Abstract
Submucosal tumors (SMT) are protuberant lesions with intact mucosa that have a wide differential. These lesions may be removed by standard polypectomy, endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), or surgically. However, in lesions that arise from the muscularis propria, full thickness resection is recommended. This can be completed using either endoscopic full thickness resection (EFTR) or submucosal tunneling endoscopic resection (STER). EFTR can be accomplished by completing a full thickness resection followed by defect closure or by securing gastrointestinal wall patency before resection. STER is an option that first creates a mucosal dissection proximal to the lesion to allow a submucosal tunnel to be created. Using this tunnel, the lesion may be resected. When comparing STER to EFTR, there was no significant difference when evaluating tumor size, operation time, rate of complications, or en bloc resection rate. However, suture time, amount of clips used, and overall hospital stay were decreased in STER. With these differences, EFTR may be more efficacious in certain parts of the gastrointestinal tract where a submucosal tunnel is harder to accomplish.
Keywords: Endoscopic full thickness resection (EFTR), submucosal tunneling endoscopic resection (STER), submucosal tumor (SMT)
Submucosal tumors (SMTs) and current treatment modalities
SMTs are defined as protuberant lesions covered with intact mucosa (1). During endoscopy, it is difficult to ascertain the etiology, but it can be speculated based on size, shape, firmness, color, and overall appearance (2). The prevalence of these lesions is equal among men and women and normally occur after the 5th decade of life (3). The differential includes: mesenchymal tumors, lymphomas, epithelial tumors, or congenital causes (2). Gastrointestinal stromal tumors (GIST) account for approximately half of incidentally found submucosal lesions in the stomach (4,5). The overall age-adjusted yearly incidence rate for GISTs is 0.68/100,000. The rate of incidence increases steadily with age, as individuals between age 20–29 have an incidence rate of 0.06/100,000, while those that are 80 and above have a rate of 2.29/100,000 (6). Given that the mucosa is intact, most SMTs are asymptomatic and found incidentally (7,8). However, when symptomatic, SMTs present commonly with GI bleeding, iron deficiency anemia, and non-specific abdominal pain secondary to mass effect (2).
SMTs are most frequently found in the stomach, followed by esophagus, duodenum, and colon (2). On endoscopy, if gastric lesions are <2 cm without any clinically malignant features, it is recommended to follow up these lesions with endoscopy or endoscopic ultrasound one to two times a year. Malignant features include irregular borders, ulceration, or growth during follow up. In cases of lesions 2–5 cm, or <2 cm but with malignant features, it is recommended to perform a detailed exam with EUS, computed tomography (CT) with contrast, or EUS guided fine needle aspiration (FNA) (2,7). When evaluating for malignancy using specific features, EUS without FNA has a sensitivity of 83–86% with a specificity of 76–80% (9). However, this technique has a low accuracy rate in the histological diagnosis of SMTs, thus biopsy is ultimately necessary (10,11).
American Society for Gastrointestinal Endoscopy (ASGE) and National Comprehensive Cancer Network (NCCN) recommend removal of SMTs for clinically relevant GISTs (>2 cm), histologically proven malignant SMTs, and mini-GISTs and small SMTs (<5 cm) with high-risk features on EUS or those that have increased in size (12). The standard procedure for GISTs is complete resection with sufficient surgical margins by laparotomy (13,14). In cases with gastric GISTs that are <5 cm, laparoscopic surgery serves as an alternative as it is safe, less invasive, and has similar long term outcomes to laparotomy with significant improvement in cosmetic results (15-18). There is limited evidence for laparoscopic surgery for small intestinal and colorectal GISTs (12). Additionally, laparoscopic surgery may be challenging when tumors are located in the gastroesophageal (GE) junction, pylorus, duodenum, or lower rectum (12). In cases where GISTs are present near the GE junction and pylorus, laparoscopic and endoscopic cooperative surgery (LECS) can be used. The concomitant use of endoscopy enables better localization and visualization, ultimately reducing unintentionally large resections that may result in deformities and gastric malfunction (12,19,20). However, LECS has the potential to cause tumor cell seeding into the peritoneal cavity by exposing the tumor surface. In an attempt to minimize intraperitoneal contamination and tumor cell seeding, combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET) and non-exposed endoscopic wall-inversion surgery (NEWS) have been developed (21,22). However, these approaches are demanding, time consuming, and cost ineffective (12). In cases of superficial SMTs originating from the muscularis mucosa, endoscopic submucosal dissection (ESD) is an option that allows en bloc resection. It was developed to allow for resection of difficult lesions that are large, irregular, have ulcerations, or difficult locations for conventional endoscopic mucosal resection (EMR); however, perforation frequently occurs for SMTs located in deeper layers (23,24). These procedures are not appropriate for SMTs with an extraluminal growth component. Additionally, they cannot guarantee a negative resection margin of muscularis based tumors as the muscular is not removed but rather undergoes partial excavation (25). These issues are addressed by endoscopic full-thickness resection (EFTR).
Endoscopic full thickness resection (EFTR)
General indications and pre-operative evaluation
There is not a clear consensus for the indications for EFTR. However, as stated above, the efficacy and safety of standard polypectomy, EMR, ESD, laparoscopy, and LECS are limited in specific settings. EFTR is useful in cases of lesions arising from the muscularis propria, locations that are difficult to assess (i.e., GE junction), high risk of adverse events (i.e., lesions in a diverticulum or in appendiceal orifice), non-lifting lesions that may be secondary to fibrosis and scarring, small subepithelial lesions such as neuroendocrine tumors, or recurrence of epithelial neoplasms following EMR or ESR (26-28). These indications arise from the fact that these particular lesions were previously contraindicated using standard modalities due to high perforation rates or inability to obtain complete resection. However, the development of resection tools and closure devices in the setting of EFTR have allowed for better resection while minimizing complications such as perforation (26). Prior to EFTR, it is essential that the patient undergo characterization of the lesion. This is accomplished using EUS to confirm that it is not a benign lesion, while also determining the size, layer of origin, involvement of adjacent structures, and regional lymphadenopathy (29). Additionally, EUS has the ability to assess lesion attachment to the muscularis propria. In one study, successful R0 resection was predicted by the observation of narrow or no lesion attachment to the fourth hypoechoic layer (30). It is also recommended to obtain CT imaging in addition to EUS prior to EFTR. It results in less procedure time, propofol, larger margin of resections, all while increasing the coincidence rate between preoperative program and actual endoscopic procedures to predict maneuvers (31). Cases with lesions that display high risk features such as irregular borders, cystic spaces, heterogeneous echotexture, and suspect lymph notes are contraindicated for EFTR as there is a high risk of lymph node metastasis or periprocedural intraperitoneal dissemination of carcinoma cells or SMTs (27,32).
Techniques
The defining feature of EFTR is defect closure in the setting of a full thickness resection. There are two main approaches to this: (I) standard EFTR which includes full thickness resection followed by defect closure or (II) prior clip assisted EFTR that secures gastrointestinal wall patency before resection (28).
Standard EFTR
Standard EFTR’s general technique is resection followed by closure of the defect. It is primarily used in gastric SMTs originating from the muscularis propria. More specifically, it is indicated for gastric SMTs that are <3 cm. Although feasible for lesions greater than 3 cm, it may be difficult for extraction through the esophagus after en bloc resection and the gastric wall defect may be difficult to close (33). In lesions with deep and broad attachment to the muscularis propria, there is a higher likelihood of developing intraprocedural events such as tension pneumoperitoneum. Although full thickness resection followed by closure can be completed effectively, it does create some difficulties (26-28,34,35). These difficulties include inability to properly close large defects, loss of insulation to maintain proper operative field, tumor seeding into the abdominal cavity, or spillage of gastrointestinal content into the abdominal cavity (28). Gas-related events, such as subcutaneous emphysema, pneumomediastinum, and pneumoperitoneum may also occur but these are not viewed as adverse effects as they are anticipated and there is rapid absorption of carbon dioxide (26,28). Standard EFTR is not recommended in the duodenum or esophagus given the limited space that restricts maneuverability. Additionally, the location of the duodenum and esophagus may result in the formation of fistulas and mediastinitis as serious adverse events. For similar reasons, it is not recommended for use in the colon with concern for leak, peritonitis, and unreliable closure (26,28).
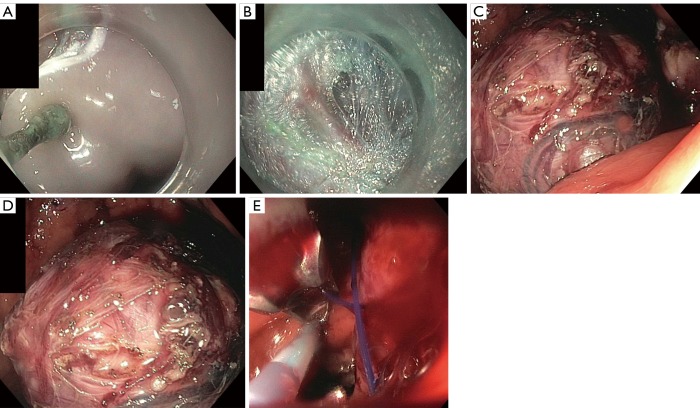
Standard EFTR utilizes similar devices and accessories as used in ESD. The lesion is marked with argon plasma coagulation (APC) and then a submucosal fluid injection is performed. Immediately after, a circumferential mucosal incision is performed. Dissection is performed with intent to accomplish en bloc resection. Given that SMTs are more difficult to assess for R0 resection as compared to epithelial lesions, it is recommended to resect the lesion with the pseudocapsule intact (26,28). This can be accomplished with electrosurgical knives that either have non-insulated or insulated tips. For lesions that are tightly adhered to the muscularis propria and serosa, intentional through-and-through perforation is required for complete removal. Dedicated forceps (Coagrasper; Olympus Corp., Tokyo, Japan) have been created to control bleeding and prophylactic coagulation. Following resection, defects can be closed either with through the scope clips with or without endoloop reinforcement, over the scope (OTS) clips, or endoscopic suturing (26,28). This procedure can be visualized in Figure 1. Figure 1A demonstrates the identification of the tumor with the submucosal injection. Figure 1B illustrates the cutting of the mucosal and submucosal layer to exposure the tumor. In Figure 1C, the resection can be seen. Figure 1D is the closure of the gastric defect with sutures. Finally, Figure 1E is the resected tumor.
Figure 1.
This is a representation of step-by-step approach in EFTR for the removal of a submucosal lesion. (A) To begin, the submucosal tumor was identified and submucosal injections were applied; (B) next, the mucosal and submucosal layers were dissccled to expose the tumor; (C) full thickness of the tumor was completed; (D) tumor is shown in its entirety; (E) the closure of the gastric defect is completed using endoscopic sutures. EFTR, endoscopic full thickness resection.
Standard EFTR with subsequent clip closure—gastric sites
Multiple studies have shown success in the resection of gastric SMTs. Ye et al. had technical success in 50 out of 51 cases (98%) with an average procedure time of 52 minutes. The mean tumor length was 2.4 cm. Patients were followed on average for 22.4 months with no residual tumor or recurrence. No serious adverse effects were observed (36). Shi et al. had similar results as they removed completed 20 en bloc resections with a rate of 100% and mean size of 1.47 cm. There were no long term complications, but five patients developed fever and abdominal pain one day after the procedure (37). Huang et al. removed 35 lesions with a mean size of 2.8 cm with no complications, contrast extravasation on postoperative contrast roentgenography on the third day, or lesion residue or recurrence at 6 month follow up (35). Zhou had 100% resection rate for 26 gastric SMTs with a mean size of 2.8 cm and no residual or recurrence at a mean follow up of 8 months, with a range of 6–24 months (27). These studies demonstrate that standard EFTR with subsequent clip closure in the setting of gastric SMTs is feasible, safe, and effective for lesions <3 cm.
Standard EFTR with subsequent clip closure—colonic sites
EFTR in the colon can be accomplished in three ways: traction of the colon with forceps or anchoring device and snare resection; suction into cap followed by snare resection; or cutting of colonic wall similar to ESD (26,28). Ahmed et al. compared traction versus suction in porcine models, with the finding that traction resulted in larger specimen with less injury to adjacent viscera (38). Closure can be completed with through the scope clips or OTS clips. von Renteln et al. identified feasibility of a grasp-and-snare technique for EFTR combined with an over-the-scope clip for defect closure. In the porcine models that had closure with OTSC, closure was successful in 9 out of the 20 cases after resecting specimens ranging from 2.4 to 5.5 cm. Three models obtained injury to adjacent organs. In models that received ligation with an endoloop before resection, there was 100% success with only one case of lumen obstruction afterwards. However, the specimens were smaller as they ranged from 1.2 to 2.2 cm (39). There are limited number of clinical trials to confirm these porcine results and ultimately determine the efficacy and safety of EFTR in the colon. Xu et al. enrolled 19 patients with colonic SMTs 3 cm or smaller. In 16 out of 18 patients, full thickness resection was successful with endoscopic closure. The other two patients required laparoscopic closure. There was no recurrence of tumor at 18-month follow up (40). Data, especially in human subjects, is very limited in regards to EFTR for colonic resections. More studies need to be completed before it can be deemed safe and effective.
Standard EFTR with subsequent suturing
There are three techniques involved with endoscopic suturing: dedicated suturing devices, through the scope catheter based devices, and multitasking platforms (41). Two OTS suturing devices include Over-Stitch (Apollo Endosurgery Inc., Austin, Tex) and EagleClaw, the latter of which is not available anymore. These are similar in that they are mounted on the tip of an endoscope and provide single knot sutures. Over-Stitch has been found to successfully close post-ESD mucosal defects in a clinical setting, while also closed gastric perforations following EFTR in porcine models (42,43). There are no clinical studies that investigated the use of OTS sutures for EFTR closure. T-Tags (TAS, Ethicon, Blue Ash, Ohio, United States) was a through the scope suture instrument that demonstrated use in several studies, but has since been removed from the market (28). Mori et al. completed a trial to compare OTS clips, hand-sewn sutures, and Double-arm-bar Suturing System (DBSS). DBSS is similar to the Over-Stitch model in that device is mounted on an endoscope and allow serial single-stitch sutures. Thirty EFTRs were completed, with 10 porcine models in each arm. Mori et al. demonstrated that there was no significant difference in leaking between the hand-sewn and DBSS, but that both hand-sewn and DBSS were able to withhold higher burst pressures when compared to the OTS clip arm (44). Based on literature review, several of the suturing techniques are no longer on the market. More clinical data is needed for newer strategies to determine their safety and efficacy.
Prior clip assisted EFTR
The use of OTS clip assisted EFTR provides a method in which the defect is secured before resection is completed. It is indicated in cases where the lesions are <1 cm in the upper GI tract and <2 cm in the colorectum. It serves the advantage of not creating a large defect that may result in perforation or contamination of the peritoneal cavity. This method should be avoided in lesions that are located in the appendiceal orifice or in the presence of a native appendix, so as to reduce risk of appendicitis.
Two non-dedicated OTS clip devices are currently being used. This includes the Padlock clip (US Endoscopy, Mentor, OH) and the OTSC (Ovesco Endoscopy AG, Tübingen, Germany). The Padlock clip is a star-shaped nitinol ring with 6 inner needles. It facilitates circumferential tissue apposition using radical compression technology. The standard is available in 9.5 and 11 mm diameter endoscope, while the Pro-Select fits an 11.5 to 14 mm endoscope. Both clips have a cap diameter of 11 mm. The trigger wire is located along the shaft of the endoscope, allowing for a free lumen for passage of accessories and necessary suctioning. The clip is deployed with a simple push button. The other non-dedicated OTS clip device is the OTSC, which consists of a cap with a premounted clip and hand wheel for release. It functions similar to a band ligation. It is available in 11, 12, and 14 mm with cap depths of either 3 or 6 mm. Additionally, it is available with three variations in clip teeth: blunt, small spikes, or spikes on elongated teeth. The procedure entails having the OTS clip advanced to the lesion, which is then retracted into the cap either using a retraction device or forceps with additional suction to create a pseudopolyp of tissue. A fluid injection may be used beforehand, but is not recommended as it is not needed and may limit the volume that is retracted. The clip is deployed and the pseudopolyp is resected with an electrosurgical snare (26,28). In cases where resection is difficult, an electrosurgical knife may be used. This technique was used in the stomach, duodenum, rectosigmoid colon, and appendiceal orifice status post appendectomy for lesions that were non-lifting, recurrent adenomas that were not amenable to standard polypectomy techniques, or lesions in difficult locations with success (45-48). Of these studies, only one was not able to achieve 100% technical success, while the fourth had a success rate of 94%. Additionally, there were no adverse events in these studies. Demonstrating that either OTS clip devices are effective for post clip assisted EFTR.
FTRD is a dedicated EFTR device that has been available in Europe since 2014 and the United States since 2017 for colorectal lesions. A recent retrospective analysis of 20 patients that underwent EFTR with FTRD of the duodenum for adenomas, subepithelial tumors, and T1 adenocarcinoma found technical success in 85% of patients with an R0 resection rate of 63.2% (49). There were no serious adverse effects; only minor bleeding on the first post-interventional day in 3 out of 19 patients, thus indicating it may be safe for upper GI lesions in the near future. FTRD functions as a one-step EFTR after placement of an OTS clip that has an integrated electrosurgical snare. It can be placed onto a colonoscope with a recommended diameter of 11.5 to 13.2 mm. It has a modified OTSC premounted on a cap that has a larger depth of 23 mm and diameter of 21 mm. Compared to the OTSC cap, it is much longer (23 vs. 6 mm), allowing for more tissue incorporation (28). The electrosurgical snare is 13 mm and integrated within the cap, while its catheter and handle run alongside the endoscope shaft. Prior to using this device, the lesion is marked with APC. After the lesion is retracted into the cap, the clip is deployed with immediate snare resection. Difficulties with this technique result from the large cap, which impairs visualization and flexibility that may result in incomplete resection. A limitation of FTRD is difficulty with lesions that are >3 cm (50). In one study, it was demonstrated that difficulty might arise even at lesions greater than 2 cm, as R0 resection rates dropped from 81.2% to 58.1% in lesions larger than 2 cm (51). However, even with these limitations, EFTR with FTRD has proven to be a feasible, safe, and effect technique for full thickness resection. In 60 patients that underwent EFTR with FTRD, 97% had technical success, full thickness was achieved in 88%, R0 resection rate was 79%, while only 7% suffered adverse events including appendicitis or minor bleeding (52). In another trial for patients with non-lifting adenomas, there was 100% technical success in 20 patients with a mean size of 26 mm resected, and only one adverse effect of abdominal pain associated with fever and leukocytosis (53). Ultimately, FTRD has proven to be an effective model for small lesions located in the lower GI tract. More data is needed to determine its role in the upper GI tract, but this is limited by its outer diameter of 21 mm and sharp edges that limit its peroral passage. Additionally, more experience in the stomach is required due to the thickness of the gastric wall (28).
Submucosal tunneling endoscopic resection (STER)
The submucosal tunneling technique was originally developed as a submucosal endoscopy with a mucosal flap safety valve (54). It was found to be feasible for natural orifice transluminal endoscopic surgery (NOTES), and subsequently developed for per oral endoscopic myotomy (POEM) in the treatment of esophageal achalasia (55-57). In a POEM, an esophageal mucosal incision is made to create a submucosal tunnel that crosses the GE junction, and thus providing a submucosal space to operate in. This ultimately led to the development of STER (58).
Indications and contraindications
STER is used for SMTs that are generally less than 3.5 cm in diameter. Larger SMTs are difficult to retrieve while they may also cause poor visualization secondary to mass effect (59). Ultimately this results in lower rates of en bloc resection and higher rates of adverse events (60-63). Additionally, larger lesions may result in piecemeal resection that may result in incomplete resection and rupture of the tumor capsule (64). As mentioned previously, EUS is not adequate in diagnosing the histology the lesion; therefore it is not appropriate to assume that a lesion is benign. Given that a submucosal space needs to be created, the best locations for STER include relatively straight and tubular structures such as the esophagus or gastric cardia (26). However, even though it is more difficult, STER is applicable in sites such as the stomach and rectum without an increase in adverse events (65,66). There are several contraindications to STER. It should not be performed in cases where the mucosa is ulcerated, as the integrity of the mucosa cannot be maintained (67). SMTs with irregular borders have a higher risk of malignancy and may be difficult to resect using STER (63,68,69). In lesions with a deep portion of the muscularis propria involved, there is a high risk of perforation, chronic fistula formation, and secondary infection (68). The most common adverse effects are pneumothorax, subcutaneous and mediastinal emphysema and pneumoperitoneum (28).
Procedure
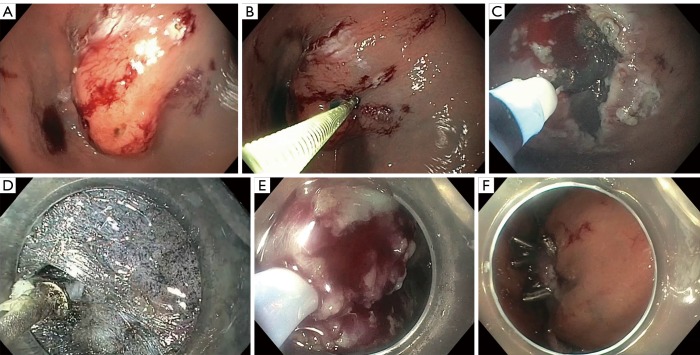
This procedure is represented in Figure 2. Figure 2A demonstrates a submucosal lesion. The steps involved with STER include first creating a submucosal fluid cushion, as demonstrated in Figure 2B. This aids in the mucosal incision with an electrosurgical knife approximately 5 cm above the proximal edge of the SMT as demonstrated in Figure 2C. Mucosotomy is usually completed with a 2 cm longitudinal incision, but inverted T and transverse incisions are also acceptable (70). The endoscope is passed through the incision with an electrosurgical knife. This can be visualized in Figure 2D. As the scope is advanced, a dye such as indigo carmine methylene blue is used to better differentiate the submucosal and muscular layers. This is to avoid injury while enlarging the tunnel mucosa. While staying close to the muscularis propria, the tunnel should be expanded 2 cm past the distal margin of the SMT to ensure appropriate working space (69). Depending on the degree of attachment to the muscularis propria, a variety of electrosurgical knives may be used for a partial or full thickness resection, similar to ESD. However, a snare may also be used in the removal of the SMT, as seen in Figure 2E. After the lesion is extracted, the mucosotomy is closed with clip placement (Figure 2F) or endoscopic suturing. By using this technique, the risk of developing mediastinitis or peritonitis, even in full resections, is decreased because the mucosa is intact.
Figure 2.
This is a representation of the step-by-step approach using STER for the removal of a submucosal lesion. (A) The submucosal lesion is identified in the fundus of the stomach; (B) a submucosal injection was completed to create a submucosal fluid cushion to aid in the incision; (C) an incision was made proximal to the submucosal lesion; (D) the scope was inserted into the proximal incision as the submucosal and muscular layers were separated using methylene blue to better differentiate the layers; (E) the lesion was resected using a snare; (F) the defect following mucosotomy was closed using the placement of clips. STER, submucosal tunneling endoscopic resection.
Lv et al. performed a meta-analysis of 28 studies between 2011–2015 to determine the efficacy and safety of STER for upper gastrointestinal SMTs. There were 1,041 patients with a complete resection rate of 97.5% and en bloc resection rate of 94.6% (71). There was no tumor recurrence or STER related deaths, however of note—there is no guidelines regarding ideal follow up. In general, there was a low rate of adverse events. The main adverse events included air leakage symptoms with a pooled estimate of 14.8%. This included events such as subcutaneous emphysema and pneumomediastinum (SEP), pneumothorax, and pneumoperitoneum. SEP was the most common complication with a prevalence of 14.8%, occurring at the GE junction 26.1% of the time. A majority of these outcomes were treated with conservative therapies. To support this, in a study completed by Chen et al., 68 of 290 patients (23.4%) suffered adverse events but only 29 (10%) required intervention (72). STER is a safe effective method for the removal of SMT originating from the muscularis propria. In a retrospective study by Chen et al., 180 patients were followed for a mean of 36 months. None of these patients had recurrence or distant metastasis (73).
Comparison of EFTR and STER
Although each has their respective limitations, both EFTR and STER have proved to be a safe, feasible, and effective method for the removal of SMTs. In a retrospective trial of 52 patients to compare EFTR to STER, there was no significant difference when evaluating tumor size, operation time, rate of complications, or en bloc resection rate. However, there was a difference when comparing suture time and number of clips for suture. Suture time was 380.6 seconds for EFTR versus 291.5 seconds for STER, while EFTR used on average 7.6 clips compared to 6.0 for STER (74). The use of a submucosal tunnel with STER promotes early wound healing, decreases the risk of gastrointestinal tract leakage and infection. In the aforementioned study, 4 of the 32 patients who underwent EFTR experienced abdominal pain and discomfort due to leakage of gas and liquid into the abdominal cavity. On the contrary, only one patient had such a complication in the STER group. In addition, the STER technique allows for better visualization, which promotes precise hemostasis, whereas with the EFTR technique it is sometimes hard to facilitate precise hemostasis (74). Another retrospective clinical study of 43 patients confirmed these results, while also demonstrating that patients who underwent EFTR had a longer hospital stay (75). An important consideration of using STER is the location of the SMT. A submucosal tunnel is harder to accomplish in certain parts of the gastrointestinal tract, which would make EFTR a more reasonable option (74).
Acknowledgments
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Wiech T, Walch A, Werner M. Histopathological classification of nonneoplastic and neoplastic gastrointestinal submucosal lesions. Endoscopy 2005;37:630-4. 10.1055/s-2005-870127 [DOI] [PubMed] [Google Scholar]
- 2.Nishida T, Kawai N, Yamaguchi S, et al. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc 2013;25:479-89. 10.1111/den.12149 [DOI] [PubMed] [Google Scholar]
- 3.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc 1991;5:20-3. 10.1007/BF00591381 [DOI] [PubMed] [Google Scholar]
- 4.Abraham SC, Krasinskas AM, Hofstetter WL, et al. "Seedling" mesenchymal tumors (Gastrointestinal stromal tumors and leiomyomas) are common incidental tumors of the esophagogastric junction. American Journal of Surgical Pathology 2007;31:1629-35. 10.1097/PAS.0b013e31806ab2c3 [DOI] [PubMed] [Google Scholar]
- 5.Kawanowa K, Sakuma Y, Sakurai S, et al. High incidence of microscopic gastrointestinal stromal tumors in the stomach. Hum Pathol 2006;37:1527-35. 10.1016/j.humpath.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 6.Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol 2005;100:162-8. 10.1111/j.1572-0241.2005.40709.x [DOI] [PubMed] [Google Scholar]
- 7.Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol 2008;13:416-30. 10.1007/s10147-008-0798-7 [DOI] [PubMed] [Google Scholar]
- 8.Nishida T, Kumano S, Sugiura T, et al. Multidetector CT of high-risk patients with occult gastrointestinal stromal tumors. AJR Am J Roentgenol 2003;180:185-9. 10.2214/ajr.180.1.1800185 [DOI] [PubMed] [Google Scholar]
- 9.Polkowski M. Endoscopic ultrasound and endoscopic ultrasound-guided fine-needle biopsy for the diagnosis of malignant submucosal tumors. Endoscopy 2005;37:635-45. 10.1055/s-2005-861422 [DOI] [PubMed] [Google Scholar]
- 10.Hwang JH, Saunders MD, Rulyak SJ, et al. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc 2005;62:202-8. 10.1016/S0016-5107(05)01567-1 [DOI] [PubMed] [Google Scholar]
- 11.Karaca C, Turner BG, Cizginer S, et al. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc 2010;71:722-7. 10.1016/j.gie.2009.10.019 [DOI] [PubMed] [Google Scholar]
- 12.Nishida T, Goto O, Raut CP, et al. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 2016;122:3110-8. 10.1002/cncr.30239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demetri GD, von Mehren M, Antonescu CR, et al. NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 2010;8 Suppl 2:S1-41; quiz S42-4. [DOI] [PMC free article] [PubMed]
- 14.ESMO/European Sarcoma Network Working Group Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii21-6. 10.1093/annonc/mdu255 [DOI] [PubMed] [Google Scholar]
- 15.Chen K, Zhou YC, Mou YP, et al. Systematic review and meta-analysis of safety and efficacy of laparoscopic resection for gastrointestinal stromal tumors of the stomach. Surg Endosc 2015;29:355-67. 10.1007/s00464-014-3676-6 [DOI] [PubMed] [Google Scholar]
- 16.Bischof DA, Kim Y, Dodson R, et al. Open versus minimally invasive resection of gastric GIST: a multi-institutional analysis of short- and long-term outcomes. Ann Surg Oncol 2014;21:2941-8. 10.1245/s10434-014-3733-3 [DOI] [PubMed] [Google Scholar]
- 17.Nishimura J, Nakajima K, Omori T, et al. Surgical strategy for gastric gastrointestinal stromal tumors: laparoscopic vs. open resection. Surg Endosc 2007;21:875-8. 10.1007/s00464-006-9065-z [DOI] [PubMed] [Google Scholar]
- 18.Novitsky YW, Kercher KW, Sing RF, et al. Long-term outcomes of laparoscopic resection of gastric gastrointestinal stromal tumors. Ann Surg 2006;243:738-45; discussion 745-7. 10.1097/01.sla.0000219739.11758.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiki N, Yamamoto Y, Fukunaga T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 2008;22:1729-35. 10.1007/s00464-007-9696-8 [DOI] [PubMed] [Google Scholar]
- 20.Niimi K, Ishibashi R, Mitsui T, et al. Laparoscopic and endoscopic cooperative surgery for gastrointestinal tumor. Ann Transl Med 2017;5:187. 10.21037/atm.2017.03.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitsui T, Niimi K, Yamashita H, et al. Non-exposed endoscopic wall-inversion surgery as a novel partial gastrectomy technique. Gastric Cancer 2014;17:594-9. 10.1007/s10120-013-0291-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoue H, Ikeda H, Hosoya T, et al. Endoscopic mucosal resection, endoscopic submucosal dissection, and beyond: full-layer resection for gastric cancer with nonexposure technique (CLEAN-NET). Surg Oncol Clin N Am 2012;21:129-40. 10.1016/j.soc.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 23.Goto O, Uraoka T, Horii J, et al. Expanding indications for ESD: submucosal disease (SMT/carcinoid tumors). Gastrointest Endosc Clin N Am 2014;24:169-81. 10.1016/j.giec.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 24.Hoteya S, Iizuka T, Kikuchi D, et al. Endoscopic submucosal dissection for gastric submucosal tumor, endoscopic sub-tumoral dissection. Dig Endosc 2009;21:266-9. 10.1111/j.1443-1661.2009.00905.x [DOI] [PubMed] [Google Scholar]
- 25.Cai M, Zhou P, Lourenco LC, et al. Endoscopic Full-thickness Resection (EFTR) for Gastrointestinal Subepithelial Tumors. Gastrointest Endosc Clin N Am 2016;26:283-95. 10.1016/j.giec.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 26.Rajan E, Wong Kee Song LM. Endoscopic Full Thickness Resection. Gastroenterology 2018;154:1925-37 e2. [DOI] [PubMed]
- 27.Zhou PH, Yao LQ, Qin XY, et al. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc 2011;25:2926-31. 10.1007/s00464-011-1644-y [DOI] [PubMed] [Google Scholar]
- 28.Schmidt A, Meier B, Caca K. Endoscopic full-thickness resection: Current status. World J Gastroenterol 2015;21:9273-85. 10.3748/wjg.v21.i31.9273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon L, Buscaglia JM. Endoscopic approach to subepithelial lesions. Therap Adv Gastroenterol 2014;7:123-30. 10.1177/1756283X13513538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bialek A, Wiechowska-Kozlowska A, Pertkiewicz J, et al. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video). Gastrointest Endosc 2012;75:276-86. 10.1016/j.gie.2011.08.029 [DOI] [PubMed] [Google Scholar]
- 31.Chu Y, Qiao X, Gao X, et al. Combined EUS and CT for evaluating gastrointestinal submucosal tumors before endoscopic resection. Eur J Gastroenterol Hepatol 2014;26:933-6. 10.1097/MEG.0000000000000136 [DOI] [PubMed] [Google Scholar]
- 32.Hwang JH, Rulyak SD, Kimmey MB, et al. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology 2006;130:2217-28. 10.1053/j.gastro.2006.04.033 [DOI] [PubMed] [Google Scholar]
- 33.Goto O, Shimoda M, Sasaki M, et al. Potential for peritoneal cancer cell seeding in endoscopic full-thickness resection for early gastric cancer. Gastrointest Endosc 2018;87:450-6. 10.1016/j.gie.2017.08.036 [DOI] [PubMed] [Google Scholar]
- 34.Feng Y, Yu L, Yang S, et al. Endolumenal endoscopic full-thickness resection of muscularis propria-originating gastric submucosal tumors. J Laparoendosc Adv Surg Tech A 2014;24:171-6. 10.1089/lap.2013.0370 [DOI] [PubMed] [Google Scholar]
- 35.Huang LY, Cui J, Lin SJ, et al. Endoscopic full-thickness resection for gastric submucosal tumors arising from the muscularis propria layer. World J Gastroenterol 2014;20:13981-6. 10.3748/wjg.v20.i38.13981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye LP, Yu Z, Mao XL, et al. Endoscopic full-thickness resection with defect closure using clips and an endoloop for gastric subepithelial tumors arising from the muscularis propria. Surg Endosc 2014;28:1978-83. 10.1007/s00464-014-3421-1 [DOI] [PubMed] [Google Scholar]
- 37.Shi Q, Chen T, Zhong YS, et al. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy 2013;45:329-34. 10.1055/s-0032-1326214 [DOI] [PubMed] [Google Scholar]
- 38.Ahmed I, Shibukawa G, Groce R, et al. Study of full-thickness endoluminal segmental resection of colon in a porcine colon model (with videos). Gastrointest Endosc 2007;65:696-702. 10.1016/j.gie.2006.10.051 [DOI] [PubMed] [Google Scholar]
- 39.von Renteln D, Schmidt A, Vassiliou MC, et al. Endoscopic full-thickness resection and defect closure in the colon. Gastrointest Endosc 2010;71:1267-73. 10.1016/j.gie.2009.12.056 [DOI] [PubMed] [Google Scholar]
- 40.Xu M, Wang XY, Zhou PH, et al. Endoscopic full-thickness resection of colonic submucosal tumors originating from the muscularis propria: an evolving therapeutic strategy. Endoscopy 2013;45:770-3. 10.1055/s-0033-1344225 [DOI] [PubMed] [Google Scholar]
- 41.Conway NE, Swanstrom LL. Endoluminal flexible endoscopic suturing for minimally invasive therapies. Gastrointest Endosc 2015;81:262-9 e19. [DOI] [PubMed]
- 42.Kantsevoy SV, Bitner M, Mitrakov AA, et al. Endoscopic suturing closure of large mucosal defects after endoscopic submucosal dissection is technically feasible, fast, and eliminates the need for hospitalization (with videos). Gastrointest Endosc 2014;79:503-7. 10.1016/j.gie.2013.10.051 [DOI] [PubMed] [Google Scholar]
- 43.Chiu PW, Phee SJ, Wang Z, et al. Feasibility of full-thickness gastric resection using master and slave transluminal endoscopic robot and closure by Overstitch: a preclinical study. Surg Endosc 2014;28:319-24. 10.1007/s00464-013-3149-3 [DOI] [PubMed] [Google Scholar]
- 44.Mori H, Kobara H, Fujihara S, et al. Feasibility of pure EFTR using an innovative new endoscopic suturing device: the Double-arm-bar Suturing System (with video). Surg Endosc 2014;28:683-90. 10.1007/s00464-013-3266-z [DOI] [PubMed] [Google Scholar]
- 45.Backes Y, Kappelle WFW, Berk L, et al. Colorectal endoscopic full-thickness resection using a novel, flat-base over-the-scope clip: a prospective study. Endoscopy 2017;49:1092-7. 10.1055/s-0043-114730 [DOI] [PubMed] [Google Scholar]
- 46.Fahndrich M, Sandmann M. Endoscopic full-thickness resection for gastrointestinal lesions using the over-the-scope clip system: a case series. Endoscopy 2015;47:76-9. [DOI] [PubMed] [Google Scholar]
- 47.Sarker S, Gutierrez JP, Council L, et al. Over-the-scope clip-assisted method for resection of full-thickness submucosal lesions of the gastrointestinal tract. Endoscopy 2014;46:758-61. 10.1055/s-0034-1365513 [DOI] [PubMed] [Google Scholar]
- 48.Al-Bawardy B, Rajan E, Wong Kee Song LM. Over-the-scope clip-assisted endoscopic full-thickness resection of epithelial and subepithelial GI lesions. Gastrointest Endosc 2017;85:1087-92. 10.1016/j.gie.2016.08.019 [DOI] [PubMed] [Google Scholar]
- 49.Bauder M, Schmidt A, Caca K. Endoscopic full-thickness resection of duodenal lesions-a retrospective analysis of 20 FTRD cases. United European Gastroenterol J 2018;6:1015-21. 10.1177/2050640618773517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aepli P, Criblez D, Baumeler S, et al. Endoscopic full thickness resection (EFTR) of colorectal neoplasms with the Full Thickness Resection Device (FTRD): Clinical experience from two tertiary referral centers in Switzerland. United European Gastroenterol J 2018;6:463-70. 10.1177/2050640617728001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt A, Beyna T, Schumacher B, et al. Colonoscopic full-thickness resection using an over-the-scope device: a prospective multicentre study in various indications. Gut 2018;67:1280-9. 10.1136/gutjnl-2016-313677 [DOI] [PubMed] [Google Scholar]
- 52.Valli PV, Mertens J, Bauerfeind P. Safe and successful resection of difficult GI lesions using a novel single-step full-thickness resection device (FTRD((R))). Surg Endosc 2018;32:289-99. 10.1007/s00464-017-5676-9 [DOI] [PubMed] [Google Scholar]
- 53.Andrisani G, Pizzicannella M, Martino M, et al. Endoscopic full-thickness resection of superficial colorectal neoplasms using a new over-the-scope clip system: A single-centre study. Dig Liver Dis 2017;49:1009-13. 10.1016/j.dld.2017.04.015 [DOI] [PubMed] [Google Scholar]
- 54.Sumiyama K, Gostout CJ, Rajan E, et al. Submucosal endoscopy with mucosal flap safety valve. Gastrointest Endosc 2007;65:688-94. 10.1016/j.gie.2006.07.030 [DOI] [PubMed] [Google Scholar]
- 55.Yoshizumi F, Yasuda K, Kawaguchi K, et al. Submucosal tunneling using endoscopic submucosal dissection for peritoneal access and closure in natural orifice transluminal endoscopic surgery: a porcine survival study. Endoscopy 2009;41:707-11. 10.1055/s-0029-1214959 [DOI] [PubMed] [Google Scholar]
- 56.Pauli EM, Moyer MT, Haluck RS, et al. Self-approximating transluminal access technique for natural orifice transluminal endoscopic surgery: a porcine survival study (with video). Gastrointest Endosc 2008;67:690-7. 10.1016/j.gie.2007.09.023 [DOI] [PubMed] [Google Scholar]
- 57.Kumta NA, Mehta S, Kedia P, et al. Peroral endoscopic myotomy: establishing a new program. Clin Endosc 2014;47:389-97. 10.5946/ce.2014.47.5.389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu MD, Cai MY, Zhou PH, et al. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 2012;75:195-9. 10.1016/j.gie.2011.08.018 [DOI] [PubMed] [Google Scholar]
- 59.Liu BR, Song JT. Submucosal Tunneling Endoscopic Resection (STER) and Other Novel Applications of Submucosal Tunneling in Humans. Gastrointest Endosc Clin N Am 2016;26:271-82. 10.1016/j.giec.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 60.Chen T, Lin ZW, Zhang YQ, et al. Submucosal Tunneling Endoscopic Resection vs Thoracoscopic Enucleation for Large Submucosal Tumors in the Esophagus and the Esophagogastric Junction. J Am Coll Surg 2017;225:806-16. 10.1016/j.jamcollsurg.2017.09.002 [DOI] [PubMed] [Google Scholar]
- 61.Inoue H, Ikeda H, Hosoya T, et al. Submucosal endoscopic tumor resection for subepithelial tumors in the esophagus and cardia. Endoscopy 2012;44:225-30. 10.1055/s-0031-1291659 [DOI] [PubMed] [Google Scholar]
- 62.Li QY, Meng Y, Xu YY, et al. Comparison of endoscopic submucosal tunneling dissection and thoracoscopic enucleation for the treatment of esophageal submucosal tumors. Gastrointest Endosc 2017;86:485-91. 10.1016/j.gie.2016.11.023 [DOI] [PubMed] [Google Scholar]
- 63.Liu BR, Song JT, Kong LJ, et al. Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc 2013;27:4354-9. 10.1007/s00464-013-3023-3 [DOI] [PubMed] [Google Scholar]
- 64.Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet 2013;382:973-83. 10.1016/S0140-6736(13)60106-3 [DOI] [PubMed] [Google Scholar]
- 65.Li QL, Chen WF, Zhang C, et al. Clinical impact of submucosal tunneling endoscopic resection for the treatment of gastric submucosal tumors originating from the muscularis propria layer (with video). Surg Endosc 2015;29:3640-6. 10.1007/s00464-015-4120-2 [DOI] [PubMed] [Google Scholar]
- 66.Hu JW, Zhang C, Chen T, et al. Submucosal tunneling endoscopic resection for the treatment of rectal submucosal tumors originating from the muscular propria layer. J Cancer Res Ther 2014;10 Suppl:281-6. 10.4103/0973-1482.151533 [DOI] [PubMed] [Google Scholar]
- 67.Du C, Linghu E. Submucosal Tunneling Endoscopic Resection for the Treatment of Gastrointestinal Submucosal Tumors Originating from the Muscularis Propria Layer. J Gastrointest Surg 2017;21:2100-9. 10.1007/s11605-017-3579-7 [DOI] [PubMed] [Google Scholar]
- 68.Ye LP, Zhang Y, Mao XL, et al. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc 2014;28:524-30. 10.1007/s00464-013-3197-8 [DOI] [PubMed] [Google Scholar]
- 69.Wang H, Tan Y, Zhou Y, et al. Submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors originating from the muscularis propria layer. Eur J Gastroenterol Hepatol 2015;27:776-80. 10.1097/MEG.0000000000000394 [DOI] [PubMed] [Google Scholar]
- 70.Du C, Ma L, Chai N, et al. Factors affecting the effectiveness and safety of submucosal tunneling endoscopic resection for esophageal submucosal tumors originating from the muscularis propria layer. Surg Endosc 2018;32:1255-64. 10.1007/s00464-017-5800-x [DOI] [PubMed] [Google Scholar]
- 71.Lv XH, Wang CH, Xie Y. Efficacy and safety of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors: a systematic review and meta-analysis. Surg Endosc 2017;31:49-63. 10.1007/s00464-016-4978-7 [DOI] [PubMed] [Google Scholar]
- 72.Chen T, Zhang C, Yao LQ, et al. Management of the complications of submucosal tunneling endoscopic resection for upper gastrointestinal submucosal tumors. Endoscopy 2016;48:149-55. [DOI] [PubMed] [Google Scholar]
- 73.Chen T, Zhou PH, Chu Y, et al. Long-term Outcomes of Submucosal Tunneling Endoscopic Resection for Upper Gastrointestinal Submucosal Tumors. Ann Surg 2017;265:363-9. 10.1097/SLA.0000000000001650 [DOI] [PubMed] [Google Scholar]
- 74.Tan Y, Tang X, Guo T, et al. Comparison between submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric stromal tumors originating from the muscularis propria layer. Surg Endosc 2017;31:3376-82. 10.1007/s00464-016-5350-7 [DOI] [PubMed] [Google Scholar]
- 75.Duan TY, Tan YY, Wang XH, et al. A comparison of submucosal tunneling endoscopic resection and endoscopic full-thickness resection for gastric fundus submucosal tumors. Rev Esp Enferm Dig 2018;110:160-5. [DOI] [PubMed] [Google Scholar]