In this editorial, we propose the application of the Six Sigma framework to reduce the problem of medication nonadherence, a global epidemic that creates a large impact on health care systems worldwide, due to increased mortality and the economic burden of hospitalization. Nonadherence may adversely affect the safety and efficacy of the treatment and may also lead to drug resistance. For example, in a recent study from this journal of nonadherence to uric acid–lowering therapy in the treatment of gout, pharmacokinetic‐pharmacodynamic modelling showed that both a single drug holiday of increasing duration and random missed doses resulted in increased concentrations of urinary uric acid, potentially leading to kidney damage. The effects were proportional to the length of the drug holiday and the number of missed doses.1 Such studies highlight the importance of precisely defining and monitoring adherence behaviours. Furthermore, in some cases of apparent resistance to therapy, the problem may not lie with the treatments themselves but with undiagnosed suboptimal adherence to medications. As an important public health consideration, interest in the problem of medication nonadherence has grown, with the number of publications increasing exponentially during the 40‐year period since the first studies in the mid‐1970s to more than 1200 articles2 in 2015.
With this increase in interest, the terminology surrounding medication adherence and nonadherence has grown and evolved over time; however, as a result, the language has become ambiguous and some of the more commonly used terms have different meanings for different people. In response to this, the European Society for Patient Adherence, Compliance and Persistence (ESPACOMP) has endorsed the ABC taxonomy to define medication adherence and developed the EMERGE reporting guidelines3 with the aim of providing a common language for researchers and clinicians, thereby standardizing medical literature and facilitating health policy decisions.4 This taxonomy defines medication adherence as the process by which patients take their medication as prescribed. It is divided into three important steps: (a) initiation, (b) implementation, and (c) discontinuation. Initiation of therapy occurs when the patient takes the first dose of a prescribed medication and is a binary event.4 Implementation of a dosing regimen is the extent to which the actual dosing regimen follows the prescribed dosing regimen (ie, dosing history).4 Discontinuation, or the end of therapy, is related to persistence, which is the time from initiation until the last dose (ie, time to event).4 There are over 700 factors associated with medication adherence,5 and these may occur at any of the steps; therefore, medication adherence is hardly predictable. Patients with low levels of trust and substantial concerns about prescribed medicines may not initiate medication despite having filled the prescription. Examples of nonadherence during implementation include not taking doses on time, missing doses, and taking extra doses to compensate for missed doses and drug holidays, while nonadherence in persistence occurs when patients discontinue treatment earlier than expected for various reasons.
With the multitude of factors affecting medication adherence, it would not be feasible to solve these with a single “magic solution.” Despite this, in recently published research, there appears to be a focus on reminding patients to take medication in real time. These reminders can range from simple pill boxes or slide devices to smart tablets that show whether the tablet has reached the stomach.6, 7 However, these tend to focus on the single problem of forgetfulness; repeated reminders may also become intrusive and cumbersome for patients who may then disengage from, or worse, discontinue therapy. These also do not consider the problem of intentional nonadherence, which occurs when the patient deliberately does not follow the treatment regimen. In addition, some studies are conceptually confusing because they do not follow the ABC taxonomy. For example, there is often a disconnect between the problem (eg, persistence) and the proposed solution (eg, improving implementation through reminders).6 The current state of adherence research is analogous to testing whether a helmet is useful when jumping out of a plane. Just as the best safety device to use depends on the emergency situation, the most appropriate solution to medication adherence depends on the underlying cause of the problem.
To properly address the issue of medication adherence, one should consider its multidimensional aspect and reduce the resulting variability in outcomes by applying the Six Sigma framework in the context of personalized medicine. First developed in the telecommunications industry, the Six Sigma framework applies the scientific method to existing systems and processes with the aim of reducing variability and improving quality. By reducing the factors that contribute to medication nonadherence, the Six Sigma approach may reduce variability in drug exposure, improve health care outcomes, and decrease costs; however, it does require a significant investment of money and time, particularly as those involved in applying the framework (eg, health care practitioners) must be trained in its use. An example of the use of Six Sigma within the health care setting is the improvement of the process relating to the timing of prophylactic antibiotic administration prior to surgery, which resulted in 86% of patients receiving antibiotics within the target of less than or equal to 60 minutes prior to surgery, as opposed to the baseline of 38%.8 Some of the tools used in Six Sigma have also been utilized to identify the reasons for failing to adhere to a diabetes programme in Colombia and to develop proposals to reduce these barriers to adherence. However, the efficacy of these proposals has not been reported.9 Recent developments in our understanding of the problem of medication nonadherence, including sound taxonomy, reliable and precise measures, and appropriate analysis, together with an increased recognition of the need for a multidisciplinary approach and tailored interventions, mean that for the first time, the necessary requirements for a Six Sigma approach to this problem are now fulfilled.
The DMAIC (Define, Measure, Analyse, Improve and Control) approach, a component of the Six Sigma framework, involves defining the problem (eg, using a robust taxonomy), measuring the performance to define the baseline, using sound analysis to determine the root causes of the problem, improving performance by addressing the root causes, and controlling the improved process: measurement‐guided adherence management (Figure 1). To date, the Six Sigma framework has not been formally applied to medication adherence; however, it would be an ideal approach to adopt in addressing the growing need for research in this area, as evidenced by a recent National Institutes of Health call for grant applications relating to patient adherence.10 For example, the most effective strategy in managing medication adherence has been found to involve providing feedback of electronic adherence data to patients,11 and although not explicitly stated, this approach constitutes the basis of the Six Sigma framework, as patients and physicians can determine the causes of nonadherence and discuss solutions. Recent publications have determined characteristics that are associated with nonadherence12, 13 and have proposed tools to estimate adherence.14 The use of Six Sigma may clarify how to interpret these results and how to apply them in measurement‐guided adherence management. Studies must be performed to determine the impact of applying Six Sigma as a new approach to ultimately determine whether it will be useful in the long term for both individual and groups of patients. If successful, utilizing the Six Sigma framework would lead to an understanding of the barriers to adherence, allow customization of the approaches to address those barriers (involving a collaboration between patients and health care practitioners), and highlight nonadherence as the norm in society, so that health care practitioners can acknowledge and anticipate this as potentially the largest source of variability in drug response within the care of their patients.
Figure 1.
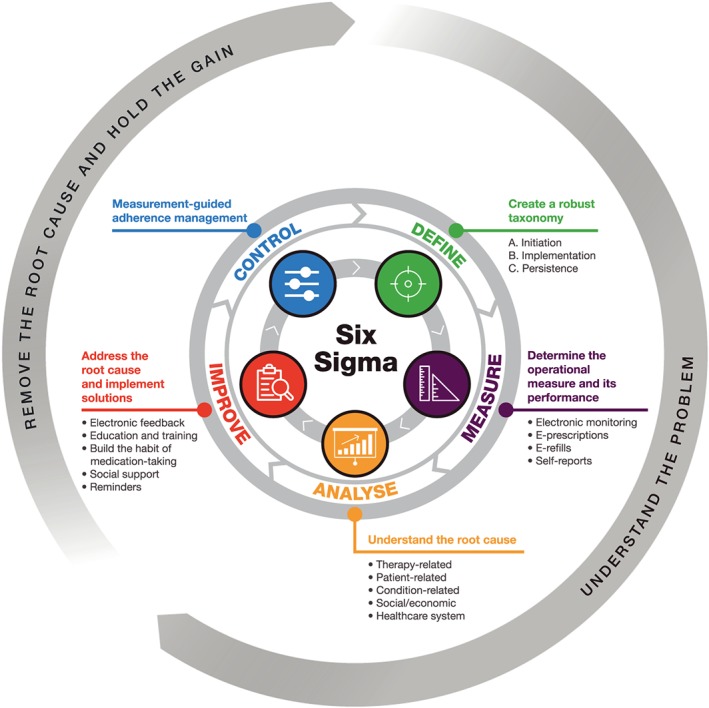
Proposed DMAIC approach for improving medication adherence
ACKNOWLEDGEMENTS
This work was supported by Pfizer Inc. Medical writing support, under the guidance of the author, was provided by Christina Viegelmann, PhD, of CMC Connect, a division of McCann Health Medical Communications Ltd, Glasgow, UK, and was funded by Pfizer Inc, New York, NY, USA, in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015; 163: 461–464).
Vrijens B. A Six Sigma framework to successfully manage medication adherence. Br J Clin Pharmacol. 2019;85:1661–1663. 10.1111/bcp.13905
REFERENCES
- 1. Hill‐McManus D, Soto E, Marshall S, et al. Impact of non‐adherence on the safety and efficacy of uric acid‐lowering therapies in the treatment of gout. Br J Clin Pharmacol. 2018;84:142‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kronish IM, Moise N. In search of a “magic pill” for medication nonadherence. JAMA Intern Med. 2017;177(5):631‐632. [DOI] [PubMed] [Google Scholar]
- 3. De Geest S, Zullig LL, Dunbar‐Jacob J, et al. ESPACOMP Medication Adherence Reporting Guideline (EMERGE). Ann Intern Med. 2018;169(1):30‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vrijens B, De Geest S, Hughes DA, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol. 2012;73:691‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choudhry NK, Krumme AA, Ercole PM, et al. Effect of reminder devices on medication adherence: the REMIND randomized clinical trial. JAMA Intern Med. 2017;177(5):624‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peters‐Strickland T, Pestreich L, Hatch A, et al. Usability of a novel digital medicine system in adults with schizophrenia treated with sensor‐embedded tablets of aripiprazole. Neuropsychiatr Dis Treat. 2016;12:2587‐2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Parker BM, Henderson JM, Vitagliano S, et al. Six sigma methodology can be used to improve adherence for antibiotic prophylaxis in patients undergoing noncardiac surgery. Anesth Analg. 2007;104:140‐146. [DOI] [PubMed] [Google Scholar]
- 9. Cancelado Carretero HM, Pachón Serna AM. Enhancement for an adherence process on a diabetes program at an IPS, through Chronic Care Model (CCM), Clinical Decision Support Systems (CDSS) and Lean Six Sigma. In: International Conference on Industrial Engineering and Operations Management, Bogota, Colombia: IEOM Society International, 2017: 1315–1333.
- 10. National Institutes of Health . Improving patient adherence to treatment and prevention regimens to promote health (R01 Clinical Trial Optional). 2018. https://grants.nih.gov/grants/guide/pa‐files/PA‐18‐722.html. Accessed 05/07/2018.
- 11. Demonceau J, Ruppar T, Kristanto P, et al. Identification and assessment of adherence‐enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta‐analysis. Drugs. 2013;73:545‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Avataneo V, De Nicolo A, Rabbia F, et al. Therapeutic drug monitoring‐guided definition of adherence profiles in resistant hypertension and identification of predictors of poor adherence. Br J Clin Pharmacol. 2018;84:2535‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gendelman O, Weitzman D, Rosenberg V, et al. Characterization of adherence and persistence profile in a real‐life population of patients treated with adalimumab. Br J Clin Pharmacol. 2018;84:786‐795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Man WH, Perez‐Pitarch A, Wilting I, et al. Development of a nomogram for the estimation of long‐term adherence to clozapine therapy using neutrophil fluorescence. Br J Clin Pharmacol. 2018;84:1228‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]


