Abstract
Nonbiaryl N-C atropisomer is an important structural scaffold, which is present in natural products, medicines and chiral ligands. However the direct enantioselective C-H amination to access optically pure N-C atropisomer is still difficult and rare. Here we report a π-π interaction and dual H-bond concerted control strategy to develop the chiral phosphoric acids (CPAs) catalyzed direct intermolecular enantioselective C-H amination of N-aryl-2-naphthylamines with azodicarboxylates as amino sources for the construction of atroposelective naphthalene-1,2-diamines. This type of N-C atropisomers is stabilized by intramolecular hydrogen bond and the method features a broad range of substrates, high yields and ee values, providing a strategy to chirality transfer via the modification of N-C atropisomers.
Subject terms: Asymmetric catalysis, Organocatalysis, Synthetic chemistry methodology
Atropisomers with a chiral C-N axis are useful for natural products synthesis and as ligands in asymmetric catalysis. Here, the authors reportt a π-π interaction and dual H-bond concerted control strategy in enantioselective C-H amination affording configurationally stable N-C atropisomers.
Introduction
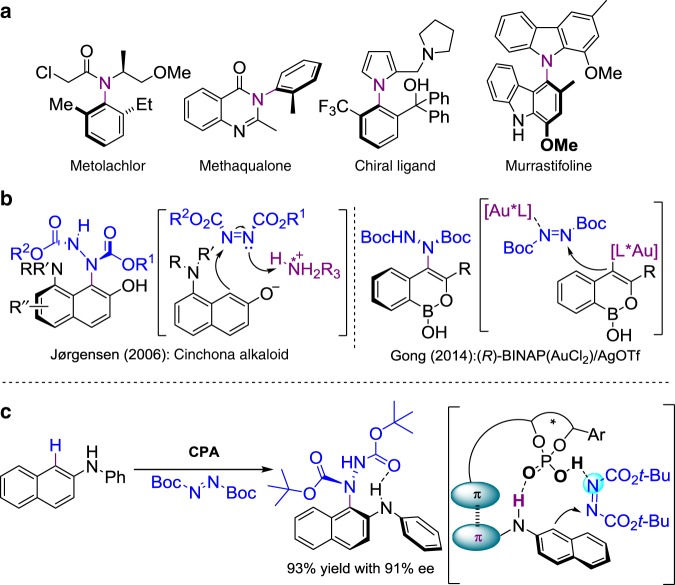
Atropisomeric compounds, in which the chirality generates from a chiral axis with highly sterically hindered rotation, are among the most useful catalysts or ligands in enantioselective catalysis and have been attracting considerable attention of chemists1–6. To date, the biaryl atropisomers, such as BINAP7,8 and BINOL9,10, have been well developed and widely used. In sharp contrast, the catalytic atroposelective construction of nonbiaryl atropisomers, which is of critical importance as medicine or chiral ligands in our lives (Fig. 1a)11–14, due to the rotational restriction around an N-C single bond, remains largely unexplored. Until recently, the preparation of these optically active N-C nonbiaryl atropisomers still depends largely on the chiral resolution and diastereoselective synthesis using chiral pool precursors15–17. Only few strategies for catalytic atroposelective construction of N-C nonbiaryl atropisomers exist and mainly consist of enantioselective cyclization18–20, N-functionalization21–27, and desymmetrization28–30 of the existing achiral N-C bond. The direct catalytic enantioselective method to build a new chiral N-C bond to form nonbiaryl N-C atropisomers still has significant synthetic challenges and has attracted great interest.
Fig. 1.
N-C nonbiaryl axially chiral structure. a N-C axially chiral compounds. b Previous N-C atropisomers with azodicarboxylates. c This work: CPA-catalyzed direct atroposelective C-H aminations via a concerted control of π-π interaction and dual H-bond strategy
Over the past three years, our laboratory has been engaged in developing efficient C-H amination methods31–34. Among them, azodicarboxylates, as a special amino source, have attracted our great interest. In the course of our investigation, we found that 2-naphthylamine derivatives could succeed in constructing C-N bonds at C1 position with azodicarboxylates catalyzed by a transition metal or Brønsted acid. Inspired by these results on the C1 position of C(sp2)-H amination, we proceeded to investigate whether the challenging nonbiaryl axially chiral N-C bonds could be formed by this direct C-H enantioselective amination, which would be an arguably ideal and attractive approach with great atom and step economy.
The previous landmark study by Jørgensen’s group35 introduced the enantioselective nonbiaryl axially chiral amination of 8-amino-2-naphthol derivatives with azodicarboxylates catalyzed by cinchona alkaloids (Fig. 1b). This study indicated that the amino group of C-8 position played an indispensable role in the stability of axially chirality and therefore the amination was limited by substrate scope. In 2014, Gong’s group36 reported the chiral Au-catalyzed cycloisomerization amination cascade reaction with azodicarboxylates to build heteroaryl atropisomers. Much disclosed in this context that the nonbiaryl atropisomers of diazenes were easily racemized and the single crystal of optically pure heteroaryl atropisomers was unable to be obtained. Despite these limits and challenges, it provided the foundation for our process.
Herein, we design a π–π interaction and dual H-bond concerted control strategy to develop the chiral phosphoric acids (CPAs) catalyzed direct intermolecular enantioselective C-H amination of N-aryl-2-naphthylamines with azodicarboxylates for the construction of N-C atroposelective nonbiaryl naphthalene-1,2-diamines (Fig. 1c).
Results
Reaction strategy
Our initial strategy was inspired by the pioneering studies of Akiyama37 and Terada38 on the use of CPAs as organocatalysts, which were widely used in organic synthesis. In line with our recent interest in the C-H amination reaction and the use of azodicarboxylates as an amino source, we believe that 2-naphthylamine as a nucleophiles derivative has a weakly acidic hydrogen atom, which could be activated and form a dual hydrogen-bonding intermediate A with azodicarboxylates under CPA as a catalyst to simultaneously activate two reaction partners and thus construct a chiral N-C axis (Fig. 2a). It should be mentioned, while chiral 2-naphthylamine derivatives such as BINAP and NOBIN39–41 are widely used as enantioselective catalysts in various reactions, the enantioselective construction of 2-naphthylamine derivatives is still rare42–47. Recently, Tan48 reported the organocatalytic arylations of 2-naphthylamine to successfully form biaryl atropisomers. However, the more challenging chiral nonbiaryl N-C atropisomers of 2-naphthylamine derivatives have not been discovered.
Fig. 2.
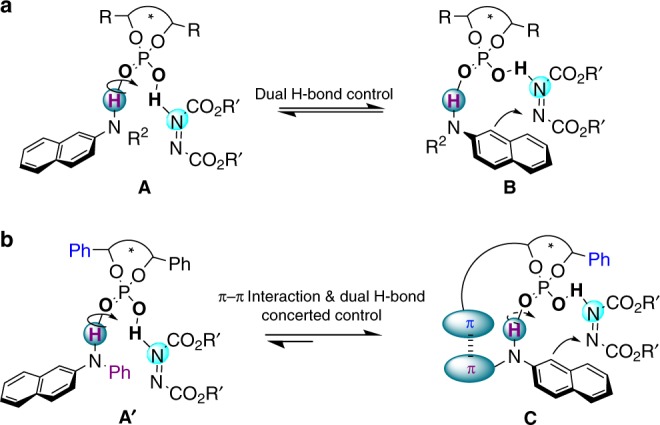
Reaction strategy. a Initial dual hydrogen-bonding strategy. b Modified strategy: concerted control of π-π interaction and dual H-bond strategy
Based on this initial strategy mentioned above, we started to evaluate our hypothesis by using N-substituted 2-naphthylamine 1 and di-tert-butyl azodicarboxylate (DBAD) 2a as model substrates. As shown in Table 1, when the substrate 1a (with hydrogen atom substituted by methyl and phenyl) was used, the reaction failed to generate any desired product using CPA1-3 as catalysts (Table 1, entries 1–3). Interestingly, when 1b and 1c (the substrate with an N-H bond) were used in the amination (Table 1, entries 4–7), the amination underwent smoothly and gave the amination products in 45–74% yields. These results showed that the N-H bond was indispensable and played a critical role in this process, which was in agreement with our initial design. However, the enantioselectivity of the desired product was very low (4–8% ee) even when the more sterically hindered Ph- or Ph3Si-substituted CPAs CPA2 and CPA3 were used. As shown in Fig. 2a, we believe the main reason is that the rotation is unhindered using only the dual H-bond to control the transition state of intermediate B. While the general approach to install some large groups at 2-naphylamine substrates (C8 position) might limit the rotation of intermediate B and partially probably improve the stereoselective control, however, it will severely limit the versatility and scope of substrates and applications of this chiral amination reaction.
Table 1.
Screening results of reaction strategy
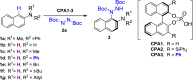
| |||||
|---|---|---|---|---|---|
| Entry | 1 | Catalyst | Dual H-Bond | π-π | 3 |
| Interactions | Yielda eeb | ||||
| 1 | 1a | CPA1 | No | No | No reaction |
| 2 | 1a | CPA2 | No | No | No reaction |
| 3 | 1a | CPA3 | No | Yes | No reaction |
| 4 | 1b | CPA3 | Yes | No | 63%, 4% ee |
| 5 | 1c | CPA1 | Yes | No | 74%, 7% ee |
| 6 | 1c | CPA2 | Yes | No | 71%, 4% ee |
| 7 | 1c | CPA3 | Yes | No | 45%, 8% ee |
| 8 | 1d | CPA3 | Yes | Yes | 45%, 47% ee |
| 9 | 1e | CPA3 | Yes | No | 19%, 9% ee |
| 10 | 1 f | CPA3 | Yes | No | 23%, 0% ee |
| 11 | 1 g | CPA3 | Yes | No | 37%, 4% ee |
All screening reactions were carried out in a 10 mL glass vial with a PTFE-lined cap on a 0.1 mmol scale. 2.0 equiv of 2a, 10 mol% catalyst, 1 mL DCM, 25 °C for 3 h
aYield represents isolated yield
bDetermined by HPLC analysis
To overcome these drawbacks and limitations, we designed a weak intermolecular interaction to help limit the contortion of intermediate B and enhance the stereoselectivity control of our C-H amination reaction. The π-π interaction49,50 as an arguably ideal modified strategy was introduced in our enantioselective axially chiral amination (Fig. 2b, c). To evaluate the efficiency of our modified design, we used N-phenyl substituted 2-naphthylamine 1d to react with DBAD in the presence of phenyl-substituted CPA3 as a catalyst (Table 1, entry 8). Remarkably, the enantioselectivity of this N-C axially chiral amination reaction was significantly promoted and gave the 47% ee with an isolated yield of 45%. We also investigated some more protecting groups of the nitrogen with the more steric hindrance, such as benzyl (1e), tert-butyl (1g), and they all gave low enantioselectivity (Table 1, entries 9–11). These results strongly support our modified design in Fig. 2b, and clearly indicate that the concerted control strategy of π-π interaction and dual H-bond between the N-phenyl of 2-naphthylamine and the aryl of CPAs are extremely important and efficient for the control of stereoselectivity in this nonbiaryl axially chiral amination reaction.
Optimization of reaction conditions
Based on our final design (Fig. 2b) and considerations mentioned above, we further attempted our chiral amination by using N-phenyl-2-naphthylamine 1d and DBAD 2a as model substrates. After an initial screening of the aryl-substituted CPA catalysts (Table 2, entries 1–6), we found that all the aryl-substituted CPAs CPA3-CPA7 showed good enantioselectivity and the catalyst CPA6 gave the desired product 3a with the best result of 94% isolated yield and 74% ee in DCM at −60 °C (Table 2, entry 4). Encouraged by this result, various solvents were screened (Table 2, entries 7–9). A remarkable solvent effect was observed. When the mixed solvent of DCM and Et2O (1:1) was used as the reaction medium, the product 3a was notably promoted to 88% ee (Table 2, entry 10). After adjusting the ratio of DCM/Et2O and changing the temperature, we found that the optimal catalytic system (standard condition) consisted of 2.0 equiv of azodicarboxylate 2a, 10 mol% of CPA6 at −70 °C in a mixed solvent (0.1 M, DCM: Et2O = 7:3) for 48 h, which resulted in 93% isolated yield and 91% ee (Table 2, entry 12).
Table 2.
Optimization of reaction conditions
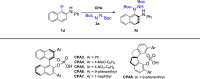
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | R | Cat | Solvent | Temp/°C | Time/h | Yield %a | ee %b |
| 1 | Ph | CPA3 | DCM(D) | −60 | 12 | 63 | 73 |
| 2 | Ph | CPA4 | DCM | −60 | 12 | 24 | 62 |
| 3 | Ph | CPA5 | DCM | −60 | 12 | 75 | 74 |
| 4 | Ph | CPA6 | DCM | −60 | 12 | 94 | 74 |
| 5 | Ph | CPA7 | DCM | −60 | 12 | 58 | 73 |
| 6 | Ph | CPA8 | DCM | −60 | 12 | 11 | 12 |
| 7 | Ph | CPA6 | Toluene | −60 | 12 | 24 | 47 |
| 8 | Ph | CPA6 | Et2O(E) | −60 | 12 | 11 | 41 |
| 9 | Ph | CPA6 | THF | −60 | 12 | <2 | – |
| 10 | Ph | CPA6 | D:E = 1:1 | −60 | 12 | 42 | 88 |
| 11 | Ph | CPA6 | D:E = 7:3 | −60 | 12 | 67 | 90 |
| 12 | Ph | CPA6 | D:E = 7:3 | −70 | 48 | 93 | 91 |
| 13 | Ph | CPA6 | D:E = 7:3 | −78 | 48 | 65 | 92 |
| 14c | Ph | CPA6 | D:E = 7:3 | −70 | 48 | 94 | 91 |
All screening reactions were carried out in a 10 mL glass vial with a PTFE-lined cap on a 0.1 mmol scale. 2.0 equiv of 2a, 10% mol catalyst, 1 mL solvent
aYield represents isolated yield
bDetermined by HPLC analysis
c20 mol% catalyst
In addition, lowering the temperature would decrease the chemical yield (Table 2, entry 13) and increasing the amount of catalyst resulted in a little change (Table 2, entry 14).
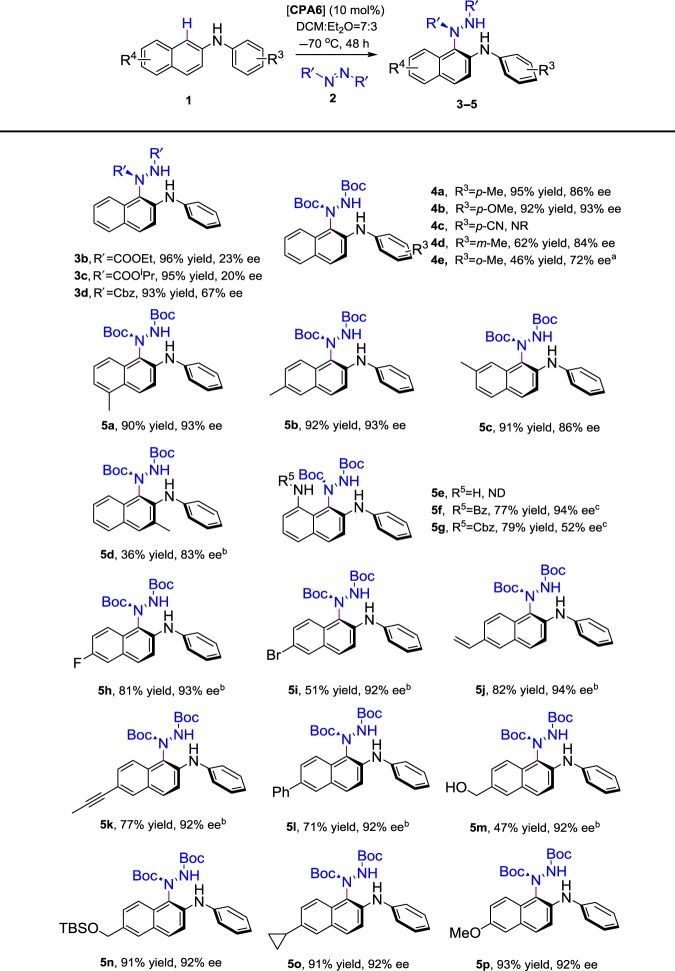
Substrate scope
With the optimized conditions at hand, we first probed the scope of different azodicarboxylate sources (Table 3b–d). Some commercially available azodicarboxylates, such as DEAD (diethyl azodicarboxylate), DIAD (diisopropyl azodicarboxylate), and dibenzyl azodicarboxylate, could react with 1d and provide amination products 3b–3d with high yields. However, it is clear that the enantioselectivity of this axially chiral amination was sensitive to the steric hindrance of azodicarboxylate, and DBAD was still the best azodicarboxylate amino source and gave the desired N-C axially chiral product 3a in 91% ee. (Table 2, entry 12). Subsequently, the effect of the substituents (R3) on the benzene ring was examined. The electron-donating groups including methyl and methoxy were fully compatible with the reaction condition and gave the axially chiral products 4a and 4b in high yields (95% and 92%) with good to excellent enantioselectivities (86% ee and 93% ee). In addition, when the substituent was on meta-position or ortho-position, the reactivity was notably decreased (Table 3, 4d, 4e).
Table 3.
Substrate scope
All yields were based on isolated products on a 0.1 mmol scale under standard reaction condition. The ee value was determined by HPLC analysis
NR no reaction, ND no detected
a−20 °C
b72 h
c−30 °C, 72 h
Encouraged by these results, we further expanded the scope of functionalized naphthalene rings in order to examine the generality of this axially chiral amination. The N-phenyl-2-naphthylamines substituted with a variety of functional groups, such as alkyl, aryl, halogens, vinyl, and alkynyl, were well tolerated in our reaction system and formed N-C axially chiral products 5a–5p in good to excellent yields and high enantioselectivities. Using methyl as a substituent at C3 position, the reactivity was notably deceased but the stereoselectivity (5d) was slightly affected. The 5, 6, or 7-methyl-substituted substrates, which were away from the reactive site, gave excellent isolated yields of 90–92% and 86–93% ee (Table 3, 5a–5c). Furthermore, we focused our attention on the amino group at the adjacent C8-position, which was of great importance in Jørgensen’s report. When 8-amino substituted N-phenyl-2-naphthylamine was used as a substrate in our reaction conditions, the reaction was very complex and the desired C-1 amination product 5e was not obtained. To our delight, when Bz was used as an N-protecting group, C-1 regioselective axially chiral amination product 5f was obtained with 77% yield and 94% ee. The absolute regioselectivity and configuration of 5g was determined by X-ray crystallographic analysis (see Supplementary Fig. 129 for details).
In addition, various substituent groups were investigated (5h–5p). The electronic properties of the substituents did not affect the stereoselectivity of the reaction and gave 92–94% ee. The substrates with electron-withdrawing groups would reduce the electron cloud density and limit the reactivity of amination reaction. For example, the 6-Br substituted N-phenyl-2-naphthylamine gave the desired product 5i in 51% isolated yield, but with excellent 92% ee.
Mechanistic study
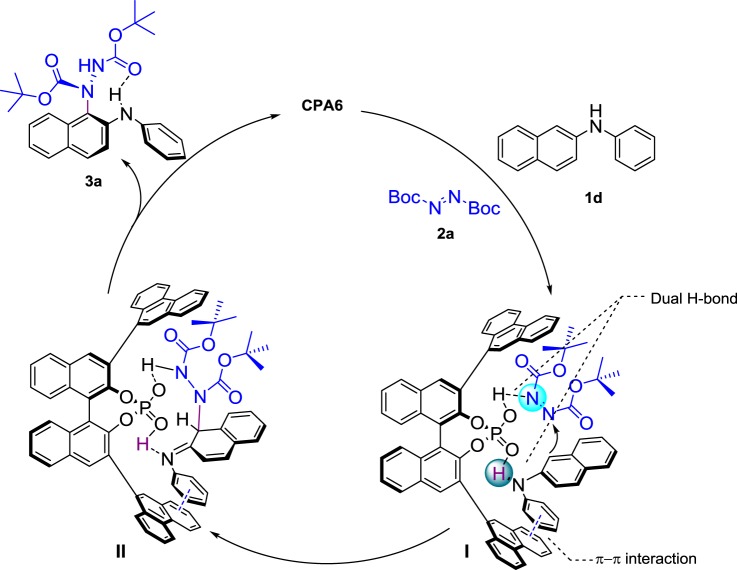
Based on our design, experimental results, and literature precedent51–53, a possible reaction pathway was proposed (Fig. 3). First, the CPA simultaneously activated N-phenyl-2-naphthylamine and azodicarboxylate through a dual hydrogen-bonding activation mode to form intermediate I. Then, the π-π interaction assisted intermediate I with the concerted control of the enantioselectivity, and the nucleophilic addition of N-phenyl-2-naphthylamine to the azodicarboxylate proceeded and formed intermediate II. In the following step, rearomatization became favorable for efficient central-to-axial chirality conversion54 and delivered the desired amination product 3a. Notably, an intramolecular hydrogen bond (2.233 Å) between the N–H and the oxygen of the carbonyl group was observed and the 1H NMR chemical shift evidently changed with temperature (see Supplementary Figs. 127–129).
Fig. 3.
Proposed reaction mechanism. The mechanism was described citing 1d as an example
Stereo-stability study
To further verify the stereo-stability of our N-C axially chiral products, a series of the half-lives of racemization (in n-hexane at 25 °C) of the substituted products were measured. For the substituent group on the benzene ring, the 4b gave 67.4 h. The substituent groups on the naphthalene ring gave 5a (103.3 h), 5b (32.1 h), 5c (63.3 h), 5 h (24.8 h), 5i (46.6 h), 5 l (52.7 h), 5 m (33.3 h), and 5p (20.6 h). The compound 5d with substituent at adjacent C3 position of naphthalene ring gave the half-life of racemization of 11.4 h. Interestingly, substituent group on C8 position of the naphthalene 5 g was very stable and gave the half-life of racemization of 5325.2 h. At solid state, we used 3a (90.10% ee) as an example, and it was 90.04% ee after 90 h at −18 °C. These results indicated that our N-C axially chiral products have suitable stability.
To get a better understanding of the stereo-stability of our N-C nonbiaryl atropisomers, a series of experiments were then carried out to examine the steric hindrance of azodicarboxylates (Fig. 4, Part I), the effect of intramolecular hydrogen bond (Fig. 4, part II), and the substituted groups of the naphthylamine (Fig. 4, part III). Based our above experiment results (3a–3d), we knew that the more steric hindrance of azodicarboxylates gave the better enantioselectivity. Furthermore, in order to confirm that the low enantioselectivity was originated from the racemization during the reaction process or the hard stereo-control, we did three parallel experiments to obtain the 3d at 12 h (64% ee), 24 h (66% ee) and 48 h (67% ee) under standard conditions, showing that the N-C nonbiaryl atropisomers were stable at reaction conditions without racemization. Compared 3a (92% ee, 70.9 h) and 3d (67% ee, 25.0 h), this evidence suggested that the large steric hindrance of azodicarboxylates (Fig. 4, part I) not only improved the enantioselectivity of reaction, but also stabilized the N-C atropisomers.
Fig. 4.
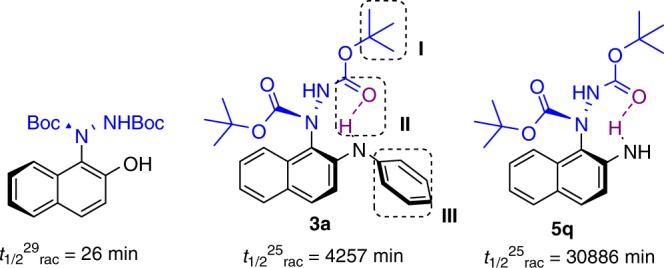
The Stereo-stability study. The half-lives of racemization of 3a and 5q were examined in n-hexane at 25 °C
The N-C nonbiaryl atropisomers of our 2-naphthylamine substrates are much more stable than 2-naphthol35 (Fig. 4), and the intramolecular hydrogen bond55,56 (conformed by X-ray) would be the important reason. Despite the N-phenyl substituted group was necessary because of π–π interaction in stereo-control in Table 1, the compound 5q (obtained by chiral resolution), which was much easier to form intramolecular hydrogen bond, was used to compare the half-lives of racemization to examine the intramolecular hydrogen bond effect on stereo-stability and gave 514.8 h. These results indicated that the intramolecular hydrogen bond (N-H…O) notably improved the stereo-stability of these N-C axially chiral products.
Synthetic applicability
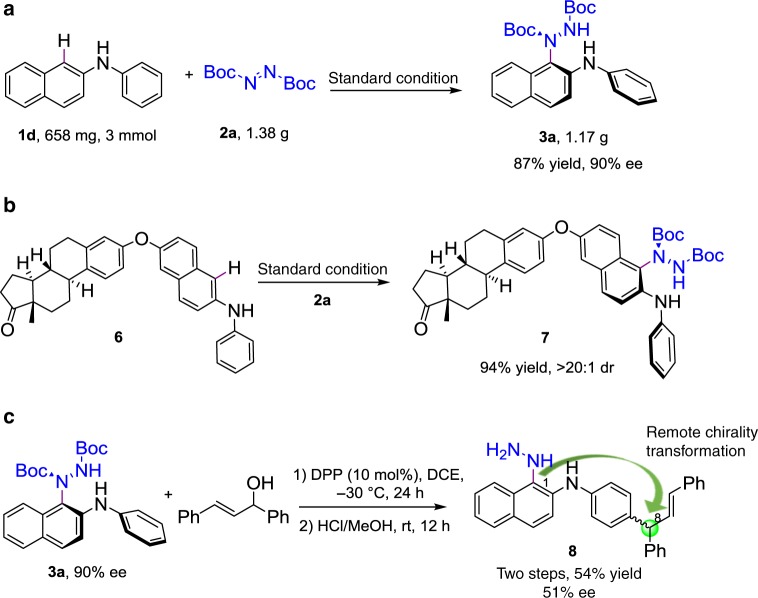
To enhance the practicality and effectiveness of our enantioselective catalytic protocol, a gram-scale reaction was carried out, as shown in Fig. 5a. The desired N-C axially chiral amination product 3a was obtained in 1.17 g (87% yield) with ideal 90% ee under standard conditions. In addition, we also tested the feasibility of this protocol in the late-stage modification of complex molecules in Fig. 5b. To our delight, the estrone derivative 6 was well tolerated and gave the desired product 7 in 94% yield with excellent stereochemical integrity and high diastereoselectivity (>20:1 dr).
Fig. 5.
Synthetic applicability. a Gram-scale synthesis of 3a. b The feasibility of complex molecule. c The chirality transfer processes
To demonstrate the synthetic utility of our N-C axially chiral amination products, based on our continuing interest in developing remote C-H functionalization, herein we designed a challenging remote chiral transformation in Fig. 5c. Allylic alkylation is an important component of chemistry and enantioselective studies, and particularly those of allylic alcohols, rely heavily on transition metals57–59. The para-position of the aniline C-H allylic alkylation was not reported. Herein, the N-C axially chiral amination product 3a was used to react with (E)-1,3-diphenylprop-2-en-1-ol, which was catalyzed by DPP (diphenyl phosphate) at −30 °C in DCE for 24 h. The desired product was obtained in 72% yield. After the removal of Boc and the N-C axial chirality, a remote chiral 1- to 8-position transformation was achieved in two steps, in 54% yield with 51% ee. Further enantioselective catalysis studies of this allylic alkylation will be reported by us.
Discussion
We have designed a π–π interaction and dual H-bond concerted control strategy and developed the CPAs catalyzed direct enantioselective nonbiaryl N-C axially chiral amination of 2-naphthylamine derivatives with azodicarboxylates as amino sources in good yields and high enantioselectivities. This type of N-C atropisomers is stabilized by intramolecular hydrogen bond and a broad range of substrates was proved to be well tolerated. Moreover, these axially chiral products could be converted into derivatives containing new chiral centers. Further mechanistic studies and the applications of this enantioselective axially chiral amination methodology in the synthesis of complex nitrogen-containing products are currently underway.
Methods
General procedure
A mixture of 2-naphthylamine derivatives 1 (0.1 mmol, 1 equiv) and CPA6 (0.01 mmol, 0.1 equiv), in DCM: Et2O = 7:3 (1 mL) was stirred at −70 ℃ for 30 min in a 10 mL glass vial (purged sealed with PTFE cap). Then azodicarboxylate (0.2 mmol, 2 equiv) was added, and the reaction mixture was stirred at −70 ℃ for 48 h. The reaction mixture was direct purified by silica gel flash chromatography to give the amination product.
Supplementary information
Acknowledgements
This work was supported by the “Thousand Youth Talents Plan”, NSFC (21672145, 51733007, 21702136), the Shuguang program (16SG10) from SEDF & SMEC, the STCSM (17JC1403700), and The Drug Innovation Major Project of the Ministry of Science and Technology of China (2018ZX09711001-005-002). We thank Prof. Yong-Qiang Tu and Prof. Wan-bin Zhang (Shanghai Jiao Tong University) for helpful suggestions and comments on this manuscript.
Author contributions
H.-Y.B. performed the experiments and analyzed the data. F.-X.T. and T.-Q.L. helped with characterizing some new compounds. G.-D.Z, J.-M.T., T.-M.D. and Z.-M.C. provided useful and valuable advice. S.-Y.Z. conceived and directed the project and wrote the paper.
Data availability
The data that support the findings of this study are available from the corresponding authors on request (DOI: 10.6084/m9.figshare.8131271). The X-ray crystallographic coordinates for structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1875011[https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=1875011&DatabaseToSearch=Published]. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information: Nature Communications thanks Jean Rodriguez and other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-10858-x.
References
- 1.Kumarasamy E, Raghunathan R, Sibi MP, Sivaguru J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. Chem. Rev. 2015;115:11239–11300. doi: 10.1021/acs.chemrev.5b00136. [DOI] [PubMed] [Google Scholar]
- 2.Wencel-Delord J, Panossian A, Leroux FR, Colobert F. Recent advances and new concepts for the synthesis of axially stereoenriched biaryls. Chem. Soc. Rev. 2015;44:3418–3430. doi: 10.1039/C5CS00012B. [DOI] [PubMed] [Google Scholar]
- 3.Smyth JE, Butler NM, Keller PA. A twist of nature – the significance of atropisomers in biological systems. Nat. Prod. Rep. 2015;32:1562–1583. doi: 10.1039/C4NP00121D. [DOI] [PubMed] [Google Scholar]
- 4.Loxq P, Manoury E, Poli R, Deydier E, Labande A. Synthesis of axially chiral biaryl compounds by asymmetric catalytic reactions with transition metals. Coord. Chem. Rev. 2016;308:131–190. doi: 10.1016/j.ccr.2015.07.006. [DOI] [Google Scholar]
- 5.Renzi P. Organocatalytic synthesis of axially chiral atropisomers. Org. Biomol. Chem. 2017;15:4506–4516. doi: 10.1039/C7OB00908A. [DOI] [PubMed] [Google Scholar]
- 6.Zilate B, Castrogiovanni A, Sparr C. Catalyst-controlled stereoselective synthesis of atropisomers. ACS Catal. 2018;8:2981–2988. doi: 10.1021/acscatal.7b04337. [DOI] [Google Scholar]
- 7.Carroll MP, Guiry PJ. P, N ligands in asymmetric catalysis. Chem. Soc. Rev. 2014;43:819–833. doi: 10.1039/C3CS60302D. [DOI] [PubMed] [Google Scholar]
- 8.Berthod M, Mignani G, Woodward G, Lemaire M. Modified BINAP: The how and the why. Chem. Rev. 2005;105:1801–1836. doi: 10.1021/cr040652w. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Yekta S, Yudin AK. Modified BINOL ligands in asymmetric catalysis. Chem. Rev. 2003;103:3155–3212. doi: 10.1021/cr020025b. [DOI] [PubMed] [Google Scholar]
- 10.Wang J-Z, et al. Symmetry in cascade chirality-transfer processes: a catalytic atroposelective direct arylation approach to BINOL derivatives. J. Am. Chem. Soc. 2016;138:5202–5205. doi: 10.1021/jacs.6b01458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi I, Suzuki Y, Kitagawa O. Asymmetric synthesis of atropisomeric compounds with an N-C chiral axis. Org. Prep. Proc. Int. 2014;46:1–23. doi: 10.1080/00304948.2014.866467. [DOI] [Google Scholar]
- 12.LaPlante SR, et al. Assessing atropisomer axial chirality in drug discovery and development. J. Med. Chem. 2011;54:7005–7022. doi: 10.1021/jm200584g. [DOI] [PubMed] [Google Scholar]
- 13.Mino T, et al. Chiral N-(tert-butyl)-N-methylaniline type ligands: Synthesis and application to palladium-catalyzed asymmetric allylic alkylation. Tetrahedron. 2015;71:5985–5993. doi: 10.1016/j.tet.2015.01.027. [DOI] [Google Scholar]
- 14.Faigl F, Erdélyi Z, Deák S, Nyerges M, Mátravölgyi B. A new pyrrolidine-derived atropisomeric amino alcohol as a highly efficient chiral ligand for the asymmetric addition of diethylzinc to aldehydes. Tetrahedron Lett. 2014;55:6891–6894. doi: 10.1016/j.tetlet.2014.10.101. [DOI] [Google Scholar]
- 15.Kamikawa K, Kinoshita S, Matsuzaka H, Uemura M. Stereoselective synthesis of axially chiral N-C bonds in N-aryl indoles. Org. Lett. 2006;8:1097–1110. doi: 10.1021/ol0529997. [DOI] [PubMed] [Google Scholar]
- 16.Kamikawa K, et al. Stereoselective synthesis of both enantiomers of N-aryl indoles with axially chiral N−C bonds. J. Org. Chem. 2007;72:3394–3402. doi: 10.1021/jo0700427. [DOI] [PubMed] [Google Scholar]
- 17.Rae J, et al. Synthesis of axially chiral C–N scaffolds via asymmetric coupling with enantiopure sulfinyl iodanes. ACS Catal. 2018;8:2805–2809. doi: 10.1021/acscatal.7b04343. [DOI] [Google Scholar]
- 18.Tanaka K, Takeishi K, Noguchi K. Enantioselective synthesis of axially chiral anilides through rhodium-catalyzed [2 + 2 + 2] cycloaddition of 1,6-diynes with trimethylsilylynamides. J. Am. Chem. Soc. 2006;128:4586–4587. doi: 10.1021/ja060348f. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y-B, Zheng S-C, Hu Y-M, Tan B. Brønsted acid-catalysed enantioselective construction of axially chiral. Nat. Commun. 2017;8:15489. doi: 10.1038/ncomms15489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Zhang J, Ma J, Cheng D-J, Tan B. Highly atroposelective synthesis of arylpyrroles by catalytic asymmetric Paal–Knorr reaction. J. Am. Chem. Soc. 2017;139:1714–1717. doi: 10.1021/jacs.6b09634. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa O, Kohriyama M, Taguchi T. Catalytic asymmetric synthesis of optically active atropisomeric anilides through enantioselective N-allylation with chiral Pd-tol-BINAP catalyst. J. Org. Chem. 2002;67:8682–8684. doi: 10.1021/jo0264271. [DOI] [PubMed] [Google Scholar]
- 22.Terauchi J, Curran DP. N-Allylation of anilides with chiral palladium catalysts: the first catalytic asymmetric synthesis of axially chiral anilides. Tetrahedron.: Asymmetry. 2003;14:587–592. doi: 10.1016/S0957-4166(03)00041-7. [DOI] [Google Scholar]
- 23.Kitagawa O, Takahashi M, Yoshikawa M, Taguchi T. Efficient synthesis of optically active atropisomeric anilides through catalytic asymmetric N-arylation reaction. J. Am. Chem. Soc. 2005;127:3676–3677. doi: 10.1021/ja042216x. [DOI] [PubMed] [Google Scholar]
- 24.Kitagawa O, et al. Highly enantioselective synthesis of atropisomeric anilide derivatives through catalytic asymmetric N-arylation: Conformational analysis and application to asymmetric enolate chemistry. J. Am. Chem. Soc. 2006;128:12923–12931. doi: 10.1021/ja064026n. [DOI] [PubMed] [Google Scholar]
- 25.Shirakawa S, Liu K, Maruoka K. Catalytic asymmetric synthesis of axially chiral o-iodoanilides by phase-transfer catalyzed alkylations. J. Am. Chem. Soc. 2012;134:916–919. doi: 10.1021/ja211069f. [DOI] [PubMed] [Google Scholar]
- 26.Liu Y-L, Feng X-Q, Du H-F. Asymmetric synthesis of axially chiral anilides by Pd-catalyzed allylic substitutions with P/olefin ligands. Org. Biomol. Chem. 2015;13:125–132. doi: 10.1039/C4OB01087F. [DOI] [PubMed] [Google Scholar]
- 27.Li S-L, et al. Atroposelective catalytic asymmetric allylic alkylation reaction for axially chiral anilides with achiral Morita–Baylis–Hillman carbonates. J. Am. Chem. Soc. 2018;140:12836–12843. doi: 10.1021/jacs.8b06014. [DOI] [PubMed] [Google Scholar]
- 28.Diener ME, Metrano AJ, Kusano S, Miller SJ. Enantioselective synthesis of 3-arylquinazolin-4(3H)-ones via peptide-catalyzed atroposelective bromination. J. Am. Chem. Soc. 2015;137:12369–12377. doi: 10.1021/jacs.5b07726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J-W, et al. Discovery and enantiocontrol of axially chiral urazoles via organocatalytic tyrosine click reaction. Nat. Commun. 2016;7:10677. doi: 10.1038/ncomms10677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirai M, et al. Catalytic enantioselective synthesis of N–C axially chiral mebroqualone and its derivatives through reductive asymmetric desymmetrization. Org. Lett. 2016;18:5700–5703. doi: 10.1021/acs.orglett.6b02865. [DOI] [PubMed] [Google Scholar]
- 31.Bai H-Y, Ma Z-G, Yi M, Lin J-B, Zhang S-Y. Palladium-catalyzed direct intermolecular amination of unactivated methylene C(sp3)–H bonds with azodiformates via bidentate-chelation assistance. ACS Catal. 2017;7:2042–2046. doi: 10.1021/acscatal.6b03621. [DOI] [Google Scholar]
- 32.Fu X, Bai H-Y, Zhu G-D, Huang Y, Zhang S-Y. Metal-controlled, regioselective, direct intermolecular α- or γ-amination with azodicarboxylates. Org. Lett. 2018;20:3469–3472. doi: 10.1021/acs.orglett.8b01183. [DOI] [PubMed] [Google Scholar]
- 33.Bai H-Y, et al. Transition metal‐controlled direct regioselective intermolecular amidation of C−H bonds with azodicarboxylates: Scope, mechanistic studies, and applications. Adv. Synth. Catal. 2018;360:4205–4214. doi: 10.1002/adsc.201800623. [DOI] [Google Scholar]
- 34.Dong J-W, Ding T-M, Zhang S-Y, Chen Z-M, Tu Y-Q. A facile approach to oximes and ethers by a tandem NO+ -initiated semipinacol rearrangement and H-elimination. Angew. Chem. Int. Ed. 2018;57:13192–13196. doi: 10.1002/anie.201807861. [DOI] [PubMed] [Google Scholar]
- 35.Brandes S, Bella M, Kjoersgaard A, Jørgensen KA. Chirally aminated 2-naphthols—organocatalytic synthesis of non-biaryl atropisomers by asymmetric Friedel-Crafts amination. Angew. Chem. Int. Ed. 2006;45:1147–1151. doi: 10.1002/anie.200503042. [DOI] [PubMed] [Google Scholar]
- 36.Guo R, et al. Asymmetric synthesis of heteroaryl atropisomers via a gold-catalyzed cycloisomerization–amination cascade reaction. Chem. Commun. 2014;50:5451–5454. doi: 10.1039/C4CC01397B. [DOI] [PubMed] [Google Scholar]
- 37.Akiyama T, Itoh J, Yokota K, Fuchibe K. Enantioselective Mannich-type reaction catalyzed by a chiral brønsted acid. Angew. Chem. Int. Ed. 2004;43:1566–1568. doi: 10.1002/anie.200353240. [DOI] [PubMed] [Google Scholar]
- 38.Uraguchi D, Terada M. Chiral brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 2004;126:5356–5357. doi: 10.1021/ja0491533. [DOI] [PubMed] [Google Scholar]
- 39.Ding K-L, Li X, Ji B-M, Guo H-C, Kitamura M. Ten years of research on NOBIN chemistry. Curr. Org. Synth. 2005;2:499–545. doi: 10.2174/157017905774322631. [DOI] [Google Scholar]
- 40.Ding K-L, Guo H-C, Li X, Yuan Y, Wang Y. Synthesis of NOBIN derivatives for asymmetric catalysis. Top. Catal. 2005;35:105–116. doi: 10.1007/s11244-005-3816-2. [DOI] [Google Scholar]
- 41.Vallavoju N, Selvakumar S, Jockusch S, Sibi M, Sivaguru PJ. Enantioselective organo-photocatalysis mediated by atropisomeric thiourea derivatives. Angew. Chem. Int. Ed. 2014;53:5604–5608. doi: 10.1002/anie.201310940. [DOI] [PubMed] [Google Scholar]
- 42.De CK, Pesciaioli F, List B. Catalytic asymmetric benzidine rearrangement. Angew. Chem. Int. Ed. 2013;52:9293–9295. doi: 10.1002/anie.201304039. [DOI] [PubMed] [Google Scholar]
- 43.Shirakawa S, Wu X, Maruoka K. Kinetic resolution of axially chiral 2-amino-1,1’-biaryls by phase-transfer-catalyzed N-allylation. Angew. Chem. Int. Ed. 2013;52:14200–14203. doi: 10.1002/anie.201308237. [DOI] [PubMed] [Google Scholar]
- 44.Li G-Q, et al. Organocatalytic aryl-aryl bond formation: An atroposelective [3,3]-rearrangement approach to BINAM derivatives. J. Am. Chem. Soc. 2013;135:7414–7417. doi: 10.1021/ja401709k. [DOI] [PubMed] [Google Scholar]
- 45.Cheng D-J, et al. Highly enantioselective kinetic resolution of axially chiral BINAM derivatives catalyzed by a brønsted acid. Angew. Chem. Int. Ed. 2014;53:3684–3687. doi: 10.1002/anie.201310562. [DOI] [PubMed] [Google Scholar]
- 46.Qi L-W, Mao J-H, Zhang J, Tan B. Organocatalytic asymmetric arylation of indoles enabled by azo groups. Nat. Chem. 2017;10:58–64. doi: 10.1038/nchem.2866. [DOI] [PubMed] [Google Scholar]
- 47.Ma C, Zhou J-Y, Zhang Y-Z, Mei G-J, Shi F. Catalytic asymmetric [2 + 3] cyclizations of azlactones with azonaphthalenes. Angew. Chem. Int. Ed. 2018;57:5398–5402. doi: 10.1002/anie.201801349. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y-H, Qi L-W, Fang F, Tan B. Organocatalytic atroposelective arylation of 2-naphthylamines as a practical approach to axially chiral biaryl amino alcohols. Angew. Chem. Int. Ed. 2017;56:16308–16312. doi: 10.1002/anie.201710537. [DOI] [PubMed] [Google Scholar]
- 49.Meyer EA, Castellano RK, Diederich F. Interactions with aromatic rings in chemical and biological recognition. Angew. Chem. Int. Ed. 2003;42:1210–1250. doi: 10.1002/anie.200390319. [DOI] [PubMed] [Google Scholar]
- 50.Neel AJ, Hilton MJ, Sigman MS, Toste FD. Exploiting non-covalent π interactions for catalyst design. Nature. 2017;543:637–646. doi: 10.1038/nature21701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lv J, Luo S. Asymmetric binary acid catalysis: chiral phosphoric acid as dual ligand and acid. Chem. Commun. 2013;49:847–858. doi: 10.1039/C2CC34288J. [DOI] [PubMed] [Google Scholar]
- 52.Mori K, Itakura T, Akiyama T. Enantiodivergent atroposelective synthesis of chiral biaryls by asymmetric transfer hydrogenation: chiral phosphoric acid catalyzed dynamic kinetic resolution. Angew. Chem. Int. Ed. 2016;55:11642–11646. doi: 10.1002/anie.201606063. [DOI] [PubMed] [Google Scholar]
- 53.Li S, Zhang J-W, Li X-L, Cheng D-J, Tan B. Phosphoric acid-catalyzed asymmetric synthesis of SPINOL derivatives. J. Am. Chem. Soc. 2016;138:16561–16566. doi: 10.1021/jacs.6b11435. [DOI] [PubMed] [Google Scholar]
- 54.Raut VS, et al. Enantioselective syntheses of furan atropisomers by an oxidative central-to-axial chirality conversion strategy. J. Am. Chem. Soc. 2017;139:2140–2143. doi: 10.1021/jacs.6b11079. [DOI] [PubMed] [Google Scholar]
- 55.Kawabata T, et al. Axially chiral binaphthyl surrogates with an inner N-H-N hydrogen bond. J. Am. Chem. Soc. 2009;131:54–55. doi: 10.1021/ja808213r. [DOI] [PubMed] [Google Scholar]
- 56.Hayashi K, et al. Insights into the origins of configurational stability of axially chiral biaryl amines with an intramolecular N−H−N hydrogen bond. J. Org. Chem. 2010;75:5031–5036. doi: 10.1021/jo100586b. [DOI] [PubMed] [Google Scholar]
- 57.Butt NA, Zhang W. Transition metal-catalyzed allylic substitution reactions with unactivated allylic substrates. Chem. Soc. Rev. 2015;44:7929–7967. doi: 10.1039/C5CS00144G. [DOI] [PubMed] [Google Scholar]
- 58.Wright TB, Evans PA. Enantioselective rhodium-catalyzed allylic alkylation of prochiral α,α-disubstituted aldehyde enolates for the construction of acyclic quaternary stereogenic centers. J. Am. Chem. Soc. 2016;138:15303–15306. doi: 10.1021/jacs.6b10099. [DOI] [PubMed] [Google Scholar]
- 59.Tang S-B, Zhang X, Tu H-F, You S-L. Regio- and enantioselective rhodium-catalyzed allylic alkylation of racemic allylic alcohols with 1,3-diketones. J. Am. Chem. Soc. 2018;140:7737–7742. doi: 10.1021/jacs.8b05126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors on request (DOI: 10.6084/m9.figshare.8131271). The X-ray crystallographic coordinates for structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 1875011[https://www.ccdc.cam.ac.uk/structures/Search?Ccdcid=1875011&DatabaseToSearch=Published]. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.