Abstract
Aims
To evaluate the real‐world effect of dipeptidyl peptidase‐4 inhibitor (DPP4i) on the incidence of autoimmune diseases (AD), including rheumatoid arthritis (RA), inflammatory bowel diseases, multiple sclerosis and systemic lupus erythematosus.
Methods
We identified new users of DPP4i (n = 497 619) or non‐DPP4i (n = 643 165) oral combination therapy between 1 January 2011 and 30 June 2015 among patients with type 2 diabetes mellitus in the Korean national health insurance claims database. Patients were followed from the date of initiation of combination therapy until AD outcome, censoring for treatment discontinuation or switching, death or end of study (31 August 2016). Adjusted hazard ratios (aHR) and 95% confidence intervals (CI) for RA, inflammatory bowel diseases, other AD (multiple sclerosis and systemic lupus erythematosus), and the composite of all outcomes were estimated using propensity score (PS)‐adjusted Cox model.
Results
In the PS‐weighted and PS‐matched analysis, the risk of incident RA was decreased for DPP4i initiators compared with non‐DPP4i initiators (aHR 0.67 [95% CI 0.49–0.92] and aHR 0.72 [95% CI 0.51–1.01], respectively). In both analyses, the risk of incident composite AD was also decreased for DPP4i initiators compared with non‐DPP4i initiators (aHR 0.82 [95% CI 0.68–0.99] and aHR 0.76 [95% CI 0.62–0.93], respectively).
Conclusions
In this large population‐based cohort study, upfront DPP4i combination therapy was associated with a lower risk of composite AD compared with initial non‐DPP4i combination therapy.
Keywords: autoimmune diseases, cohort study, dipeptidyl peptidase IV inhibitors, rheumatoid arthritis, type 2 diabetes mellitus
What is already known about this subject
Dipeptidyl peptidase (DPP)‐4 inhibitors slow the DPP‐4 enzyme‐mediated degradation of the incretin hormones, resulting in stimulating insulin release, delaying satiety, decreasing glucagon release, and preserving β‐cell mass.
Altered levels of DPP‐4 expression and DPP‐4 enzyme activity have been reported in several immune‐mediated diseases, including rheumatoid arthritis, inflammatory bowel diseases, multiple sclerosis and systemic lupus erythematosus.
What this study adds
In this large‐scale real‐world study, we showed that the use of DPP‐4 inhibitors was associated with a lower risk of incident composite autoimmune disease compared with the use of non‐DPP‐4 inhibitor.
Sex was found to be an effect modifier in IBD; especially, a much higher protective effect was observed in males by DPP‐4 inhibitor use than in females.
1. INTRODUCTION
Dipeptidyl peptidase (DPP)‐4 inhibitors have been widely used in type 2 diabetes mellitus. They act by slowing the DPP‐4 enzyme‐mediated degradation of the incretin hormones. The accumulation of incretins led to better glycaemic control by stimulating insulin release, delaying satiety, decreasing glucagon release and preserving β‐cell mass.1, 2
DPP‐4 is a ubiquitous protease with many substrates, and its function is not restricted to incretin regulation. In clinical settings, altered levels of DPP‐4 expression and DPP‐4 enzyme activity have been reported in several immune‐mediated diseases, including rheumatoid arthritis (RA), inflammatory bowel diseases (IBD), multiple sclerosis (MS) and systemic lupus erythematosus (SLE).3, 4, 5, 6, 7 These results indicate that DPP‐4 mediates immune function and disease pathogenesis via the development, maturation and migration of T cells, cytokine secretion, T cell‐dependent antibody production, and immunoglobulin isotype switching of B cells.8, 9, 10 Accumulating clinical evidence suggests that DPP‐4 may be a novel target for the treatment of autoimmune diseases.10, 11 Currently, however, clinical evidence showing the epidemiological effect of DPP‐4 inhibitors on autoimmune diseases is scarce. In addition, several studies rather raised concerns about severe joint pain related to the use of DPP‐4 inhibitors.12, 13
Therefore, the aim of this study was to investigate the relative risk of autoimmune diseases among users of DPP‐4 inhibitors compared with non‐DPP‐4‐inhibiting oral hypoglycaemic agents, using a nationwide healthcare claims database in Korea. Specifically, we examined the risk of RA, IBD, MS, SLE and a composite outcome.
2. MATERIALS AND METHODS
2.1. Data source
Data for this population‐based study were collected from the Health Insurance Review & Assessment services (HIRA) database, the national insurance claims database covering the entire Korean population (~50 million people). The HIRA is a government‐affiliated agency that reviews and assesses healthcare costs and service quality, as well as operates healthcare information system to support research.14 All Koreans have a unique identification number, which is mandatory for all administrative purposes, including any contact with the healthcare system. Comprehensive data related to demographics, diagnoses and procedures performed during outpatient visits or inpatient stays, and complete prescription records are available in the HIRA database and can be tracked longitudinally. The diagnoses were coded according to the International Classification of Disease, 10th Revision (ICD‐10). All database records were de‐identified in compliance with the Act on the Protection of Personal Information Maintained by Public Agencies and, therefore, this study was exempt from institutional review board approval.
2.2. Study population
We conducted an active‐comparator new‐user cohort study. The study population included new users of DPP‐4 inhibitors or non‐DPP‐4 inhibitor oral combination therapy among patients aged 18 years or older diagnosed with type 2 diabetes mellitus between 1 January 2011 and 30 June 2015 (Figure 1). Although DPP‐4 inhibitors were approved for the treatment of type 2 diabetes as monotherapy or combination therapy, only combination therapy has been covered by health insurance in Korea. Therefore, DPP‐4 inhibitors are more commonly used as a combination therapy with other oral hypoglycaemic agents. Accordingly, patients with at least 1 prescription for a combination therapy of oral hypoglycaemic agents during the cohort registration period were first identified and classified into 2 exposure groups: (i) new users of DPP‐4 inhibitors combination therapy and (ii) new users of non‐DPP‐4 inhibitor oral combination therapy. The earliest start date of a combination therapy of oral hypoglycaemic agents was considered as the cohort entry date. We defined type 2 diabetes mellitus by at least 1 diagnosis of type 2 diabetes mellitus (ICD‐10 codes E11–14) during the cohort enrolment period. Patients with type 1 diabetes mellitus with a prescription for insulin alone during the 365‐day period before cohort entry (baseline period) were excluded. We defined new use of DPP‐4 inhibitors or non‐DPP‐4 inhibitor combination therapy as no prior prescription claims for any combination therapy of oral hypoglycaemic agents during the 365‐day baseline period. This new‐user design synchronizes duration of use at the beginning of follow‐up, thereby reducing survivor bias.15 To only include patients who were newly treated with incretin‐based therapy, patients using DPP‐4 inhibitors and glucagon‐like peptide 1 agonists during the baseline period were excluded. To identify incident autoimmune diseases, we further excluded patients with an outpatient or inpatient diagnosis of autoimmune diseases (RA, IBD, MS and SLE) during the baseline period.
Figure 1.
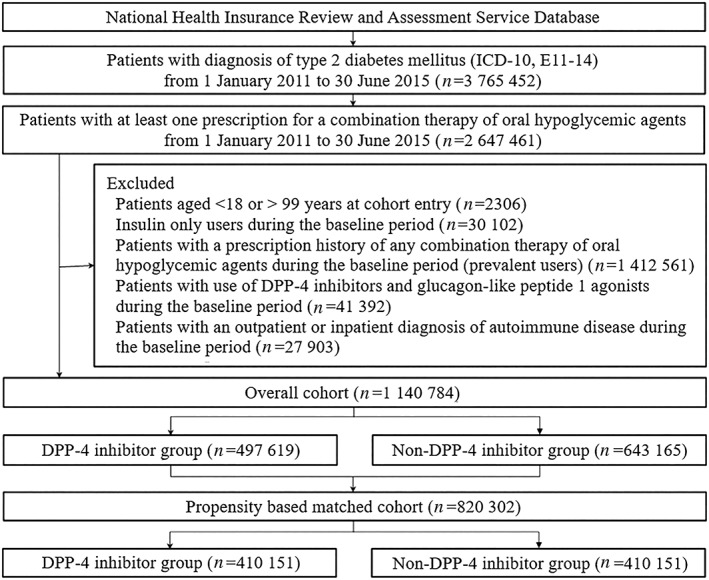
Cohort selection. DPP4, dipeptidyl peptidase‐4
Each patient was followed from cohort entry date until the earliest outcome of interest; treatment discontinuation or switch; death (in‐hospital death); or the end of study database (31 August 2016; Supplement 1). Patients were allowed to have a gap of up to 30 days between prescription fill dates in the calculation of continuous therapy.
2.3. Outcomes
The outcomes assessed were incidence of RA, IBD, other autoimmune diseases including MS and SLE, and a composite outcome of RA, IBD, MS and SLE. A patient was considered to have a first event if they were hospitalized or had 3 or more outpatient visits with a primary diagnosis of each outcome (ICD‐10 codes for RA: M05–06; for IBD: K50–51; for MS: G35; for SLE: M32).16, 17, 18 The outcome date refer to the earliest date of outcome in a patient.
2.4. Adjustment for confounders
The presence of risk factors was assessed using claims data from the 365‐day baseline period prior to cohort entry. The following potential confounders were determined: age; sex; year of cohort entry; diabetes mellitus‐related hospitalization; the number of oral hypoglycaemic drugs at cohort entry; and microvascular complications of diabetes mellitus (retinopathy, neuropathy, and nephropathy). Comorbidities were determined by 2 or more claims with ICD‐10 codes for the following conditions during the baseline period: hypertension, ischaemic heart disease, heart failure, stroke, dyslipidaemia, chronic kidney disease, chronic liver disease, obesity, alcohol abuse, cancer and human immunodeficiency virus infection. The Charlson comorbidity index was also calculated using a method proposed by Quan et al,19 which the weighted sum of 17 categories of comorbidity defined using ICD‐10 diagnoses codes, with a higher score indicating a more severe burden of comorbidity and a higher mortality risk. Indicators of healthcare resource utilization during the baseline period were also determined: the number of hospitalizations, visits to the emergency department, and other outpatient visits.
We used propensity scores (PS) to control for confounding: PS‐weighted and PS‐matched analyses. Using baseline variables, we predicted the probability of a patient initiating combination therapy with DPP‐4 inhibitors vs non‐DPP‐4 inhibitors using a logistic regression model. The variables included in the PS adjustment were age, sex, index year, diabetes‐related hospitalization, number of oral hypoglycaemic drugs at cohort entry, microvascular complications, comorbidities and healthcare utilization (Table 1). For the PS‐weighted analysis, we assigned a weight of 1 to DPP‐4 inhibitor combination therapy and a weight of (propensity score/[1 − propensity score]) to non‐DPP‐4 inhibitor combination therapy. Such a weighted analysis resulted in pseudo‐populations of non‐DPP‐4 inhibitor combination therapy initiators with potential confounder distribution similar to that of DPP‐4 inhibitor combination therapy initiators.20, 21 Thus, our weighted analysis addressed the question: what would have happened to patients who were treated with upfront DPP‐4 inhibitor combination therapy if they had initiated non‐DPP‐4 inhibitor combination therapy instead?22 PS‐matched analysis indicated a 1:1 greedy propensity score matched with the results in a previous study.23 Matching minimizes confounding by restricting comparisons to the single best match for each DPP‐4 inhibitor combination.
Table 1.
Patient characteristics: DPP‐4i vs non‐DPP‐4i combination therapy cohorts before and after propensity matching
| All patients | Propensity‐based matched patients | |||||
|---|---|---|---|---|---|---|
| Characteristics | DPP4i group (n = 497 619) | Non‐DPP4i group (n = 643 165) | Standardized difference | DPP4i group (n = 410 151) | Non‐DPP4i group (n = 410 151) | Standardized difference |
| Mean (SD) or % | Mean (SD) or % | |||||
| Demographics | ||||||
| Age | 57.1 (12.8) | 58.8 (13.1) | 0.13 | 57.7 (12.9) | 57.7 (12.9) | 0.00 |
| Age group | ||||||
| 18–44 | 16.8 | 14.2 | 15.8 | 15.7 | ||
| 45–64 | 54.4 | 51.0 | 53.6 | 53.0 | ||
| 65+ | 28.8 | 34.8 | 30.6 | 31.3 | ||
| Female | 41.5 | 42.1 | 0.01 | 41.6 | 41.7 | 0.00 |
| Index year | ||||||
| 2011 | 14.6 | 34.6 | 0.01 | 17.7 | 18.2 | 0.00 |
| 2012 | 19.4 | 25.8 | 22.8 | 23.3 | ||
| 2013 | 24.1 | 18.2 | 25.6 | 25.5 | ||
| 2014 | 26.1 | 14.6 | 22.7 | 22.3 | ||
| 2015 | 15.7 | 6.9 | 11.2 | 10.7 | ||
| Indicators of diabetes severity | ||||||
| Diabetes‐related hospitalization | 3.4 | 4.3 | 0.05 | 3.4 | 3.5 | 0.00 |
| No. of oral hypoglycaemic drugs at cohort entry | ||||||
| 2 | 91.1 | 97.1 | 0.26 | 96.5 | 95.7 | 0.04 |
| 3 | 8.6 | 2.9 | 3.4 | 4.2 | ||
| 4 + | 0.2 | 0.0 | 0.1 | 0.1 | ||
| Microvascular complication | ||||||
| Retinopathy | 7.9 | 8.9 | 0.04 | 8.2 | 8.3 | 0.00 |
| Neuropathy | 11.5 | 14.3 | 0.08 | 12.3 | 12.5 | 0.01 |
| Nephropathy | 4.9 | 4.2 | 0.03 | 4.5 | 4.5 | 0.00 |
| Oral hypoglycaemic drugs at cohort entry | ||||||
| Metformin | 94.5 | 94.4 | 0.01 | 94.2 | 95.4 | 0.05 |
| Sulfonylureas | 12.9 | 89.0 | 2.35 | 8.4 | 88.2 | 2.70 |
| Thiazolidinediones | 0.9 | 8.0 | 0.35 | 0.4 | 9.0 | 0.42 |
| DPP4i | 100.0 | 0.0 | n/a | 100.0 | 0.0 | n/a |
| SGLT‐2i | 0.0 | 0.9 | 0.13 | 0.0 | 1.4 | 0.17 |
| Meglitinides | 0.3 | 2.6 | 0.19 | 0.2 | 2.4 | 0.19 |
| a‐glucosidase inhibitors | 0.4 | 8.1 | 0.38 | 0.3 | 8.0 | 0.39 |
| Comorbidities | ||||||
| Hypertension | 49.8 | 52.1 | 0.05 | 50.7 | 51.0 | 0.01 |
| Ischaemic heart disease | 10.7 | 9.7 | 0.03 | 10.1 | 10.3 | 0.01 |
| Heart failure | 2.6 | 2.4 | 0.02 | 2.5 | 2.5 | 0.00 |
| Stroke | 5.7 | 6.5 | 0.04 | 5.9 | 6.0 | 0.00 |
| Dyslipidaemia | 51.0 | 42.4 | 0.17 | 47.9 | 48.0 | 0.00 |
| Chronic kidney disease | 1.4 | 1.3 | 0.01 | 1.3 | 1.3 | 0.00 |
| Chronic liver disease | 21.1 | 19.1 | 0.05 | 20.4 | 20.5 | 0.00 |
| Obesity | 0.2 | 0.2 | 0.01 | 0.2 | 0.2 | 0.00 |
| Alcohol abuse | 5.5 | 6.0 | 0.02 | 5.7 | 5.7 | 0.00 |
| Cancer | 4.9 | 4.8 | 0.01 | 4.8 | 4.9 | 0.00 |
| HIV infection | 0.0 | 0.0 | 0.00 | 0.0 | 0.0 | 0.00 |
| Charlson comorbidity index | 2.0 (1.7) | 2.1 (1.9) | 0.04 | 2.0 (1.8) | 2.0 (1.8) | 0.01 |
| Healthcare utilization | ||||||
| No. of physician visits | 22.5 (23.8) | 23.9 (25.5) | 0.06 | 23.1 (24.4) | 23.2 (24.5) | 0.01 |
| No. of emergency room visits | 0.2 (0.6) | 0.2 (0.6) | 0.01 | 0.2 (0.6) | 0.2 (0.6) | 0.00 |
| No. of hospitalizations | 0.5 (1.5) | 0.7 (1.9) | 0.09 | 0.5 (1.5) | 0.6 (1.6) | 0.01 |
DPP4i, dipeptidyl peptidase‐4 inhibitors; n/a, not applicable; s.d., standard deviation; SGLT‐2i, sodium‐glucose co‐transporter‐2 inhibitors; HIV, human immunodeficiency virus.
2.5. Statistical analyses
Differences in baseline characteristics were compared between groups exposed to DPP‐4 inhibitors and non‐DPP‐4 inhibitor using standardized differences. Standardized differences of <0.10 denoted balance in baseline characteristics between the groups.24
Incidence rates and hazard ratios (HR) with 95% confidence intervals (CI) of a study outcome were calculated in DPP‐4 inhibitor vs non‐DPP‐4 inhibitor groups in both PS‐weighted and PS‐matched cohorts using Cox proportional hazards models. Group imbalance between baseline variables was further adjusted by multivariable Cox model. Kaplan–Meier curves were plotted for the cumulative incidence of each outcome in the PS‐matched cohorts. We performed sensitivity analyses to evaluate outcomes when the study cohort was restricted to patients who received a prescription of each therapy for 30 consecutive days following the cohort entry date. To estimate the sex‐specific HRs and 95% CIs, we stratified the analysis by sex. All analyses were performed with SAS, version 9.4 (SAS Institute).
2.6. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,25 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.26
3. RESULTS
A total of 1 140 784 patients were included in the cohort, including 497 619 patients who started a combination therapy with a DPP‐4 inhibitor drug and 643 165 patients who started a combination therapy with non‐DPP‐4 inhibitor drugs alone. After the PS matching, 410 151 pairs of DPP‐4 inhibitor and non‐DPP‐4 inhibitor initiators were included (Figure 1).
Compared with patients using non‐DPP‐4 inhibitors, DPP‐4 inhibitor initiators were more likely to be younger, treated with a number of oral hypoglycaemic drugs at cohort entry, and diagnosed with dyslipidaemia at baseline. Types of oral hypoglycaemic drugs at cohort entry varied between DPP‐4 inhibitor and non‐DPP‐4 inhibitor initiators. After matching, all the baseline characteristics were balanced, with the exception of variables of oral hypoglycaemic drug types at cohort entry (Table 1). The mean ± standard deviation follow‐up time was 1.68 ± 1.55 years.
Table 2 shows the incidence rates of autoimmune disease per 1000 person–years and corresponding HRs for initiators of DPP‐4 inhibitor combination compared with non‐DPP‐4 inhibitor combination therapy. In the PS‐weighted and PS‐matched analysis, the risk of incident RA was decreased for DPP‐4 inhibitor initiators compared with non‐DPP‐4 inhibitor initiators (0.08 vs 0.11 per 1000 person–years; adjusted HR [aHR], 0.67 [95% CI 0.49–0.92] and 0.09 vs 0.12; aHR 0.72 [95% CI 0.51–1.01], respectively). In both analysis, the risk of incident composite autoimmune disease was also decreased in the group of DPP‐4 inhibitor initiators compared with non‐DPP‐4 inhibitor initiators (0.24 vs 0.29 per 1000 person–years; aHR 0.82 [95% CI 0.68–0.99] and 0.24 vs 0.32; aHR 0.76 [95% CI 0.62–0.93], respectively). Figure 2 shows the Kaplan–Meier curves comparing the cumulative incidence of autoimmune disease between the PS‐matched DPP‐4 inhibitor and non‐DPP4 inhibitor groups.
Table 2.
Number of events, person–years, event rates and adjusted hazard ratios of DPP‐4i vs non‐DPP‐4i in the entire population as well as propensity‐based matched patients
| All patients | DPP4i group | Non‐DPP4i group | ||||||
|---|---|---|---|---|---|---|---|---|
| (n = 497 619) | (n = 643 165) | |||||||
| Cases | Person–years | Incidence (per 1000 person–years) | Cases | Person–years | Incidence (per 1000 person–years) | HR | (95% CI) | |
| Rheumatoid arthritis | 65 | 775 741 | 0.08 | 120 | 1 138 608 | 0.11 | 0.67 | (0.49–0.92) |
| Inflammatory bowel disease | 100 | 775 672 | 0.13 | 175 | 1 138 499 | 0.15 | 0.94 | (0.72–1.24) |
| Other AD (SLE + MS) | 25 | 775 789 | 0.03 | 39 | 1 138 728 | 0.03 | 0.87 | (0.51–1.49) |
| Composite AD | 190 | 775 559 | 0.24 | 334 | 1 138 239 | 0.29 | 0.82 | (0.68–0.99) |
| Matched patients | (n = 410 151) | (n = 410 151) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Person–years | Incidence (per 1000 person–years) | Cases | Person–years | Incidence (per 1000 person–years) | HR | (95% CI) | |
| Rheumatoid arthritis | 59 | 669 397 | 0.09 | 80 | 647 925 | 0.12 | 0.72 | (0.51–1.01) |
| Inflammatory bowel disease | 84 | 669 336 | 0.13 | 101 | 647 880 | 0.16 | 0.81 | (0.61–1.08) |
| Other AD (SLE + MS) | 19 | 669 448 | 0.03 | 28 | 647 984 | 0.04 | 0.67 | (0.37–1.19) |
| Composite AD | 162 | 669 236 | 0.24 | 209 | 647 718 | 0.32 | 0.76 | (0.62–0.93) |
AD, autoimmune diseases; CI, confidence interval; DPP4i, dipeptidyl peptidase‐4 inhibitors; HR, hazard ratio; MS, multiple sclerosis; SLE, systemic lupus erythematosus.
Figure 2.
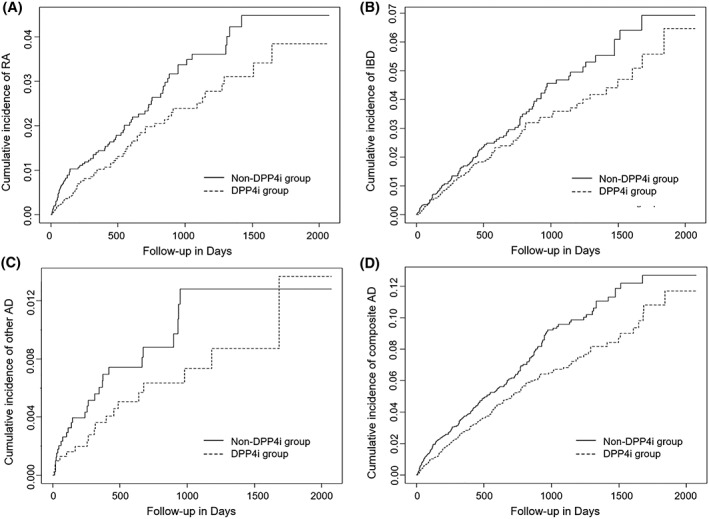
Kaplan–Meier curves for cumulative incidence of autoimmune disease: PS‐matched analysis. A, Rheumatoid arthritis (RA); B, inflammatory bowel diseases (IBD); C, other autoimmune disease (AD) including multiple sclerosis (MS) and systemic lupus erythematosus (SLE); D, composite outcome (RA, IBD, MS and SLE); DPP4i, dipeptidyl peptidase‐4 inhibitors
Sensitivity analyses on patients with a prescription for 30 consecutive days after cohort entry date revealed more pronounced HRs in the PS‐weighted analysis for DPP‐4 inhibitor initiators compared to non‐DPP‐4 inhibitor initiators; the risk of incident RA and composite autoimmune disease were decreased for DPP‐4 inhibitor initiators compared with non‐DPP‐4 inhibitor initiators (0.08 vs 0.10 per 1000 person–years; aHR 0.45 [95% CI 0.26–0.78] and 0.23 vs 0.26; aHR,0.65 [95% CI 0.48–0.87], respectively). However, significant association was not found in the PS‐matched analysis (Table 3).
Table 3.
Sensitivity analyses examining association between treatments with DPP‐4i vs non‐DPP‐4i and autoimmune disease among patients with a prescription of each therapy for 30 consecutive days following the cohort entry date
| All patients | DPP4i group | Non‐DPP4i group | ||||||
|---|---|---|---|---|---|---|---|---|
| (n = 467 213) | (n = 614 440) | |||||||
| Cases | Person–years | Incidence (per 1000 person–years) | Cases | Person–years | Incidence (per 1000 person–years) | HR | (95% CI) | |
| Rheumatoid arthritis | 61 | 774 510 | 0.08 | 109 | 1 137 478 | 0.10 | 0.45 | (0.26–0.78) |
| Inflammatory bowel disease | 95 | 774 444 | 0.12 | 161 | 1 137 366 | 0.14 | 0.67 | (0.45–1.01) |
| Other AD (SLE + MS) | 19 | 774 556 | 0.02 | 31 | 1 137 597 | 0.03 | 1.21 | (0.59–2.49) |
| Composite AD | 175 | 774 339 | 0.23 | 301 | 1 137 145 | 0.26 | 0.65 | (0.48–0.87) |
| PS‐matched analysis | (n = 382 535) | (n = 382 535) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cases | Person–years | Incidence (per 1000 person–years) | Cases | Person–years | Incidence (per 1000 person–years) | HR | (95% CI) | |
| Rheumatoid arthritis | 56 | 665 448 | 0.08 | 73 | 634 092 | 0.12 | 0.83 | (0.38–1.82) |
| Inflammatory bowel disease | 80 | 665 390 | 0.12 | 89 | 634 055 | 0.14 | 0.89 | (0.44–1.77) |
| Other AD (SLE + MS) | 15 | 665 498 | 0.02 | 20 | 634 158 | 0.03 | 0.38 | (0.08–1.76) |
| Composite AD | 151 | 665 296 | 0.23 | 182 | 633 920 | 0.29 | 0.80 | (0.49–1.31) |
AD, autoimmune diseases; CI, confidence interval; DPP4i, dipeptidyl peptidase‐4 inhibitors; HR, hazard ratio; MS, multiple sclerosis; SLE, systemic lupus erythematosus.
The risk of incident composite autoimmune disease was nonsignificantly lower in the DPP‐4 inhibitor group (aHR 0.81 [95% CI 0.61–1.09]) among females, while the risk of incident composite autoimmune disease (aHR 0.71 [95% CI 0.53–0.95]) among males remained reduced for DPP‐4 inhibitor compared with non‐DPP‐4 inhibitor group. Lower aHR values for IBD in the DPP‐4 inhibitor group among males were observed: aHR 0.63 (95% CI 0.43–0.94). There was no evidence of interaction between DPP‐4 inhibitor use and sex on the incidence of autoimmune disease (Table 4).
Table 4.
Sex‐specific incidence rates and hazard ratios for DPP‐4i vs non‐DPP‐4i in propensity‐based matched patients
| DPP4i group | Non‐DPP4i group | HR | (95% CI) | P for interaction | |||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Person–years | Incidence (per 1000 person–years) | Cases | Person–years | Incidence (per 1000 person–years) | ||||
| Rheumatoid arthritis | |||||||||
| Male (n = 478 871) | 30 | 381 496 | 0.08 | 39 | 358 524 | 0.11 | 0.73 | (0.45–1.18) | .94 |
| Female (n = 341 431) | 29 | 289 901 | 0.10 | 41 | 289 401 | 0.14 | 0.71 | (0.44–1.15) | |
| Inflammatory bowel disease | |||||||||
| Male (n = 478 871) | 41 | 381 456 | 0.11 | 61 | 358 495 | 0.17 | 0.63 | (0.43–0.94) | .07 |
| Female (n = 341 431) | 43 | 287 880 | 0.15 | 40 | 289 386 | 0.14 | 1.09 | (0.71–1.67) | |
| Other AD (SLE + MS) | |||||||||
| Male (n = 478 871) | 8 | 381 512 | 0.02 | 6 | 358 564 | 0.02 | 1.28 | (0.44–3.68) | .16 |
| Female (n = 341 431) | 11 | 287 935 | 0.04 | 22 | 289 419 | 0.08 | 0.51 | (0.25–1.04) | |
| Composite AD | |||||||||
| Male (n = 478 871) | 79 | 381 419 | 0.21 | 106 | 358 432 | 0.29 | 0.71 | (0.53–0.95) | .50 |
| Female (n = 341 431) | 83 | 287 817 | 0.29 | 103 | 289 286 | 0.36 | 0.81 | (0.61–1.09) | |
AD, autoimmune diseases; CI, confidence interval; DPP4i, dipeptidyl peptidase‐4 inhibitors; HR, hazard ratio; MS, multiple sclerosis; SLE, systemic lupus erythematosus.
4. DISCUSSION
In this large‐scale real‐world study, we showed that the use of DPP‐4 inhibitors was associated with a lower risk of incident RA and composite autoimmune disease compared with the use of non‐DPP‐4 inhibitors, using PS‐weighted and PS‐matched analysis. For the first time, we reported the sex‐specific incidence of RA and other autoimmune diseases and found that the risk of incident IBD and composite autoimmune disease was significantly lower among the males in the DPP‐4 inhibitor group.
Our results are consistent with the results of a population‐based cohort study using US insurance claims data. The study by Kim et al. showed that upfront DPP‐4 inhibitor combination therapy was associated with a decreased risk of RA and composite autoimmune disease compared with initial non‐DPP‐4 inhibitor combination therapy. However, the long‐term effects of DPP‐4 inhibitor were not completely assessed, because the maximum duration of patient follow‐up was relatively short at 1 year.27 By contrast, our study consisted of a larger number of study patients and longer follow‐up periods (maximum follow‐up of 5.67 years), and revealed the protective effect of DPP‐4 inhibitors on autoimmune diseases in a real‐world population setting.
Although the underlying mechanisms have not been fully elucidated, several studies have shown that DPP‐4 can influence the proliferation and chemotaxis of T lymphocytes through 3 main functions: adenosine deaminase binding; peptidase activity; and extracellular matrix binding.28, 29, 30 Other studies have revealed that inhibition of human peripheral blood mononuclear cell proliferation with sitagliptin was dose‐dependent and that sitaglitin concentrations of 50 μg/mL could modulate the differentiation of T helper (Th) 17/Th1 cells in regulatory cells producing transforming growth factor‐β1 reducing the production of interleukin‐6, interferon‐γ and interleukin‐17 using human and animal models.31, 32, 33
Meanwhile, studies have shown that DPP‐4 inhibitors are associated with the development of joint pain and drug‐related joint inflammation.13, 34 Accordingly, the US Food and Drug Administration warned of a strong temporal relationship between DPP‐4 inhibitor use and severe arthralgia based on 33 case reports of severe joint pain in August 2015.35 However, a population‐based cohort study found that DPP‐4 inhibitor use in patients with type 2 diabetes mellitus did not increase the risk of incidence of severe joint pain or nonspecific arthropathies in a maximum follow‐up of 5 years, regardless of sex or age.36 The present results indicate a similar trend in terms of arthropathies in that the use of DPP‐4 inhibitors reduced or at least did not increase the risk of RA development. Further pharmacoepidemiological and pathological studies are needed to confirm the results of the present study.
Based on the previous studies showing that the prevalence of RA and clinical characteristics of SLE varied according to sex,37, 38, 39 stratified analysis by sex was carried out. In our study, sex was found to be an effect modifier in IBD; especially, a much higher protective effect was observed in males by DPP‐4 inhibitor use than in females. The risk of IBD incidence was reduced by 37% (95% CI 6–57%) by using DPP‐4 inhibitors in males, but the protective effect of DPP‐4 inhibitors was not found in females. The mechanisms associated with sex differences remain to be investigated.
Results only using patients with a prescription for 30 consecutive days were similar to those using the whole sample in the PS‐weighted analysis, where the use of DPP‐4 inhibitors was associated with a lower risk of incident RA and composite autoimmune disease compared with the use of non‐DPP‐4 inhibitors. However, in the case of PS‐matched analysis, results using the new cohort failed to show significant associations; this was attributable to insufficient statistical power due to the reduced sample size.
The strengths of our study include its large sample size as well as the national health insurance claims data representing the entire population of approximately 50 million Korean residents. In addition, this study provides the first insights into the risk of autoimmune diseases in users of DPP‐4 inhibitors among Asian population. Since DPP‐4 inhibitors were associated with better glucose‐lowering efficacy in Asians than in other ethnic groups,40 this study may have important implications for our understanding of the clinical role of DPP‐4 inhibitors in autoimmune diseases.
Our study also has a few limitations. We mainly relied on diagnostic codes for outcome ascertainment; thus, misclassification is possible, but unlikely to vary with treatment choice. In addition, to minimize the potential for outcome misclassification, all the outcomes were defined as hospitalization or 3 or more outpatient visits with a primary diagnosis of each outcome.16, 17, 18 Moreover, although we adjusted for many confounders, the results were affected by unmeasured confounders, which is a universal limitation associated with all observational studies.
In conclusion, upfront DPP‐4 inhibitor combination therapy was associated with a lower risk of composite autoimmune diseases (RA, IBD, MS and SLE) compared with initial non‐DPP‐4 inhibitor combination therapy.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
All authors have contributed significantly to the work and have read and approved the manuscript for publication. J.S. and H.G. were responsible for the study concept and design. J.S. and J.Y. participated in data collection. J. S. analysed the data. J.S., J.Y. and H.G. contributed to the manuscript writing and discussion.
Supporting information
Figure S1.
Distribution of patients who were censored according to each censored point: treatment discontinuation or switch, death, and end of study database.
Seong J‐M, Yee J, Gwak HS. Dipeptidyl peptidase‐4 inhibitors lower the risk of autoimmune disease in patients with type 2 diabetes mellitus: A nationwide population‐based cohort study. Br J Clin Pharmacol. 2019;85:1719–1727. 10.1111/bcp.13955
REFERENCES
- 1. Mannucci E, Rotella CM. Future perspectives on glucagon‐like peptide‐1, diabetes and cardiovascular risk. Nutr Metab Cardiovasc Dis. 2008;18:639e45. [DOI] [PubMed] [Google Scholar]
- 2. Cornell S. Differentiating among incretin therapies: a multiple‐target approach to type 2 diabetes. J Clin Pharm Ther. 2012;37:510e24. [DOI] [PubMed] [Google Scholar]
- 3. Gotoh H, Hagihara M, Nagatsu T, Iwata H, Miura T. Activities of dipeptidyl peptidase II and dipeptidyl peptidase IV in synovial fluid from patients with rheumatoid arthritis and osteoarthritis. Clin Chem. 1989;35(6):1016‐1018. [PubMed] [Google Scholar]
- 4. Sromova L, Mareckova H, Sedova L, Balaziova E, Sedo A. Dipeptidyl peptidase‐IV in synovial fluid and in synovial fluid mononuclear cells of patients with rheumatoid arthritis. Clin Chim Acta. 2010;411(15‐16):1046‐1050. [DOI] [PubMed] [Google Scholar]
- 5. Hildebrandt M, Rose M, Rüter J, Salama A, Mönnikes H, Klapp BF. Dipeptidyl peptidase IV (DP IV, CD26) in patients with inflammatory bowel disease. Scand J Gastroenterol. 2001;36(10):1067‐1072. [DOI] [PubMed] [Google Scholar]
- 6. Reinhold D, Bank U , Täger M, et al. DP IV/CD26, APN/CD13 and related enzymes as regulators of T cell immunity: implications for experimental encephalomyelitis and multiple sclerosis. Front Biosci. 2008;13(13):2356‐2363. [DOI] [PubMed] [Google Scholar]
- 7. Kobayashi H, Hosono O, Mimori T, et al. Reduction of serum soluble CD26/dipeptidyl peptidase IV enzyme activity and its correlation with disease activity in systemic lupus erythematosus. J Rheumatol. 2002;29(9):1858‐1866. [PubMed] [Google Scholar]
- 8. Lambeir AM, Durinx C, Scharpé S, De Meester I. Dipeptidyl‐peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40(3):209‐294. [DOI] [PubMed] [Google Scholar]
- 9. Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008;29(6):295‐301. [DOI] [PubMed] [Google Scholar]
- 10. Yazbeck R, Howarth GS, Abbott CA. Dipeptidyl peptidase inhibitors, an emerging drug class for inflammatory disease? Trends Pharmacol Sci. 2009;30(11):600‐607. [DOI] [PubMed] [Google Scholar]
- 11. Ohnuma K, Hosono O, Dang NH, Morimoto C. Dipeptidyl peptidase in autoimmune pathophysiology. Adv Clin Chem. 2011;53:51‐84. [DOI] [PubMed] [Google Scholar]
- 12. Mascolo A, Rafaniello C, Sportiello L, et al. Dipeptidyl peptidase (DPP)‐4 inhibitor‐induced arthritis/arthralgia: a review of clinical cases. Drug Saf. 2016;39(5):401‐407. [DOI] [PubMed] [Google Scholar]
- 13. Saito T, Ohnuma K, Suzuki H, et al. Polyarthropathy in type 2 diabetes patients treated with DPP4 inhibitors. Diabetes Res Clin Pract. 2013;102(1):e8‐e12. [DOI] [PubMed] [Google Scholar]
- 14. The Health Insurance Review & Assessment services . HIRA System. Healthcare big data analysis. http://www.hira.or.kr/eng/ebook/00_Page_img/extra/00.pdf. Accessed November 7, 2017.
- 15. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol. 2003;158(9):915‐920. [DOI] [PubMed] [Google Scholar]
- 16. Yen YF, Chuang PH, Jen IA, et al. Incidence of autoimmune diseases in a nationwide HIV/AIDS patient cohort in Taiwan, 2000‐2012. Ann Rheum Dis. 2017;76(4):661‐665. [DOI] [PubMed] [Google Scholar]
- 17. Choi CW, Eun SH, Choi KH, Bae JM. Increased risk of comorbid rheumatic disorders in vitiligo patients: a nationwide population‐based study. J Dermatol. 2017;44(8):909‐913. [DOI] [PubMed] [Google Scholar]
- 18. Kok VC, Horng JT, Hung GD, et al. Risk of autoimmune disease in adults with chronic insomnia requiring sleep‐inducing pills: a population‐based longitudinal study. J Gen Intern Med. 2016;31(9):1019‐1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD‐9‐CM and ICD‐10 administrative data. Med Care. 2005;43(11):1130‐1139. [DOI] [PubMed] [Google Scholar]
- 20. Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680‐686. [DOI] [PubMed] [Google Scholar]
- 21. Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity‐based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262‐270. [DOI] [PubMed] [Google Scholar]
- 22. Stürmer T, Rothman KJ, Glynn RJ. Insights into different results from different causal contrasts in the presence of effect‐measure modification. Pharmacoepidemiol Drug Saf. 2006;15(10):698‐709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parsons LS. Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Presented at the Twenty‐Sixth Annual SAS Users Group International Conference (SUGI 26), Long Beach, California, 22–25 April 2001. Paper 214–26.
- 24. Sung YK, Cho SK, Choi CB, Bae SC. Prevalence and incidence of rheumatoid arthritis in South Korea. Rheumatol Int. 2013;33(6):1525‐1532. [DOI] [PubMed] [Google Scholar]
- 25. Southan C, Sharman JL, Benson HE, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res. 2016;44(D1):D1054‐D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alexander SP, Fabbro D, Kelly E, et al. THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: enzymes. Br J Pharmacol. 2017;174:S272‐S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SC, Schneeweiss S, Glynn RJ, Doherty M, Goldfine AB, Solomon DH. Dipeptidyl peptidase‐4 inhibitors in type 2 diabetes may reduce the risk of autoimmune diseases: a population‐based cohort study. Ann Rheum Dis. 2015;74(11):1968‐1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gorrell MD, Gysbers V, McCaughan GW. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol. 2001;54(3):249‐264. [DOI] [PubMed] [Google Scholar]
- 29. Bengsch B, Seigel B, Flecken T, Wolanski J, Blum HE, Thimme R. Human Th17 cells express high levels of enzymatically active dipeptidylpeptidase IV (CD26). J Immunol. 2012;188(11):5438‐5447. [DOI] [PubMed] [Google Scholar]
- 30. Salgado FJ, Pérez‐Díaz A, Villanueva NM, Lamas O, Arias PN. CD26: a negative selection marker for human Treg cells. Cytometry A. 2012;81:843‐855. [DOI] [PubMed] [Google Scholar]
- 31. Pinheiro MM, Stoppa CL, Valduga CJ, et al. Sitagliptin inhibit human lymphocytes proliferation and Th1/Th17 differentiation in vitro. Eur J Pharm Sci. 2017;100:17‐24. [DOI] [PubMed] [Google Scholar]
- 32. Tian L, Gao J, Hao J, et al. Reversal of new‐onset diabetes mellitus through modulating inflammation and stimulating beta‐cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology. 2010;151(7):3049‐3060. [DOI] [PubMed] [Google Scholar]
- 33. Dobrian AD, Ma Q, Lindsay JW, et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2011;300(2):E410‐E421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crickx E, Marroun I, Veyrie C, et al. DPP4 inhibitor‐induced polyarthritis: a report of three cases. Rheumatol Int. 2014;34(2):291‐292. [DOI] [PubMed] [Google Scholar]
- 35. Administration USFaD . FDA Drug Safety Communication: FDA warns that DPP‐4 inhibitors for type 2 diabetes mellitus may cause severe joint pain, 2015. Available from: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM460038.pdf, Accessed 24 May, 2018.
- 36. Hou WH, Chang KC, Li CY, Ou HT. Dipeptidyl peptidase‐4 inhibitor use is not associated with elevated risk of severe joint pain in patients with type 2 diabetes mellitus: a population‐based cohort study. Pain. 2016;157(9):1954‐1959. [DOI] [PubMed] [Google Scholar]
- 37. Aranow C, Del Guidice J, Barland P, Weinstein A. Systemic lupus erythematosus disease severity in men and women: a case‐control study. J Rheumatol. 2002;29(8):1674‐1677. [PubMed] [Google Scholar]
- 38. Soto ME, Vallejo M, Guillen F, Simon JA, Arena E, Reyes PA. Gender impact in systemic lupus erythematosus. Clin Exp Rheumatol. 2004;22:713‐721. [PubMed] [Google Scholar]
- 39. Mamdani M, Sykora K, Li P, et al. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005;330(7497):960‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kim YG, Hahn S, Oh TJ, Kwak SH, Park KS, Cho YM. Differences in the glucose‐lowering efficacy of dipeptidyl peptidase‐4 inhibitors between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetologia. 2013;56(4):696‐708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Distribution of patients who were censored according to each censored point: treatment discontinuation or switch, death, and end of study database.


