig. 1, Fig. 3). Vitelline duct not observed. Proximal portion of uterus broad, convoluted, travels anteriorly, right of vas deferens to right posterior portion of ovary, narrows slightly, becoming increasingly convoluted, extending right, curving posteriorly to distal portion opening dorsally in prominent, muscular female genital pore 256 from right body margin, in line with oötype, well anterior to male genital pore, 487 from posterior body extremity (Fig. 1, Fig. 3). Vitelline follicles conspicuous along lateral outer margin of lateral nerve to level of ovary on left of body, and anterior portion of uterus on right. Vitelline follicles also present in anterior portion, anterior to dorsal nerve commissure. Excretory vesicle not observed.
Type host: Dugong dugon (Müller, 1776).
Host details: adult male, unknown age, D2-condition, 273 cm; tail width 90 cm (specimen number MM313).
Site in host: heart washings.
Type locality: The Strand, Townsville, Queensland, Australia (19.25 S, 146.8333 E).
Type material: holotype (Queensland Museum G237841).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 1999), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID). The LSID for Cardicola dhangali n. sp. is http://zoobank.org/urn:lsid:zoobank.org:pub:FC2C3CA7-503A-4846-AA28-D56E9B00B7B.
Etymology: the new species name is derived from the traditional language (Kala Lagaw Ya) of the Western and Central islands of the Torres Strait for dugong: ‘Dhangal’.
3.2. Remarks
Cardicola dhangali n. sp. differs from all other known Cardicola species by its parasitism of the only known mammalian host for the genus, Dugong dugon, its highly convoluted uterus (Fig. 1, Fig. 3), and the possession of antero-lateral projections on the posterior caecal branches immediately post-bifurcation (Fig. 1). It is similar in morphological aspects to Cardicola chaetodontis Yamaguti (1970) found in the gills and heart of butterflyfishes, Cardicola aurata Holzer et al. (2008) from Sparus aurata, Cardicola currani Bullard and Overstreet (2004) from Sciaenops ocellatus, Cardicola forsteri Cribb et al. (2000), Cardicola jiigurru Yong et al. (2016) from Chanos chanos, and Cardicola palmeri Bullard and Overstreet (2004) from Pogonias cromis. The majority of Cardicola species possess female and male genital pores positioned to the left of the body mid-line. The genital pores of C. chaetodontis (sensu Yamaguti, 1970) and the new species are positioned to the right of the body mid-line. Of the male and female genital pores in Cardicola species, the male genital pore alone of C. dhangali n. sp., C. aurata, C. currani, C. forsteri, C. jiigurru, C. palmeri opens posterior to the remainder of the reproductive system (see Cribb et al., 2000; Bullard and Overstreet, 2004; Holzer et al., 2008; Yong et al., 2016).
Cardicola dhangali n. sp. can be distinguished from C. chaetodontis (Yamaguti, 1970; Nolan and Cribb, 2006) by the position of the male genital pore (not posterior to the reproductive system in the latter), the morphology and position of the ovary, which is bi-lobed and medial in C. chaetodontis, but irregularly lobate and positioned medial to left of the body-mid line in the new species, and the extent and pathway of the posterior caecal branches. The posterior caecal branches terminate overlapping with the ovary of Cardicola dhangali n. sp. (Fig. 1), yet terminate short of the ovary in C. chaetodontis (see Yamaguti, 1970; Nolan and Cribb, 2006). The posterior caecal branches are characteristically sigmoidally convoluted in Cardicola dhangali n. sp. (Fig. 1), yet relatively straight in C. chaetodontis (see Yamaguti, 1970; Nolan and Cribb, 2006).
Cardicola dhangali n. sp. differs from C. aurata, C. currani, C. forsteri, C. jiigurru, and C. palmeri in the position of the male genital pore on the right side of the body. In addition, Cardicola dhangali n. sp. differs from C. aurata in the size and shape of the seminal vesicle, which is much larger in the latter, the morphology of the ejaculatory duct, which is armed in C. aurata, and there are fewer tegumental spines per row (4–11, and 11–14, respectively) in C. aurata and C. forsteri. Cardicola dhangali n. sp. differs further from C. currani, C. jiigurru, and C. palmeri by the size, or size and shape of its tegumental spines. These are shortest in C. jiigurru (<1 μm long); much larger and re-curved in C. currani and C. palmeri. These three species are also further differentiated from Cardicola dhangali n. sp. by the position or morphology of the ovary, which is medial in C. currani and C. palmeri, rather than medial to left of the body in the new species, and is clearly bi-lobed in C. jiigurru. The testis of C. aurata, C. chaetodontis, C. currani, and C. jiigurru is inter-caecal, whereas the testis of the new species extends extra-caecally. For additional morphological comparisons see Table 1.
Table 1.
Comparison between Cardicola dhangali n. sp. and its most morphologically similar congeners; percentages calculated from total body length, following (in part) Nolan et al. (2014). Measurements shown in μm.
| Species | Body | Body length/width | Spine length | Spines per row | Oral sucker | Oesophagus % | Anterior caeca length % | Posterior caeca/anterior caeca | Testis length/width | Testis length % | Testis width % | Ovary length % | Ovary position | Male genital pores position | Female genital pore position |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. dhangali n. sp. | 3690 × 1105 | 3.3 | 7–8 | 7–14 | Absent | 29.02 | 9.73, 10.27 | 6.79, 8.50 | 3.54 | 64.71 | 18.26 | 8.86 | Medial and left | Right | Right |
| C. aurataa | 1093–1321 × 248–284 | 3.8–4.9 | 4–5 | 4–11 | Present | – | – | 3.10–3.60 | – | – | – | – | – | Left-medially | Left |
| hC. chaetodontisb | 1150–1850 × 180–200 | – | 5–7 | – | Absent | – | – | – | – | – | – | – | – | Right | Right |
| hC. chaetodontisc | 899–1396 × 119–257 | 4.3–70 | – | 5–6 | Present | 33–37 | 2–5 | 5.4–12.5 | 3.1–5.5 | 36–38 | 41–66 | 7–16 | Right to medial | Left | Left |
| C. curranid | 1375–2853 × 442–663 | 2.6–5.0 | 19–25 | 2–4 | Absent | 31–40 | 9–18 | 1.8–4.2 | – | 24–37 | 25–59 | – | Medial | Left | Left |
| C. forsterie,f | 2512–4670 × 570–1070(f) | 4.34–5.79(e) | – | – | Present | 29–33(f) | – | 4–7(f) | – | 72–75(f) | – | – | – | Left | Left |
| C. jiigurrug | 1785–2505 × 132–160 | 13.2–15.7 | <1 | – | Present | 28.2–36.3 | 15.9–22.0 | 2.2–4.1 | – | – | – | – | – | Left | Left |
| C. palmerid | 1449–2357 × 867–1105 | 1.9–2.9 | 33–38 | 3–5 | Absent | 44–52 | 19–26 | 1.0–1.8 | – | 14–21 | 42–61 | – | Medial | Left | Left |
aHolzer et al. (2008); bYamaguti (1970); cNolan and Cribb, (2006); dBullard and Overstreet (2004); eShirakashi et al. (2012); fNolan et al. (2014); gYong et al. (2016); hCardicola chaetodontis considered here as two potentially separate species based on the morphological differences in Yamaguti (1970) and Nolan and Cribb, (2006).
4. Discussion
Infection of dugong (Dugong dugon) with an aporocotylid blood fluke likely represents a host-switch event from an actinopterygian fish. While this new host record presents an exciting discovery, it is less surprising that the new species was morphologically attributed to Cardicola, because it is the richest and least host-specific of the marine aporocotylid genera. Cardicola is unusual compared to most other aporocotylid genera in that it exhibits lower host-specificity; thirty-five Cardicola species have been described from 14 actinopterygian fish families, in contrast to its sister taxon, Paradeontacylix (see Holzer et al., 2008; Yong et al., 2016, 2018), of which eight (of the nine described) species only infect carangid fishes (e.g. Hutson and Whittington, 2006; Repullés-Albelda et al., 2008; WoRMS Editorial Board, 2018). Cardicola continues to exhibit a remarkable rate of discovery, with 24 new species attributed to this genus since 2000 (Yong et al., 2018; WoRMS Editorial Board, 2018). This is likely a consequence of multiple factors, including the circulatory system historically being overlooked in routine fish dissections (Cribb and Bray, 2011), increased dedicated taxonomic research effort in the northern (e.g., Bullard and Overstreet, 2004; Bullard et al., 2012; Bullard, 2010, 2013) and southern hemispheres (e.g., Nolan and Cribb, 2006, 2014; Yong et al., 2016, 2018), and importance in aquaculture (e.g., Cribb et al., 2000; Ogawa et al., 2010). Recently, Nolan et al. (2014) predicted that continued discovery of new Cardicola species would likely reveal a series of radiations in association with particular fish taxa, as well as evidence of host-switching.
Sirenian ancestry is remote from that of cetaceans or pinnipeds and sirenians re-evolved an aquatic lifestyle independently of, although simultaneously with, cetaceans (Domning, 2009). Thus, it is unclear whether dedicated parasitological necropsies will reveal more aporocotylid richness in non-sirenian marine mammal groups. Indeed, unlike most other taxa of marine mammals, modern sirenians, and especially dugongs, inhabit warm, shallow waters where many aporocotylid species occur in fish hosts. Given this, host-switching of aporocotylids into marine mammals is more likely to involve sirenians than cetaceans or pinnipeds. Alternatively, given the low host specificity of Cardicola and the shared habitat with dugongs, it is plausible that this discovery may be a case of accidental infection. Parasitological surveys of a broad range of marine mammalian taxa are probably not feasible because traditional approaches to study parasites are impractical for large marine mammals, most of which are protected (Hermosilla et al., 2015). Thus, rare opportunities to acquire quality morphological and molecular material, such as those from fresh strandings, must be seized to enable the elucidation of the likelihood and frequency of host-switching events and co-evolution in the Aporocotylidae.
The location of blood fluke eggs in the definitive host influences transmission and pathogenesis and could potentially assist with screening marine mammals for blood flukes. Successful parasite transmission requires eggs or miracidia to escape from the closed circulatory system. Some venous-dwelling schistosome eggs concentrate in the nasal circulation of birds and terrestrial mammals, which are presumably released when the host submerges its head during drinking, feeding or diving (Platt and Brooks, 1997). Adults of Schistosoma mansoni Sambon, 1907 produce eggs that pass into the intestinal lumen for release into the environment, while many eggs are carried to the liver, where they become trapped, often causing severe pathology (Pearce and MacDonald, 2002). Disease manifestation from egg accumulation has led to the hypothesis that hosts that succumb to infection in aquatic environments could release eggs via predation, scavengers, or decomposition (Platt and Brooks, 1997). Indeed, the arterial-dwelling spirorchiids release eggs in the direction of blood flow, which results in the wide dissemination of eggs within the host (Platt and Brooks, 1997). Aporocotylid miracidia are believed to primarily escape to the external environment through the gills, while a few species’ eggs probably traverse the gut or depend on the death of the host for release (Lester et al., 2009; Bray et al., 2012). There are numerous reports of aporocotylid eggs trapped in the heart of fish where they can become encapsulated and die (Overstreet and Thulin, 1989; Ogawa et al., 1989; Bullard and Overstreet, 2002, 2008; Lester et al., 2009; Yong et al., 2013) and endocarditis has been associated with infections in aquaculture (Warren et al., 2017). Plausible routes to the external environment for aporocotylids of dugongs could include the lungs, nostrils and excretory or reproductive systems. Aporocotylid eggs observed trapped in dugong ovaries (Marsh et al., 1984) indicate that host death may also be a feasible mechanism for release of eggs and transmission to susceptible intermediate hosts. If blood flukes utilize mammalian excretory systems for release of eggs like their schistosome relatives, non-invasive detection could be feasible from collection of fecal deposits and the application of environmental DNA techniques.
5. Conclusions
Infection of dugongs with the aporocotylid blood fluke, Cardicola dhangali n. sp., most likely represents a host-switch event from an actinopterygian fish. Given current research interest in aporocotylid discovery and the evolutionary history of the Schistosomatoidea, it is of intrinsic value to present this description of Cardicola dhangali n. sp. so that researchers can maximize future sampling opportunities of sirenians and other marine mammals that might act as hosts.
Conflicts of interest
All authors declare no conflicts of interest.
Acknowledgements
We thank RA Bray and GA Boxshall at the Natural History Museum for discussion and technical support. This research was supported through a James Cook University Special Studies Program (sabbatical) awarded to KSH. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijppaw.2019.06.009.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Bray R.A., Cribb T.H., Littlewood D.T.J. Sasala nolani gen. n., sp. n. (Digenea: Aporocotylidae) from the body-cavity of the guineafowl puffer fish Arothron meleagris (Lacepede) (Tetraodontiformes: tetraodontidae) from off moorea. Fr. Polyn. Zootaxa. 2012;3334:29–41. [Google Scholar]
- Brant S.V., Loker E.S. Can specialized pathogens colonize distantly related hosts? Schistosome evolution as a case study. PLoS Pathog. 2005;1(3):e38. doi: 10.1371/journal.ppat.0010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brant S.V., Morgan J.A.T., Mkoji G.M., Snyder S.D., Rajapakse R.P.V.J., Eric S., Loker E.S. An approach to revealing blood fluke life cycles, taxonomy, and diversity: provision of key reference data including dna sequence from single life cycle stages. J. Parasitol. 2006;92:77–88. doi: 10.1645/GE-3515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard S.A. A new species of Cardicola Short, 1953 (Digenea: Aporocotylidae) from the heart and branchial vessels of two surfperches (Perciformes: embiotocidae) in the eastern Pacific Ocean off California. Am. Soc. Parasitol. 2010;96:382–388. doi: 10.1645/GE-2325.1. [DOI] [PubMed] [Google Scholar]
- Bullard S.A. Cardicola langeli sp. n. (Digenea: Aporocotylidae) from heart of sheepshead, Archosargus probatocephalus (Actinopterygii: sparidae) in the Gulf of Mexico, with an updated list of hosts, infection sites and localities for Cardicola spp. Folia Parasitol. 2013;60:17–27. doi: 10.14411/fp.2013.003. [DOI] [PubMed] [Google Scholar]
- Bullard S.A., Baker T., de Buron I. New species of Cardicola (Digenea: Aporocotylidae) from heart of atlantic croaker, Micropogonias undulatus (Perciformes:Sciaenidae), of the south atlantic bight. J. Parasitol. 2012;98:328–332. doi: 10.1645/GE-2893.1. [DOI] [PubMed] [Google Scholar]
- Bullard S.A., Overstreet R.M. Potential pathological effects of blood flukes (Digenea: sanguinicolidae) on pen-reared marine fishes. Proc. Gulf Caribb. Fish. Inst. 2002;52:10–25. [Google Scholar]
- Bullard S.A., Overstreet R.M. Two new species of Cardicola (Digenea: sanguinicolidae) in drums (sciaenidae) from Mississippi and Louisiana. J. Parasitol. 2004;90:128–136. doi: 10.1645/GE-106R. [DOI] [PubMed] [Google Scholar]
- Bullard S.A., Overstreet R.M. Digeneans as enemies of fishes. In: Eiras J.C., Segner H., Wahli T., Kapoor B.G., editors. Vol. 2. Science Publishers; Enfield: 2008. pp. 817–976. (Fish Diseases). [Google Scholar]
- Cribb T.H., Adlard R.D., Hayward C.J., Bott N.J., Ellis D., Evans D., Nowak B.F. The life cycle of Cardicola forsteri (Trematoda: Aporocotylidae), a pathogen of ranched southern bluefin tuna, Thunnus maccoyi. Int. J. Parasitol. 2011;41:861–870. doi: 10.1016/j.ijpara.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Cribb T.H., Bray R.A. Trematode families and genera: have we found them all? Trends Parasitol. 2011;27:149–154. doi: 10.1016/j.pt.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Cribb T.H., Chick R.C., O'Connor W., O'Connor S., Johnson D., Sewell K.B., Cutmore S.C. Evidence that blood flukes (Trematoda: Aporocotylidae) of chondrichthyans infect bivalves as intermediate hosts: indications of an ancient diversification of the Schistosomatoidea. Int. J. Parastiol. 2017;47:885–891. doi: 10.1016/j.ijpara.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Cribb T.H., Crespo-Picazo J.L., Cutmore S.C., Stacy B.A., Chapman P.A., García-Párraga D. Elucidation of the first definitively identified life cycle for a marine turtle blood fluke (Trematoda: Spirorchiidae) enables informed control. Int. J. Parasitol. 2017;47:61–67. doi: 10.1016/j.ijpara.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Cribb T.H., Daintith M., Munday B. A new blood-fluke, Cardicola forsteri, (Digenea : sanguinicolidae) of southern blue-fin tuna (Thunnus maccoyii) in aquaculture. T. Roy. Soc. South. Aust. 2000;124:117–120. [Google Scholar]
- de Buron I., Colon B.L., Siegel S.V., Oberstaller J., Rivero A., Kyle D.E. First evidence of polychaete intermediate hosts for Neospirorchis spp. marine turtle blood flukes (Trematoda: spirorchiidae) Int. J. Parasitol. 2018 doi: 10.1016/j.ijpara.2018.08.002. [DOI] [PubMed] [Google Scholar]
- Domning D.P. Sirenian evolution. In: Würsig B., Perrin W., Würsig B., Thewissen J.G.M., editors. Encyclopedia of Marine Mammals. second ed. Academic Press; 2009. pp. 1016–1019. [Google Scholar]
- Eros C., Marsh H., Bonde R., O'Shea T., Beck C., Recchia C., Dobbs K., Turner M., Lemm S., Pears R., Bowater R. Townsville: Great Barrier Reef Marine Park Authority. second ed. Research Publication; 2007. Procedures for the salvage and necropsy of the dugong (Dugong dugon) No. 85. [Google Scholar]
- Evans W.A., Heckmann R.A. The life history of Sanguinicola Klamathensis life. Science. 1973;13:1285–1291. [Google Scholar]
- Hermosilla C., Silva L.M.R., Prieto R., Kleinertz S., Taubert A., Silva M.A. Endo- and ectoparasites of large whales (Cetartiodactyla: balaenopteridae, Physeteridae): overcoming difficulties in obtaining appropriate samples by non- and minimally-invasive methods. Int. J. Parasitol. Parasites Wildl. 2015;4:414–420. doi: 10.1016/j.ijppaw.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer A.S., Montero F.E., Repullés A., Nolan M.J., Sitja-Bobadilla A., Alvarez-Pellitero P., Zarza C., Raga J.A. Cardicola aurata sp. n. (Digenea: sanguinicolidae) from mediterranean Sparus aurata L. (Teleostei: sparidae) and its unexpected phylogenetic relationship with Paradeontacylix McIntosh, 1934. Parasitol. Int. 2008;57:472–482. doi: 10.1016/j.parint.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Hutson K.S., Whittington I.D. Paradeontacylix godfreyi n. sp. (Digenea: sanguinicolidae) from the heart of wild Seriola lalandi (Perciformes: carangidae) in southern Australia. Zootaxa. 2006;1151:55–68. [Google Scholar]
- ICZN International Code of Zoological Nomenclature . Fourth Edition. 1999. The International Trust for Zoological Nomenclature, London, UK. 306 pp. [Google Scholar]
- Kirk R.S., Lewis J.W. The life-cycle and morphology of Sanguinicola inermis plehn, 1905 (Digenea: sanguinicolidae) Syst. Parasitol. 1993;25:125–133. doi: 10.1017/s0031182000060868. [DOI] [PubMed] [Google Scholar]
- Køie M. The redia, cercaria and early stages of Aporocotyle simplex Odhner, 1900 (Sanguinicolidae) – a digenetic trematode which has a polychaete annelid as the only intermediate host. Ophelia. 1982;21:115–145. [Google Scholar]
- Lester R.J.G., Rawlinson S.E., Weaver L.C. Movement of sea mullet Mugil cephalus as indicated by a parasite. Fish. Res. 2009;96:129–132. [Google Scholar]
- Marsh H., Heinsohn G.E., Channells P.W. Changes in the ovaries and uterus of the dugong, Dugong dugon (Sirenia: Dugongidae), with age and reproductive activity. Aust. J. Zool. 1984;32:743–766. [Google Scholar]
- McVay M.J., Bakenhaster M., Bullard S.A. Cardicola laruei Short, 1953 (Digenea: Aporocotylidae) from heart of seatrout, Cynoscion spp., (Perciformes: sciaenidae) in the Gulf of Mexico and Atlantic Ocean: taxonomic redescription, first observations of egg and miracidium, and comments on geographic distribution and host specificity. Comp. Parasitol. 2011;78:291–305. [Google Scholar]
- Nolan M.J., Cribb T.H. Cardicola Short, 1953 and Braya n. gen. (Digenea: sanguinicolidae) from five families of tropical Indo-Pacific fishes. Zootaxa. 2006;1265:1–80. [Google Scholar]
- Nolan M.J., Miller T.L., Cutmore S.C., Cantacessi C., Cribb T.H. Cardicola beveridgei n. sp. (Digenea: Aporocotylidae) from the mangrove jack, Lutjanus argentimaculatus (perciformes: lutjanidae), and C. bullardi n. sp. from the Australian spotted mackerel, Scomberomorus munroi (perciformes: scombridae), from the northern great barrier reef. Parasitol. Int. 2014;63:735–745. doi: 10.1016/j.parint.2014.06.006. [DOI] [PubMed] [Google Scholar]
- Ogawa K., Hattori K., Hatai K., Kubota S. Histopathology of cultured marine fish, Seriola purpurascens (Carangidae) infected with Paradeontacylix spp. (Trematoda: sanguinicolidae) in its vascular system. Fish Pathol. 1989;24:75–81. [Google Scholar]
- Ogawa K., Tanaka S., Sugihara Y., Takami I. A new blood fluke of the genus Cardicola (Trematoda: sanguinicolidae) from Pacific bluefin tuna Thunnus orientalis (Temminck and Schlegel, 1844) cultured in Japan. Parasitol. Int. 2010;59:44–48. doi: 10.1016/j.parint.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Orélis-Ribeiro R., Arias C.R., Halanych K.M., Cribb T.H., Bullard S.A. Diversity and ancestry of flatworms infecting blood of the nontetrapod craniates “fishes”. Adv. Parasitol. 2014;85:1–64. doi: 10.1016/B978-0-12-800182-0.00001-5. [DOI] [PubMed] [Google Scholar]
- Overstreet R.M., Thulin J. Response by Plectropomus leopardus and other serranid fishes to Pearsonellum corventum (Digenea: sanguinicolidae), including melanomacrophage centres in the heart. Aust. J. Zool. 1989;37:129–142. [Google Scholar]
- Pearce E.J., MacDonald A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- Platt T.R., Blair D., Purdie J., Melville L. Griphobilharzia amoena n. gen. n. sp. (Digenea: Schistosomatidae), a parasite of the freshwater crocodile Crocodylus johnstoni (Reptilia: crocodylia) from Australia, with the erection of a new subfamily, Griphobilhar ziinae. J. Parasitol. 1991;77:65–68. [Google Scholar]
- Platt T.R., Brooks D.R. Evolution of the schistosomes (Digenea: Schistosomatoidea): the origin of dioecy and colonization of the venous system. J. Parasitol. 1997;83:1035–1044. [PubMed] [Google Scholar]
- Repullés-Albelda A., Montero F.E., Holzer A.S., Ogawa K., Hutson K.S., Raga J.A. Speciation of the Paradeontacylix spp. (sanguinicolidae) of Seriola dumerili. Two new species of the genus Paradeontacylix from the mediterranean. Parasitol. Int. 2008;57:405–414. doi: 10.1016/j.parint.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Schell S.C. The life history of Sanguinicola idahoensis sp. n. (Trematoda: sanguinicolidae), a blood parasite of steelhead trout Salmo gairdneri richardson. J. Parasitol. 1974;60:561–566. [PubMed] [Google Scholar]
- Shirakashi S., Kishimoto Y., Kinami R., Katano H., Ishimaru K., Murata O., Itoh N., Ogawa K. Morphology and distribution of blood fluke eggs and associated pathology in the gills of cultured Pacific bluefin tuna, Thunnus orientalis. Parasitol. Int. 2012;61:242–249. doi: 10.1016/j.parint.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Snyder S.D. Phylogeny and paraphyly among tetrapod blood flukes (Digenea: Schistosomatidae and spirorchiidae) Int. J. Parasitol. 2004;34:1385–1392. doi: 10.1016/j.ijpara.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sugihara Y., Yamada T., Tamaki A., Yamanishi R., Kanai K. Larval stages of the bluefin tuna blood fluke Cardicola opisthorchis (Trematoda: Aporocotylidae) found from Terebella sp. (Polychaeta: terebellidae) Parasitol. Int. 2014;63:295–299. doi: 10.1016/j.parint.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Warren M.B., Orélis-Ribeiro R., Ruiz C.F., Dang B.T., Arias C.R., Bullard S.A. Endocarditis associated with blood fluke infections (Digenea: Aporocotylidae: Psettarium cf. anthicum) among aquacultured cobia (Rachycentron canadum) from Nha Trang Bay. Vietnam Aquacult. 2017;468:549–557. [Google Scholar]
- WoRMS Editorial Board . 2018. World Register of Marine Species. Checklist dataset. accessed via GBIF.org on 2018-10-26. [DOI] [Google Scholar]
- Yamaguti S. vol 436p. Keigaku Pub. Co.; Tokyo: 1970. (Digenetic Trematodes of Hawaiian Fishes). [Google Scholar]
- Yong R.Q.-Y., Cutmore S.C., Miller T.L., Adlard R.D., Cribb T.H. The ghost of parasites past: eggs of the blood fluke Cardicola chaetodontis (Aporocotylidae) trapped in the heart and gills of butterflyfishes (Perciformes: chaetodontidae) of the great barrier reef. Parasitology. 2013;140:1186–1194. doi: 10.1017/S0031182013000681. [DOI] [PubMed] [Google Scholar]
- Yong R.Q.-Y., Cutmore S.C., Wee N.Q.-X., Cribb T.H. A complex of Cardicola (Digenea: Aporocotylidae) species infecting the milkfish, Chanos chanos (Gonorynchiformes), with descriptions of two new species. Syst. Parasitol. 2016;93:831–846. doi: 10.1007/s11230-016-9673-5. [DOI] [PubMed] [Google Scholar]
- Yong R.Q.-Y., Cutmore S.C., Cribb T.H. Two new species of Cardicola (trematoda: Aporocotylidae) from the damselfish Abudefduf whitleyi (perciformes: pomacentridae) and the triggerfish Sufflamen chrysopterum (tetraodontiformes: balistidae) Mar. Biodivers. 2018:1–11. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.