Abstract
Background
Although device-based optimization has been developed to overcome the limitations of conventional optimization methods in cardiac resynchronization therapy (CRT), few real-world data supports the results of clinical trials that showed the efficacy of automatic optimization algorithms. We investigated whether CRT using the adaptive CRT algorithm is comparable to non-adaptive biventricular (BiV) pacing optimized with electrocardiogram or echocardiography-based methods.
Methods
Consecutive 155 CRT patients were categorized into 3 groups according to the optimization methods: non-adaptive BiV (n = 129), adaptive BiV (n = 11), and adaptive left ventricular (LV) pacing (n = 15) groups. Additionally, a subgroup of patients (n = 59) with normal PR interval and left bundle branch block (LBBB) was selected from the non-adaptive BiV group. The primary outcomes included cardiac death, heart transplantation, LV assist device implantation, and heart failure admission. Secondary outcomes were electromechanical reverse remodeling and responder rates at 6 months after CRT.
Results
During a median 27.5-month follow-up, there was no significant difference in primary outcomes among the 3 groups. However, there was a trend toward better outcomes in the adaptive LV group compared to the other groups. In a more rigorous comparisons among the patients with normal PR interval and LBBB, similar patterns were still observed.
Conclusion
In our first Asian-Pacific real-world data, automated dynamic CRT optimization showed comparable efficacy to conventional methods regarding clinical outcomes and electromechanical remodeling.
Keywords: Cardiac Resynchronization Therapy, Automatic Optimization, Left Ventricular Pacing
Graphical Abstract
INTRODUCTION
Cardiac resynchronization therapy (CRT) is established as a standard treatment for advanced heart failure (HF) patients with electrical dyssynchrony.1 However, about a third of patients still do not get enough benefits from CRT.2,3,4,5 Therefore, a multi-disciplinary and comprehensive approach is needed throughout the pre-implant, implant, and post-implant phases to achieve maximal responses to CRT.6,7,8,9 Optimization of atrioventricular (AV) and ventriculo-ventricular (VV) delays is one of the most important parts of post-implant management because it is closely related with effective biventricular (BiV) pacing percentage. Echocardiography has been widely used to guide CRT optimization, but its effectiveness is still questionable.10,11,12 Echo-based AV or VV optimization is a time-consuming process requiring additional experts and costs. Moreover, repeated echo-guided optimizations according to the constantly changing clinical and cardiac conditions are nearly impossible to be performed. Recently, device-based autonomic optimization methods have been developed to facilitate the process. Results from the Adaptive CRT trial, which investigated the effectiveness of the adaptive CRT (aCRT) optimization algorithm (Medtronic Inc., Minneapolis, MN, USA), showed that autonomic optimization using the aCRT algorithm was comparable or superior to echo-based ones in many clinical and echocardiographic endpoints.13,14,15,16,17 To date, however, there are few real-world data regarding the effectiveness of automatic CRT optimization using the aCRT algorithm.18 We hypothesized that the aCRT optimization algorithm would work as well as, or better than, the conventional optimization methods in real-world clinical practices as well. Therefore, we tried to compare the efficacy of CRT using different optimization methods: conventional non-adaptive vs. automatic adaptive optimization.
METHODS
Patient population
We included consecutive patients older than 18 years who received CRT (de novo or upgrade) for advanced HF at our center from March 2012 to April 2018. All patients had the following characteristics: left ventricular (LV) ejection fraction (EF) ≤ 35% on transthoracic echocardiogram, QRS duration more than 120 ms on 12-lead electrocardiogram, and drug-refractory systolic HF19 with New York Heart Association (NYHA) class II, III, or ambulatory IV symptoms. Patients were excluded who met any of the following criteria: 1) narrow QRS (< 120 ms) or 2) multi-point pacing CRT. Baseline characteristics including NYHA functional status, demographics, and medications were collected through careful reviews of electronic medical records.
aCRT algorithm and classification of patients
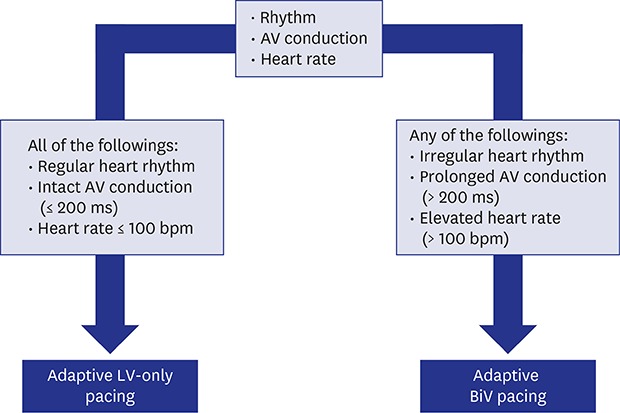
The aCRT algorithm was designed to update varying AV conduction and heart rate every minute to optimize pacing configuration automatically (Supplementary Fig. 1). The algorithm provides two different pacing modes: 1) “adaptive LV-only pacing” mode is applied to optimize AV delay and minimize RV pacing when AV conduction is normal and heart rate ≤ 100 bpm, or 2) “adaptive BiV pacing” mode works to adjust AV and VV delay automatically in the situation of impaired AV conduction or heart rate > 100 bpm. Patients were categorized into 3 groups according to the optimization method as follows: non-adaptive BiV, adaptive BiV, and adaptive LV groups. In the non-adaptive BiV group, BiV pacing was delivered at fixed AV and VV intervals determined by conventional (ECG- or echo-based) optimization methods. For the adaptive groups, AV or VV intervals were automatically optimized by the aCRT algorithm. Of patients in the adaptive groups, those with LV-only pacing ≥ 50% were categorized into the adaptive LV group, and the remaining into the adaptive BiV group.20 Considering that all patients in the adaptive LV group had left bundle branch block (LBBB) and favorable factors for CRT response as essential requirements for the adaptive algorithm (sinus rhythm and normal AV delay), a subgroup of patients with sinus rhythm, normal PR intervals (≤ 200 ms), and LBBB were selected from the non-adaptive BiV group as a matched group for additional comparison to the adaptive LV group.
Electrocardiographic and echocardiographic data collection
Baseline electrocardiographic parameters were collected including baseline rhythm, PR interval, and QRS duration and morphology. QRS morphology was classified as either LBBB or non-LBBB. The LBBB morphology was defined when all of the following criteria were met: 1) QRS duration ≥ 140 ms (men) or ≥ 130 ms (women), 2) QS or rS in lead V1 and V2, and 3) mid-QRS notching or slurring in two or more of leads V1,V2, V5, V6, I, and aVL.21 In pacing-dependent patients with previous pacemakers upgraded to CRT, paced-QRS complexes were used for the assessment of baseline QRS duration and morphology. Baseline PR intervals were measured except for the patients with persistent atrial fibrillation (AF). Echocardiographic parameters such as LV end-systolic volume (LVESV), LV end-diastolic volume (LVEDV), left ventricular ejection fraction (LVEF), and left atrial volume index were assessed by Simpson's biplane method using commercially available equipment, such as Vivid 7 (GE Healthcare, Chicago, IL, USA), Acuson 512 (Siemens, Munich, Germany) or Sonos 5500 (Philips, Andover, MA, USA).
Device implantation and optimization
CRT devices were implanted under local anesthesia using a transvenous approach. The LV lead was preferably placed into the anterolateral, lateral, or posterolateral LV walls in the left anterior oblique (LAO) view, and into the basal or mid-LV segments in the right anterior oblique (RAO) view. If the transvenous approach had failed, an epicardial LV lead was screwed into the lateral wall mid-LV segment via thoracoscopic surgery. The position of the right ventricular (RV) and LV leads and their pacing electrodes were confirmed by LAO and RAO fluoroscopic images.22 In the non-adaptive BiV group, AV and VV delays were determined to show the greatest stroke volume or narrowest QRS duration during the index hospitalization before discharge. For the adaptive BiV and adaptive LV groups, the aCRT optimization algorithm was activated immediately after the implantation. CRT pacing percentages were obtained at every planned or unplanned visit to our device clinic. In the adaptive groups, CRT pacing percentages were calculated as the sum of BiV and LV-only pacing percentages. For the non-adaptive BiV group, CRT pacing percentages were equal to BiV pacing percentages.
Study outcomes
The primary endpoint was a composite clinical outcome including cardiac death, heart transplantation, LV assist device implantation for destination therapy, and HF-related hospitalization after CRT implantation. Secondary outcomes consisted of electromechanical reverse remodeling and responder rates at 6 months after CRT implantation. For assessment of reverse remodeling, absolute (differences between pre-CRT and post-CRT values) and relative changes (absolute changes/pre-CRT values ×100, %) in the ECG and echocardiographic parameters were calculated. The definitions for CRT responder were as follows: 1) ‘clinical responder’ for survivors at 6 months with any improvement in NYHA class, 2) ‘echocardiographic responder’ for patients with a relative decrease in LVESV by ≥ 15%, and 3) ‘super-responder’ for clinical responders with a relative decrease in LVESV by ≥ 30% or with LVEF ≥ 45%.
Statistical analysis
Continuous variables are presented as medians (25th–75th percentiles) and compared using Mann-Whitney U tests or Kruskal-Wallis tests. Categorical variables are shown as numbers with percentages, and comparisons between groups were performed using χ2 tests or Fisher's exact test. For analysis of the primary outcome, event-free survival was estimated by the Kaplan-Meier method and compared with the log-rank test. Logistic regression models were used to determine the independent predictors of a super-responder. Variables with P values < 0.2 in univariate analysis and adaptive LV pacing were included in multivariate analysis. To focus on the evaluation of the efficacy of LV-only pacing, baseline characteristics and study outcomes were compared between the subgroup of the non-adaptive BiV and the adaptive LV group using the same statistical methods. Statistical analyses were performed with the IBM SPSS Statistics version 23 (IBM Corporation, Armonk, NY, USA). All tests were two-tailed, and a P value < 0.05 was considered statistically significant.
Ethics statement
The Institutional Review Board (IRB) at Samsung Medical Center approved the study protocol (IRB No. 2018-04-138). Informed consent was waived by the board.
RESULTS
Patients and baseline characteristics of total patients
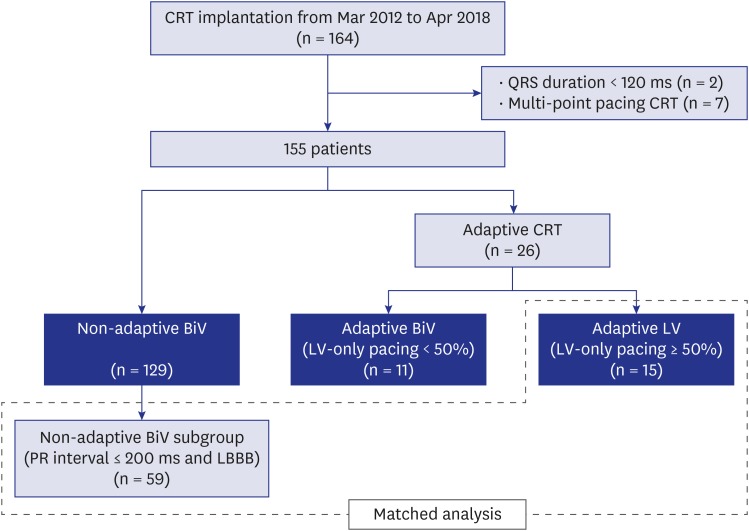
We identified 164 CRT patients during the study period. In the final analysis, 155 patients were included after 2 patients with narrow QRS and 7 patients treated with LV multi-point pacing function were excluded (Fig. 1). Forty-two patients (27.1%) had the ischemic etiology of HF, and 17 patients (11.0%) had persistent AF. Most of the CRT devices were CRT-defibrillator (94.2%), and 98.1% of patients had their LV leads in the lateral LV wall. Of the 155 patients included in the present study, 129 patients were optimized with conventional non-adaptive methods and the remaining 26 with the aCRT algorithm. Among 26 patients optimized with the aCRT algorithm, 11 were categorized into the adaptive BiV group, and the remaining 15 into the adaptive LV group. A subgroup of patients with normal PR intervals and LBBB were selected from the non-adaptive BiV group as the matched group (n = 59) (Fig. 1). There were no significant differences in baseline demographics among the groups. Due to the application criteria of adaptive LV-only pacing, LBBB was more frequent in the adaptive LV group than the non-adaptive BiV group (100% vs. 72.1%; P = 0.03) and the PR interval was significantly shorter in the adaptive LV group than the non-adaptive BiV group (174 ms vs. 196 ms; P = 0.01). The median CRT pacing percentage during the first 6 months after CRT was higher in the non-adaptive BiV group than the adaptive LV group (99.6% vs. 98.2%; P = 0.01) (Table 1).
Fig. 1. Flow diagram of the study population. Patients were matched according to baseline PR interval and QRS morphology.
CRT = cardiac resynchronization therapy, BiV = biventricular, LV = left ventricular, LBBB = left bundle branch block.
Table 1. Baseline characteristics.
| Characteristics | Non-adaptive BiV (n = 129) | Adaptive BiV (n = 11) | Adaptive LV (n = 15) | P valuea | Matched non-adaptive BiV (n = 59) | Adaptive LV (n = 15) | P valuea | |
|---|---|---|---|---|---|---|---|---|
| Demographics and medical history | ||||||||
| Gender, men | 83 (64.3) | 9 (81.8) | 9 (60.0) | 0.50 | 35 (59.3) | 9 (60.0) | > 0.999 | |
| Age, yr | 66 (58–75) | 74 (63–83) | 67 (57–69) | 0.66 | 69 (62–77) | 67 (61–71) | 0.54 | |
| BMI, kg/m2 | 22.8 (20.7–25.6) | 22.8 (20.2–23.9) | 24.6 (21.8–25.8) | 0.55 | 22.6 (20.1–25.5) | 24.4 (21.1–25.7) | 0.44 | |
| NYHA class | 3 (3–3) | 3 (3–3) | 3 (2–3) | 0.09 | 3 (3–3) | 3 (2–3) | 0.06 | |
| Hypertension | 71 (55.0) | 8 (72.7) | 9 (60.0) | 0.52 | 33 (55.9) | 9 (60.0) | > 0.999 | |
| Diabetes | 45 (34.9) | 2 (18.2) | 7 (46.7) | 0.33 | 22 (37.3) | 7 (46.7) | 0.56 | |
| Ischemic CMP | 32 (24.8) | 4 (36.4) | 6 (40.0) | 0.27 | 12 (20.3) | 6 (40.0) | 0.18 | |
| Persistent AF | 16 (12.4) | 1 (9.1) | 0 (0) | 0.38 | 7 (11.9) | 0 (0) | 0.33 | |
| Medication | ||||||||
| Beta blocker | 84 (65.1) | 6 (54.5) | 10 (66.7) | 0.75 | 35 (59.3) | 10 (66.7) | 0.77 | |
| RAS blocker | 105 (81.4) | 11 (100) | 12 (80.0) | 0.35 | 46 (78.0) | 12 (80.0) | > 0.999 | |
| Aldosterone antagonist | 81 (62.8) | 5 (45.5) | 8 (53.3) | 0.50 | 39 (66.1) | 8 (53.3) | 0.38 | |
| Echocardiographic findings | ||||||||
| LVEF, % | 24.2 (20.0–27.7) | 22.5 (22.3–29.3) | 23.4 (17.0–28.4) | 0.56 | 23.5 (19.8–28.1) | 23.4 (17.0–28.4) | 0.48 | |
| LVEDV, mLb | 244 (192–283) | 250 (219–295) | 274 (232–291) | 0.42 | 234 (189–278) | 274 (232–291) | 0.14 | |
| LVESV, mLb | 180 (150–233) | 177 (162–220) | 213 (170–227) | 0.46 | 178 (140–226) | 213 (170–227) | 0.19 | |
| Electrocardiographic findings | ||||||||
| LBBB | 93 (72.1) | 9 (81.8) | 15 (100) | 0.03 | - | - | - | |
| QRS width, ms | 170 (152–182) | 184 (168–222) | 169 (152–172) | 0.30 | 166 (152–189) | 169 (152–172) | 0.55 | |
| PR interval, ms | 196 (172–224) | 184 (174–202) | 174 (156–188) | 0.02 | 174 (152–189) | 174 (156–188) | 0.97 | |
| Indication for CRT | ||||||||
| De novo | 106 (82.2) | 8 (72.7) | 15 (100) | 0.09 | 51 (86.4) | 15 (100) | 0.20 | |
| Upgrade | 23 (17.8) | 3 (27.3) | 0 (0) | 8 (13.6) | 0 (0) | |||
| Type of CRT | ||||||||
| CRT-D | 121 (93.8) | 10 (90.9) | 15 (100) | 0.61 | 56 (94.9) | 15 (100) | > 0.999 | |
| CRT-P | 8 (6.2) | 1 (9.1) | 0 (0) | 3 (5.1) | 0 (0) | |||
| LV leads | ||||||||
| Lateral position | 126 (98.4) | 11 (100) | 15 (100) | > 0.999 | 58 (98.3) | 15 (100) | > 0.999 | |
| Bipolar lead | 44 (34.1) | 7 (63.6) | 3 (20.0) | 0.07 | 18 (30.5) | 3 (20.0) | 0.53 | |
| Epicardial lead | 7 (5.4) | 0 (0) | 0 (0) | > 0.999 | 3 (5.1) | 0 (0) | > 0.999 | |
| CRT pacing, % | 99.6 (97.0–100) | 99.6 (97.1–99.9) | 98.2 (97.7–98.5) | 0.03 | 99.7 (97.0–100) | 98.2 (97.7–98.5) | 0.01 | |
Values are presented as median (interquartile range) or number of patients (%).
BiV = biventricular, LV = left ventricular, BMI = body mass index, NYHA = New York Heart Association, CMP = cardiomyopathy, AF = atrial fibrillation, RAS = renin-angiotensin system, LVEF = left ventricular ejection fraction, LVEDV = left ventricular end-diastolic volume, LVESV = left ventricular end-diastolic volume, LBBB = left bundle branch block, CRT = cardiac resynchronization therapy, CRT-D = cardiac resynchronization therapy-defibrillator, CRT-P = cardiac resynchronization therapy-pacemaker.
aP value refers to the difference among the three groups by Kruskal-Wallis test or χ2 tests; bLV volumes were available in 137 patients (88.4%) in total population, and 65 patients (87.8%) in matched population.
Study outcomes in total study population
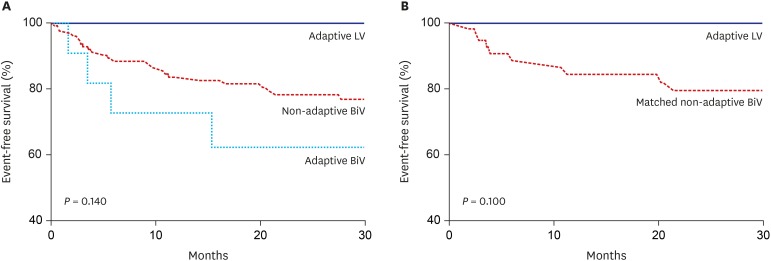
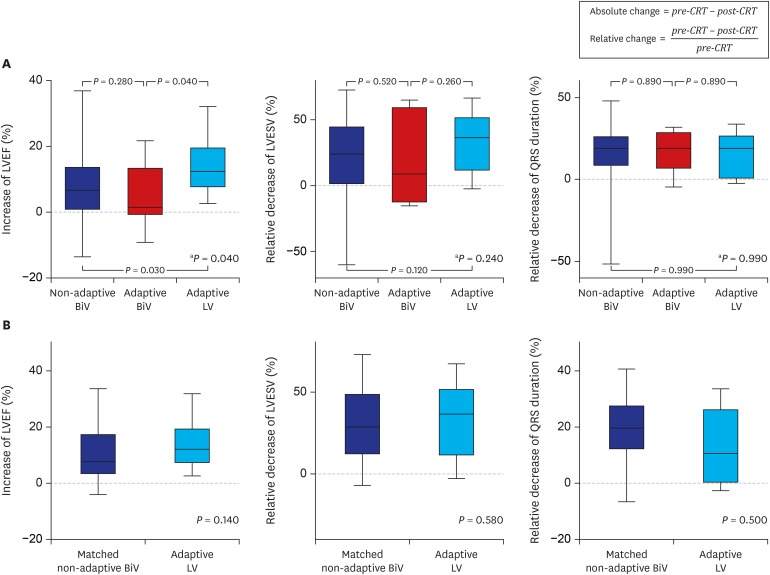
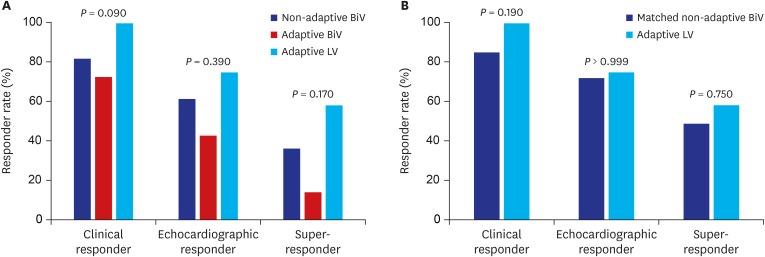
There was no statistically significant difference in the primary clinical composite outcome during a median follow-up of 27.5 months among the groups (26.4% for the non-adaptive BiV vs. 36.4% for the adaptive BiV vs. 0% for the adaptive LV group; P = 0.14) (Table 2 and Fig. 2). Cardiac death, heart transplantation, LV assist device, or HF-related hospitalization rates were not significantly different among the groups as the primary composite outcomes, but the adaptive LV group seemed to show the best clinical outcome without any adverse clinical events (Table 2). In secondary outcomes, patients in the adaptive LV group showed the highest increase in LVEF at 6 months after CRT implantation (12.4% for the adaptive LV vs. 6.6% for the non-adaptive BiV vs. 1.5% for the adaptive BiV group; P = 0.04). In addition, the changes in LVESV and QRS duration tended to be greatest in the adaptive LV group. Although the 3 groups showed no significant differences in the clinical, echocardiographic, and super responder rates, there was a tendency toward a greater response rate in the adaptive LV compared to the other groups (58.3% for the adaptive LV vs. 36.3% for the non-adaptive BiV vs. 14.3% for the adaptive BiV group; P = 0.17 for super-responder rate) (Figs. 3 and 4, and Table 3). Using multivariate logistic regression analysis, we identified that baseline LBBB was significantly associated with super-responder (odds ratio [OR], 6.89; 95% confidence interval [CI], 1.44–33.1; P = 0.02). For the analysis, men, hypertension, LVEDV, LBBB, PR interval and adaptive LV pacing were included for multivariate analysis in the total population, while men, hypertension, diabetes, LVEDV, and adaptive LV pacing were included in the matched population (Table 4).
Table 2. Primary clinical outcomes.
| Variables | Non-adaptive BiV (n = 129) | Adaptive BiV (n = 11) | Adaptive LV (n = 15) | P valuea | Matched non-adaptive BiV (n = 59) | Adaptive LV (n = 15) | P valuea |
|---|---|---|---|---|---|---|---|
| Composite outcome | 34 (26.4) | 4 (36.4) | 0 (0) | 0.14 | 13 (22.2) | 0 (0) | 0.10 |
| Cardiac death | 7 (5.4) | 1 (9.1) | 0 (0) | 0.65 | 3 (5.1) | 0 (0) | 0.43 |
| HTPL or LVAD | 14 (10.9) | 0 (0) | 0 (0) | 0.24 | 7 (11.9) | 0 (0) | 0.22 |
| HF-hospitalization | 30 (23.3) | 4 (36.4) | 0 (0) | 0.14 | 11 (18.6) | 0 (0) | 0.14 |
| Follow-up duration, mon | 27.5 (10.0–46.3) | 36.1 (19.3–58.4) | 23.1 (6.2–39.4) | 0.30 | 26.8 (5.6–51.6) | 23.1 (6.2–39.4) | 0.56 |
Values are presented as number of patients (%).
BiV = biventricular, LV = left ventricular, HTPL = heart transplantation, LVAD = left ventricular assist device, HF = heart failure.
aP value refers to the difference between the groups by log-rank test.
Fig. 2. Comparison of survival curves for primary outcomes. (A) Total population. (B) Matched population. The primary outcome was a composite of clinical events including cardiac death, heart transplantation, left ventricular assist device implantation for destination therapy, and heart failure-related rehospitalization.
LV = left ventricular, BiV = biventricular.
Fig. 3. Changes in LVEF, LVESV, and QRS duration at 6 months after CRT. (A) Total population. (B) Matched population.
CRT = cardiac resynchronization therapy, LVEF = left ventricular ejection fraction, BiV = biventricular, LV = left ventricular, LVESV = left ventricular end-systolic volume.
aP value refers to the difference among the 3 groups by Kruskal-Wallis test.
Fig. 4. Responder rates. (A) Total population. (B) Matched population.
BiV = biventricular, LV = left ventricular.
Table 3. Secondary outcomes.
| Variables | Non-adaptive BiV | Adaptive BiV | Adaptive LV | P valuea | Matched non-adaptive BiV | Adaptive LV | P value | |
|---|---|---|---|---|---|---|---|---|
| Absolute change | ||||||||
| LVEF, % | 6.6 (0.9, 13.4) | 1.5 (−0.3, 13.0) | 12.4 (7.7, 18.2) | 0.04 | 7.8 (3.8, 17.3) | 12.4 (7.7, 18.2) | 0.14 | |
| LVESV, mL | 42.2 (4.0, 72.7) | 26.4 (−13.6, 74.7) | 63.7 (31.6, 107.1) | 0.20 | 50.9 (23.2, 72.7) | 63.7 (31.6, 107.1) | 0.36 | |
| QRS width, ms | 30 (14, 44) | 29 (16, 54) | 30 (2, 44) | 0.89 | 35 (20, 46) | 30 (2, 44) | 0.49 | |
| Relative change | ||||||||
| LVESV, % | 24.0 (1.9, 43.8) | 8.9 (−8.3, 38.4) | 36.8 (17.1, 49.6) | 0.24 | 28.6 (12.8, 47.5) | 36.8 (17.1, 49.6) | 0.58 | |
| QRS width, % | 19.0 (8.8, 26.1) | 19.0 (8.4, 27.7) | 18.9 (1.2, 25.6) | 0.99 | 20.9 (12.4, 27.3) | 18.9 (1.2, 25.6) | 0.50 | |
| Responder rate | ||||||||
| Clinical | 100 (82.0) | 8 (72.7) | 14 (100) | 0.09 | 46 (85.2) | 14 (100) | 0.19 | |
| EchoCG | 56 (61.5) | 3 (42.9) | 9 (75.0) | 0.39 | 31 (72.1) | 9 (75.0) | > 0.999 | |
| Super | 33 (36.3) | 1 (14.3) | 7 (58.3) | 0.17 | 21 (48.8) | 7 (58.3) | 0.75 | |
Values are presented as median (interquartile range) or number of patients (%).
The values in absolute and relative changes represent the ‘increment’ of LVEF or ‘decrement’ of LVESV or QRS width. In total population, LVEF was available in 126 patients (81.3%) and LV volumes were available in 110 patients (71.0%). In matched population, LVEF was available in 60 patients (81.1%) and LV volumes were available in 55 patients (74.3%).
BiV = biventricular, LV = left ventricular LVEF = left ventricular ejection fraction, LVESV = left ventricular end-diastolic volume, EchoCG = echocardiographic.
aP value refers to the difference among the three groups by Kruskal-Wallis test or χ2 tests.
Table 4. Predictors for super-responder.
| Variables | Total population | Matched population | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysisa | Univariate analysis | Multivariate analysisa | ||||||
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | ||
| Demographics and medical history | |||||||||
| Gender, men | 0.43 (0.19–0.96) | 0.04 | 0.65 (0.25–1.67) | 0.37 | 0.19 (0.06–0.62) | 0.01 | 0.22 (0.06–0.80) | 0.02 | |
| Age, yr | 0.99 (0.95–1.02) | 0.38 | 0.97 (0.92–1.02) | 0.23 | |||||
| NYHA class | 0.66 (0.33–1.31) | 0.23 | 0.87 (0.32–2.41) | 0.79 | |||||
| Hypertension | 0.53 (0.24–1.16) | 0.11 | 0.73 (0.29–1.82) | 0.50 | 0.30 (0.10–0.95) | 0.04 | 0.36 (0.10–1.32) | 0.12 | |
| Diabetes | 0.64 (0.28–1.47) | 0.30 | 0.37 (0.12–1.13) | 0.08 | 0.33 (0.09–1.27) | 0.33 | |||
| Ischemic CMP | 0.69 (0.28–1.70) | 0.42 | 1.20 (0.32–4.52) | 0.79 | |||||
| Persistent AF | 0.45 (0.09–2.30) | 0.34 | 0.96 (0.13–7.36) | 0.97 | |||||
| Medication | |||||||||
| Beta blocker | 1.08 (0.47–2.46) | 0.86 | 1.45 (0.48–4.38) | 0.51 | |||||
| RAS blocker | 1.52 (0.49–4.66) | 0.47 | 1.05 (0.27–4.12) | 0.95 | |||||
| Aldosterone antagonist | 1.76 (0.78–3.95) | 0.17 | 1.45 (0.48–4.38) | 0.51 | |||||
| Echocardiographic findings | |||||||||
| LVEF, % | 0.99 (0.93–1.06) | 0.79 | 1.03 (0.94–1.13) | 0.56 | |||||
| LVEDV, mL | 1.00 (0.99–1.00) | 0.12 | 1.00 (0.99–1.00) | 0.23 | 0.99 (0.98–1.00) | 0.08 | 0.99 (0.98–1.00) | 0.13 | |
| LVESV, mL | 1.00 (0.99–1.00) | 0.16 | 0.99 (0.98–1.00) | 0.09 | |||||
| Electrocardiographic findings | |||||||||
| LBBB | 11.08 (2.46–49.80) | 0.002 | 6.89 (1.44–33.10) | 0.02 | |||||
| QRS width, ms | 0.99 (0.98–1.01) | 0.48 | 1.01 (0.98–1.04) | 0.53 | |||||
| PR interval, ms | 0.99 (0.98–1.00) | 0.17 | 1.00 (0.99–1.01) | 0.40 | 1.00 (0.98–1.02) | 0.92 | |||
| Device parameter | |||||||||
| De novo CRT | 2.15 (0.65–7.10) | 0.21 | 1.63 (0.25–10.58) | 0.61 | |||||
| Adaptive LV pacing | 2.64 (0.78–8.93) | 0.12 | 1.77 (0.46–6.81) | 0.40 | 1.47 (0.40–5.35) | 0.56 | 1.79 (0.37–8.66) | 0.47 | |
| CRT pacing, % | 1.03 (0.98–1.08) | 0.27 | 1.03 (0.97–1.10) | 0.37 | |||||
OR = odds ratio, CI = confidence interval, NYHA = New York Heart Association, CMP = cardiomyopathy, AF = atrial fibrillation, RAS = renin-angiotensin system, LVEF = left ventricular ejection fraction, LVEDV = left ventricular end-diastolic volume, LVESV = left ventricular end-diastolic volume, LBBB = left bundle branch block, CRT = cardiac resynchronization therapy, LV = left ventricular.
aMen, hypertension, LVEDV, LBBB, PR interval and adaptive LV pacing were included for multivariate analysis in the total population, while men, hypertension, diabetes, LVEDV, and adaptive LV pacing were included in the matched population.
Comparison of the non-adaptive BiV subgroup to the adaptive LV group
Considering the better prognosis of CRT patients with normal PR intervals or typical LBBB morphology than those without,23,24 the matched subgroup (n = 59) of the non-adaptive BiV group with normal PR intervals and LBBB were compared to the adaptive LV group to enhance comparability (Fig. 1). There was no significant difference in baseline characteristics except for a higher CRT pacing percentage (99.7% vs. 98.2%; P = 0.01) in the matched non-adaptive BiV subgroup (Table 1). However, a tendency toward better outcomes in the adaptive LV than the matched non-adaptive BiV subgroup was consistent throughout the primary and secondary outcomes (Figs. 2B, 3B, and 4B and Tables 2 and 3). Multivariate analysis showed women gender was the only factor associated with super-responder (OR, 6.5; 95% CI, 1.4–31.0; P = 0.02) (Table 4).
DISCUSSION
In the present study, we investigated whether the automated dynamic optimization algorithm (aCRT) is as effective as conventional ECG or Echo-based optimizations in real-world practice. The main findings of this study were: 1) CRT patients optimized with the aCRT algorithm showed similar outcomes in clinical events and electromechanical remodeling compared to those optimized with conventional methods, 2) adaptive LV pacing showed comparable efficacy to non-adaptive BiV pacing even when the analysis was confined to patients with normal PR intervals and LBBB, and 3) there was a consistent tendency toward better outcomes in the adaptive LV group compared to the other groups regarding both primary and secondary outcomes.
Optimal programming of AV and VV delays is one of the most important strategies to improve the efficacy of CRT.8,25,26 Until recently, echo-based optimization was the most popular method.27,28,29 However, this method is difficult to utilize in routine clinical practice because of its innate limitations. Reproducibility is low due to inter- and intra-operator variability.11 and echocardiographic measurement needs to be tediously repeated in multiple combinations of various AV and VV timing. Additionally, the optimal AV or VV delay can vary with cardiac remodeling and the patient's hemodynamic status. Several studies have already shown significant changes of the optimal AV interval values between the initial and the follow-up period.30 Thus, fixed intervals optimized in resting position cannot accommodate the constantly changing patient status. Furthermore, the results of multicenter trials showed that routine use of echocardiographic optimization was no more effective than empirical programming.11,31
As a consequence, device-based automatic optimization methods have emerged as alternatives to conventional methods. SmartDelayTM (Boston Scientific, Natick, MA, USA) and QuickOptTM (Abbott, Sylmar, CA, USA), which are intracardiac electrogram-based algorithms, were developed to optimize AV or AV/VV intervals. However, automatic updates of optimal AV and VV delays were not possible in these manually operated algorithms, and previous trials using these algorithms have failed to show superiority over echo-based optimization.11,31 In contrast, the aCRT algorithm automatically adjusts pacing configuration by updating the patient's current AV conduction and heart rate every minute. It aims to create a well-balanced intra-LV fusion in 2 situations. When AV conduction is normal, the algorithm induces fusion between intrinsic activation conducting through the AV node and stimulated activation from the LV electrode (adaptive RV-synchronized LV-only pacing mode). Otherwise, it leads to the fusion between 2 stimulated activations from the RV and LV electrodes (adaptive BiV pacing mode) (Supplementary Fig. 1).
In the Adaptive CRT trial, a prospective, multi-center randomized double-blinded study, the aCRT arm showed comparable efficacy to the echo-optimized BiV arm in improvement of clinical composite score, cardiac performance, and HF or ventricular arrhythmia episodes at 6-month postrandomization.14 Moreover, sub-analyses of the Adaptive CRT trial demonstrated superiority of the aCRT algorithm over echo-optimized BiV pacing in terms of 6-month response rate, mortality, HF-related hospitalization, AF incidence, and device longevity. Furthermore, a higher LV pacing percentage was independently associated with superior clinical outcomes as similarly as the adaptive LV group of our data.15,16,20,32 There are possible explanations for further benefits of LV-only pacing over BiV pacing. First, the aCRT algorithm can minimize unnecessary RV pacing. Previous studies have shown that chronic RV pacing could induce ventricular dyssynchrony and new-onset LV systolic dysfunction, known as pacing-induced cardiomyopathy, even in patients without underlying cardiac dysfunction.33,34,35 Second, acute hemodynamic benefits of RV-synchronized LV-only pacing have been demonstrated in previous studies.36,37,38,39
More recent studies have also shown favorable outcomes of other device-based automatic algorithms such as the SyncAV algorithm (Abbott) and SonR algorithm (Sorin Group, Saluggia, Italy) in terms of QRS narrowing or clinical improvement.40,41 However, further real-world data are needed, considering that most of the evidence supporting the efficacy of automatic device-based optimization algorithms has been based on clinical trials.
It is known that regional or ethnic differences may have significant impact on the etiology, treatment, and prognosis of HF.42,43,44,45 However, there is a scarcity of real-world data on the efficacy of aCRT, especially in the Asia-Pacific region. Even in the Adaptive CRT trial, only a few patients from the Asia-Pacific region were included.14 Our first Asian-Pacific real-world data showed that aCRT was comparable in clinical outcomes and electromechanical remodeling to conventional optimization methods. Additionally, beneficial effects of aCRT seemed to be more noticeable in the adaptive LV group as in line with the previous randomized trial data. Patients in our study had relatively wider QRS duration (167.9 ms vs. 154.8 ms), greater LVESV index (114.8 mL/m2 vs. 72.4 mL/m2), and lower LVEF (24.0% vs. 29.8%) than those in the Adaptive CRT trial. Nonetheless, our results demonstrated that the efficacy of aCRT optimization was consistent regardless of ethnic differences or worse HF clinical features.
Another notable finding of our study was that the non-adaptive BiV pacing group tended to show poorer outcomes even with a significantly higher CRT pacing percentage than the adaptive LV group. This might imply a greater significance of the pacing quality than the pacing quantity in CRT. Several conventional CRT studies showed that BiV pacing percentage reports could be overestimated due to fusion or pseudo-fusion pacing.9,46 In other words, pacing percentage does not always guarantee actual BiV capture, particularly in CRT patients with fixed programming. This might be another explanation to why the adaptive LV group showed a tendency toward better outcomes than the non-adaptive BiV group in our results, despite the lower pacing percentage.
This study has several limitations. First, this is a single-center study with a small number of patients, especially in both adaptive groups. So, this sample size could be insufficient to acquire substantial statistical power. For this reason, we are planning a multicenter registry study. Second, the optimization method was not randomized. However, to minimize selection bias and balance intergroup differences, we selected matched subgroup patients. Third, follow-up echocardiographic data were not available in all patients due to the retrospective study design. Lastly, the criteria of defining the adaptive LV group (LV-only pacing ≥ 50%) might be arbitrary. However, this criteria was based on the previous study showing that RV-synchronized LV pacing ≥ 50% was independently associated with favorable clinical outcomes in the aCRT arm.20 In our study, patients in the adaptive LV group showed a median LV pacing percentage of 97.7%.
This is the first Asia-Pacific real-world data investigating the efficacy of the aCRT algorithm. The aCRT algorithm showed a trend toward better performance in terms of clinical outcomes and electromechanical remodeling compared to the conventional optimization methods requiring extra time and cost for cumbersome measurements. Further studies including a large number of patients are needed to confirm the beneficial effects of dynamic AV/VV optimization and RV-synchronized LV-only pacing in real-world practice.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Park SJ, Kim JS.
- Data curation: Gwag HB, Park SJ.
- Formal analysis: Gwag HB, Park SJ.
- Investigation: Gwag HB, Park Y, Lee SS.
- Supervision: Park SJ, Kim JS.
- Validation: Gwag HB, Park SJ.
- Visualization: Gwag HB, Park SJ.
- Writing - original draft: Gwag HB.
- Writing - review & editing: On YK, Park KM.
SUPPLEMENTARY MATERIAL
The adaptive CRT algorithm to choose the pacing mode.
References
- 1.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WT, Gras D, Yu CM, Guzzo L, Gupta MS, Committee FS, et al. Rationale and design of a randomized clinical trial to assess the safety and efficacy of frequent optimization of cardiac resynchronization therapy: the Frequent Optimization Study Using the QuickOpt Method (FREEDOM) trial. Am Heart J. 2010;159(6):944–948.e1. doi: 10.1016/j.ahj.2010.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117(20):2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 4.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 5.León AR, Abraham WT, Brozena S, Daubert JP, Fisher WG, Gurley JC, et al. Cardiac resynchronization with sequential biventricular pacing for the treatment of moderate-to-severe heart failure. J Am Coll Cardiol. 2005;46(12):2298–2304. doi: 10.1016/j.jacc.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Hayes DL, Boehmer JP, Day JD, Gilliam FR, 3rd, Heidenreich PA, Seth M, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011;8(9):1469–1475. doi: 10.1016/j.hrthm.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Koplan BA, Kaplan AJ, Weiner S, Jones PW, Seth M, Christman SA. Heart failure decompensation and all-cause mortality in relation to percent biventricular pacing in patients with heart failure: is a goal of 100% biventricular pacing necessary? J Am Coll Cardiol. 2009;53(4):355–360. doi: 10.1016/j.jacc.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53(9):765–773. doi: 10.1016/j.jacc.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Daubert JC, Saxon L, Adamson PB, Auricchio A, Berger RD, Beshai JF, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace. 2012;14(9):1236–1286. doi: 10.1093/europace/eus222. [DOI] [PubMed] [Google Scholar]
- 10.Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Europace. 2013;15(8):1070–1118. doi: 10.1093/europace/eut206. [DOI] [PubMed] [Google Scholar]
- 11.Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, et al. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation. 2010;122(25):2660–2668. doi: 10.1161/CIRCULATIONAHA.110.992552. [DOI] [PubMed] [Google Scholar]
- 12.Gras D, Gupta MS, Boulogne E, Guzzo L, Abraham WT. Optimization of AV and VV delays in the real-world CRT patient population: an international survey on current clinical practice. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S236–S239. doi: 10.1111/j.1540-8159.2008.02294.x. [DOI] [PubMed] [Google Scholar]
- 13.Krum H, Lemke B, Birnie D, Lee KL, Aonuma K, Starling RC, et al. A novel algorithm for individualized cardiac resynchronization therapy: rationale and design of the adaptive cardiac resynchronization therapy trial. Am Heart J. 2012;163(5):747–752.e1. doi: 10.1016/j.ahj.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Martin DO, Lemke B, Birnie D, Krum H, Lee KL, Aonuma K, et al. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm. 2012;9(11):1807–1814. doi: 10.1016/j.hrthm.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Singh JP, Abraham WT, Chung ES, Rogers T, Sambelashvili A, Coles JA, Jr, et al. Clinical response with adaptive CRT algorithm compared with CRT with echocardiography-optimized atrioventricular delay: a retrospective analysis of multicentre trials. Europace. 2013;15(11):1622–1628. doi: 10.1093/europace/eut107. [DOI] [PubMed] [Google Scholar]
- 16.Starling RC, Krum H, Bril S, Tsintzos SI, Rogers T, Hudnall JH, et al. Impact of a novel adaptive optimization algorithm on 30-day readmissions: evidence from the adaptive CRT trial. JACC Heart Fail. 2015;3(7):565–572. doi: 10.1016/j.jchf.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 17.Yamasaki H, Lustgarten D, Cerkvenik J, Birnie D, Gasparini M, Lee KL, et al. Adaptive CRT in patients with normal AV conduction and left bundle branch block: does QRS duration matter? Int J Cardiol. 2017;240:297–301. doi: 10.1016/j.ijcard.2017.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Hsu JC, Birnie DH, Stadler RW, Cerkvenik J, Feld GK, Birgersdotter-Green U. Adaptive cardiac resynchronization therapy is associated with decreased risk of incident atrial fibrillation compared to standard biventricular pacing: a real-world analysis of 37,450 patients followed by remote monitoring. Heart Rhythm. 2019;16(7):983–989. doi: 10.1016/j.hrthm.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 19.Ahn MS, Yoo BS, Yoon J, Lee SH, Kim JY, Ahn SG, et al. Prognostic effect of guideline-directed therapy is more noticeable early in the course of heart failure. J Korean Med Sci. 2019;34(17):e133. doi: 10.3346/jkms.2019.34.e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birnie D, Lemke B, Aonuma K, Krum H, Lee KL, Gasparini M, et al. Clinical outcomes with synchronized left ventricular pacing: analysis of the adaptive CRT trial. Heart Rhythm. 2013;10(9):1368–1374. doi: 10.1016/j.hrthm.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107(6):927–934. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 22.Rad MM, Blaauw Y, Dinh T, Pison L, Crijns HJ, Prinzen FW, et al. Left ventricular lead placement in the latest activated region guided by coronary venous electroanatomic mapping. Europace. 2015;17(1):84–93. doi: 10.1093/europace/euu221. [DOI] [PubMed] [Google Scholar]
- 23.Olshansky B, Day JD, Sullivan RM, Yong P, Galle E, Steinberg JS. Does cardiac resynchronization therapy provide unrecognized benefit in patients with prolonged PR intervals? The impact of restoring atrioventricular synchrony: an analysis from the COMPANION Trial. Heart Rhythm. 2012;9(1):34–39. doi: 10.1016/j.hrthm.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 24.Gervais R, Leclercq C, Shankar A, Jacobs S, Eiskjaer H, Johannessen A, et al. Surface electrocardiogram to predict outcome in candidates for cardiac resynchronization therapy: a sub-analysis of the CARE-HF trial. Eur J Heart Fail. 2009;11(7):699–705. doi: 10.1093/eurjhf/hfp074. [DOI] [PubMed] [Google Scholar]
- 25.Stellbrink C, Breithardt OA, Franke A, Sack S, Bakker P, Auricchio A, et al. Impact of cardiac resynchronization therapy using hemodynamically optimized pacing on left ventricular remodeling in patients with congestive heart failure and ventricular conduction disturbances. J Am Coll Cardiol. 2001;38(7):1957–1965. doi: 10.1016/s0735-1097(01)01637-0. [DOI] [PubMed] [Google Scholar]
- 26.Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation. 1999;99(23):2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 27.Gorcsan J, 3rd, Abraham T, Agler DA, Bax JJ, Derumeaux G, Grimm RA, et al. Echocardiography for cardiac resynchronization therapy: recommendations for performance and reporting--a report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J Am Soc Echocardiogr. 2008;21(3):191–213. doi: 10.1016/j.echo.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Sawhney NS, Waggoner AD, Garhwal S, Chawla MK, Osborn J, Faddis MN. Randomized prospective trial of atrioventricular delay programming for cardiac resynchronization therapy. Heart Rhythm. 2004;1(5):562–567. doi: 10.1016/j.hrthm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Gold MR, Niazi I, Giudici M, Leman RB, Sturdivant JL, Kim MH, et al. A prospective comparison of AV delay programming methods for hemodynamic optimization during cardiac resynchronization therapy. J Cardiovasc Electrophysiol. 2007;18(5):490–496. doi: 10.1111/j.1540-8167.2007.00770.x. [DOI] [PubMed] [Google Scholar]
- 30.Brabham WW, Gold MR. The role of AV and VV optimization for CRT. J Arrhythm. 2013;29(3):153–161. [Google Scholar]
- 31.Abraham WT, Gras D, Yu CM, Calo L, Islam N, Klein N, et al. Results from the FREEDOM Trial: Assess the Safety and Efficacy of Frequent Optimization of Cardiac Resynchronization Therapy. SP08. Late-Breaking Clinical Trials. Washington, D.C.: Heart Rhythm Society; 2010. [Google Scholar]
- 32.Birnie D, Hudnall H, Lemke B, Aonuma K, Lee KL, Gasparini M, et al. Continuous optimization of cardiac resynchronization therapy reduces atrial fibrillation in heart failure patients: results of the adaptive cardiac resynchronization therapy trial. Heart Rhythm. 2017;14(12):1820–1825. doi: 10.1016/j.hrthm.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Kiehl EL, Makki T, Kumar R, Gumber D, Kwon DH, Rickard JW, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13(12):2272–2278. doi: 10.1016/j.hrthm.2016.09.027. [DOI] [PubMed] [Google Scholar]
- 34.Kim JH, Kang KW, Chin JY, Kim TS, Park JH, Choi YJ. Major determinant of the occurrence of pacing-induced cardiomyopathy in complete atrioventricular block: a multicentre, retrospective analysis over a 15-year period in South Korea. BMJ Open. 2018;8(2):e019048. doi: 10.1136/bmjopen-2017-019048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gwag HB, Chun KJ, Hwang JK, Park KM, On YK, Kim JS, et al. Comparison of de novo versus upgrade cardiac resynchronization therapy; focused on the upgrade for pacing-induced cardiomyopathy. Yonsei Med J. 2017;58(4):703–709. doi: 10.3349/ymj.2017.58.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kass DA, Chen CH, Curry C, Talbot M, Berger R, Fetics B, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99(12):1567–1573. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- 37.van Gelder BM, Bracke FA, Meijer A, Pijls NH. The hemodynamic effect of intrinsic conduction during left ventricular pacing as compared to biventricular pacing. J Am Coll Cardiol. 2005;46(12):2305–2310. doi: 10.1016/j.jacc.2005.02.098. [DOI] [PubMed] [Google Scholar]
- 38.Lee KL, Burnes JE, Mullen TJ, Hettrick DA, Tse HF, Lau CP. Avoidance of right ventricular pacing in cardiac resynchronization therapy improves right ventricular hemodynamics in heart failure patients. J Cardiovasc Electrophysiol. 2007;18(5):497–504. doi: 10.1111/j.1540-8167.2007.00788.x. [DOI] [PubMed] [Google Scholar]
- 39.Varma N, Jia P, Ramanathan C, Rudy Y. RV electrical activation in heart failure during right, left, and biventricular pacing. JACC Cardiovasc Imaging. 2010;3(6):567–575. doi: 10.1016/j.jcmg.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varma N, O'Donnell D, Bassiouny M, Ritter P, Pappone C, Mangual J, et al. Programming cardiac resynchronization therapy for electrical synchrony: reaching beyond left bundle branch block and left ventricular activation delay. J Am Heart Assoc. 2018;7(3):e007489. doi: 10.1161/JAHA.117.007489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritter P, Delnoy PP, Padeletti L, Lunati M, Naegele H, Borri-Brunetto A, et al. A randomized pilot study of optimization of cardiac resynchronization therapy in sinus rhythm patients using a peak endocardial acceleration sensor vs. standard methods. Europace. 2012;14(9):1324–1333. doi: 10.1093/europace/eus059. [DOI] [PubMed] [Google Scholar]
- 42.Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm. 2009;6(3):325–331. doi: 10.1016/j.hrthm.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 43.Lam CS, Teng TK, Tay WT, Anand I, Zhang S, Shimizu W, et al. Regional and ethnic differences among patients with heart failure in Asia: the Asian sudden cardiac death in heart failure registry. Eur Heart J. 2016;37(41):3141–3153. doi: 10.1093/eurheartj/ehw331. [DOI] [PubMed] [Google Scholar]
- 44.Gijsberts CM, Benson L, Dahlström U, Sim D, Yeo DP, Ong HY, et al. Ethnic differences in the association of QRS duration with ejection fraction and outcome in heart failure. Heart. 2016;102(18):1464–1471. doi: 10.1136/heartjnl-2015-309212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Atherton JJ, Hayward CS, Wan Ahmad WA, Kwok B, Jorge J, Hernandez AF, et al. Patient characteristics from a regional multicenter database of acute decompensated heart failure in Asia Pacific (ADHERE International-Asia Pacific) J Card Fail. 2012;18(1):82–88. doi: 10.1016/j.cardfail.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 46.Hernández-Madrid A, Facchin D, Klepfer RN, Ghosh S, Matía R, Moreno J, et al. Device pacing diagnostics overestimate effective cardiac resynchronization therapy pacing results of the hOLter for Efficacy analysis of CRT (OLÉ CRT) study. Heart Rhythm. 2017;14(4):541–547. doi: 10.1016/j.hrthm.2017.01.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The adaptive CRT algorithm to choose the pacing mode.