Summary
Background
A ring-shaped, contraceptive vaginal system designed to last 1 year (13 cycles) delivers an average of 0·15 mg segesterone acetate and 0·013 mg ethinylestradiol per day. We evaluated the efficacy of this contraceptive vaginal system and return to menses or pregnancy after use.
Methods
In two identically designed, multicentre, open-label, single-arm, phase 3 trials (one at 15 US academic and community sites and one at 12 US and international academic and community sites), participants followed a 21-days-in, 7-days-out segesterone acetate and ethinylestradiol contraceptive vaginal system schedule for up to 13 cycles. Participants were healthy, sexually active, non-pregnant, non-sterilised women aged 18–40 years. Women were cautioned that any removals during the 21 days of cyclic use should not exceed 2 h, and used daily paper diaries to record vaginal system use. Consistent with regulatory requirements for contraceptives, we calculated the Pearl Index for women aged 35 years and younger, excluding adjunctive contraception cycles, as the primary efficacy outcome measure. We also did intention-to-treat Kaplan-Meier life table analyses and followed up women who did not use hormonal contraceptives or desired pregnancy after study completion for 6 months for return to menses or pregnancy. The trials are registered with ClinicalTrials.gov, numbers NCT00455156 and NCT00263341.
Findings
Between Dec 19, 2006, and Oct 9, 2009, at the 15 US sites, and between Nov 1, 2006, and July 2, 2009, at the 12 US and international sites we enrolled 2278 women. Our overall efficacy analysis included 2265 participants (1130 in the US study and 1135 in the international study) and 1303 (57·5%) participants completed up to 13 cycles. The Pearl Index for the primary efficacy group was 2·98 (95% CI 2·13–4·06) per 100 woman-years, and was well within the range indicative of efficacy for a contraceptive under a woman's control. The Kaplan-Meier analysis revealed the contraceptive vaginal system was 97·5% effective, which provided further evidence of efficacy. Pregnancy occurrence was similar across cycles. All 290 follow-up participants reported return to menses or became pregnant (24 [63%] of 38 women who desired pregnancy) within 6 months.
Interpretation
The segesterone acetate and ethinylestradiol contraceptive vaginal system is an effective contraceptive for 13 consecutive cycles of use. This new product adds to the contraceptive method mix and the 1-year duration of use means that women do not need to return to the clinic or pharmacy for refills every few months.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health, the US Agency for International Development, and the WHO Reproductive Health Research Department.
Introduction
Despite global efforts to support family planning goals of women and families, over 200 million women and girls in low-income and middle-income countries who want to delay or avoid pregnancy do not have access to modern contraceptive methods that they find acceptable.1, 2 An unmet contraceptive need persists in the USA, where 45% of all pregnancies are unintended.3 To address this need, the Population Council developed a 1-year (13-cycle), ring-shaped, contraceptive vaginal system that is under a woman's control.
Proof of concept for development of a vaginal contraceptive was first shown in the late 1960s by Mishell and Lumkin.4 The vaginal mucosa is efficient for systemic absorption of contraceptive steroids, including orally inactive steroids.4 The contraceptive vaginal ring delivers a novel progestin, segesterone acetate—also known as Nestorone—with ethinylestradiol, an approved component of many oral contraceptives. Segesterone acetate is a 19-norprogesterone derivative and a new chemical entity for which there are considerable non-clinical5, 6 and clinical trial data.7, 8 This drug is not absorbed orally but is effective when administered via non-oral routes.5, 6 In the contraceptive vaginal system, the two hormones are delivered via a silicone-based, soft ring containing a segesterone acetate and ethinylestradiol core and a segesterone-acetate-only core (a total of 103 mg segesterone acetate and 17·4 mg ethinylestradiol). After an initial burst from steroid accumulation on the ring surface, drug is released constantly for consistent blood levels (an average daily dose of 0·15 mg segesterone acetate and 0·013 mg ethinylestradiol),9 in contrast to daily steroid fluctuations with oral contraceptives.10
Research in context.
Evidence before this study
Although several contraceptive methods are available, including combined hormonal contraceptives, many women and girls worldwide do not have access to modern contraceptives that address changing needs over their reproductive life span, and many unintended pregnancies still occur. To address this need, the Population Council developed a ring-shaped, contraceptive vaginal system containing segesterone acetate and ethinylestradiol that is under a woman's control, releases a consistent level of hormones over 1 year of cyclic use, and does not require refrigeration during periods of non-use. Results of pharmacokinetic and phase 2 studies showed absorption of segesterone acetate and ethinylestradiol through the vaginal mucosa with serum levels that were adequate for ovulation suppression and pregnancy prevention. Data from these studies also showed that women were able to use the product without difficulty and found it acceptable. Based on the positive results from these earlier studies and the potential of the product to add to the method mix of safe, effective, and acceptable contraceptives, the Population Council pursued full development of the segesterone acetate and ethinylestradiol contraceptive vaginal system.
Added value of this study
Results of our pivotal phase 3 studies showed the efficacy and safety of the segesterone acetate and ethinylestradiol contraceptive vaginal system, with an overall Pearl Index of 2·98 and a contraceptive efficacy rate of 97·5% based on a life table analysis. These results and accompanying data were crucial components of a US Food and Drug Administration review leading to regulatory approval in August, 2018. Since this study included seven non-US sites, these data can be considered applicable to global populations.
Implications of all the available evidence
The segesterone acetate and ethinylestradiol contraceptive vaginal system is a new, safe, and effective combined hormonal contraceptive that is under a woman's control. The fact that one contraceptive vaginal system is effective for a full year of cyclic use and does not require refrigeration during periods of non-use might facilitate contraceptive accessibility and help to address a global unmet contraceptive need.
An open-label, pharmacokinetic study of the segesterone acetate and ethinylestradiol contraceptive vaginal system in 39 women verified rapid absorption of both steroids through the vaginal mucosa after insertion, with steady-state, systemic levels of segesterone acetate and ethinylestradiol achieved by day 4 of use.11 Mean serum segesterone acetate and ethinylestradiol concentrations peak immediately after the first contraceptive vaginal system insertion in cycle 1 and decline rapidly to a steady state by 96 h. Peak and steady state levels fall modestly in subsequent cycles.
The segesterone acetate and ethinylestradiol contraceptive vaginal system has several potential advantages for women across diverse settings. The cyclic regimen of 21 days in and 7 days out provides regular withdrawal bleeds, a pattern familiar to and desirable for many women.12 Using the same contraceptive vaginal system for thirteen 28-day cycles (1 year) enhances convenience and addresses a common access issue that can be a factor in unintended pregnancies (difficulties returning to health-care facilities or pharmacies for refills or new prescriptions for hormonal contraceptives requiring timely use). Additionally, the segesterone acetate and ethinylestradiol contraceptive vaginal system does not require refrigeration before dispensing or during non-use, facilitating storage and distribution, an access element affecting providers and women across many regions.
Here, we present data from two 13-cycle, open-label trials designed to assess the contraceptive efficacy of the segesterone acetate and ethinylestradiol contraceptive vaginal system and document return to menses or pregnancy after use following study completion.
Methods
Study design and participants
Consistent with US Food and Drug Administration (FDA) guidance, contraceptive development for a new chemical entity should include two pivotal, open-label, phase 3 studies. Hence, we conducted two studies with matching protocols and combined the results to show efficacy. One study was done by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Contraceptive Clinical Trial Network (CCTN) at 15 US academic and community sites and the other (international and US) study was done by the Population Council (the study sponsor) at 12 academic and community sites including five in the USA, and one each in Brazil, Chile, Dominican Republic, Finland, Hungary, Sweden, and Australia.
Study protocols were approved by the Institutional Review Boards (IRBs) of the Population Council and the NICHD Coordinating Center, and the IRB or Ethical Committee at each site. An independent Data Safety Monitoring Board (DSMB) established by the NICHD regularly reviewed pregnancy and safety data. All potential participants provided written informed consent before screening and any study procedures.
Participants were healthy, sexually active, non-pregnant, non-sterilised women aged 18–40 years, who did not intend to become pregnant over the next 13 months. Participants also had to have an intact uterus and both ovaries, have a history of regular menstrual cycles that usually occurred every 28 days (±7 days) when not using hormonal contraception (if post-partum or post-abortal, participants had to have a history of regular menstrual cycles of 21–35 days and resumption of at least one cycle with a cycle length consistent with past cycles), be willing to discontinue their current contraceptive method to participate in the study, and, in the opinion of the investigator, be able to comply with the protocol (eg, live within the clinic catchment area or within a reasonable distance of the clinic). Exclusion criteria were known hypersensitivity to oestrogens or progestins; known hypersensitivity to silicone rubber; known or suspected pregnancy; history of infertility of longer than 1 year in the woman or her male partner; history of vasectomy or sterility in male partner; tubal ligation (sterilisation) in women; undiagnosed abnormal genital bleeding; and undiagnosed vaginal discharge or vaginal lesions or abnormalities. Participants diagnosed at screening with a chlamydia or gonococcus infection could be included in the trial after treatment; partner treatment was also recommended. Investigators determined whether participants were at high risk for reinfection (eg, because of multiple sex partners or an untreated partner) and whether such participants could be included. In accordance with primary investigator or medical designee assessment and local standards of practice, women with a history of genital herpes could be included if outbreaks were infrequent. Further exclusion criteria were a history of pelvic inflammatory disease since last pregnancy episode; a history of toxic shock syndrome, and in accordance with the Bethesda system of classification, women with a current abnormal Pap smear suggestive of high-grade precancerous lesion(s), including high-grade squamous intraepithelial lesions, were excluded. Women with low-grade squamous intraepithelial lesions or atypical squamous cells of undetermined significance or who were high-risk and humam papillomavirus-positive could participate if further evaluated with colposcopy and biopsy and no evidence of a lesion with severity greater than cervical intraepithelial neoplasia (CIN) I was present or endocervical curettage was negative. Women with a biopsy finding of CIN I were followed up for this finding per standard of care; and women were excluded if treatment was indicated. In accordance with other Pap classification systems, women with high grade dysplasia were excluded, women with low grade dysplasia or CIN I interpretation on Pap smear could participate if further evaluated with colposcopy and, as needed, biopsy with endocervical curettage, women could participate if colposcopy findings were negative, provided there was appropriate follow-up in accordance with local standards of care, and women whose colposcopy results indicated need for biopsy could participate as long as biopsy results indicated there was no lesion with a severity greater than CIN I and endocervical curettage results were negative. Additional exclusion criteria were cystoceles or rectoceles or another anatomical abnormality that would preclude use of a vaginal ring; women planning to undergo major surgery; smoking in women who were 35 years and older or who would be 35 years of age during the course of the trial (women <35 years who smoked 15 cigarettes or more per day were evaluated by the primary investigator for inclusion based on risk factors that would increase their risk for cardiovascular disease and thromboembolism, eg, lipid levels, glucose level, blood pressure, body-mass index [BMI], family history of cardiovascular disease at a young age); breastfeeding; current or previous thrombophlebitis or thromboembolic disorders; history of venous thrombosis or embolism in a first-degree relative younger than 55 years of age suggesting a familial defect in the blood coagulation system, which in the opinion of the primary investigator, suggested use of a hormonal contraceptive could pose a significant risk; cerebrovascular or cardiovascular disease; history of retinal vascular lesions, or unexplained partial or complete loss of vision; known or suspected carcinoma of the breast; carcinoma of the endometrium or other known or suspected oestrogen-dependent neoplasia; previous history of any other carcinoma unless in remission for more than 5 years; current or history of medically diagnosed severe depression, which, in the opinion of the investigator, could be exacerbated by use of a hormonal contraceptive; headaches with focal neurological symptoms; severe constipation; history of cholestatic jaundice of pregnancy or jaundice with previous steroid use; benign or malignant liver tumours or active liver disease; diastolic blood pressure greater than 85 mm Hg or systolic blood pressure greater than 135 mm Hg after 5–10 min rest; known or suspected alcoholism or drug abuse; abnormal serum chemistry values according to the physician's judgment; participation in another clinical trial involving an investigational drug within the last 30 days (before screening); BMI greater than 29 kg/m2; use of liver enzyme inducers on a regular basis; use of monthly injectable contraceptives unless suspended 2 months before initiation of treatment; use of medroxyprogesterone acetate unless suspended 6 months before treatment; current use of implanted hormonal contraceptives (participants using this method who requested removal for reasons unrelated to the purpose of enrolment in this study could be considered for participation); current use of a non-hormonal intrauterine device (participants with intrauterine devices who requested removal for reasons unrelated to the purpose of enrolment in this study could be considered for participation); known HIV infection; and women at high risk of contracting HIV, for example, women with multiple sex partners who needed to use condoms consistently or injection drug users. If women enrolled in the study did use condoms to protect against sexually transmitted infections, they were instructed that this occasional use should be with non-nonoxynyl-9 containing condoms and they should record condom use in their diaries. Women found to have a sexually transmitted infection at screening were treated before inclusion in the study (with the exception of those infected with HIV). Women were not enrolled if they chronically used concomitant medications known to induce cytochrome P450 liver enzymes, especially cytochrome P450 3A4.
6 months after the trial commenced, the DSMB recommended exclusion of women with a BMI greater than 29·0 kg/m2 rather than exclusion based on weight because of the well characterised risk of venous thromboembolism in obese women13 and the occurrence of non-fatal venous thromboembolisms in two participants with BMI greater than 29·0 kg/m2 reported early in the study.
Participants were recruited to the trial through IRB-approved local advertising.
Procedures
Participants were screened after expressing interest and consenting to trial involvement. Screening involved clinical tests, physical examination, including a gynaecological examination, and obtaining a medical history, including history of pregnancy. After screening, all eligible participants began the study identically, regardless of their previous contraceptive method use. Contraceptive vaginal system use was initiated at the baseline visit, which occurred between menstrual days 2 and 5, and after a negative urine pregnancy test. Urine pregnancy tests were repeated at all subsequent visits (cycles 3, 6, 9, and 13), and at the final study termination visit (1–2 weeks after cycle 13). Women who discontinued the study early had pregnancy tests at their last study visit.
The day of contraceptive vaginal system insertion became day 1 of the first treatment cycle. Site staff counselled participants on proper use of the contraceptive vaginal system, provided written instructions that outlined the 21-days-in and 7-days-out regimen, and reinforced directions about keeping the contraceptive vaginal system in place continuously during the first 21 days of each cycle. Women were cautioned that any removals of the contraceptive vaginal system during the 21 days of cyclic use should not exceed 2 h. These instructions were based on early work with segesterone acetate implants, which suggested that ovulation was likely when segesterone acetate serum levels drop below 100 pmol/L,8, 14 and pharmacokinetic data confirming that segesterone acetate levels decline rapidly with contraceptive vaginal system removal for longer than 2 h.11 Correct use of the contraceptive vaginal system was reviewed at all subsequent visits, and during telephone contact that occurred midway between scheduled visits. Participants having difficulty following these instructions were counselled at additional timepoints. A contraceptive vaginal system could be replaced if accidentally lost.
Participants used a daily diary card during the study to record dates when the contraceptive vaginal system was in or out of place, any duration of removal longer than 2 h during a period of contraceptive vaginal system use, and any bleeding or spotting during each 28-day cycle. Participants also noted dates when they were sexually active, used condoms or other contraceptives, including emergency contraceptive pills, experienced expulsions (complete or partial) or any other problems with the contraceptive vaginal system, any health issues, and use of other medications. Study personnel reviewed diary entries at all participant visits. Participants removed the contraceptive vaginal system for the last time on day 22 of cycle 13.
Participants not continuing hormonal contraception at study end were followed up for 6 months to document spontaneous return to menses. Women who desired pregnancy were followed up for pregnancy occurrence up to 6 months. All women in the follow-up study received urine pregnancy test kits at their last study visits, with instructions to test within 2 to 3 weeks after that visit, and monthly thereafter, if they had no bleeding or had pregnancy symptoms. Participants were contacted every 2 months to determine if they had resumed spontaneous menses or had become pregnant. Patients were asked to return to the study site if the pregnancy test was positive for pregnancy confirmation and were referred for prenatal care as required.
Outcomes
The primary efficacy endpoint was the Pearl Index for women in the efficacy population who were aged 35 years or younger. The Pearl Index is considered a standard measure for determining contraceptive efficacy and reflects the incidence of pregnancy per 100 woman-years. The acceptable range of Pearl Index efficacy includes values where the upper boundary of the 95% CI is less than 5. We analysed an intention-to-treat Kaplan-Meier life table as a secondary efficacy outcome to evaluate the cumulative probability of pregnancy by cycle. Adverse events, physical examinations, and laboratory assessments were also done and have been reported elsewhere.9
Statistical analysis
We based the sample size on FDA recommendations and European Medicines Agency harmonisation guidelines that specify 20 000 treatment cycles of contraceptive vaginal system exposure with at least 400 women completing 1 year of treatment for contraceptives that contain a new chemical entity. The sample size for each phase 3 trial was established at around 1200 women, for a total of 2400 women, and, assuming that only 45% to 55% of participants would complete 1 year of contraceptive vaginal system use, with an exposure of 9000 to 11 000 cycles per study. Consistent with regulatory guidance, we planned to combine efficacy data from the two trials to show contraceptive efficacy.
All participants who documented contraceptive vaginal system use in diaries after initiation of study participation (except for cycles occurring after pregnancy or during the 6-month follow-up) were included in the efficacy population. The Pearl Index was calculated as (total number of on-treatment pregnancies ÷ number of on-treatment cycles) × 1300. For the numerator of the Pearl Index calculation, we included all pregnancies that occurred during any of 13 cycles, provided the participant's estimated date of conception confirmed that the pregnancy occurred after onset of contraceptive vaginal system use or within 7 days after the participant's final use. Site investigators confirmed each pregnancy and the study sponsor reviewed all pregnancy data. We calculated the estimated conception date from one of the following (if available) in this order: gestational age as determined by ultrasound or quantitative β-human chorionic gonadotropin (if no ultrasound), estimated date of delivery as entered into the obstetric record, or date of last menses.
For the denominator of the Pearl Index calculation, we counted all treatment cycles, regardless of diary documentation of coitus, except those cycles in which participants recorded or reported use of adjunctive contraception and those cycles that occurred after a conception. We calculated 95% CIs with a Poisson distribution and exact confidence limits.
We also analysed the Pearl Index among subgroups within the entire efficacy population. We analysed the Pearl Index in accordance with treatment adherence defined as participants' diary data indicating continuous contraceptive vaginal system use for 21 days versus ring removal for more than 2 h during use in any cycle. Additionally, we calculated a Pearl Index for subgroups based on age, race, ethnicity, geographical region, and parity. For modelling purposes, we combined race into three categories (only black, only white, and other [including mixed]).
On the basis of the range of pregnancy rates for these subgroups, we did a post-hoc analysis to explore the effect of a participant's desire to have children after they completed the study. We used the efficacy population for this analysis, which was based on participant responses to the screening question, “Do you wish to have children after the study is over?” Potential answers were “yes”, “no”, or “unsure”. We used Poisson regression modelling to examine the association between select baseline characteristics (ethnicity, race, age group, parity, and geographical location) and pregnancy, adjusted by participants' desire to have children. Baseline characteristics were selected a priori and tested in separate models that adjusted for that characteristic and the desire for children after the study.
For the Kaplan-Meier survival analysis, we followed all participants until they either had an outcome of pregnancy or were censored at the time of their last follow-up. The unit of time in our analysis was the cycle, with pregnancies recorded by cycle of conception. Unlike the Pearl Index calculation, cycles based on use of adjunctive contraception were not excluded.
We calculated the proportion of participants in the return to menses or pregnancy follow-up cohort who had a spontaneous menses at least 18 days after their final contraceptive vaginal system removal or became pregnant within 6 months, respectively.
Data for the efficacy analyses were finalised as SAS version 5 transport datasets. Statistical analyses and displays were created with SAS version 9.2 or later, and the life table analysis was done with SAS PROC LIFETEST. The trials are registered with ClinicalTrials.gov, numbers NCT00455156 and NCT00263341.
Role of the funding source
The NICHD was involved in the conduct of the study. The US Agency for International Development (USAID) and WHO had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
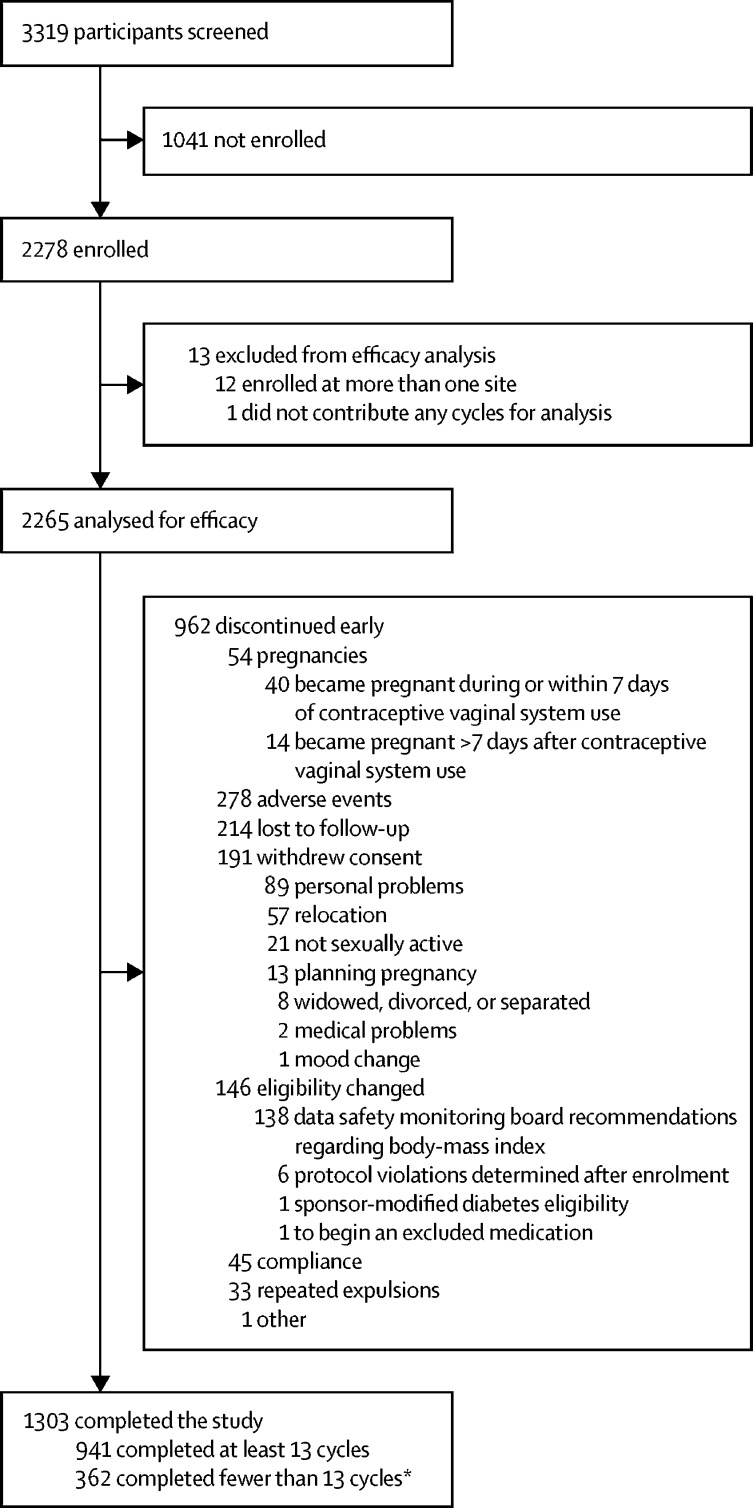
Between Dec 19, 2006, and Oct 9, 2009, at the 15 US sites in the NICHD CCTN study, and between Nov 1, 2006, and July 2, 2009, at the 12 (international) sites in the Population Council study we enrolled 2278 women (figure 1). Although 362 participants did not complete all 13 cycles, some of these participants were enrolled within 6 months of drug expiration and their participation was limited to 6 months by design. Our overall efficacy analysis included 2265 participants (1130 in the US study and 1135 in the international study; appendix p 2) and 1303 (57·5%) participants completed up to 13 cycles (figure 1). 278 (12·3%) of 2265 women discontinued the study early because of adverse events considered related to treatment and 214 (9·4%) were lost to follow-up (figure 1). The median follow-up time was 11 cycles (IQR 6–13).
Figure 1.
Trial profile
*All contraceptive vaginal systems expired by Dec 31, 2008, and subjects were required to discontinue treatment at that time.
Mean age was 26·7 years (SD 5·1; range 18–40) with 1586 (70·0%) of 2265 participants ranging in age from 20 to 29 years (table 1; appendix p 2). BMI was greater than 20 kg/m2 but no more than 27 kg/m2 in 1561 (68·9%) of 2265 participants. 1739 (76·8%) participants identified as white, and 651 (28·7%) identified their ethnicity as Hispanic or Latina.
Table 1.
Demographic and baseline characteristics
| Participants (n=2265) | ||
|---|---|---|
| Age (years) | 26·7 (5·1) | |
| 18–19 | 140 (6·2%) | |
| 20–24 | 842 (37·2%) | |
| 25–29 | 744 (32·8%) | |
| 30–35 | 385 (17·0%) | |
| 36–40 | 154 (6·8%) | |
| BMI (kg/m2) | 24·0 (3·6) | |
| ≤20 | 248 (10·9%) | |
| >20 to ≤25 | 1231 (54·3%) | |
| >25 to ≤27 | 330 (14·6%) | |
| >27 to ≤29 | 256 (11·3%) | |
| >29 | 200 (8·8%) | |
| Race* | ||
| White | 1739 (76·8%) | |
| Black | 418 (18·5%) | |
| Asian | 106 (4·7%) | |
| American Indian or Native Alaskan | 27 (1·2%) | |
| Native Hawaiian or Pacific Islander | 15 (0·7%) | |
| Other or unknown | 114 (5·0%) | |
| Ethnicity | ||
| Hispanic or Latina | 651 (28·7%) | |
| Non-Hispanic or non-Latina | 1614 (71·3%) | |
| Marital status | ||
| Never married | 1585 (70·0%) | |
| Married | 535 (23·6%) | |
| Divorced | 95 (4·2%) | |
| Separated | 48 (2·1%) | |
| Widowed | 2 (0·1%) | |
| Education | ||
| College degree or higher | 948 (41·9%) | |
| Some college | 751 (33·2%) | |
| High school diploma or equivalent | 419 (18·5%) | |
| Less than high school | 147 (6·5%) | |
| Parity >0 | 793 (35·0%) | |
| Desired children after the study | ||
| Yes | 1207 (53·3%) | |
| No | 619 (27·3%) | |
| Unsure | 439 (19·4%) | |
| Smoking | ||
| Never smoked | 1590 (70·2%) | |
| Former smoker | 331 (14·6%) | |
| Current smoker | 343 (15·1%) | |
| Unknown | 1 (<0·1%) | |
| Alcohol use history | ||
| Never drank | 584 (25·8%) | |
| Former drinker | 106 (4·7%) | |
| Current drinker | 1575 (69·5%) | |
Data are mean (SD) or n (%). BMI=body-mass index.
Multiple races allowed per participant.
The Pearl Index for the primary efficacy group was 2·98 (95% CI 2·13–4·06) per 100 woman-years (table 2), which is well within the range indicative of efficacy for a contraceptive under a woman's control. This calculation was based on 40 pregnancies that occurred in participants who were aged 35 years or younger and became pregnant during or within 7 days of last contraceptive vaginal system use. In 33 of these pregnancies, the study investigators made the diagnosis and dated the pregnancies by ultrasound examination. The remaining pregnancies were confirmed and dated by serum β-human chorionic gonadotropin or participants' self-report of their last menstrual period. The number of cycles used in the primary Pearl Index calculation was based on 2111 women aged 35 years or younger who contributed 17 427 cycles of use. We removed 1978 cycles from the Pearl Index calculation because of use of adjunctive contraception.
Table 2.
Pearl Indices for primary endpoint and other subgroups
| Number of participants | Number of pregnancies | Cycles | Pearl Index (95% CI)* | ||
|---|---|---|---|---|---|
| Primary endpoint | |||||
| Age ≤35 years (primary endpoint) | 2111 | 40 | 17 427 | 2·98 (2·13–4·06) | |
| Intention-to-treat subgroup analyses (n=2265)† | |||||
| Age (years) | |||||
| 18–19 | 140 | 7 | 1117 | 8·15 (3·50–15·8) | |
| 20–24 | 842 | 22 | 6773 | 4·22 (2·69–6·24) | |
| 25–29 | 744 | 8 | 6298 | 1·65 (0·75–3·07) | |
| 30–35 | 385 | 3 | 3239 | 1·20 (0·30–3·12) | |
| ≥36 | 154 | 1 | 1318 | 0·99 (0·06–4·34) | |
| Body-mass index (kg/m2) | |||||
| ≤29 | 2065 | 39 | 17 763 | 2·85 (2·05–3·85) | |
| >29 | 200 | 2 | 982 | 2·65 (0·44–8·18) | |
| Race | |||||
| Only black | 319 | 11 | 2009 | 7·12 (3·70–12·2) | |
| Only white | 1613 | 19 | 13 983 | 1·77 (1·09–2·68) | |
| Other or unknown (including mixed) | 333 | 11 | 2753 | 5·19 (2·70–8·90) | |
| Ethnicity | |||||
| Hispanic | 651 | 27 | 5809 | 6·04 (4·04–8·62) | |
| Non-Hispanic | 1614 | 14 | 12 936 | 1·41 (0·79–2·28) | |
| Site | |||||
| US | 1519 | 26 | 11 766 | 2·87 (1·91–4·12) | |
| Non-US | 746 | 15 | 6979 | 2·79 (1·61–4·46) | |
| European | 309 | 1 | 2764 | 0·47 (0·03–2·07) | |
| Non-European | 1956 | 40 | 15 981 | 3·25 (2·35–4·37) | |
| Parity | |||||
| 0 | 1472 | 14 | 12 280 | 1·48 (0·83–2·40) | |
| Parity >0 | 793 | 27 | 6465 | 5·43 (3·63–7·74) | |
| Education | |||||
| Grade school | 147 | 8 | 1224 | 8·50 (3·88–15·8) | |
| High school graduate | 419 | 13 | 3531 | 4·79 (2·63–7·88) | |
| Some college | 751 | 11 | 5747 | 2·49 (1·29–4·26) | |
| College graduate or more | 948 | 9 | 8243 | 1·42 (0·68–2·56) | |
| Adherence | |||||
| Contraceptive vaginal system never removed temporarily when scheduled to be in* | 1706 | 24 | 14 843 | 2·10 (1·37–3·06) | |
| Contraceptive vaginal system removed at least once temporarily when scheduled to be in* | 421 | 16 | 3530 | 5·89 (3·46–9·27) | |
Data are n, unless otherwise indicated.
Subject diary cards were used to determine if the contraceptive vaginal system was removed temporarily (between 2 h and 23 h) on a day when the system was scheduled to be in. Some subjects did not return diary cards, and thus could not be grouped by temporary system removal; thus, the numbers of pregnancies and cycles do not sum to the total number of participants.
41 pregnancies included in these analyses.
The Pearl Index was 2·10 (95% CI 1·37–3·06) for women who did not record any contraceptive vaginal system removals for longer than 2 h during the 21-day periods of cyclic use compared with 5·89 (3·46–9·27) for women who recorded contraceptive vaginal system removal with duration longer than 2 h (table 2). The Pearl Index was highest (8·15, 95% CI 3·50–15·8) among the youngest women (aged 18–19 years) and decreased as age increased, with a Pearl Index of 0·99 (95% CI 0·06–4·34) among women older than 35 years. The Pearl Index was not influenced by BMI. Women who reported their race as only white had a Pearl Index of 1·77 (1·09–2·68), women who reported their race as only black had a Pearl Index of 7·12 (3·70–12·2), and women who reported their race as other, including mixed race or unknown, had a Pearl Index of 5·19 (2·70–8·90). Hispanic women had a Pearl Index of 6·04 (4·04–8·62) and non-Hispanic women had a Pearl Index of 1·41 (0·79–2·28). Women from the European sites had a Pearl Index of 0·47 (0·03–2·07), whereas women from non-European sites had a Pearl Index of 3·25 (2·35–4·37). The nulliparous subgroup had a Pearl Index of 1·48 (0·83–2·40), whereas the subgroup of women with a parity of one or more had a Pearl Index of 5·43 (3·63–7·74).
On the basis of a post-hoc analysis evaluating the effect of participants' desire to have children after they completed the study, there were no Pearl Index differences across the three response categories (yes, no, or unsure) for desiring children (data not shown). The desire for children was significantly higher among non-parous women, but their overall pregnancy rate was significantly lower compared with parous women (data not shown). However, after adjusting for desire for more children, black women were more than twice as likely to become pregnant during the trial than were white women, and Hispanic women desiring pregnancy were almost five times more likely to become pregnant during the study than were non-Hispanic women (table 3). Among all women in the study, those who did not have children (nulliparous subgroup) were significantly more likely to desire children after the study (p<0·0001) and were significantly less likely to become pregnant during the study (Pearl Index for nulliparous women 1·48, 95% CI 0·83–2·40, vs 5·43, 3·63–7·74 for women with one or more children). Therefore, for women who did not have children, there was an inverse association between desire to have children and becoming pregnant during the study.
Table 3.
Exploratory analysis evaluating the effect of desire for children after the study on pregnancy rate by race and ethnicity
| Pearl Index ratio (95% CI) | p value | |
|---|---|---|
| Black vs white race | 2·25 (1·09–4·62) | 0·027 |
| Hispanic vs non-Hispanic ethnicity | 4·81 (1·53–15·13) | 0·0076 |
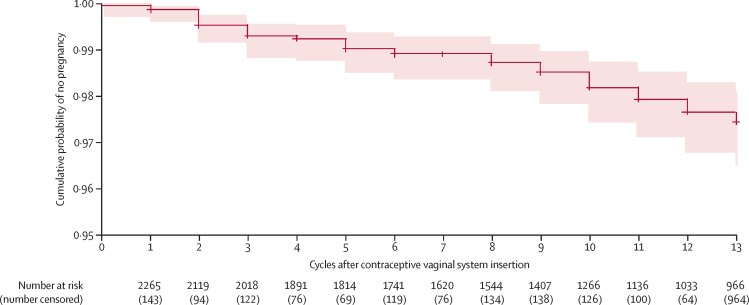
The Kaplan-Meier life table estimate of the cumulative probability of not becoming pregnant during or within 7 days of last contraceptive vaginal system use was 0·9745 (95% CI 0·9649–0·9814; table 4; figure 2).
Table 4.
Kaplan-Meier intention-to-treat life table analysis of pregnancy
|
At risk (effective sample size) |
Number of pregnancies*(failure) |
Number of discontinuations (censored) |
Probability |
Cumulative probability |
|||
|---|---|---|---|---|---|---|---|
| Pregnancy (failure) | No pregnancy (survival) | No pregnancy (95% CI)† (survival) | Pregnancy (failure) | ||||
| 1 | 2265 | 2 | 143 | 0·0009 | 0·9991 | 0·9987 (0·9959–0·9996) | 0·0013 |
| 2 | 2119 | 7 | 94 | 0·0033 | 0·9967 | 0·9954 (0·9914–0·9975) | 0·0046 |
| 3 | 2018 | 5 | 122 | 0·0025 | 0·9975 | 0·9929 (0·9883–0·9957) | 0·0071 |
| 4 | 1891 | 2 | 76 | 0·0005 | 0·9995 | 0·9924 (0·9876–0·9953) | 0·0076 |
| 5 | 1814 | 4 | 69 | 0·0022 | 0·9978 | 0·9902 (0·9848–0·9937) | 0·0098 |
| 6 | 1741 | 2 | 119 | 0·0011 | 0·9989 | 0·9891 (0·9834–0·9928) | 0·0109 |
| 7 | 1620 | 0 | 76 | 0·0000 | 1·0000 | 0·9891 (0·9834–0·9928) | 0·0109 |
| 8 | 1544 | 3 | 134 | 0·0019 | 0·9981 | 0·9871 (0·9810–0·9913) | 0·0129 |
| 9 | 1407 | 3 | 138 | 0·0021 | 0·9979 | 0·9850 (0·9783–0·9897) | 0·0150 |
| 10 | 1266 | 5 | 126 | 0·0032 | 0·9968 | 0·9819 (0·9744–0·9873) | 0·0181 |
| 11 | 1136 | 3 | 100 | 0·0026 | 0·9974 | 0·9793 (0·9711–0·9852) | 0·0207 |
| 12 | 1033 | 3 | 64 | 0·0029 | 0·9971 | 0·9765 (0·9675–0·9830) | 0·0235 |
| 13 | 966 | 2 | 964 | 0·0021 | 0·9979 | 0·9745 (0·9649–0·9814) | 0·0255 |
Only pregnancies occurring within 7 days of last contraceptive vaginal system use were included.
The 95% CI was calculated with complementary log-log transformation.
Figure 2.
Cumulative probability of no pregnancy
Cross marks indicate censoring of patients and the red shaded area indicates 95% CI.
290 participants entered the 6-month follow-up for return to menses or pregnancy—147 from the US study and 143 from the international study. All participants in this follow-up study reported return to normal menses more than 18 days after last contraceptive vaginal system use or pregnancy. Pregnancy was reported within 6 months of exiting the study in 24 (63·2%) of 38 participants from the group who desired pregnancy.
Adverse events most frequently reported by women using the contraceptive vaginal system included headache, nausea, vaginal discharge or vulvovaginal mycotic infection, and abdominal pain. Few (<1·5%) women discontinued use of the contraceptive vaginal system because of these complaints. Four (0·2%) women had venous thromboembolism, three of whom had risk factors for thrombosis (one with factor V Leiden mutation and two with BMI >29·0 kg/m2).9
Discussion
The segesterone acetate and ethinylestradiol contraceptive vaginal system provides user-controlled, reversible, hormonal contraception in a convenient, novel contraceptive vaginal system that can be reused for a full year. The results from our two pivotal phase 3 studies established a Pearl Index of 2·98 (95% CI 2·13–4·06), which is consistent with that of other more recently approved combined hormonal contraceptives under a woman's control.15, 16, 17, 18 Our intention-to-treat life table analysis indicating that the contraceptive vaginal system is 97·5% effective in preventing pregnancy provides further evidence of efficacy. We found no trend for a change in pregnancy risk across 13 cycles, confirming that a single vaginal system delivers consistent contraceptive efficacy for a full year.
Pearl Indices were variable among subgroups. Participants removing the contraceptive vaginal system for longer than 2 h during cyclical use had a higher Pearl Index (5·89, 95% CI 3·46–9·27) than did women who used it properly (2·10, 1·37–3·06), indicative of reduced efficacy. The higher Pearl Index in women who removed the contraceptive vaginal system for longer than 2 h during ring-in days of cyclic use was likely due to segesterone acetate serum levels inadequate for ovulation suppression,11 and might suggest escape ovulation. Although the risk of escape ovulation has been described for oral contraceptives with peak–trough pharmacokinetics,19 further pharmacokinetic research is required to uncover whether this phenomenon might also occur with steady-state contraceptives (ie, injectables, implants, patches, and vaginal rings). Similar to findings from other investigations, the youngest participants in our study had the highest contraceptive failure rates.20, 21 The higher pregnancy rate at US versus European sites is also consistent with results from other contraceptive studies,22, 23 and might reflect regional differences in following instructions for contraceptive use.
Our post-hoc regression analysis indicated higher pregnancy rates for black and Hispanic women desiring pregnancy after the study. This finding reflects the conclusions of other investigators who have considered ethnicity, race, poverty level, and education relative to adherence in contraceptive clinical trials.24, 25, 26 One interpretation is that social and cultural challenges might affect adherence and reporting of contraceptive use, especially for black and Hispanic women considering future pregnancy.27 Based on new research findings of greater reported happiness among newly pregnant Hispanic and black women compared with white women,28 some women might have been considering pregnancy even though enrolled in a contraceptive trial or they might have been more accepting of unintended pregnancy. Overall, factors affecting adherence in clinical trials among various populations are complex and require further research.
Some aspects of contraceptive adherence might be specific to vaginal rings. Results of an acceptability study with women in our international study revealed that women who removed the contraceptive vaginal system for longer than 2 h did so primarily to wash the contraceptive vaginal system or for intercourse.29 These women were more likely to be black or Hispanic and more likely to become pregnant (OR 4·07, 95% CI 1·58–10·50).29 This finding requires additional research, but is important to consider for counselling.
Our results also raise the methodological issue of obtaining accurate reports about contraceptive use during clinical trials and in real-life settings. Women in these trials used paper diaries to record information required for calculating pregnancy rates, which did not reveal between-group differences in adherence and highlight the challenges that investigators must confront when trying to show product efficacy in clinical trials.24, 25, 26, 27 Investigators need more innovative technologies and strategies for obtaining accurate information about participant motivation and behaviours in contraceptive trials, especially for user-controlled methods.
The strengths of this efficacy analysis are its large sample size, the two-study, multicentre, multicountry participant population, the frequency of pregnancy testing, and the ongoing follow-up and surveillance. Although 962 (42%) patients discontinued the study, this was expected, was taken into account when determining the sample size, and was similar to reports from other contraceptive clinical trials and real-world settings.30 A limitation of the study is possible under-reporting or inaccuracies in the participant-completed paper diaries for the use of the contraceptive vaginal system and back-up contraception. Another important limitation relates to the DSMB decision to limit enrolment of and discontinue from the study women with a BMI greater than 29·0 kg/m2, which might influence the generalisability of the data such that additional research is warranted. Similarly, studies with women from sub-Saharan African and south Asia might be warranted as part of introduction of this contraceptive vaginal system in these regions.
We have focused here on the efficacy of the segesterone acetate and ethinylestradiol contraceptive vaginal system; safety data have been published separately.9 Authors reported an adverse event profile, including type and incidence, consistent with that of other combined hormonal contraceptives. Four venous thrombotic events occurred in the 2308 women in the safety population, and three of these women had thrombosis risk factors.9 Thus, women with known risk factors for venous thrombotic events (eg, BMI >29·0 kg/m2, first degree relative with venous thrombotic event, or thrombophilia) might not be eligible for this contraceptive vaginal system, as their pre-existing risk could be enhanced by ethinylestradiol use. Additionally, the bleeding pattern observed with the segesterone acetate and ethinylestradiol contraceptive vaginal system was consistent with a planned hormonal withdrawal bleed during the 7-day ring-out period, with few women having unscheduled (breakthrough) bleeding per cycle.
The segesterone acetate and ethinylestradiol contraceptive vaginal system represents a new contraceptive category recently approved by the FDA as Annovera (TherapeuticsMD; Boca Raton, FL, USA). It is a highly effective, procedure-free, and woman-controlled contraceptive that can be used cyclically for one year. Women and health-care professionals in low-resource settings should find the absence of need for refrigeration advantageous. Importantly, this novel 13-cycle contraceptive is another birth control option that might help address the persistent, unmet, global, contraceptive need.
Data sharing
We will not be sharing data at this time.
Acknowledgments
Acknowledgments
We thank The Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (NICHD), the US Agency for International Development (USAID), and WHO for funding the phase 3 studies. We also acknowledge all participating study investigators (appendix p 1) and coordinators at the 27 clinical sites for conduct of the two phase 3 clinical trials and the over 2200 women participants from eight countries. We further acknowledge the medical writing assistance of Kathleen Ohleth (Precise Publications; Bedminster. NJ, USA) supported by TherapeuticsMD (Boca Raton, FL, USA). The NICHD (contract no HHSN27500403372) funded and conducted the US study and USAID (grant no GPO-A-00-04-00019-00) funded the international study, which was conducted by the Population Council. WHO Department of Reproductive Health and Research funded two international study sites. Medical writing support for manuscript submission and resubmission was supported by TherapeuticsMD. The authors acknowledge the major contribution of Daniel R Mishell Jr (deceased), from the Department of Obstetrics and Gynecology, University of Southern California, Keck School of Medicine (Los Angeles, CA, USA) who invented the concept of the vaginal system to deliver contraceptive steroids, did many of the clinical studies for the segesterone acetate and ethinylestradiol contraceptive vaginal system, and was a principle investigator for the 300 B phase 3 study analysed in this Article while a member of the International Committee for Contraceptive Research (ICCR) of the Population Council. The authors also gratefully acknowledge the contribution of Horacio B Croxatto, from the University of Chile (Santiago, Chile), who established the clinical centre in Chile, participated in all pivotal clinical studies for this ring, and provided guidance for the full development of this new contraceptive while a member of the ICCR.
Contributors
DFA, RBM, LB, CLW, PD, DA, JTJ, VB, ALN, GB, EB, DJP, RS-W, and DLB contributed to the design of the phase 3 studies. DFA, RBM, LB, CLW, PD, DA, JTJ, VB, ALN, EB, GB, DJP, and RS-W were involved in site data collection. RBM, MP, CD, NK, GWC, RS-W, and DLB participated in data analysis or interpretation. All authors contributed to manuscript writing or substantial editing and review and approved the final draft of the manuscript.
Declaration of interests
DFA within the past 3 years has received research support from Actavis, Bayer Healthcare, Endoceutics, Glenmark, Merck, Radius Health, Shionogi, and TherapeuticsMD; and has served as a consultant to AbbVie, Actavis, Agile Therapeutics, Bayer Healthcare, Endoceutics, Exeltis, InnovaGyn, Merck, Pfizer, Radius Health, Sermonix, Shionogi, Teva Women's Healthcare, and TherapeuticsMD. RBM is an employee of Population Council. CLW consults for or is on the advisory board of Allergan, Bayer Healthcare, Cooper Surgical, and Merck; and has received research support from Agile Therapeutics, Estetra SPRL, Leon Farma, and Medicines360. DA has received grant support from WHO for the conduct of this study. JTJ is on the advisory board of AbbVie, Bayer Healthcare, Merck, Population Council, and Sebela; and has received research support from AbbVie, Bayer Healthcare, Dare Bioscience, Estetra SRPL, Medicines360, Merck, National Institutes of Health (NIH), and National Institute of Child Health and Human Development (NICHD). ALN consults for or is on the advisory board of Agile Therapeutics, AMAG Technology, Bayer Healthcare, ContraMed/Sebela, Cooper Surgical, Merck, and Pharmanest; has received research support from Estetra, EvoFem, FHI (MonaLisa), and Mathra; and has served on the speaker's bureau of Agile Therapeutics, Avion, Bayer Healthcare, Cooper Surgical, and Merck. DJP consults for Agile Therapeutics and AMAG Technology; has received research support from Population Council; has served on the speaker's bureau of AMAG Technology and TherapeuticsMD; and is currently Chief Executive Officer of Sermonix with stock or stock options. MP is an employee of Population Council. CD is an employee of Health Decisions, which received research support from NICHD. NK is an employee of Population Council. GWC is an employee of Population Council. RS-W is an employee of Population Council. DLB is the Program Chief of the Contraceptive Development Program at the NICHD and directs the Contraceptive Clinical Trial Network. In that capacity, she was involved in a leadership role in the design of the study, conduct of the study, data analysis, interpretation of data, writing of the manuscript, and the decision to submit the paper for publication. All other authors declare no competing interests.
Supplementary Material
References
- 1.Starbird E, Norton M, Marcus R. Investing in family planning: key to achieving the sustainable development goals. Glob Health Sci Pract. 2016;4:191–210. doi: 10.9745/GHSP-D-15-00374. [DOI] [PMC free article] [PubMed] [Google Scholar]; E Starbird, M Norton, R Marcus. Investing in family planning: key to achieving the sustainable development goals. Glob Health Sci Pract. 2016; 4: 191–210 [DOI] [PMC free article] [PubMed]
- 2.Alkema L, Kantorova V, Menozzi C, Biddlecom A. National, regional, and global rates and trends in contraceptive prevalence and unmet need for family planning between 1990 and 2015: a systematic and comprehensive analysis. Lancet. 2013;381:1642–1652. doi: 10.1016/S0140-6736(12)62204-1. [DOI] [PubMed] [Google Scholar]; L Alkema, V Kantorova, C Menozzi, A Biddlecom. National, regional, and global rates and trends in contraceptive prevalence and unmet need for family planning between 1990 and 2015: a systematic and comprehensive analysis. Lancet. 2013; 381: 1642–52 [DOI] [PubMed]
- 3.Kavanaugh ML, Jerman J. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018;97:14–21. doi: 10.1016/j.contraception.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]; ML Kavanaugh, J Jerman. Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception. 2018; 97: 14–21 [DOI] [PMC free article] [PubMed]
- 4.Mishell DR, Jr, Lumkin ME. Contraceptive effect of varying dosages of progestogen in silastic vaginal rings. Fertil Steril. 1970;21:99–103. doi: 10.1016/s0015-0282(16)37333-2. [DOI] [PubMed] [Google Scholar]; Mishell DR Jr. ME Lumkin. Contraceptive effect of varying dosages of progestogen in silastic vaginal rings. Fertil Steril. 1970; 21: 99–103 [DOI] [PubMed]
- 5.Kumar N, Koide SS, Tsong Y, Sundaram K. Nestorone: a progestin with a unique pharmacological profile. Steroids. 2000;65:629–636. doi: 10.1016/s0039-128x(00)00119-7. [DOI] [PubMed] [Google Scholar]; N Kumar, SS Koide, Y Tsong, K Sundaram. Nestorone: a progestin with a unique pharmacological profile. Steroids. 2000; 65: 629–36 [DOI] [PubMed]
- 6.Kumar N, Fagart J, Liere P. Nestorone as a novel progestin for nonoral contraception: structure-activity relationships and brain metabolism studies. Endocrinology. 2017;158:170–182. doi: 10.1210/en.2016-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]; N Kumar, J Fagart, P Liere. et al. Nestorone as a novel progestin for nonoral contraception: structure-activity relationships and brain metabolism studies. Endocrinology. 2017; 158: 170–82 [DOI] [PMC free article] [PubMed]
- 7.Díaz S, Schiappacasse V, Pavez M. Clinical trial with nestorone subdermal contraceptive implants. Contraception. 1995;51:33–38. doi: 10.1016/0010-7824(94)00006-i. [DOI] [PubMed] [Google Scholar]; S Díaz, V Schiappacasse, M Pavez. et al. Clinical trial with nestorone subdermal contraceptive implants. Contraception. 1995; 51: 33–38 [DOI] [PubMed]
- 8.Haukkamaa M, Laurikka-Routti M, Heikinheimo O, Moo-Young A. Contraception with subdermal implants releasing the progestin ST-1435: a dose-finding study. Contraception. 1992;45:49–55. doi: 10.1016/0010-7824(92)90140-o. [DOI] [PubMed] [Google Scholar]; M Haukkamaa, M Laurikka-Routti, O Heikinheimo, A Moo-Young. Contraception with subdermal implants releasing the progestin ST-1435: a dose-finding study. Contraception. 1992; 45: 49–55 [DOI] [PubMed]
- 9.Gemzell-Danielsson K, Sitruk-Ware R, Creinin MD. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019;99:323–328. doi: 10.1016/j.contraception.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; K Gemzell-Danielsson, R Sitruk-Ware, MD Creinin. et al. Segesterone acetate/ethinyl estradiol 12-month contraceptive vaginal system safety evaluation. Contraception. 2019; 99: 323–28 [DOI] [PMC free article] [PubMed]
- 10.Brache V, Payán LJ, Faundes A. Current status of contraceptive vaginal rings. Contraception. 2013;87:264–272. doi: 10.1016/j.contraception.2012.08.037. [DOI] [PubMed] [Google Scholar]; V Brache, LJ Payán, A Faundes. Current status of contraceptive vaginal rings. Contraception. 2013; 87: 264–72 [DOI] [PubMed]
- 11.Creasy G, Brache V, Croxatto H. User controlled long acting reversible contraception: the pharmacokinetic profile of the nestorone/ethinyl estradiol contraceptive vaginal ring (NES/EE-CVR) a 1-year cyclical re-useable vaginal ring. Eur J Contracept Reprod Health Care. 2014;19:S85. [Google Scholar]; G Creasy, V Brache, H Croxatto. et al. User controlled long acting reversible contraception: the pharmacokinetic profile of the nestorone/ethinyl estradiol contraceptive vaginal ring (NES/EE-CVR) a 1-year cyclical re-useable vaginal ring. Eur J Contracept Reprod Health Care. 2014; 19: S85
- 12.Polis CB, Hussain R, Berry A. There might be blood: a scoping review on women's responses to contraceptive-induced menstrual bleeding changes. Reprod Health. 2018;15:114. doi: 10.1186/s12978-018-0561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; CB Polis, R Hussain, A Berry. There might be blood: a scoping review on women's responses to contraceptive-induced menstrual bleeding changes. Reprod Health. 2018; 15: 114 [DOI] [PMC free article] [PubMed]
- 13.Yang G, De Staercke C, Hooper WC. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012;2:499–509. doi: 10.4236/ojpm.2012.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]; G Yang. De Staercke C. WC Hooper. The effects of obesity on venous thromboembolism: a review. Open J Prev Med. 2012; 2: 499–509 [DOI] [PMC free article] [PubMed]
- 14.Brache V, Massai R, Mishell DR. Ovarian function during use of Nestorone(R) subdermal implants. Contraception. 2000;61:199–204. doi: 10.1016/s0010-7824(00)00092-5. [DOI] [PubMed] [Google Scholar]; V Brache, R Massai, DR Mishell. et al. Ovarian function during use of Nestorone(R) subdermal implants. Contraception. 2000; 61: 199–204 [DOI] [PubMed]
- 15.Portman DJ, Kaunitz AM, Howard B, Weiss H, Hsieh J, Ricciotti N. Efficacy and safety of an ascending-dose, extended-regimen levonorgestrel/ethinyl estradiol combined oral contraceptive. Contraception. 2014;89:299–306. doi: 10.1016/j.contraception.2014.01.013. [DOI] [PubMed] [Google Scholar]; DJ Portman, AM Kaunitz, B Howard, H Weiss, J Hsieh, N Ricciotti. Efficacy and safety of an ascending-dose, extended-regimen levonorgestrel/ethinyl estradiol combined oral contraceptive. Contraception. 2014; 89: 299–306 [DOI] [PubMed]
- 16.Lybrel (90 mcg levonorgestrel and 20 mcg ethinyl estradiol) tablets Prescribing Information. Wyeth Pharmaceuticals; Philadelphia: 2010. [Google Scholar]; Lybrel (90 mcg levonorgestrel and 20 mcg ethinyl estradiol) tablets. Prescribing Information. Philadelphia: Wyeth Pharmaceuticals, 2010.
- 17.Kroll R, Reape KZ, Margolis M. The efficacy and safety of a low-dose, 91-day, extended-regimen oral contraceptive with continuous ethinyl estradiol. Contraception. 2010;81:41–48. doi: 10.1016/j.contraception.2009.07.003. [DOI] [PubMed] [Google Scholar]; R Kroll, KZ Reape, M Margolis. The efficacy and safety of a low-dose, 91-day, extended-regimen oral contraceptive with continuous ethinyl estradiol. Contraception. 2010; 81: 41–48 [DOI] [PubMed]
- 18.Lo Loestrin Fe (norethindrone acetate and ethinyl estradiol tablets ethinyl estradiol tablets and ferrous fumarate tablets) Prescribing Information. Warner Chilcott; Rockaway: 2010. [Google Scholar]; Lo Loestrin Fe (norethindrone acetate and ethinyl estradiol tablets, ethinyl estradiol tablets and ferrous fumarate tablets). Prescribing Information. Rockaway: Warner Chilcott, 2010.
- 19.Chowdhury V, Joshi UM, Gopalkrishna K, Betrabet S, Mehta S, Saxena BN. ‘Escape’ ovulation in women due to the missing of low dose combination oral contraceptive pills. Contraception. 1980;22:241–247. doi: 10.1016/s0010-7824(80)80003-5. [DOI] [PubMed] [Google Scholar]; V Chowdhury, UM Joshi, K Gopalkrishna, S Betrabet, S Mehta, BN Saxena. ‘Escape’ ovulation in women due to the missing of low dose combination oral contraceptive pills. Contraception. 1980; 22: 241–47 [DOI] [PubMed]
- 20.Winner B, Peipert JF, Zhao Q. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012;366:1998–2007. doi: 10.1056/NEJMoa1110855. [DOI] [PubMed] [Google Scholar]; B Winner, JF Peipert, Q Zhao. et al. Effectiveness of long-acting reversible contraception. N Engl J Med. 2012; 366: 1998–2007 [DOI] [PubMed]
- 21.Kazerooni R, Blake A, Thai J. Predictors of pregnancy in female veterans receiving a hormonal contraceptive pill, patch, or ring. Ann Pharmacother. 2015;49:1284–1290. doi: 10.1177/1060028015607825. [DOI] [PubMed] [Google Scholar]; R Kazerooni, A Blake, J Thai. Predictors of pregnancy in female veterans receiving a hormonal contraceptive pill, patch, or ring. Ann Pharmacother. 2015; 49: 1284–90 [DOI] [PubMed]
- 22.Lobo Abascal P, Luzar-Stiffler V, Giljanovic S, Howard B, Weiss H, Trussell J. Differences in reporting Pearl Indices in the United States and Europe: focus on a 91-day extended regimen combined oral contraceptive with low-dose ethinyl estradiol supplementation. Eur J Contracept Reprod Health Care. 2016;21:88–91. doi: 10.3109/13625187.2015.1059416. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lobo Abascal P. V Luzar-Stiffler, S Giljanovic, B Howard, H Weiss, J Trussell. Differences in reporting Pearl Indices in the United States and Europe: focus on a 91-day extended regimen combined oral contraceptive with low-dose ethinyl estradiol supplementation. Eur J Contracept Reprod Health Care. 2016; 21: 88–91 [DOI] [PMC free article] [PubMed]
- 23.Barnett C, Hagemann C, Dinger J, Do Minh T, Heinemann K. Fertility and combined oral contraceptives—unintended pregnancies and planned pregnancies following oral contraceptive use—results from the INAS-SCORE study. Eur J Contracept Reprod Health Care. 2017;22:17–23. doi: 10.1080/13625187.2016.1241991. [DOI] [PubMed] [Google Scholar]; C Barnett, C Hagemann, J Dinger. Do Minh T. K Heinemann. Fertility and combined oral contraceptives—unintended pregnancies and planned pregnancies following oral contraceptive use—results from the INAS-SCORE study. Eur J Contracept Reprod Health Care. 2017; 22: 17–23 [DOI] [PubMed]
- 24.Westhoff CL, Torgal AT, Mayeda ER, Shimoni N, Stanczyk FZ, Pike MC. Predictors of noncompliance in an oral contraceptive clinical trial. Contraception. 2012;85:465–469. doi: 10.1016/j.contraception.2011.09.019. [DOI] [PubMed] [Google Scholar]; CL Westhoff, AT Torgal, ER Mayeda, N Shimoni, FZ Stanczyk, MC Pike. Predictors of noncompliance in an oral contraceptive clinical trial. Contraception. 2012; 85: 465–69 [DOI] [PubMed]
- 25.Rocca CH, Harper CC. Do racial and ethnic differences in contraceptive attitudes and knowledge explain disparities in method use? Perspect Sex Reprod Health. 2012;44:150–158. doi: 10.1363/4415012. [DOI] [PubMed] [Google Scholar]; CH Rocca, CC Harper. Do racial and ethnic differences in contraceptive attitudes and knowledge explain disparities in method use?. Perspect Sex Reprod Health. 2012; 44: 150–58 [DOI] [PubMed]
- 26.Gerlinger C, Trussell J, Mellinger U. Different Pearl Indices in studies of hormonal contraceptives in the United States: impact of study population. Contraception. 2014;90:142–146. doi: 10.1016/j.contraception.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; C Gerlinger, J Trussell, U Mellinger. et al. Different Pearl Indices in studies of hormonal contraceptives in the United States: impact of study population. Contraception. 2014; 90: 142–46 [DOI] [PMC free article] [PubMed]
- 27.Trussell J, Portman D. The creeping Pearl: why has the rate of contraceptive failure increased in clinical trials of combined hormonal contraceptive pills? Contraception. 2013;88:604–610. doi: 10.1016/j.contraception.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; J Trussell, D Portman. The creeping Pearl: why has the rate of contraceptive failure increased in clinical trials of combined hormonal contraceptive pills?. Contraception. 2013; 88: 604–10 [DOI] [PMC free article] [PubMed]
- 28.Kemet S, Lundsberg LS, Gariepy AM. Race and ethnicity may not be associated with risk of unintended pregnancy. Contraception. 2018;97:313–318. doi: 10.1016/j.contraception.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]; S Kemet, LS Lundsberg, AM Gariepy. Race and ethnicity may not be associated with risk of unintended pregnancy. Contraception. 2018; 97: 313–18 [DOI] [PMC free article] [PubMed]
- 29.Stifani BM, Plagianos M, Vieira CS, Merkatz RB. Factors associated with nonadherence to instructions for using the Nestorone/ethinyl estradiol contraceptive vaginal ring. Contraception. 2018;97:415–421. doi: 10.1016/j.contraception.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; BM Stifani, M Plagianos, CS Vieira, RB Merkatz. Factors associated with nonadherence to instructions for using the Nestorone/ethinyl estradiol contraceptive vaginal ring. Contraception. 2018; 97: 415–21 [DOI] [PMC free article] [PubMed]
- 30.Trussell J, Vaughan B. Contraceptive failure, method-related discontinuation and resumption of use: results from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999;31:64–72. [PubMed] [Google Scholar]; J Trussell, B Vaughan. Contraceptive failure, method-related discontinuation and resumption of use: results from the 1995 National Survey of Family Growth. Fam Plann Perspect. 1999; 31: 64–72 [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
We will not be sharing data at this time.