Abstract
We aimed to identify independent predictors of cardiac mortality and hospitalization for heart failure (HHF) from a real-world, multi-ethnic Asian registry [the Singapore Myocardial Infarction Registry] of ST-segment elevation myocardial infarction (STEMI) patients treated by primary percutaneous coronary intervention. 11,546 eligible STEMI patients between 2008 and 2015 were identified. In-hospital, 30-day and 1-year cardiac mortality and 1-year HHF rates were 6.4%, 6.8%, 8.3% and 5.2%, respectively. From the derivation cohort (70% of patients), age, Killip class and cardiac arrest, creatinine, hemoglobin and troponin on admission and left ventricular ejection fraction (LVEF) during hospitalization were predictors of in-hospital, 30-day and 1-year cardiac mortality. Previous ischemic heart disease (IHD) was a predictor of in-hospital and 30-day cardiac mortality only, whereas diabetes was a predictor of 1-year cardiac mortality only. Age, previous IHD and diabetes, Killip class, creatinine, hemoglobin and troponin on admission, symptom-to-balloon-time and LVEF were predictors of 1-year HHF. The c-statistics were 0.921, 0.901, 0.881, 0.869, respectively. Applying these models to the validation cohort (30% of patients) showed good fit and discrimination (c-statistic 0.922, 0.913, 0.903 and 0.855 respectively; misclassification rate 14.0%, 14.7%, 16.2% and 24.0% respectively). These predictors could be incorporated into specific risk scores to stratify reperfused STEMI patients by their risk level for targeted intervention.
Subject terms: Interventional cardiology, Risk factors
Introduction
Despite prompt reperfusion of ST-segment elevation myocardial infarction (STEMI) by primary percutaneous coronary intervention (PPCI), morbidity and mortality remain significant1,2. However, not all STEMI patients have the same prognosis. Those in the low-risk category have excellent prognosis3, whereas those with high-risk features have significantly worse outcomes with the risk of all-cause mortality reaching up to 35% at 30-day in those in the highest risk category4. Therefore early risk-stratification of STEMI patients is important to guide in-patient management and follow-up in order to improve clinical outcomes.
There are several risk scores available which can be used to risk-stratify STEMI patients at the time of hospitalization, and guide management5. The most extensively investigated, and validated risk scores are the Global Registry of Acute Coronary Events (GRACE)6,7, and the Thrombolysis in Myocardial Infarction (TIMI)4 risk scores, with the GRACE score performing better than the TIMI risk score in a recent meta-analysis8. The GRACE score can be used for all types of acute coronary syndromes (ACS), and can predict in-hospital and 6-month mortality. On the other hand, there is a specific TIMI risk score for STEMI4 to predict 30-day mortality. However, the derivation and validation cohorts for these risk scores consisted predominantly of non-Asian patients treated by thrombolysis.
About 60% of the world population resides in the Asia-Pacific region1 and Asians are known to have a different and higher cardiovascular risk profile than the western population9. However, there has been limited research conducted in the ACS population in this region so far due to factors such as language and cultural barriers, potential ethical issues, and differences in regulatory processes in individual Asian countries10, but efforts are under way with the establishment of the Asia-Pacific Real world evIdenCe on Outcome and Treatment of ACS (APRICOT) project1.
Singapore is a multi-ethnic country with Chinese (≈74%), Malays (≈13%) and Indians (≈9%) accounting for the majority of the population. It has a state-funded, mandatory, acute myocardial infarction (AMI) registry, called the Singapore Myocardial Infarction Registry (SMIR)11, with a dedicated team to collect data from all hospitals. We used this unbiased, real-world registry to determine independent variables on admission that would predict cardiac mortality during hospitalization, at 30-day and 1-year and 1-year hospitalization for heart failure (HHF) in patients presenting with a STEMI and reperfused by PPCI. These variables would subsequently be incorporated in risk scores to stratify reperfused STEMI patients by their risk level for targeted intervention early during hospitalization.
Results
Study population and baseline characteristics
A total of 11,546 STEMI patients reperfused by PPCI from 2008 to 2015 were identified in the SMIR. The overall cardiac mortality rate during hospitalization was 6.4% (741/11,546) and was 6.8% (780/11,546) at 30 days and 8.3% (956/11,546) at 1 year. Data on 1-year HHF was available from 2008 to 2013 only (n = 7,446). The 1-year HHF rate was 5.4% (399/7,446).
There were 8,082 reperfused STEMI patients in the derivation cohort. The median age was 57.6 (50.7–66.1) years old and 85.2% were male. The majority were Chinese (61.4%), followed by Malays (20.1%) and Indians (16.8%). Around a third of the cohort had a history of diabetes (28.1%) and half had a history of hypertension (52.2%), dyslipidemia (46.0%) and were current smokers (49.1%). 4.0% suffered a cardiac arrest in the ambulance or on admission to the emergency department and 8.7% were in cardiogenic shock at presentation. Half of the patients presented with an anterior STEMI (49.8%), and half had an abnormal initial troponin T or I (48.7%). The median symptom-to-balloon (S2B) time was 183 (120–310) minutes and the door-to-balloon (D2B) time was 65 (50–88) minutes.
There were 3,464 reperfused STEMI patients in the validation cohort. There was no significant difference in baseline characteristics between the derivation cohort and the validation cohort as summarized in Table 1 except for body mass index.
Table 1.
Baseline characteristics in the derivation and validation cohort.
| Derivation cohort (n = 8082) | Validation cohort (n = 3464) | P | |
|---|---|---|---|
| Age in years, median (IQR) | 57.6 (50.7–66.1) | 57.9 (50.9–66.0) | 0.536 |
| Age group, n (%) | 0.597 | ||
| <40 | 331 (4.1) | 148 (4.3) | |
| 40–59 | 4369 (54.1) | 1833 (52.9) | |
| 60–69 | 1952 (24.2) | 857 (24.7) | |
| 70–79 | 1016 (12.6) | 461 (13.3) | |
| > = 80 | 414 (5.1) | 165 (4.8) | |
| Gender, n (%) | 0.361 | ||
| Male | 6885 (85.2) | 2928 (84.5) | |
| Female | 1197 (14.8) | 536 (15.5) | |
| Ethnicity, n (%) | 0.072 | ||
| Chinese | 4960 (61.4) | 2167 (62.6) | |
| Malay | 1622 (20.1) | 722 (20.8) | |
| Indian | 1360 (16.8) | 515 (14.9) | |
| Others | 140 (1.7) | 60 (1.7) | |
| History of diabetes, n (%) | 2273 (28.1) | 980 (28.3) | 0.853 |
| History of hypertension, n (%) | 4220 (52.2) | 1796 (51.9) | 0.72 |
| History of dyslipidemia, n (%) | 3712 (46.0) | 1588 (45.9) | 0.902 |
| History of IHD, n (%) | 1212 (15.0) | 532 (15.4) | 0.63 |
| Current smoker, n (%) | 3926 (49.1) | 1718 (50.1) | 0.34 |
| BMI in kg/m2, median (IQR) | 24.6 (22.4–27.3) | 24.4 (22.2–27.1) | 0.034 |
| BMI group, n (%) | 0.041 | ||
| < = 23 | 2255 (30.9) | 1032 (32.9) | |
| >23 | 5041 (69.1) | 2101 (67.1) | |
| Killip class on admission, n (%) | 0.297 | ||
| I | 6703 (83.0) | 2849 (82.3) | |
| II | 385 (4.8) | 158 (4.6) | |
| III | 288 (3.6) | 148 (4.3) | |
| IV | 705 (8.7) | 309 (8.9) | |
| Cardiac arrest in ambulance/on admission, n (%) | 319 (4.0) | 155 (4.5) | 0.19 |
| Anterior STEMI on admission, n (%) | 4022 (49.8) | 1736 (50.1) | 0.73 |
| Creatinine on admission in µmol/L, median (IQR) | 90 (76–109) | 90 (77–109) | 0.718 |
| Creatinine group, n (%) | 0.615 | ||
| <70 | 1177 (14.6) | 519 (15.1) | |
| 70–105 | 4622 (57.3) | 1944 (56.5) | |
| 106–140 | 1501 (18.6) | 675 (19.6) | |
| 141–176 | 364 (4.5) | 152 (4.4) | |
| 177–353 | 269 (3.3) | 100 (2.9) | |
| > = 354 | 135 (1.7) | 54 (1.6) | |
| Hemoglobin on admission in g/dL, median (IQR) | 14.6 (13.5–15.7) | 14.7 (13.4–15.7) | 0.58 |
| Hemoglobin group, n (%) | 0.633 | ||
| <10 | 185 (2.3) | 87 (2.5) | |
| 10–11 | 547 (6.8) | 258 (7.5) | |
| 12–13 | 2031 (25.2) | 863 (25.1) | |
| 14–16 | 3693 (45.8) | 1563 (45.4) | |
| > = 17 | 1609 (20.0) | 674 (19.6) | |
| Elevated first troponin T/I on admission, n (%) | 3938 (48.7) | 1649 (47.6) | 0.269 |
| Blood sugar within 72 h from STEMI onset in mmol/L, median (IQR) | 8.5 (6.7–12.2) | 8.4 (6.7–12.1) | 0.287 |
| Total cholesterol within 72 h from STEMI onset in mmol/L, median (IQR) | 5.1 (4.3–6.0) | 5.1 (4.3–6.0) | 0.514 |
| HDL cholesterol within 72 h from STEMI onset in mmol/L, median (IQR) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 0.431 |
| LDL cholesterol within 72 h from STEMI onset in mmol/L, median (IQR) | 3.4 (2.6–4.1) | 3.3 (2.6–4.1) | 0.264 |
| Triglyceride within 72 h from STEMI onset in mmol/L, median (IQR) | 1.4 (1.0–2.0) | 1.4 (1.0–2.0) | 0.98 |
| HbA1c on admission in %, median (IQR) | 6.1 (5.7–7.6) | 6.1 (5.7–7.7) | 0.97 |
| S2B time in minutes, median (IQR) | 183 (120–310) | 180 (119–298) | 0.118 |
| S2B time group, n (%) | 0.205 | ||
| < = 180 | 3738 (49.2) | 1654 (50.5) | |
| >180 | 3865 (50.8) | 1622 (49.5) | |
| D2B in minutes, median (IQR) | 65 (50–88) | 64 (49–87) | 0.435 |
| Lowest LVEF during hospitalization in %, median (IQR) | 45 (35–55) | 45 (35–55) | 0.874 |
| LVEF group, n (%) | 0.372 | ||
| <40 | 2244 (29.5) | 963 (30.0) | |
| 40–50 | 3153 (41.5) | 1286 (40.1) | |
| >50 | 2201 (29.0) | 960 (29.9) | |
| Aspirin given during hospitalization, n (%) | 7710 (95.4) | 3278 (94.6) | 0.078 |
| Beta blocker given during hospitalization, n (%) | 6793 (84.1) | 2854 (82.4) | 0.027 |
| Lipid lowering therapy/statin given during hospitalization, n (%) | 7710 (95.4) | 3282 (94.8) | 0.134 |
| ACEI/ARB given during hospitalization, n (%) | 5932 (73.4) | 2537 (73.2) | 0.86 |
| Other anti-platelet given during hospitalization, n (%) | 7800 (96.5) | 3327 (96.1) | 0.22 |
| Aspirin given at discharge among those discharged alive, n (%) | 7323 (97.1) | 3102 (97.0) | 0.935 |
| Beta blocker given at discharge among those discharged alive, n (%) | 6592 (87.4) | 2766 (86.5) | 0.229 |
| Lipid lowering therapy/statin given at discharge among those discharged alive, n (%) | 7377 (97.8) | 3141 (98.3) | 0.115 |
| ACEI/ARB given at discharge among those discharged alive, n (%) | 5568 (73.8) | 2390 (74.8) | 0.299 |
| Other anti-platelet given at discharge among those discharged alive, n (%) | 7405 (98.1) | 3150 (98.5) | 0.163 |
IQR: interquartile range; IHD: ischemic heart disease; BMI: body mass index; STEMI: ST-segment elevation myocardial infarction; S2B: symptom-to-balloon; D2B: door-to-balloon; LVEF: left ventricular ejection fraction; ACE: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker.
Predictors of in-hospital, 30-day and 1-year cardiac mortality
Variables included in the univariable logistic regression are summarized in the Table 2. Variables included in the multivariable logistic regression are summarized in Table 3. Age, Killip class, cardiac arrest, creatinine, hemoglobin and troponin on admission and left ventricular ejection fraction (LVEF) during hospitalization were predictors of cardiac mortality at all 3 time points (in-hospital, 30-day and 1 year). Previous ischemic heart disease (IHD) - defined as a history of previous myocardial infarction, PCI or coronary artery bypass graft surgery - was a predictor of in-hospital and 30-day cardiac mortality only, whereas diabetes was a predictor of 1-year cardiac mortality only.
Table 2.
Univariable regression model for prediction of cardiac mortality based on the derivation cohort.
| In-hospital cardiac mortality | 30-day cardiac mortality | 1-year cardiac mortality | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95%CI) | P | Odds Ratio (95%CI) | P | Odds Ratio (95%CI) | P | |
| Age (years) | <0.001 | <0.001 | <0.001 | |||
| <40 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| 40–59 | 2.95 (1.08–8.00) | 2.64 (1.08–6.47) | 3.21 (1.31–7.85) | |||
| 60–69 | 5.21 (1.91–14.22) | 4.27 (1.73–10.53) | 5.86 (2.39–14.38) | |||
| 70–79 | 12.53 (4.60–34.14) | 10.59 (4.30–26.08) | 14.04 (5.72–34.45) | |||
| > = 80 | 21.75 (7.89–59.95) | 18.11 (7.26–45.15) | 23.59 (9.50–58.60) | |||
| Gender | <0.001 | <0.001 | <0.001 | |||
| Male | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Female | 2.52 (2.05–3.09) | 2.42 (1.98–2.96) | 2.48 (2.07–2.98) | |||
| Ethnicity | 0.105 | 0.105 | 0.072 | |||
| Chinese | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Malay | 1.01 (0.80–1.27) | 1.05 (0.84–1.31) | 1.09 (0.90–1.33) | |||
| Indian | 0.77 (0.59–1.01) | 0.77 (0.59–1.01) | 0.77 (0.61–0.98) | |||
| Others | 1.52 (0.85–2.73) | 1.44 (0.80–2.57) | 1.23 (0.70–2.15) | |||
| History of diabetes | <0.001 | <0.001 | <0.001 | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 2.10 (1.74–2.52) | 1.98 (1.66–2.37) | 2.18 (1.86–2.57) | |||
| History of hypertension | <0.001 | <0.001 | <0.001 | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 1.75 (1.45–2.12) | 1.75 (1.45–2.10) | 2.01 (1.70–2.38) | |||
| History of dyslipidemia | 0.168 | 0.316 | 0.002 | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 1.14 (0.95–1.36) | 1.09 (0.92–1.31) | 1.28 (1.09–1.50) | |||
| History of IHD | 0.014 | 0.011 | <0.001 | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 1.34 (1.06–1.70) | 1.34 (1.07–1.69) | 1.75 (1.44–2.12) | |||
| Current smoker | <0.001 | <0.001 | <0.001 | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 0.53 (0.43–0.64) | 0.61 (0.50–0.74) | 0.59 (0.49–0.70) | |||
| BMI (kg/m2) | 0.057 | 0.026 | 0.016 | |||
| < = 23 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| >23 | 0.80 (0.63–1.01) | 0.78 (0.62–0.97) | 0.79 (0.65–0.96) | |||
| Killip class on admission | <0.001 | <0.001 | <0.001 | |||
| I | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| II | 2.12 (1.36–3.31) | 2.33 (1.55–3.51) | 2.27 (1.59–3.25) | |||
| III | 7.88 (5.68–10.92) | 7.66 (5.59–10.52) | 7.52 (5.64–10.02) | |||
| IV | 15.24 (12.31–18.87) | 13.58 (11.03–16.73) | 11.58 (9.55–14.05) | |||
| Cardiac arrest in ambulance/on admission | <0.001 | <0.001 | <0.001 | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 12.87 (10.05–16.50) | 11.07 (8.65–14.18) | 9.37 (7.37–11.91) | |||
| Anterior STEMI | 0.001 | <0.001 | <0.001 | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 1.37 (1.14–1.65) | 1.44 (1.21–1.72) | 1.37 (1.16–1.60) | |||
| Creatinine on admission (µmol/L) | <0.001 | <0.001 | <0.001 | |||
| <70 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| 70–105 | 1.26 (0.83–1.92) | 1.23 (0.83–1.82) | 1.11 (0.80–1.55) | |||
| 106–140 | 4.83 (3.19–7.33) | 4.57 (3.09–6.75) | 3.82 (2.74–5.33) | |||
| 141–176 | 10.87 (6.87–17.20) | 9.59 (6.19–14.87) | 8.02 (5.47–11.76) | |||
| 177–353 | 17.71 (11.15–28.14) | 15.10 (9.69–23.52) | 13.08 (8.85–19.31) | |||
| > = 354 | 9.68 (5.43–17.26) | 7.99 (4.53–14.10) | 9.50 (5.87–15.37) | |||
| Hemoglobin on admission (g/dL) | <0.001 | <0.001 | <0.001 | |||
| <10 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| 11–11 | 0.86 (0.57–1.31) | 0.84 (0.55–1.27) | 0.78 (0.53–1.15) | |||
| 12–13 | 0.36 (0.24–0.53) | 0.34 (0.23–0.50) | 0.32 (0.23–0.46) | |||
| 14–16 | 0.16 (0.11–0.23) | 0.16 (0.11–0.23) | 0.14 (0.10–0.21) | |||
| > = 17 | 0.14 (0.09–0.21) | 0.16 (0.11–0.25) | 0.14 (0.10–0.21) | |||
| Elevated first troponin T/I on admission | <0.001 | <0.001 | <0.001 | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 2.08 (1.72–2.52) | 2.11 (1.75–2.54) | 2.15 (1.82–2.54) | |||
| S2B (minutes) | 0.534 | 0.287 | 0.178 | |||
| < = 180 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| >180 | 1.06 (0.88–1.29) | 1.11 (0.92–1.34) | 1.12 (0.95–1.33) | |||
| Lowest LVEF during hospitalization (%) | <0.001 | <0.001 | <0.001 | |||
| <40 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| 40–50 | 0.12 (0.09–0.17) | 0.14 (0.11–0.19) | 0.15 (0.12–0.19) | |||
| >50 | 0.10 (0.07–0.15) | 0.10 (0.06–0.14) | 0.12 (0.09–0.16) | |||
CI: confidence interval; IHD: ischemic heart disease; BMI: body mass index; STEMI: ST-segment elevation myocardial infarction; S2B: symptom-to-balloon; LVEF: left ventricular ejection fraction.
Table 3.
Multivariable regression model for prediction of cardiac mortality based on the derivation cohort.
| In-hospital cardiac mortality | 30-day cardiac mortality | 1-year cardiac mortality | ||||
|---|---|---|---|---|---|---|
| Odds Ratio (95%CI) | P | Odds Ratio (95%CI) | P | Odds Ratio (95%CI) | P | |
| Age (years) | <0.001 | <0.001 | <0.001 | |||
| <40 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| 40–59 | 4.26 (0.58–31.41) | 2.91 (0.70–12.16) | 3.84 (0.93–15.93) | |||
| 60–69 | 5.92 (0.80–44.08) | 3.42 (0.81–14.48) | 5.55 (1.33–23.20) | |||
| 70–79 | 9.46 (1.26–70.99) | 5.94 (1.39–25.39) | 9.57 (2.27–40.33) | |||
| > = 80 | 18.48 (2.44–140.17) | 10.71 (2.47–46.46) | 17.26 (4.04–73.63) | |||
| Gender | Not significant | Not significant | Not significant | |||
| Male | ||||||
| Female | ||||||
| History of diabetes | Not significant | Not significant | 0.014 | |||
| No | 1.00 (reference) | |||||
| Yes | 1.33 (1.06–1.67) | |||||
| History of hypertension | Not significant | Not significant | Not significant | |||
| No | ||||||
| Yes | ||||||
| History of dyslipidemia | NA | NA | Not significant | |||
| No | ||||||
| Yes | ||||||
| History of IHD | 0.001 | 0.006 | Not significant | |||
| No | 1.00 (reference) | 1.00 (reference) | ||||
| Yes | 0.55 (0.38–0.80) | 0.62 (0.44–0.87) | ||||
| Current smoker | Not significant | Not significant | Not significant | |||
| No | ||||||
| Yes | ||||||
|
BMI (kg/m2) < = 23 >23 |
Not significant | Not significant | Not significant | |||
| Killip class on admission | <0.001 | <0.001 | <0.001 | |||
| I | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| II | 1.07 (0.59–1.93) | 1.20 (0.71–2.03) | 1.13 (0.73–1.76) | |||
| III | 2.77 (1.79–4.31) | 2.70 (1.79–4.09) | 2.42 (1.68–3.48) | |||
| IV | 4.24 (3.11–5.77) | 3.75 (2.79–5.05) | 3.27 (2.50–4.27) | |||
| Cardiac arrest in ambulance/on admission | <0.001 | <0.001 | <0.001 | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 6.61 (4.52–9.65) | 5.09 (3.52–7.37) | 4.55 (3.20–6.46) | |||
| Anterior STEMI | Not significant | Not significant | Not significant | |||
| No | ||||||
| Yes | ||||||
| Creatinine on admission (µmol/L) | <0.001 | <0.001 | <0.001 | |||
| <70 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| 70–105 | 1.26 (0.73–2.16) | 1.19 (0.73–1.95) | 1.11 (0.75–1.64) | |||
| 106–140 | 2.36 (1.36–4.09) | 2.29 (1.39–3.77) | 1.92 (1.28–2.89) | |||
| 141–176 | 3.74 (2.01–6.96) | 3.16 (1.77–5.62) | 2.55 (1.57–4.13) | |||
| 177–353 | 5.04 (2.67–9.52) | 4.08 (2.26–7.38) | 3.25 (1.97–5.38) | |||
| > = 354 | 4.37 (1.97–9.70) | 2.94 (1.36–6.38) | 3.79 (2.05–6.99) | |||
| Hemoglobin on admission (g/dL) | <0.001 | 0.001 | <0.001 | |||
| <10 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| 10–11 | 1.94 (1.05–3.59) | 1.57 (0.88–2.80) | 1.47 (0.87–2.46) | |||
| 12–13 | 1.13 (0.62–2.06) | 0.87 (0.50–1.53) | 0.96 (0.59–1.59) | |||
| 14–16 | 0.73 (0.39–1.35) | 0.59 (0.33–1.05) | 0.67 (0.40–1.12) | |||
| > = 17 | 0.77 (0.38–1.56) | 0.81 (0.43–1.54) | 0.89 (0.50–1.57) | |||
| Elevated first troponin T/I on admission | <0.001 | <0.001 | <0.001 | |||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| Yes | 1.69 (1.27–2.25) | 1.76 (1.35–2.30) | 1.68 (1.34–2.12) | |||
|
S2B (minutes) < = 180 >180 |
Not significant | Not significant | Not significant | |||
| Lowest LVEF during hospitalization (%) | <0.001 | <0.001 | <0.001 | |||
| <40 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |||
| 40–50 | 0.21 (0.15–0.30) | 0.24 (0.18–0.33) | 0.25 (0.19–0.32) | |||
| >50 | 0.25 (0.16–0.38) | 0.22 (0.14–0.34) | 0.27 (0.19–0.37) | |||
CI: confidence interval; IHD: ischemic heart disease; BMI: body mass index; STEMI: ST-segment elevation myocardial infarction; S2B: symptom-to-balloon; LVEF: left ventricular ejection fraction; NA: not applicable.
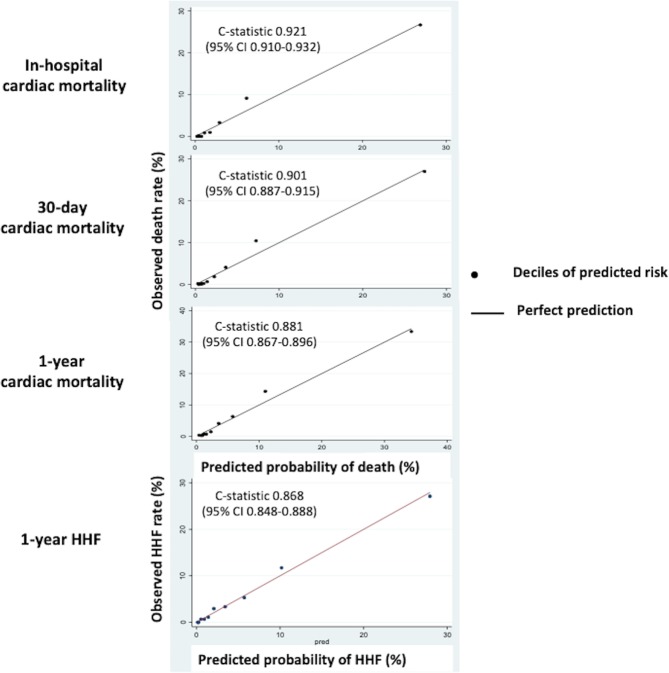
Calibration of the predictions from these models was assessed by comparing the average predictions to the actual cardiac mortality across deciles and was found to be excellent as shown in Fig. 1. Internal validation showed good fit of the models with a c-statistic of 0.921 (95%CI 0.910–0.932) for in-hospital cardiac mortality, 0.901 (95%CI 0.887–0.915) for 30-day cardiac mortality and 0.881 (95%CI 0.867–0.896) for 1-year cardiac mortality with bootstrapping techniques.
Figure 1.
Internal validation of the models in the derivation cohort. There was good calibration of the models for in-hospital, 30-day and 1-year cardiac death and 1-year HHF with c-statistic of 0.921, 0.901, 0.881 and 0.868 respectively.
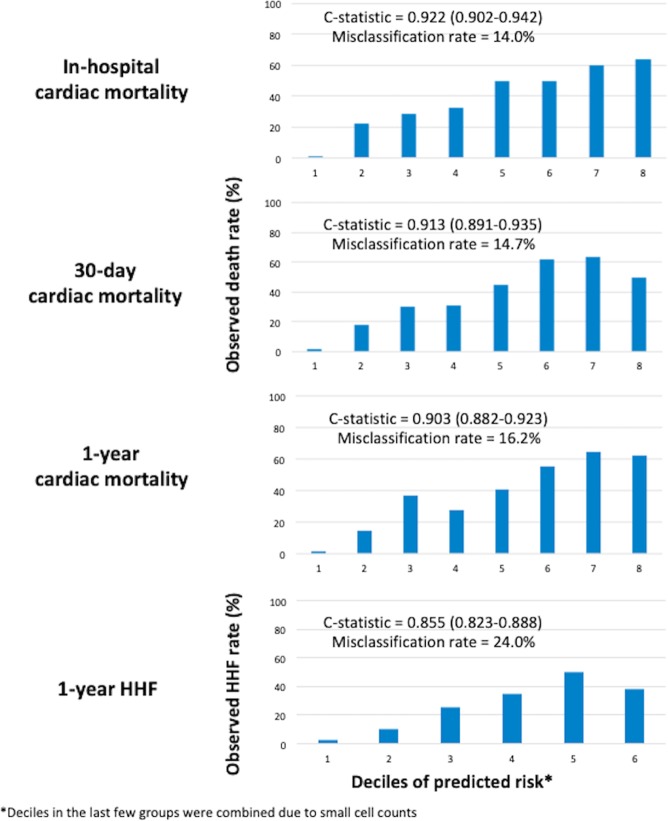
External validation was performed in the validation cohort of 3,464 patients. The model performed very well as shown in Fig. 2 with a c-statistic of 0.922 (95%CI 0.902–0.942) for in-hospital cardiac mortality, 0.913 (95%CI 0.891–0.935) for 30-day cardiac mortality and 0.903 (95%CI 0.882–0.923) for 1-year cardiac mortality. The misclassification rate for in-hospital, 30-day and 1-year cardiac mortality was 14.0%, 14.7% and 16.2% respectively.
Figure 2.
External validation of the models in the validation cohort. There was good discrimination of the models for in-hospital, 30-day and 1-year cardiac death and 1-year HHF with c-statistic of 0.922, 0.913, 0.903 and 0.855 respectively.
Predictors of 1-year hospitalization for heart failure
The derivation cohort consisted of 5,395 reperfused STEMI patients and the validation cohort consisted of 2,280 reperfused STEMI patients.
Age, previous IHD and diabetes, Killip class, creatinine, hemoglobin and troponin on admission, S2B time and LVEF were predictors of 1-year HHF (Table 4).
Table 4.
Univariable and multivariable regression model for prediction of heart failure hospitalization based on the derivation cohort.
| Heart failure hospitalization | Heart failure hospitalization | |||
|---|---|---|---|---|
| Odds Ratio (95%CI) | P | Odds Ratio (95%CI) | P | |
| Age (years) | <0.001 | 0.025 | ||
| <40 | 1.00 (reference) | 1.00 (reference) | ||
| 40–59 | 1.83 (0.74–4.54) | 1.93 (0.67–5.58) | ||
| 60–69 | 3.55 (1.42–8.84) | 2.49 (0.84–7.33) | ||
| 70–79 | 4.67 (1.84–11.82) | 2.46 (0.80–7.53) | ||
| > = 80 | 8.40 (3.18–22.15) | 4.38 (1.37–14.04) | ||
| Gender | <0.001 | Not significant | ||
| Male | 1.00 (reference) | |||
| Female | 2.28 (1.72–3.02) | |||
| Ethnicity | 0.095 | NA | ||
| Chinese | 1.00 (reference) | |||
| Malay | 1.40 (1.05–1.88) | |||
| Indian | 1.24 (0.90–1.70) | |||
| Others | 0.70 (0.22–2.24) | |||
| History of diabetes | <0.001 | <0.001 | ||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 3.33 (2.62–4.24) | 2.07 (1.54–2.77) | ||
| History of hypertension | <0.001 | Not significant | ||
| No | 1.00 (reference) | |||
| Yes | 2.73 (2.08–3.57) | |||
| History of dyslipidemia | <0.001 | Not significant | ||
| No | 1.00 (reference) | |||
| Yes | 2.02 (1.58–2.59) | |||
| History of IHD | <0.001 | <0.001 | ||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 3.14 (2.42–4.08) | 2.14 (1.56–2.94) | ||
| Current smoker | 0.008 | Not significant | ||
| No | 1.00 (reference) | |||
| Yes | 0.72 (0.57–0.92) | |||
| BMI (kg/m2) | 0.32 | NA | ||
| < = 23 | 1.00 (reference) | |||
| >23 | 0.88 (0.67–1.14) | |||
| Killip class on admission | <0.001 | <0.001 | ||
| I | 1.00 (reference) | 1.00 (reference) | ||
| II | 4.70 (3.23–6.84) | 2.85 (1.84–4.41) | ||
| III | 5.66 (3.57–8.96) | 1.95 (1.10–3.47) | ||
| IV | 5.20 (3.72–7.27) | 2.72 (1.81–4.08) | ||
| Cardiac arrest in ambulance/on admission | 0.344 | NA | ||
| No | 1.00 (reference) | |||
| Yes | 1.39 (0.70–2.78) | |||
| Anterior STEMI | <0.001 | Not significant | ||
| No | 1.00 (reference) | |||
| Yes | 1.83 (1.43–2.34) | |||
| Creatinine on admission (µmol/L) | <0.001 | <0.001 | ||
| <70 | 1.00 (reference) | 1.00 (reference) | ||
| 70–105 | 0.89 (0.60–1.30) | 1.15 (0.73–1.82) | ||
| 106–140 | 1.75 (1.14–2.67) | 1.63 (0.98–2.73) | ||
| 141–176 | 3.97 (2.35–6.71) | 3.20 (1.71–6.02) | ||
| 177–353 | 6.46 (3.79–11.02) | 2.50 (1.24–5.03) | ||
| > = 354 | 0.37 (0.05–2.74) | 0.15 (0.02–1.31) | ||
| Hemoglobin on admission (g/dL) | <0.001 | 0.003 | ||
| <10 | 1.00 (reference) | 1.00 (reference) | ||
| 10–11 | 1.25 (0.60–2.60) | 1.57 (0.57–4.28) | ||
| 12–13 | 0.52 (0.26–1.04) | 0.91 (0.34–2.39) | ||
| 14–16 | 0.28 (0.14–0.56) | 0.62 (0.23–1.63) | ||
| > = 17 | 0.31 (0.15–0.64) | 0.93 (0.34–2.56) | ||
| Elevated first troponin T/I on admission | <0.001 | 0.049 | ||
| No | 1.00 (reference) | 1.00 (reference) | ||
| Yes | 2.26 (1.77–2.90) | 1.35 (1.00–1.81) | ||
| S2B (minutes) | <0.001 | 0.003 | ||
| < = 1800 | 1.00 (reference) | 1.00 (reference) | ||
| >18 | 1.70 (1.31–2.20) | 1.57 (1.16–2.12) | ||
| Lowest LVEF during hospitalization (%) | <0.001 | <0.001 | ||
| <40 | 1.00 (reference) | 1.00 (reference) | ||
| 40–50 | 0.17 (0.13–0.23) | 0.25 (0.18–0.34) | ||
| >50 | 0.03 (0.02–0.06) | 0.04 (0.02–0.10) | ||
CI: confidence interval; IHD: ischemic heart disease; BMI: body mass index; STEMI: ST-segment elevation myocardial infarction; S2B: symptom-to-balloon; LVEF: left ventricular ejection fraction; NA: not applicable
Calibration of the predictions from this model was assessed by comparing the average predictions to the actual HHF across deciles and was found to be excellent (Fig. 1). Internal validation showed good fit of the models with a c-statistic of 0.868 (95%CI 0.848–0.888) for 1-year HHF.
External validation showed that the model performed very well with a c-statistic of 0.855 (95%CI 0.823–0.888), with misclassification rate of 24.0% (Fig. 2).
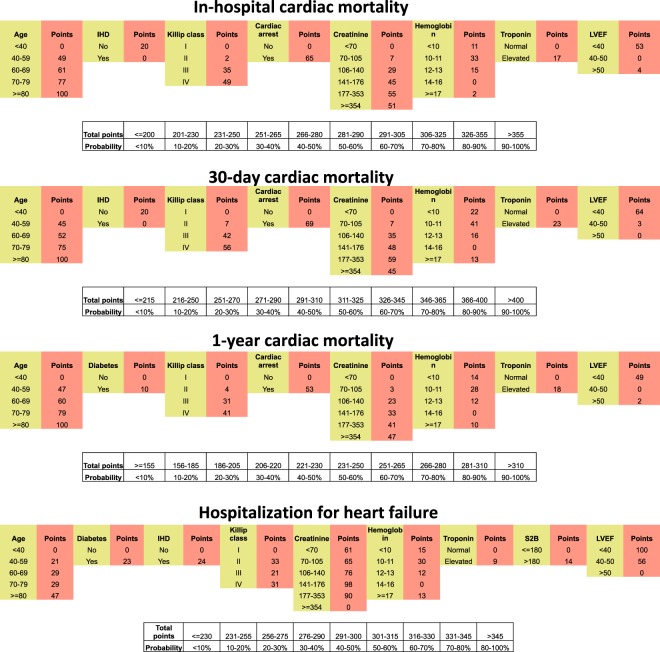
Figure 3 shows the variables that made up each risk score and the score associated with each variable. These risk scores could potentially be incorporated into a calculator to facilitate usage in the clinical setting.
Figure 3.
Nomograms to predict cardiac mortality and 1-year HHF for individual patients.
Discussion
The strength of the SMIR is that it is a mandatory registry and as such, all STEMI patients treated by PPCI between 2008 and 2013 were captured in a cohort of multi-ethnic Asian STEMI patients, comprising predominantly of Chinese, Malays and Indians, reperfused by PPCI in the current era. Unlikely other risk scores predicting all-cause mortality, we have identified independent predictors of cardiac mortality. All these variables are available either at the time of admission or within the first few hours following admission and can be used to predict in-hospital, 30-day and 1-year cardiac mortality and 1-year HHF early during hospitalization.
The potential immediate utility of these predictors is to identify those low risk patients who could be discharged early (e.g. after 48–72 hours). Secondly, it could potentially be used to identify those at high risk and who would benefit from more aggressive up-titration of their prognostic medications (beta-blockers, angiotensin converting enzyme inhibitors, aldosterone antagonists etc.) and more frequent follow-ups. Last but not least, this score could also be used when counseling patients prior to discharge regarding their prognosis.
The initial derivation cohort of the GRACE registry consisted of 13,708 patients recruited between 1999 and 2001 and only a third presented with a STEMI and only 15.2% received reperfusion therapy6. Furthermore, they only reported in-hospital and 6 months mortality. Although the recruitment was done in 14 countries, none were from Asia. However, the GRACE2 was subsequently expanded to more countries, including hospitals in Asia12. The risk profiles and prognosis for STEMI and NSTEMI are different13,14 and therefore developing risk scores specific for STEMI and NSTEMI may provide a more accurate prediction of their prognosis. The TIMI risk score has a dedicated score for STEMI4. The derivation cohort in the TIMI STEMI risk score was recruited from >800 hospitals4. However, it was not clear how many patients were Asians. Although 15,078 patients were enrolled between 1997 and 1998, they only included patients presenting within 6 hours of symptoms onset and only reported 30-day mortality4. The derivation cohort of the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) score15 included 2,082 STEMI patients recruited from 1997 to 1999 into a randomized controlled trial. Patients presenting within 12 hours of symptoms onset were included and those in cardiogenic shock at presentation were excluded and therefore patients in this cohort were not representative of the real world population. The Primary Angioplasty in Myocardial Infarction II (PAMI-II) criteria16 (for 6-month mortality) and Zwolle PPCI index17 (30-day mortality) can also be used to identify low risk patients for early discharge2 but they were both derived from non-Asian cohorts with patients recruited in the 1990s, with the former comprising of 3,252 patients from an RCT and the latter including 1,791 patients from a registry for their derivation cohorts. The Korean Acute Myocardial Infarction Registry (KAMIR)18 recently reported a risk score for Asians, derived from a cohort of 14,885 patients but it was not clear how the risk score was derived18. There was only one Asian ethnic group represented and they combined both STEMI and NSTEMI patients who either underwent invasive reperfusion strategy or were medically managed. They only reported 1-year mortality and they showed that the KAMIR score performed better than the GRACE score both in the NSTEMI and STEMI, highlighting the need for a risk score that is specific to the local population18. Most recently, the Acute Coronary Treatment and Intervention Outcomes Network (ACTION) risk score used to predict in-hospital mortality in ACS was reported19. They included a total of 243,440 patients and unlike the SMIR, entry in the former registry was voluntary. Moreover, they consisted predominantly of Caucasians and African Americans and they combined both STEMI and NSTEMI in the model. Although they showed that STEMI had worse outcomes in their model, it is likely that the other components of their score would have carried different weight for STEMI and NSTEMI if they were analyzed separately. The SMIR STEMI risk score is novel as it included all consecutive STEMI patients treated by PPCI as part of a compulsory nationwide registry and it can predict in-hospital, 30-day and 1-year cardiac mortality in a specific group of patients (STEMI patients reperfused by PPCI) in a multi-ethnic Asian cohort. Table 5 summarizes the prognostic variables included in the SMIR STEMI risk score in comparison to variables in the GRACE, TIMI STEMI, CADILLAC, PAMI-II, Zwolle, KAMIR and ACTION scores. All variables were present in at least one of the other risk scores in one form or another.
Table 5.
Established risk score from GRACE, TIMI STEMI, CADILLAC, PAMI, Zwolle, KAMIR and ACTION.
| Outcome | SMIR | GRACE12 | TIMI-STEMI4 | CADILLAC15 | PAMI-II16 | Zwolle17 | KAMIR18 | ACTION19 |
|---|---|---|---|---|---|---|---|---|
| In-hospital, 30-day and 1 year cardiac mortality and 1-year HHF | 6-month all-cause mortality | 1-year all-cause mortality | 1-year all-cause mortality | 6-month all-cause mortality | 30-day all-cause mortality | 1-year AMI mortality | In-hospital all-cause mortality | |
| c-statistic | 0.88–0.92 | 0.82 | 0.65 | 0.83 | 0.78 | 0.91 | 0.83 | 0.88 |
| Age | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| History of diabetes | Yes | Yes | Yes | Yes (admission glucose) | ||||
| History of hypertension | Not significant | Yes | ||||||
| History of IHD | Yes | Yes (angina) | ||||||
| BMI/ weight | Not significant | Yes (weight) | ||||||
| Killip class | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Cardiac arrest | Yes | Yes | Yes | |||||
| Anterior STEMI | Not significant | Yes (ST-segment deviation) | Yes (Anterior or LBBB) | Yes (Anterior or LBBB) | Yes | |||
| Creatinine | Yes | Yes | Yes (eGFR <60 ml/min) | Yes | Yes (CrCl) | |||
| Hemoglobin | Yes | Yes (Anemia) | ||||||
| Troponin T/I | Yes | Yes | Yes | |||||
| Ischemic time | Yes (>3 hours) | Yes (>4 hours) | Yes (>4 hours) | |||||
| LVEF during hospitalization | Yes (baseline LVEF <40%) | Yes (baseline LVEF <40%) | Yes (baseline LVEF <40%) | |||||
| Heart rate | Not available | Yes | Yes | Yes | Yes | |||
| Blood pressure | Not available | Yes | Yes | Yes | ||||
| 3-vessel disease | Not available | Yes | Yes | |||||
| Post PPCI TIMI flow | Not available | Yes | Yes |
HHF: hospitalization for heart failure; STEMI: ST-segment elevation myocardial infarction; BMI: body mass index; IHD: ischemic heart disease; LVEF: left ventricular ejection fraction; PPCI: primary percutaneous coronary intervention; TIMI: thrombolysis in myocardial infarction.
Limitations
We did not perform a direct comparison of the performance of the SMIR STEMI risk score against the GRACE score, which is currently the most popular, due to data on heart rate and blood pressure not being collected on admission. Less than 3% of the reperfused STEMI patients underwent thrombolysis from 2008 to 2015 and were not included in this analysis. Therefore whether the SMIR STEMI risk score would perform well in those Asian countries where thrombolysis is still widely used for STEMI1 remains to be investigated. Angiographic data were not available and were not included in the score. Furthermore procedural data (culprit only PCI or multi-vessel PCI) were also not available. However, a previous study20 have shown that the angiographic variables provided only a minor improvement in the c-statistic of a risk score to predict in-hospital mortality in patients undergoing PCI procedures. We did not include events occurring during hospitalization that could have improved the risk prediction for 30-day and 1-year cardiac mortality, as we wanted to keep the model simple, for easy adoption in the clinical setting. Only the admission troponin was included in the model as the discharge troponin was performed at variable time point and the assays used were not standardized among the various hospitals. Therefore it was not possible to provide reliable data regarding how the latter correlated with outcomes. The follow-up period was 1 year only and a longer period of follow-up would have provided further valuable information on the longer-term outcomes of these patients. As the risk score for HHF excluded patients who died due to non-HF causes within 1 year from STEMI discharge and without HF admission, the competing risk from non-HF death may have biased the HHF risk score and undermined its usefulness. Last but not least, we used the ICD codes to identify HHF endpoints and the latter has been shown to fail to capture all HHF events in western health care system21. Whether this holds true in the Singapore health care system with a dedicated team to collect all outcomes in this compulsory nationwide registry remains to be seen.
Conclusion
We used a real-world, national AMI registry to identify independent predictors of in-hospital, 30-day and 1-year cardiac mortality and 1-year HHF in a multi-ethnic Asian STEMI patients reperfused by PPCI. We have provided the variables and their associated scores that could be incorporated into a risk score to risk-stratify patients and guide duration of hospital stay, short and medium term management and follow-up, to improve outcomes in these patients.
Methods
Population
This was a retrospective study on data collected prospectively between 2008 and 2015 by the National Registry of Diseases Office in SMIR. Data collection on all AMI cases from all public and private hospitals is mandated and funded by the state and yearly reports are generated for the Ministry of Health11,22,23. Ethics approval was obtained from the SingHealth Centralized Institutional Review Board and the requirement for patient consent was waived. The study was conducted according to the Declaration of Helsinki.
Inclusion and Exclusion criteria
The main inclusion criteria were patients presenting to hospital with a STEMI within 12 hours of symptoms onset and were reperfused by PPCI. Patients with a STEMI but not reperfused by PPCI or those with a LBBB were excluded as shown in the flow chart in Fig. 4.
Figure 4.
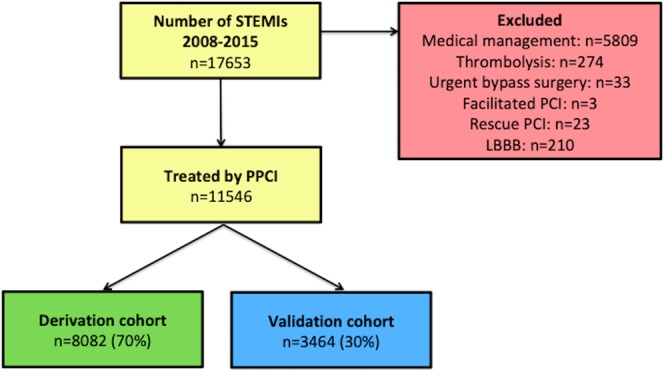
Flow chart of STEMI patients from the SMIR included in this study.
Clinical outcomes of interest
The main outcomes of interest were in-hospital cardiac mortality, 30-day cardiac mortality, 1-year cardiac mortality and 1-year HHF. Specifically for HHF, the data was available for 2008 to 2013 only. Patients who died during hospitalization for STEMI were excluded for this outcome. Among patients discharged alive, patients who died due to HF within 1 year from STEMI discharge but without HF admission were included. However, patients who died due to non-HF causes within 1 year from STEMI discharge and without HF admission were excluded.
Data collection
Registry coordinators confirmed all diagnosis of STEMI from physical and electronic medical records and data on patients’ demographics, comorbidities, location of STEMI on ECG, initial blood results such as hemoglobin, creatinine and troponin T or I were extracted. Details on Killip class and cardiac arrest in the ambulance or on admission (prior to transfer to the cardiac catheterization laboratory) and S2B/ D2B times were also recorded. The International Classification of Diseases 9th Revision (ICD-9 Clinical Modification) code 410 was used to identify STEMI cases diagnosed from 2008 to 2011, whereas ICD-10 codes I21 and I22 were used for STEMI cases diagnosed from 2012 to 2015. Data on admission heart rate and blood pressure were not available in this registry.
STEMI was defined by a typical chest pain of 20 minutes and significant ST-segment elevation (0.1 or 0.2 mV on two adjacent limb or precordial leads), and with a corresponding rise in cardiac biomarkers. Troponin T or I performed on admission was defined as abnormal if they were greater than the 99th percentile of the reference range from each hospital laboratory.
Cardiac mortality was identified as death occurring from a cardiac cause and defined by the ICD codes (ICD-9: 391–398, 402, 410–429; ICD-10: I00-I52 except I26). The time of death was extracted from death certificates obtained from the Registry of Births and Deaths. As the reporting of death is mandatory for all Singapore residents and the vital statuses of study population (patients with STEMI in January 2008 to December 2015) were matched till 30 June 2017, a date that is beyond one year from the last STEMI case included in this study, no patient was lost to follow-up for all mortality outcomes.
HHF was identified using ICD codes for heart failure admission (ICD-9: 412, 414.10, 414.19, 414.8, 428, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32, 428.33, 428.40, 428.41, 428.42, 428.43, 428.0, 428.9; ICD-10: I25.2, I25.3, I25.5, I50.1, I50.20, I50.21, I50.22, I50.23, I50.30, I50.31, I50.32, I50.33, I50.40, I50.41, I50.42, I50.43, I50.9). No patient was lost to follow-up for HHF as all admitted patients will have a discharge diagnosis. However, as the admission data was available till 31 December 2014, 1-year HHF could be matched for patients with STEMI in 2008 to 2013 only.
Statistical analysis
Continuous variables were expressed as medians with interquartile ranges and categorical variables were expressed as percentages. All analysis was performed using StataCorp. 2013. Stata Statistical Software: Release 13 (College Station, TX: StataCorp LP). The statistical methodology was similar to that used for the GRACE score derivation6. Random selection was performed to divide the cohort into two groups with 70% of the cohort included in the derivation cohort and 30% in the validation cohort. Variables that were prognostic from the GRACE, TIMI STEMI and CADILLAC15 risk scores and that were available from the SMIR were included in the univariable logistic regression. Other potentially prognostic variables such as cardiovascular risk variables, ethnicity, gender, S2B and D2B were also included in the univariable logistic regression model. Odd ratios with 95% confidence interval (95%CI) were used to assess the relationship between the included variables and cardiac mortality during hospitalization, within 30 days and within 1 year and 1-year HHF.
Multivariable stepwise logistic regression with backward elimination was subsequently used to determine the significant predictors of cardiac mortality and 1-year HHF among all the variables included in the univariable logistic regression. Variables that were significant with a P value of <0.05 were included in the final multivariable logistic models. Missing data were handled as case deletion without any imputation. Internal validity was assessed using bootstrapping techniques on the derivation cohort. External validity was assessed using the validation cohort. C-statistic (area under the curve) with 95%CI derived from Hosmer-Lemeshow test was used to assess the goodness of fit of the final multivariable logistic models. A model with a c-statistic of >0.800 was considered to have good fit. Misclassification rate derived from linear discriminant analysis was used to assess the discriminatory power of the final multivariable logistic models. The final multivariable logistic models were used to develop nomograms for patient risk6.
Acknowledgements
The authors are grateful for the efforts of all the SMIR Coordinators, Data Manager and Quality Assurance staff from NRDO. Prof Mark Y. Chan receives salary support from a National Medical Research Council Clinician Scientist Award (NMRC/CSA-INV/0001/2016). Prof D J Hausenloy is supported by the Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021) and the EU-CARDIOPROTECTION CA16225 Cooperation in Science and Technology (COST) Action.
Author Contributions
H.B. and D.J.H. conceived the idea. M.Y.C., N.F., D.C.F., W.C.L., S.T.L., A.D., H.C.T., J.W.T., K.L.T., A.S.W., P.E.W., K.K.Y., T.S.C. and T.H.K. contributed to patient enrolment in the registry. H.Z. and L.L.F. performed statistical analysis. H.B., H.Z., M.Y.C., N.F., D.C.F., W.C.L., S.T.L., A.D., H.C.T., J.W.T., K.L.T., A.S.W., P.E.W., K.K.Y., L.L.F., T.S.C., T.H.K. and D.J.H. prepared the manuscript. M.Y.C., A.S., J.W.T., K.K.Y. and D.J.H. critically reviewed the manuscript. All authors approved the final manuscript. The raw data is available and stored at the National Registry of Disease Office, Health Promotion Board.
Competing Interests
Dr. Yeo received honoraria from Boston Scientific, Abbott Vascular, and St. Jude Medical; is a consultant for Boston Scientific; is a proctor for Abbott Vascular; received research grant support from Medtronic; and received speaker fees from St. Jude Medical. Dr. Wong is a Senior Consultant at the National Heart Center Singapore and is the CEO/CTO of Innoheart Pre-clinical CRO Singapore.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chan MY, et al. Acute coronary syndrome in the Asia-Pacific region. International journal of cardiology. 2016;202:861–869. doi: 10.1016/j.ijcard.2015.04.073. [DOI] [PubMed] [Google Scholar]
- 2.Steg PG, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. European heart journal. 2012;33:2569–2619. doi: 10.1093/eurheartj/ehs289. [DOI] [PubMed] [Google Scholar]
- 3.Buccheri S, et al. Risk stratification after ST-segment elevation myocardial infarction. Expert Rev Cardiovasc Ther. 2016;14:1349–1360. doi: 10.1080/14779072.2017.1256201. [DOI] [PubMed] [Google Scholar]
- 4.Morrow DA, et al. TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–2037. doi: 10.1161/01.CIR.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 5.Bawamia B, Mehran R, Qiu W, Kunadian V. Risk scores in acute coronary syndrome and percutaneous coronary intervention: a review. Am Heart J. 2013;165:441–450. doi: 10.1016/j.ahj.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 6.Granger CB, et al. Predictors of hospital mortality in the global registry of acute coronary events. Archives of internal medicine. 2003;163:2345–2353. doi: 10.1001/archinte.163.19.2345. [DOI] [PubMed] [Google Scholar]
- 7.Eagle KA, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA: the journal of the American Medical Association. 2004;291:2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 8.D’Ascenzo F, et al. TIMI, GRACE and alternative risk scores in Acute Coronary Syndromes: a meta-analysis of 40 derivation studies on 216,552 patients and of 42 validation studies on 31,625 patients. Contemp Clin Trials. 2012;33:507–514. doi: 10.1016/j.cct.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Hussain SM, Oldenburg B, Wang Y, Zoungas S, Tonkin AM. Assessment of cardiovascular disease risk in South asian populations. Int J Vasc Med. 2013;2013:786801. doi: 10.1155/2013/786801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwong JS, Yu CM. The need for multicentre cardiovascular clinical trials in Asia. Nature reviews. Cardiology. 2013;10:355–362. doi: 10.1038/nrcardio.2013.49. [DOI] [PubMed] [Google Scholar]
- 11.Singapore Myocardial Infarction Registry Report No. 3: Trends In Acute Myocardial Infarction In Singapore 2007–2013. (https://www.nrdo.gov.sg/publications/ami 2015).
- 12.Fox KA, et al. The Global Registry of Acute Coronary Events, 1999 to 2009–GRACE. Heart. 2010;96:1095–1101. doi: 10.1136/hrt.2009.190827. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Garcia C, et al. Long-term prognosis of first myocardial infarction according to the electrocardiographic pattern (ST elevation myocardial infarction, non-ST elevation myocardial infarction and non-classified myocardial infarction) and revascularization procedures. The American journal of cardiology. 2011;108:1061–1067. doi: 10.1016/j.amjcard.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Kumar A, Cannon CP. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin Proc. 2009;84:917–938. doi: 10.4065/84.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halkin A, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. Journal of the American College of Cardiology. 2005;45:1397–1405. doi: 10.1016/j.jacc.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 16.Addala S, et al. Predicting mortality in patients with ST-elevation myocardial infarction treated with primary percutaneous coronary intervention (PAMI risk score) The American journal of cardiology. 2004;93:629–632. doi: 10.1016/j.amjcard.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 17.De Luca G, et al. Prognostic assessment of patients with acute myocardial infarction treated with primary angioplasty: implications for early discharge. Circulation. 2004;109:2737–2743. doi: 10.1161/01.CIR.0000131765.73959.87. [DOI] [PubMed] [Google Scholar]
- 18.Lee KH, et al. New horizons of acute myocardial infarction: from the Korea Acute Myocardial Infarction Registry. Journal of Korean medical science. 2013;28:173–180. doi: 10.3346/jkms.2013.28.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNamara RL, et al. Predicting In-Hospital Mortality in Patients With Acute Myocardial Infarction. Journal of the American College of Cardiology. 2016;68:626–635. doi: 10.1016/j.jacc.2016.05.049. [DOI] [PubMed] [Google Scholar]
- 20.Peterson ED, et al. Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. Journal of the American College of Cardiology. 2010;55:1923–1932. doi: 10.1016/j.jacc.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of heart failure diagnoses in administrative databases: a systematic review and meta-analysis. PloS one. 2014;9:e104519. doi: 10.1371/journal.pone.0104519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao, F. et al. Influence of Ethnicity, Age, and Time on Sex Disparities in Long-Term Cause-Specific Mortality After Acute Myocardial Infarction. Journal of the American Heart Association5 (2016). [DOI] [PMC free article] [PubMed]
- 23.de Carvalho LP, et al. Differences in late cardiovascular mortality following acute myocardial infarction in three major Asian ethnic groups. European heart journal. Acute cardiovascular care. 2014;3:354–362. doi: 10.1177/2048872614527007. [DOI] [PubMed] [Google Scholar]