Abstract
Aims
Early pain after laparoscopy is often severe. Oxycodone is a feasible analgesic option after laparoscopy, but there are sparse data on epidural administration. The aim was to evaluate the analgesic efficacy and pharmacokinetics of a single dose of epidural oxycodone as a part of multimodal analgesia after gynaecological laparoscopy.
Methods
Women (n = 60), aged 23–71 years, undergoing elective gynaecological laparoscopy, were administrated either epidural oxycodone 0.1 mg kg−1 and intravenous (i.v.) saline (EPI‐group n = 31), or epidural saline and i.v. oxycodone 0.1 mg kg−1 (IV‐group = 29) in a randomised, double blind, active control, double dummy clinical trial. A pharmacokinetic model was developed using population modelling of plasma and cerebrospinal fluid (CSF) concentrations obtained in these patients and data of 2 published studies. The primary outcome was the amount of i.v. fentanyl for rescue analgesia during the first 4 hours.
Results
Twenty of the 31 patients in the EPI‐group and 26 of the 29 patients in the IV‐group needed i.v. fentanyl for rescue analgesia, P = .021. The median (interquartile range) number of fentanyl doses were 1.0 (1.0–3.0) in the EPI‐group and 2.5 (1.0–4.0) doses in the IV‐group, P = .008. Plasma concentrations were similar, but CSF concentrations were 100‐fold higher in the EPI‐group. The population model indicated that 60% of oxycodone injected into the epidural space enters into CSF and 40% is absorbed into the systemic circulation.
Conclusions
The data support superiority of epidural administration of oxycodone compared to i.v. administration during the first hours after laparoscopic surgery. This is likely to be based on enhanced permeation into the central nervous system after epidural administration.
Keywords: analgesia, epidural, oxycodone, pharmacokinetics
What is already known about this subject
Early pain after laparoscopic surgery is often substantial and effective analgesia is required.
Oxycodone is a highly efficient opioid analgesic especially in visceral pain, but epidural administration has not been established.
In laparotomy patients, epidural oxycodone is superior to intravenous administration, but there are few data concerning laparoscopic surgery.
What this study adds
This study shows that epidural administration of oxycodone is a feasible administration route in acute pain management in those patients with an epidural catheter.
Plasma concentrations of oxycodone were similar after epidural and intravenous administration, but cerebrospinal fluid concentrations were 100‐fold higher after epidural injection indicating rapid central nervous system penetration.
In acute pain management, 0.1 mg kg−1 of oxycodone seems to be an optimal initial dose for epidural administration, as the majority of patients needed none or only 1 dose of rescue analgesic during the early recovery phase.
1. INTRODUCTION
A laparoscopic approach is used increasingly in major surgery also. Contrary to common belief, pain after laparoscopic surgery can be substantial, particularly in the first postoperative hours.1, 2 Thus, efficient pain treatment is needed to allow calm recovery. Pain after laparoscopic surgery is derived from multiple origins. Initially, it is nociceptive somatic pain from abdominal wall and visceral intra‐abdominal organs. Later, surgical trauma induces inflammatory pain that is often the main pain component after the early hours.3 Oxycodone is a potent opioid analgesic for nociceptive pain and highly efficient in visceral pain, and therefore a feasible component in multimodal pain management in early postoperative pain after laparoscopic surgery.3, 4, 5
Intravenous (i.v.) administration of opioids is often used in acute postoperative pain management. Data concerning fentanyl and particularly morphine indicate that intrathecal administration is a highly effective6, 7 but few data are available for epidural oxycodone. After laparoscopic hysterectomy i.v. oxycodone has been shown to be more potent than i.v. morphine.4, 5 Our recent data indicate that oxycodone could also be a feasible opioid in epidural analgesia.8, 9 In our previous clinical trial, epidural oxycodone provided superior early postoperative analgesia to i.v. oxycodone after gynaecological laparotomy9 but no such data are available for laparoscopic surgery. Moreover, central nervous system pharmacokinetics (PK) of oxycodone is sparsely described.
In this clinical trial, our primary aim was to assess the analgesic efficacy of a single dose of epidural oxycodone as a part of multimodal analgesia in early postoperative pain management after gynaecological laparoscopy. Secondly, we have assessed the PK of epidural oxycodone in cerebrospinal fluid (CSF) and plasma, and a population PK‐model was developed to describe CSF and plasma concentrations after these 2 administration routes. Our study hypothesis was that epidural oxycodone would provide superior analgesic efficacy compared to i.v. administration.
2. METHODS
The Research Ethics Committee of the Hospital District of Northern Savo, Kuopio, Finland approved the study protocol (ref: 83//2014). The study was registered in EudraCT (ref: 2014–004313‐82) and the Finnish Medicines Agency was notified (ref: 115/2014). The study was conducted in accordance with the Declaration of Helsinki between May 2015 and December 2017 at the Kuopio University Hospital and had institutional approval. The study design was a prospective, randomised, double‐blind, active control, double‐dummy clinical trial with 2 parallel groups. This study is a part of our study project where we evaluate the use of epidural oxycodone in different experimental and clinical situations.
We enrolled 60 patients aged 23–71 years scheduled for elective gynaecological laparoscopy with planned epidural analgesia for postoperative pain management. We did not enrol patients who were unwilling to participate, underwent major oncologic surgery, had allergy/hypersensitivity to oxycodone, paracetamol or dexketoprofen, or any ingredients in the formulations, had reduced respiratory function, had defects in the vertebral column that were likely to complicate the placement of epidural catheter, were pregnant or nursing, had a bleeding disorder or were on an anticoagulant therapy, had participated in a drug trial during the previous month, or who had used oxycodone or MAO‐, CYP3A‐ or CYP2D6 inhibitors during the previous 4 weeks.
Seventy‐nine patients were asked and 60 agreed to participate. The reasons for declining were: did not want any additional procedures (n = 5), feared the postpuncture headache (n = 4), were afraid of stinging (n = 1), had a severe illness (n = 1), was allergic to ketoprofen (n = 1) and no specific reason (n = 7).
After informed consent, participants were randomised with a random organisation generator (www.randomisation.com) into 2 parallel groups. The patients were administered either epidural oxycodone 0.1 mg kg−1 (Oxanest 10 mg mL−1; Takeda, Helsinki, Finland) and i.v. saline (EPI‐group) or epidural saline and i.v. oxycodone 0.1 mg kg−1 (IV‐group) immediately after arriving into the postanaesthesia care unit and after the baseline pain assessment. The oxycodone and saline containing syringes were prepared by a study nurse who did not otherwise participate in the study or patient care. The study drug formulations were both clear and colourless liquids, thus ensuring blinding. A flow chart is presented in Figure 1.
Figure 1.
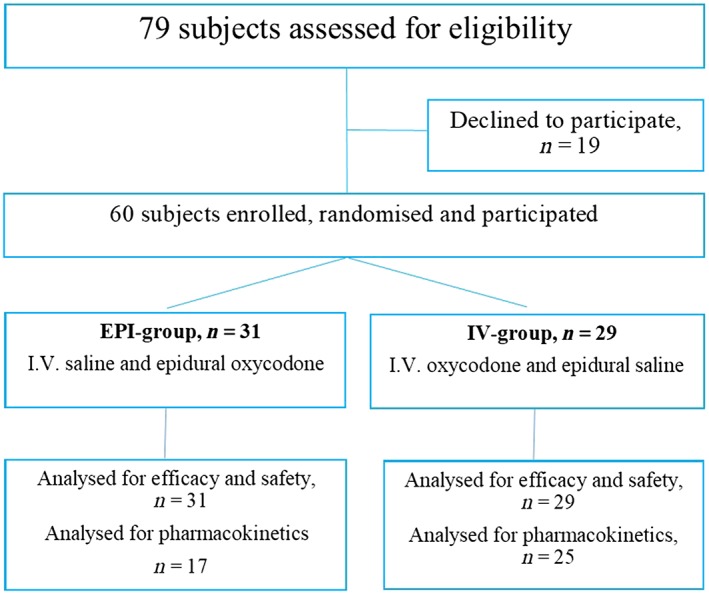
Flow chart
2.1. Anaesthesia and surgery
The endotracheal anaesthesia protocol was standardised. Briefly, 10 mg diazepam and 1 g paracetamol were given by mouth for premedication. An epidural catheter was placed at interspace Th10‐Th12 before anaesthesia induction and tested for i.v. or spinal misplacement with a lidocaine‐epinephrine admixture. General endotracheal anaesthesia with propofol, rocuronium, remifentanil and sevoflurane was administrated to patients. At the end of the anaesthesia, propofol infusion and sevoflurane inhalation were discontinued, muscle relaxation was reversed with sugammadex 1–2 mg kg−1, and the tracheal tube was removed when train‐of‐4 ratio was 0.9 or higher. Remifentanil infusion was continued at a rate of 100 μg h−1 until the study drug administration.
Drug injection oxycodone hydrochloride trihydrate 0.1 mg kg−1 was diluted to 10 mL with normal saline and 10 mL of normal saline was used as placebo. The EPI‐group received 1 dose of epidural oxycodone and i.v. placebo, and the IV‐group received 1 dose of epidural placebo and i.v. oxycodone. The study drugs were given simultaneously as 5‐minute infusions after the patient had arrived in the postanaesthesia care unit, had emerged from anaesthesia to respond to verbal commands and had evaluated pain with an 11‐point numeric rating scale (NRS, 0 = no pain, 10 = most pain) at rest, during coughing and wound compression. The wound area was compressed with a 20 N force (2 kg pressure with 3 fingers for a 10 cm2 area).2
For background analgesia all patients were given i.v. paracetamol 1 g 3 times a day and i.v. dexketoprofen 50 mg 3 times a day. The first dose of i.v. paracetamol was given 15 minutes and the first dose of dexketoprofen 60 minutes after the study drugs administration. For rescue analgesia, patients were given i.v. fentanyl 50 μg when pain at rest was ≥3/10 and/or during coughing/wound compression ≥5/10.
Pain was assessed continuously and recorded at 30 minutes intervals during the first 4 hours and after that at every 6 hours for the next 20 hours. Arterial blood pressure, heart rate, respiratory rate, peripheral capillary oxygen saturation, exhaled carbon dioxide and sedation score with a 10‐point Richmond agitation sedation scale (−5 = unarousable, 4 = combative) were monitored for the first 24 hours.
After the first 4 hours, patients were admitted to postoperative ward and postoperative analgesia was continued with an epidural infusion of an admixture of levobupivacaine (0.6 mg mL−1), fentanyl (4 μg mL−1) and epinephrine (2 μg mL−1) as a standard treatment of the hospital. Infusion rate was 2–8 mL h−1 and 2 mL boluses of the triple mixture were given as needed to keep the pain scores <3/10 at rest and < 5/10 during coughing and wound compression. No more oxycodone was given to the patients before the end of the 24‐hour study period. Patients' satisfaction with the analgesia was assessed at 24 hours with an 11‐point NRS (0 = totally dissatisfied, 10 = totally satisfied).
2.2. Efficacy and safety outcomes
The primary outcome measure was the total dose of rescue fentanyl during the first 4 postoperative hours. The secondary outcomes were the time from the study drug administration to the first dose of rescue fentanyl, pain scores, summed pain intensity (SPI) and the incidence of adverse effects during the first 24 postoperative hours. SPI was determined calculating the area under the curve (AUC) for pain scores using the trapezoidal rule. Adverse effects were actively asked for and recorded at each time of pain evaluation.
2.3. Pharmacokinetic outcomes
A paired blood (5 mL) and CSF sample (1 mL) was collected from 42 patients at a random time during the first 4 hours after the test drug injection. A lumbar puncture was performed at L4‐L5 with a 27G pencil‐point needle for CSF oxycodone assay and a blood sample was collected from the contralateral arm to the study compound administration. The oxycodone and metabolite (oxymorphone, noroxycodone and noroxymorphone) concentrations in plasma and CSF were measured with an ultraperformance liquid chromatographic system described earlier.9 The lower limit of quantification was 0.05 ng mL−1 for oxycodone and oxymorphone, 0.2 ng mL−1 for noroxycodone and 0.5 ng mL−1 for noroxymorphone, the accuracy of the assay 80–120% and the coefficient of variation below 20%.
2.4. Statistical analysis
The sample size calculation was based on our pilot pharmacokinetic study where the mean (standard deviation) need for rescue i.v. fentanyl was with epidural oxycodone 0.08 (0.10) mg and with i.v. oxycodone 0.16 (0.66) mg during the first hours after gynaecological laparoscopy.8 To show a 0.08‐mg difference in rescue i.v. fentanyl, 30 subjects per group would be needed to achieve 0.8 power at α = 0.05 (2‐sided test). To allow dropouts, the original aim was to recruit 35 subjects in both groups, but for logistic reasons, a total of 60 subjects were enrolled.
The data were recorded and analysed using SPSS software (IBM SPSS Statistics 25, International Business Machines Corporation, Armonk, NY, USA). Distribution of continuous data were checked visually, and normal distribution assumption was checked with Shapiro–Wilk test. Analysis of normal distributed continuous data were performed with 2‐sample t‐test assuming equal variances. Equality of variances was tested with Levine's test. Mann–Whitney U‐test was used when continuous data was not normally distributed. For multiple comparisons the Bonferroni correction was applied. Categorical data were analysed using the χ2 test. Data are presented as number of cases and mean (standard deviation), and when data were not normal distributed median (interquartile range) are presented. A P‐value of <.05 was considered statistically significant.
2.5. Population PK analysis
Data from the current analysis were pooled with those from earlier analyses that included 48 women, aged 24–67 years, undergoing elective gynaecological surgery. Either intravenous oxycodone or epidural oxycodone was administered as a single dose of 0.1 mg kg–1. An epidural catheter for drug administration was placed at T12‐L1 and a spinal catheter for CSF sampling at L3‐L4 for 30 women, and a paired blood and CSF sample was collected from 18 women with a lumbar puncture at L4‐L5. Plasma and CSF were collected for the analysis of oxycodone at 2, 5, 15, 30 and 45 minutes, and 1, 2, 4, 8, 12 and 24 hours.8, 9
A 2‐compartment (central V1 and peripheral V2) linear disposition model was used to fit oxycodone plasma concentration (Cp, ng mL−1) data. This analysis was parameterised in terms of central volume (V1, L), peripheral volume (V2, L), clearance (CL, L h−1) and intercompartment clearance (Q, L h−1) and solved using differential equations. A third compartment was used to model CSF concentration (C CSF , ng mL−1). Input from the epidural space to the central compartment or CSF was characterised using a rate constant (Ka), parameterised as an absorption half‐time (TABS):
The CSF compartment was assigned a volume of 150 mL10 and was linked to the central compartment using an intercompartment clearance (Q CSF , L h−1). A partition coefficient (PC) was used to describe the ratio between CSF and plasma concentration at steady‐state (Figure 2). Allometry was used to scale PK parameter estimates to a 70‐kg person.11, 12
Figure 2.
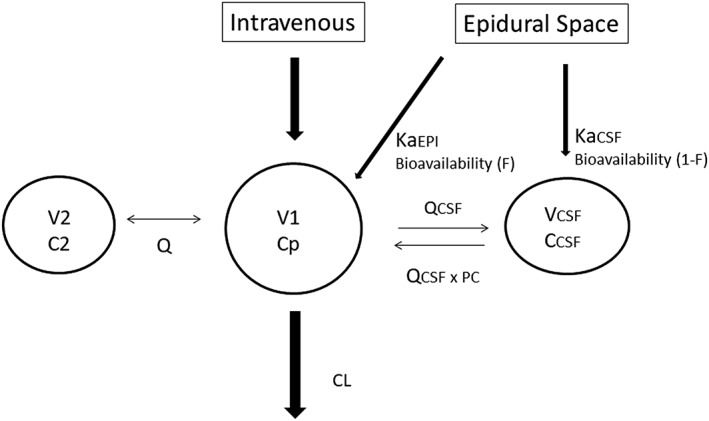
Pharmacokinetic schematic model. A 2‐compartment (central V1 and peripheral V2) linear disposition model was used to fit oxycodone plasma concentration (Cp, ng mL−1) data. Drug is cleared (CL, clearance) from the central compartment (V1, Cp). A third compartment was used to model cerebrospinal fluid (CSF) concentration (CCSF, ng mL−1). Input from the epidural space to the central compartment (KaEPI, L−1) or CSF (KaCSF, L−1) was characterised using rate constants (Ka). The CSF compartment was given a volume of 150 mL and was linked to the central compartment using an intercompartment clearance (QCSF, L h−1). A partition coefficient (PC) was used to describe the ratio between CSF and plasma concentration at steady‐state
Population parameter estimates were obtained using NONMEM 7.3 (Globomax LLC, Hanover, MD, USA). This model accounts for population parameter variability (between subjects) and residual variability (random effects) as well as parameter differences predicted by covariates (fixed effects). The population parameter variability was modelled in terms of random effect (η) variables. Each of these variables was assumed to have mean 0 and a variance denoted by ω2, which was estimated. The between‐subject variability in model parameters was modelled by exponentiating random effects.
The covariance of clearance and distribution volume variability was incorporated into the model. Residual unidentified variability (RUV) was modelled using both proportional and additive residual errors for plasma and CSF data. The between‐subject variability (ηRUV,i) of the RUV was also estimated. The population mean parameters, between‐subject variance and residual variance were estimated using the first order conditional interaction estimate method using with differential equations (ADVAN6 TOL5 of NONMEM VII). Convergence criterion was 3 significant digits. Model selection required an improvement in the NONMEM objective function between nested models, equating to a reduction >3.84 based on a χ2 distribution (α < 0.05). Bootstrap methods were used to evaluate uncertainty associated with parameter estimates.13 A total of 100 replications were used to estimate parameter confidence intervals. Visual predictive checks were used to evaluate how well the model predicted the distribution of observed concentrations in both plasma and CSF.14
2.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,15 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.16
3. RESULTS
All patients completed the 24 hours follow up and thus, for logistic reasons, the study was terminated when 60 subjects had been studied. There were no drop‐outs or protocol violations likely to influence the results and all patients were included in to the analysis, 31 patients in the EPI‐group and 29 patients in the IV‐group. Patient characteristics are presented in Table 1. Intraoperative remifentanil dose was similar in the 2 groups: median (interquartile range) 1.2 (0.82–1.9) mg in the EPI‐group and 1.5 (0.90–1.9) mg in the IV‐group, P = .89.
Table 1.
Patient characteristics. Data are mean (standard deviation), or median (interquartile range) or number of cases
| Variable | EPI‐group | IV‐group |
|---|---|---|
| n = 31 | n = 29 | |
| Age (y) | 48 (37–60) | 55 (45–66) |
| Weight (kg) | 72 (13) | 74 (15) |
| Height (cm) | 162 (4.9) | 165 (6.9) |
| BMI (kg m −2 ) | 27 (4.3) | 27 (4.7) |
| ASA, I/II/III | 6/21/4 | 7/18/4 |
| Duration of surgery (min) | 210 (98) | 220 (111) |
| Intraoperative bleeding (mL) | 130 (50–550) | 150 (44–210) |
ASA = American Society of Anesthesiologists physical status classification; BMI = body mass index
A total of 20 of the 31 patients in the EPI‐group and 26 of the 29 patients in the IV‐group required rescue i.v. fentanyl during the first 4 postoperative hours, P = .021. The total number of rescue fentanyl doses was 47 in the EPI‐group and 81 in the IV‐group. The median number of fentanyl doses was significantly less, 1.0 (1.0–3.0) doses, after epidural oxycodone than that after i.v. oxycodone, 2.5 (1.0–4.0) doses, P = .008, (Figure 3).
Figure 3.
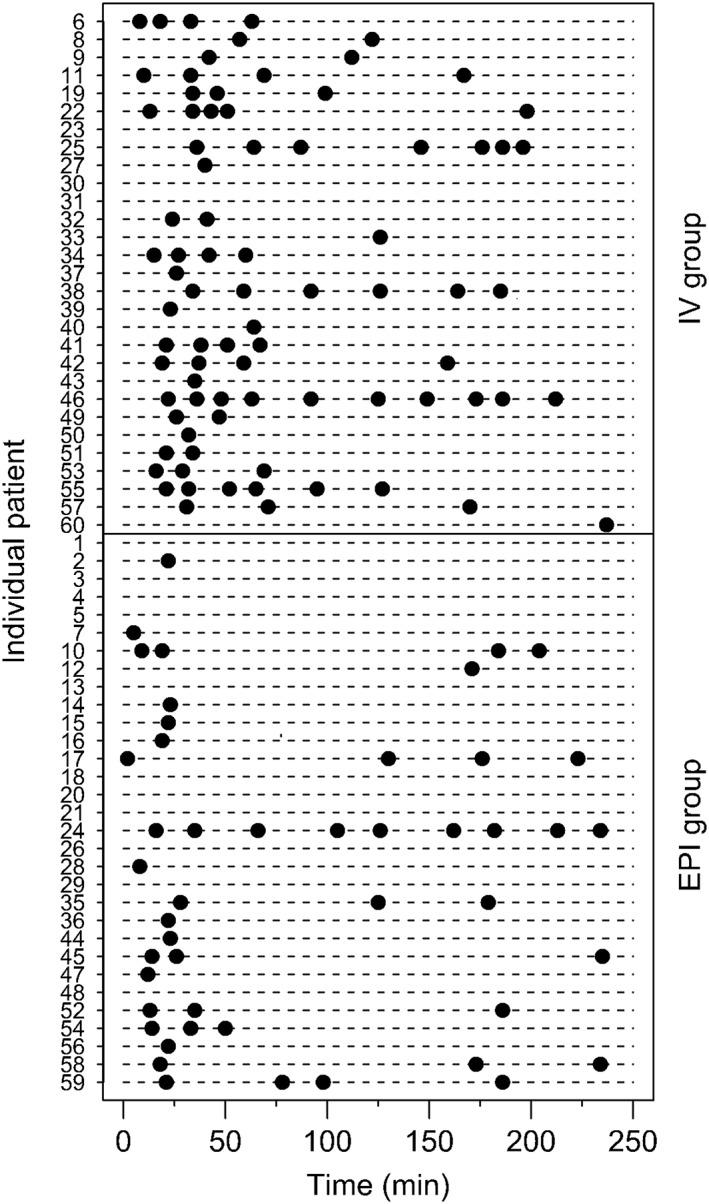
Fentanyl doses during the first 4 hours
Among those patients who needed rescue analgesia, the mean time to the first dose of rescue fentanyl was less in the EPI‐group, 24 (35) minutes, compared to the IV‐group, 40 (46) minutes, P = .003. In the EPI‐group half of the patients with rescue analgesic (10/20) received only a single dose of fentanyl during the first 23 minutes after the study drug administration, and 1 patient received a single dose at 171 minutes. In the IV‐group, 8 patients received a single dose and 18 received 2–10 doses of fentanyl until comfortable. Nineteen patients in the EPI‐group and 13 patients in the IV‐group needed rescue fentanyl during the first 30 minutes after the study drugs administration, P = .21.
There were no differences in baseline pain scores between the 2 groups. The pain scores at rest, during coughing and during wound compression at 30–60 minutes after study drug administration were lower in the EPI‐group compared to the IV‐group, Table 2. The mean of SPI (AUC for pain scores) for the first 4 postoperative hours was lower in the EPI‐group at rest, 301 (271), during couching, 615 (421), and during wound compression, 671 (447), than in the IV‐group, 511 (596; P = .001), 766 (322; P = .023), and 846 (344; P = .038), respectively.
Table 2.
Pain scores in the 2 groups during the first 4 postoperative hours. Data are mean (standard deviation)
| EPI‐group | IV‐group | P‐value with Bonferroni correction | |
|---|---|---|---|
| n = 31 | n = 29 | ||
| Baseline | |||
| • rest | 5.8 (2.3) | 5.6 (2.1) | |
| • coughing | 6.0 (2.3) | 5.8 (2.2) | |
| • wound compression | 6.0 (2.3) | 6.0 (2.3) | |
| At 30 min | |||
| • rest | 2.0 (2.1) | 3.8 (2.6) | 0.01 |
| • coughing | 2.9 (2.2) | 4.5 (2.6) | 0.04 |
| • wound compression | 2.6 (2.1) | 4.9 (2.5) | 0.001 |
| At 60 min | |||
| • rest | 0.6 (1.3) | 2.8 (2.6) | 0.001 |
| • coughing | 1.8 (2.0) | 3.7 (1.8) | 0.001 |
| • wound compression | 1.9 (2.3) | 4.0 (1.7) | 0.001 |
| At 2 h | |||
| • rest | 0.7 (1.2) | 1.3 (1.4) | 0.24 |
| • coughing | 2.1 (2.1) | 2.4 (1.7) | 0.56 |
| • wound compression | 2.4 (2.3) | 2.8 (1.8) | 0.46 |
| At 4 h | |||
| • rest | 1.4 (1.5) | 1.2 (1.5) | 0.6 |
| • coughing | 3.0 (2.2) | 2.7 (1.8) | 0.64 |
| • wound compression | 3.6 (2.4) | 2.9 (1.9) | 0.25 |
Patient satisfaction for postoperative analgesia was similarly high in both groups: 9.8 (0.4) in the EPI‐group and 9.9 (0.4) in the IV‐group, P = .39.
In the EPI‐group 25 patients had a total of 44 adverse effects, and in the IV‐group 18 patients had a total of 26 adverse effects, P = .11 (Table 3). The most common adverse effects were: postoperative nausea and vomiting (n = 10 in the EPI‐group and n = 9 in the IV‐group), and pruritus (n = 17 and n = 8). Six patients in the EPI‐group and 4 in the IV‐group had a respiratory rate <10 breaths min−1, but no interventions were needed, and the recovery was uneventful in all 10 patients.
Table 3.
Adverse effects during the first 24 postoperative hours. Data are number of cases
| EPI‐group | IV‐group | |
|---|---|---|
| n = 31 | n = 29 | |
| Patients with adverse effects | 25 | 18 |
| Total number of adverse effects | 44 | 26 |
| Postoperative nausea and vomiting | 10 | 9 |
| Pruritus | 17 | 8 |
| Respiratory rate <10 breaths min −1 | 6 | 4 |
| Headache | 4 | 3 |
| Dizziness | 3 | 1 |
| Numbness | 4 | 1 |
The plasma oxycodone concentrations were similar in the EPI‐group (n = 17) and in the IV‐group (n = 25), but the CSF oxycodone concentrations were much higher in the EPI‐group than those in the IV‐group (Figure 4).
Figure 4.
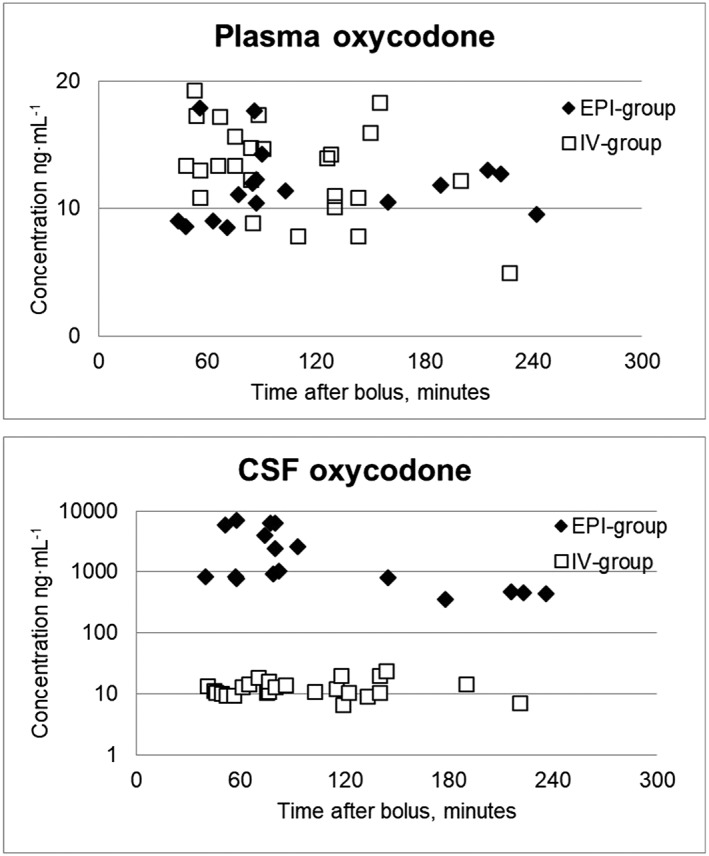
Plasma and cerebrospinal fluid (CSF) oxycodone concentrations in the EPI‐group (n = 17) and in the IV‐group (n = 25)
Noroxycodone was the main metabolite in both groups: in the EPI‐group the median noroxycodone concentrations in plasma was 2.8 (range, 1.0–6.9) ng mL−1 and in CSF 4.0 (1.2–19.4) ng mL−1, and in the IV‐group 4.5 (range, 2.6–8.6) ng mL−1 and in CSF 0.4 (0.0–0.9) ng mL−1. Oxymorphone was detected in CSF in all patients in the EPI‐group, 0.24 (0.07–0.99) ng mL−1 but only in 11 of the 25 patients in the IV‐group, 0.0 (0.0–0.25) ng mL−1, respectively. Plasma concentrations of oxymorphone were low in both groups. Noroxymorphone was detected in CSF in only 1 patient in IV‐group and in none in the EPI‐group. In both groups plasma noroxymorphone was low, median 0.0 (0.0–1.3) ng mL−1 in the EPI‐group and 0.7 (0.0–1.9) ng mL−1 in the IV‐group.
3.1. Population PK
The final pooled data comprised 790 observations (392 plasma, 260 CSF) from 90 patients. Three oxycodone concentrations were reported as less than the lower limit of quantification for either plasma or CSF and these values were replaced by lower limit of quantification/2 (Beal method M5).17 Population parameter estimates and their variability are shown in Table 4. The correlation of between subject variability is shown in Table 5. Figures 5 and 6 serve as visual predictive checks. The 95% predictive intervals encompass most data observations.
Table 4.
Oxycodone population parameter estimates
| Parameter | Estimate | 95%CI | CV (%) |
|---|---|---|---|
| V1 (L 70 kg−1) | 131 | 100, 167 | 62.9 |
| V2 (L 70 kg−1) | 82.6 | 63.6, 124 | 30.9 |
| CL (L h−1 70 kg−1) | 53.2 | 47.7, 59.4 | 42 |
| Q (L h−1 70 kg−1) | 147 | 85, 408 | 142 |
| VCSF (L) | 0.15 (fixed) | ‐ | |
| TABSEPI (h) | 0.404 | 0.221, 2.126 | 220 |
| FEPI | 0.39 | 0.17, 0.58 | 34 |
| TABSCSF (h) | 1.13 | 0.86, 1.55 | 51.6 |
| FCSF | 0.61 | ‐ | ‐ |
| PC | 0.94 | 0.91, 1.0 | ‐ |
| QCSF (L h−1 70 kg−1) | 0.57 | 0.38, 0.74 | 77.5 |
| Plasma additive RUV (ng mL−1) | 0.073 | 0.005, 0.169 | ηRUV 0.292 |
| Plasma proportional RUV (%) | 14.8 | 11.8, 18.7 | |
| CSF additive RUV (ng mL−1) | 3.48 | 1.70, 6.64 | ηRUV 0.28 |
| CSF proportional RUV (%) | 16.8 | 12.0, 39.2 |
95%CI (confidence interval) is precision of estimated parameter estimated by bootstrap analysis.
CV is between subject variability expressed as coefficient of variation. CL is clearance; CSF is cerebrospinal fluid; EPI is epidural; F is fraction; PC is partition coefficient; Q is inter‐compartment clearance; RUV is residual unidentified variability; TABS is an absorption half‐time; V1 is central volume; V2 is peripheral volume; η is random effect variable.
Table 5.
The correlation of between pharmacokinetic subject variability. CL is clearance; CSF is cerebrospinal fluid; EPI is epidural; F is fraction; Q is inter‐compartment clearance; TABS is an absorption half‐time; V1 is central volume; V2 is peripheral volume.
| CL | V1 | V2 | Q | TABSEPI | QCSF | TABSCSF | FEPI | |
|---|---|---|---|---|---|---|---|---|
| CL | 1 | |||||||
| V1 | 0.852 | 1 | ||||||
| V2 | 0.217 | −0.083 | 1 | |||||
| Q | −0.056 | 0.013 | 0.171 | 1 | ||||
| TABS EPI | 0.281 | 0.065 | −0.345 | −0.528 | 1 | |||
| Q CSF | 0.627 | 0.877 | −0.198 | −0.311 | 0.071 | 1 | ||
| TABS CSF | 0.289 | 0.330 | 0.041 | −0.804 | 0.212 | −0.67 | 1 | |
| F EPI | −0.267 | −0.410 | 0.617 | 0.742 | −0.647 | −0.652 | −0.670 | 1 |
Figure 5.
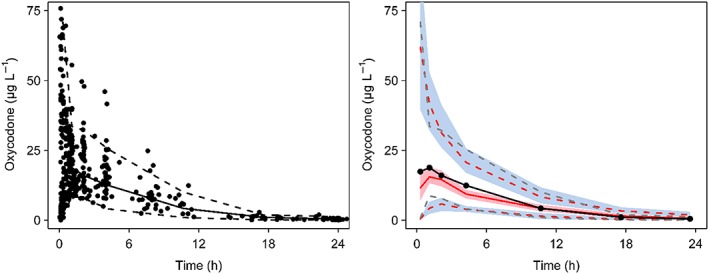
Visual predictive check for the pharmacokinetic models for plasma oxycodone showing median (solid) and 90% intervals (dashed lines). All plots show median (solid) and 90% intervals (dashed lines). Left hand plot shows all prediction corrected observed plasma concentrations. Right hand plot shows prediction corrected percentiles (10%, 50%, and 90%) for observations (black dashed lines) and predictions (pink dashed lines) with 95% confidence intervals for prediction percentiles (median, pink shading; 5th and 95th blue shading)
Figure 6.
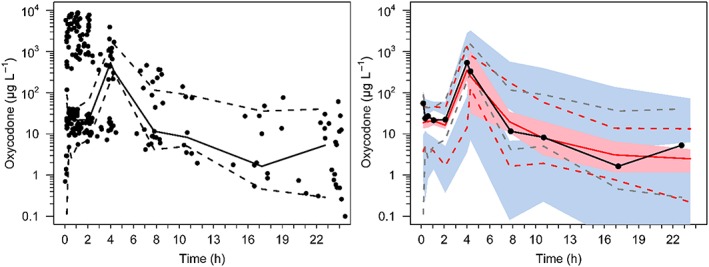
Visual predictive check for the pharmacokinetic models for cerebrospinal fluid oxycodone showing median (solid) and 90% intervals (dashed lines). All plots show median (solid) and 90% intervals (dashed lines). Left hand plot shows all prediction corrected observed CSF concentrations. Right hand plot shows prediction corrected percentiles (10%, 50%, and 90%) for observations (black dashed lines) and predictions (pink dashed lines) with 95% confidence intervals for prediction percentiles (median, pink shading; 5th and 95th blue shading)
4. DISCUSSION
In this clinical trial, epidural oxycodone was superior to i.v. oxycodone during the first 4 hours in postoperative analgesia in women having gynaecological laparoscopic surgery. The patients in the EPI‐group needed less rescue fentanyl and had lower early pain scores compared to the patients in the IV‐group. The novelty of this study was the population‐PK model indicating that 60% of oxycodone injected epidural space may enter CSF and 40% is absorbed into the systemic circulation.
The efficacy data support our previous findings that epidural oxycodone may perform better than the same dose given i.v.8, 9 Moreover, in the present study, half of the patients with epidural oxycodone who needed rescue analgesia during the first 4 postoperative hours received just a single dose of fentanyl during the first 23 minutes. Among those 9 with several doses, half of the rescue analgesic doses were administered during the first 30 minutes and a second peak of rescue fentanyl was at 3–4 hours after the epidural bolus of oxycodone. This may indicate that the onset of action of epidural oxycodone may take up to 30 minutes. The effective dose for pain relief for 50% of laparoscopy patients is approximately 0.1 mg kg−1 and the duration of analgesic action at this dose is a few hours. This was consistent with findings in laparotomy patients also.9
The optimal dosing of epidural oxycodone in postoperative pain has not been established. In this study, at an epidural oxycodone 0.1 mg kg−1 the majority of 31 patients needed none (n = 11) or only 1 dose (n = 11) of rescue fentanyl during the first 4 postoperative hours, indicating that this could be sufficient initial dose in laparoscopic surgery. Earlier, we found that all laparotomy patients needed rescue fentanyl after a same epidural dose of oxycodone and thus effective dose for pain relief for 50% of patients after laparotomy seems to be higher than 0.1 mg kg−1. In that study, after the initial titration with 1–3 rescue fentanyl doses to comfort, most patients with epidural oxycodone did not need any further doses of rescue analgesia during the next 3 hours, supporting what was found in this study.9
The optimal timing of epidural oxycodone injection is also an issue. In this and our earlier study, the time of the first dose of rescue fentanyl has ranged between 5 and 25 minutes in the EPI‐groups if the few outliers are excluded. This is similar or shorter to that after i.v. administration.9 There were no differences in baseline pain scores between the 2 groups. Thus, the observed difference between administration routes in the onset of analgesic action may be explained by epidural absorption. After epidural administration oxycodone plasma concentration peaks at 2 hours and Cmax is half of that compared to i.v. administration.8 Experimental data in sheep show that it takes just 7 minutes to reach 50% equilibration in deep brain compartment after i.v. oxycodone injection.18, 19 After epidural administration, CSF concentrations peak earlier, 0.6 hours vs 1.1 hours, and Cmax in CSF is 100–300‐fold higher than that after i.v. administration, respectively.8 These data may explain the relatively slow onset but a lasting analgesic action of epidural oxycodone. Taken together, we assume that the onset of analgesic action of epidural oxycodone is similar or slower than that after a same dose i.v. Later, when the epidural oxycodone has had enough time, 30 minutes or more, to penetrate into the CSF and then into the dorsal horn of the spinal cord the analgesia persists longer than that after a same dose i.v. However, this hypothesis should be evaluated in further studies.
Six previous studies have evaluated the epidural administration of oxycodone, of which 3 have compared oxycodone with morphine.8, 9, 20, 21, 22, 23 These data indicate that when administrated epidurally, at least 2‐times higher dose of oxycodone is needed to provide similar analgesia compared to morphine.20, 21, 22 This is not expected based on the physiochemical properties of these 2 compounds as liposolubility and protein‐binding of oxycodone are similar to morphine.24 However, experimental pharmacodynamics data are consistent with the clinical trials as, in intrathecal administration, higher doses of oxycodone are needed to attain similar analgesic efficacy to morphine.25 This is interesting because both experimental data, and clinical data on laparotomy and laparoscopy patients indicate that i.v. and subcutaneously administered oxycodone is more potent than morphine, but this seems not to be the case with epidural administration.4, 5, 25 Morphine is commonly used at dose of 2.5–4 mg for a single dose epidural analgesia. If the potency of epidural oxycodone to morphine is ≥1:2, it seems that the dosage used here (0.1 mg kg−1) is rather conservative, although sufficient for most patients for the first 4 postoperative hours. If a more prolonged analgesic action is targeted a higher initial dose or preferably a small top‐up doses or an infusion of epidural oxycodone should be used. Optimal dosing protocols for epidural oxycodone administration have to be assessed in further clinical trials.
Oxycodone pharmacokinetic parameter estimates are similar to those reported by others e.g. CL 35 l h−1 70 kg−1, CV 24.3%; V1 26 L 70 kg−1, CV 45.9%; V2 129 L 70 kg−1, CV 17.9%; Q 206 L h 70 kg−1, CV 41%).25, 26 The pharmacokinetic CSF data presented here are consistent with our previous findings.8, 9 Our data indicate that oxycodone plasma concentrations are similar after i.v. and epidural administration but oxycodone CSF concentrations are at least 100‐fold higher after epidural administration than after i.v. administration. Interestingly, after epidural administration, the concentrations of oxymorphone and noroxycodone in CSF were higher than in plasma. CYP enzymes are expressed in human brain and spinal cord, and they may have contributed to the surprisingly high CSF concentrations of metabolites observed in this and in our previous studies.8, 9, 27
Both administration routes were well tolerated. There was more pruritus after epidural oxycodone than after i.v. oxycodone. This is consistent with the knowledge on other intrathecal opioids.28 Whether oxycodone induces less pruritus than epidural morphine remains open. Yanagidate and Dohi found that pruritus and nausea were less common with epidural oxycodone than epidural morphine in patients having gynaecological surgery.21 In parturients undergoing caesarean delivery, the incidence of pruritus was similar after epidural oxycodone and epidural morphine.19
One of the strengths of this study was that the patients received a constant background multimodal analgesic regimen with paracetamol and dexketoprofen. Postoperative pain after laparoscopy comprises of nociceptive, incisional and visceral pain components and therefore, a multimodal pain management protocol should be preferred.3 Other strengths of this study were that we included only laparoscopy patients and that intraoperative care was standardised. This was important as surgical technique and anaesthetic regimen may affect the severity of postoperative pain and adverse effects in gynaecological surgery.29, 30, 31
The main limitation of this study is that this was a single dose and a single dose level study. Thus, it does not allow to make any conclusion as to what would be the optimal dose of epidural oxycodone from efficacy and safety perspectives. However, our data may indicate that 0.1 mg kg−1 could be sufficient for most laparoscopy patients but too low for laparotomy patients.9 One of the limitations is also the timing of epidural administration. Further studies should evaluate whether an earlier administration would have resulted more effective postoperative pain relief. Another limitation is small sample size that does not allow us to make any firm conclusion on AEs and safety of epidural oxycodone. However, no specific safety issues have risen in the present study or our earlier trials with epidural oxycodone.8, 9 Moreover, our experimental toxicity data indicate that the cytotoxicity on neural tissue may be similar or less with oxycodone than that with morphine.32
5. CONCLUSIONS
It was found that a single dose of epidural oxycodone 0.1 mg kg−1 was more effective than i.v. oxycodone in early analgesia after laparoscopic surgery as a part of multimodal pain treatment protocol. Most patients needed none or only 1 dose of rescue analgesic in the EPI‐group and the pain scores were lower during the first 4 postoperative hours compared to IV‐group. The population PK model indicated that 60% of oxycodone injected into the epidural space enters into CSF and 40% is absorbed into the systemic circulation.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
P.P.: data collection, analysis and interpretation of data, writing of the first draft of the manuscript, critical evaluation of the intellectual content of the final version; H.K.: study design, analysis and interpretation of data, writing of the manuscript, critical evaluation of the intellectual content of the final version; B.A: PK data analysis and interpretation of data, critical evaluation of the intellectual content of the final version; J.H: PK data analysis and interpretation of data, critical evaluation of the intellectual content of the final version; H.H.: drug concentrations measurement, analysis and interpretation of data, critical evaluation of the intellectual content of the final version; V.‐P.R.: study design, analysis and interpretation of data, writing of the manuscript, critical evaluation of the intellectual content of the final version; M.K.: principal investigator, study design, data collection, data analysis and interpretation of data, writing of the manuscript, final approval of the version to be published.
DATA AVAILABILITY STATEMENT
The data are not publicly available due to privacy or ethical restrictions.
ACKNOWLEDGEMENTS
We thank Marko Lamminsalo for his help in the compilation of data file for population PK analysis and RN Petri Toroi for his help in conducting this study.
Piirainen P, Kokki H, Anderson BJ, et al. Analgesic efficacy and pharmacokinetics of epidural oxycodone in pain management after gynaecological laparoscopy—A randomised, double blind, active control, double‐dummy clinical comparison with intravenous administration. Br J Clin Pharmacol. 2019;85:1798–1807. 10.1111/bcp.13971
The authors confirm that the Principal Investigator for this paper is Merja Kokki and that she had direct clinical responsibility for patients.
Trial approval: EudraCT reference number: 2014–004313‐82.
REFERENCES
- 1. Ekstein P, Szold A, Sagie B, Werbin N, Klausner JM, Weinbroum AA. Laparoscopic surgery may be associated with severe pain and high analgesia requirements in the immediate postoperative period. Ann Surg. 2006;243(1):41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kokki M, Broms S, Eskelinen M, Rasanen I, Ojanperä I, Kokki H. Analgesic concentrations of oxycodone‐‐a prospective clinical PK/PD study in patients with laparoscopic cholecystectomy. Basic Clin Pharmacol Toxicol. 2012;110(5):469–475. [DOI] [PubMed] [Google Scholar]
- 3. Sjövall S, Kokki M, Kokki H. Laparoscopic surgery: a narrative review of pharmacotherapy in pain management. Drugs. 2015;75(16):1867–1889. [DOI] [PubMed] [Google Scholar]
- 4. Lenz H, Sandvik L, Qvigstad E, Bjerkelund CE, Raeder J. A comparison of intravenous oxycodone and intravenous morphine in patient‐controlled postoperative analgesia after laparoscopic hysterectomy. Anesth Analg. 2009;109(4):1279–1283. [DOI] [PubMed] [Google Scholar]
- 5. Kalso E, Pöyhiä R, Onnela P, Linko K, Tigerstedt I, Tammisto T. Intravenous morphine and oxycodone for pain after abdominal surgery. Acta Anaesthesiol Scand. 1991;35(7):642–646. [DOI] [PubMed] [Google Scholar]
- 6. Salomäki TE, Laitinen JO, Nuutinen LS. A randomized double‐blind comparison of epidural versus intravenous fentanyl infusion for analgesia after thoracotomy. Anesthesiology. 1991;75(5):790–795. [DOI] [PubMed] [Google Scholar]
- 7. Hendolin H, Nuutinen L, Kokki H, Tuomisto L. Does morphine premedication influence the pain and consumption of postoperative analgesics after total knee arthroplasty? Acta Anaesthesiol Scand. 1996;40(1):81–85. [DOI] [PubMed] [Google Scholar]
- 8. Kokki M, Välitalo P, Kuusisto M, et al. Central nervous system penetration of oxycodone after intravenous and epidural administration. Br J Anaesth. 2014;112(1):133–140. [DOI] [PubMed] [Google Scholar]
- 9. Piirainen P, Kokki H, Hautajärvi H, Ranta VP, Kokki M. The analgesic efficacy and pharmacokinetics of epidural oxycodone after gynaecological laparotomy: a randomized, double‐blind, double‐dummy comparison with intravenous administration. Br J Clin Pharmacol. 2018;84(9):2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alperin N, Bagci AM, Lee SH, Lam BL. Automated quantitation of spinal CSF volume and measurement of craniospinal CSF redistribution following lumbar withdrawal in idiopathic intracranial hypertension. AJNR am J Neuroradiol. 2016;37(10):1957–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anderson BJ, Holford NH. Mechanism‐based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48(1):303–332. [DOI] [PubMed] [Google Scholar]
- 12. Holford NH, Anderson BJ. Why standards are useful for predicting dose. Br J Clin Pharmacol. 2017;83(4):685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1(1):54–77. [Google Scholar]
- 14. Post TM, Freijer JI, Ploeger BA, Danhof M. Extensions to the visual predictive check to facilitate model performance evaluation. J Pharmacokinet Pharmacodyn. 2008;35(2):185–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46:D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol. 2017;174:S272‐S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beal SL. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn. 2001;28(5):481–504. [DOI] [PubMed] [Google Scholar]
- 18. Villesen HH, Foster DJ, Upton RN, Somogyi AA, Martinez A, Grant C. Cerebral kinetics of oxycodone in conscious sheep. J Pharm Sci. 2006;95(8):1666–1676. [DOI] [PubMed] [Google Scholar]
- 19. Bäcklund M, Lindgren L, Kajimoto Y, Rosenberg PH. Comparison of epidural morphine and oxycodone for pain after abdominal surgery. J Clin Anesth. 1997;9(1):30–35. [DOI] [PubMed] [Google Scholar]
- 20. Sng BL, Kwok SC, Mathur D, et al. Comparison of epidural oxycodone and epidural morphine for post‐caesarean section analgesia: a randomised controlled trial. Indian J Anaesth. 2016;60(3):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yanagidate F, Dohi S. Epidural oxycodone or morphine following gynaecological surgery. Br J Anaesth. 2004;93(3):362–367. [DOI] [PubMed] [Google Scholar]
- 22. Olczak B, Kowalski G, Leppert W, et al. Analgesic efficacy and safety of epidural oxycodone in patients undergoing total hip arthroplasty: a pilot study. J Pain Res. 2017;10:2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pöyhiä R, Seppälä T. Liposolubility and protein binding of oxycodone in vitro. Pharmacol Toxicol. 1994;74(1):23–27. [DOI] [PubMed] [Google Scholar]
- 24. Pöyhiä R, Kalso EA. Antinociceptive effects and central nervous system depression caused by oxycodone and morphine in rats. Pharmacol Toxicol. 1992;70(2):125–130. [DOI] [PubMed] [Google Scholar]
- 25. Saari TI, Ihmsen H, Neuvonen PJ, Olkkola KT, Schwilden H. Oxycodone clearance is markedly reduced with advancing age: a population pharmacokinetic study. Br J Anaesth. 2012;108(3):491–498. [DOI] [PubMed] [Google Scholar]
- 26. Choi BM, Lee YH, An SM, Lee SH, Lee EK, Noh GJ. Population pharmacokinetics and analgesic potency of oxycodone. Br J Clin Pharmacol. 2017;83(2):314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McMillan DM, Tyndale RF. CYP‐mediated drug metabolism in the brain impacts drug response. Pharmacol Ther. 2018;184:189–200. [DOI] [PubMed] [Google Scholar]
- 28. Ballantyne JC, Loach AB, Carr DB. Itching after epidural and spinal opiates. Pain. 1988;33(2):149–160. [DOI] [PubMed] [Google Scholar]
- 29. Garry R, Fountain J, Mason S, et al. The eVALuate study: two parallel randomised trials, one comparing laparoscopic with abdominal hysterectomy, the other comparing laparoscopic with vaginal hysterectomy. BMJ. 2004;328(7432):129–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pokkinen SM, Kalliomäki ML, Yli‐Hankala A, Nieminen K. Less postoperative pain after laparoscopic hysterectomy than after vaginal hysterectomy. Arch Gynecol Obstet. 2015;292(1):149–154. [DOI] [PubMed] [Google Scholar]
- 31. Pokkinen SM, Yli‐Hankala A, Kalliomäki ML. The effects of propofol vs. sevoflurane on post‐operative pain and need of opioid. Acta Anaesthesiol Scand. 2014;58(8):980–985. [DOI] [PubMed] [Google Scholar]
- 32. Kokki M, Pesonen M, Vehviläinen P, Litmala O, Pasanen M, Kokki H. Cytotoxicity of oxycodone and morphine in human neuroblastoma and mouse motoneuronal cells: a comparative approach. Drugs R D. 2016;16(2):155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are not publicly available due to privacy or ethical restrictions.


