Abstract
Hypoxia-inducible factors (HIFs), a family of transcription factors activated by hypoxia, consist of three α-subunits (HIF1α, HIF2α and HIF3α) and one β-subunit (HIF1β), which serves as a heterodimerization partner of the HIFα subunits. HIFα subunits are stabilized from constitutive degradation by hypoxia largely through lowering the activity of the oxygen-dependent prolyl hydroxylases that hydroxylate HIFα, leading to their proteolysis. HIF1α and HIF2α are expressed in different tissues and regulate target genes involved in angiogenesis, cell proliferation and inflammation, and their expression is associated with different disease states. HIFs have been widely studied because of their involvement in cancer, and HIF2α-specific inhibitors are being investigated in clinical trials for the treatment of kidney cancer. Although cancer has been the major focus of research on HIF, evidence has emerged that this pathway has a major role in the control of metabolism and influences metabolic diseases such as obesity, type 2 diabetes mellitus and non-alcoholic fatty liver disease. Notably increased HIF1α and HIF2α signalling in adipose tissue and small intestine, respectively, promotes metabolic diseases in diet-induced disease models. Inhibition of HIF1α and HIF2α decreases the adverse diet-induced metabolic phenotypes, suggesting that they could be drug targets for the treatment of metabolic diseases.
Hypoxia-inducible factors (HIFs) are members of the basic helix-loop-helix Per-Arnt-Sim (bHLH-PAS) transcription factor superfamily and consist of a heterodimer of an oxygen-sensitive α-subunit and a constitutively expressed β-subunit (HIF1β)1,2. HIF1β was described as the aryl-hydrocarbon receptor (AHR) nuclear translocator (ARNT) because it was first discovered as a dimerization partner for the AHR3. Three oxygen-sensitive HIFα subunits are found in mammals — HIF1α, HIF2α and HIF3α — with HIF1α and HIF2α being the most widely studied α-subunits. The functions of HIF3α are less well established, but a number of alternatively spliced variants from HIF3A could generate dominant negative inhibitors of HIF1α and HIF2α4. Whether these splice variants are of biological importance is still unknown.
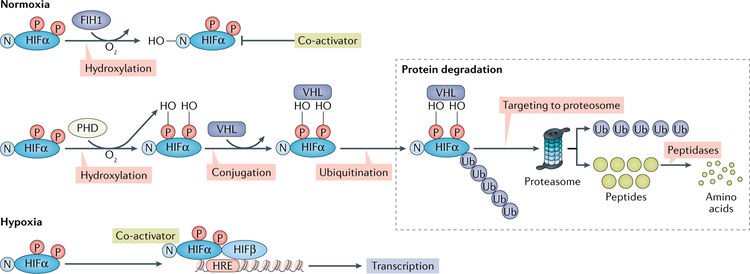
Under normoxia, HIFα proteins are rapidly hydroxylated by a group of prolyl hydroxylase domain (PHD) enzymes: PHD1, PHD2 and PHD3 (FIG. 1). Once hydroxylated, HIFα is subjected to conjugation with the E3 ubiquitin ligase complex containing the von Hippel-Lindau disease tumour suppressor (VHL) protein, leading to rapid degradation of HIFα5. In a second mode of HIFα regulation, hydroxylation of an HIFα asparaginyl residue by factor inhibiting HIF1 (FIH1; also known as HIF1AN) inactivates HIFα transcriptional activity by preventing interaction of the transcriptional co-activator cAMP-response element binding protein (CREB)-binding protein (CBP) and histone acetyltransferase p300 (p300 HAT) with HIFα, thus impeding transcription6. PHDs and FIH1 are O2-dependent oxygenases. Conversely, during hypoxia, the HIFα subunits are not hydroxylated and are stabilized by the limited oxygen that is a co-substrate for PHDs and FIH1. This effect decreases the rate of HIFα protein hydroxylation by PHDs and FIH1 and leads to protein stabilization and CBP-p300 co-activator complex augmented transcriptional activation, respectively, and increases levels of HIFα and the activation of HIF target gene expression7–9.
Fig. 1 |. Hypoxia-inducible factor-α proteins are hydroxylated under normoxic conditions by prolyl hydroxylase domain enzymes.
The hydroxylated hypoxia-inducible factor-α (HIFα) is then conjugated by the von Hippel-Lindau disease tumour suppressor (VHL) protein, leading to rapid degradation by the proteasome. HIFα can also be hydroxylated at an asparaginyl residue by the factor inhibiting HIF1 (FIH1) enzyme, which inactivates HIFα transcriptional activity by preventing it from interacting with its transcriptional co-activators. Both prolyl hydroxylase domains (PHDs) and FIH1 are O2-dependent oxygenases that are active under normoxia. Under conditions of hypoxia, HIFα subunits are not hydroxylated and the protein is stabilized, leading to the accumulation of HIFα proteins and activation of HIF target gene expression. HIFα requires the dimerization partner HIF1β to activate the transcription of HIF target genes. HRE, HIF regulating element; N, nitrate; -OH, hydroxylation; P, phosphate; Ub, ubiquitin.
HIFs interact with upstream binding sites (called HIF regulatory elements) of target genes to activate transcription. No evidence exists of any agonist binding sites on HIF that serve to activate transcription as found with other ligand-dependent transcription factors such as the AHR, another member of the bHLH-PAS superfamily10,11. By contrast, HIF1β, which serves as the binding partner for all HIFα subunits and the AHR, and other bHLH-PAS transcription factors such as period (PER) and simple minded (SIM), is constitutively expressed in most tissues10,12.
HIF1α and HIF2α are differentially expressed in various tissues and cell types; HIF1α is expressed in many tissues and cells, whereas HIF2α has a more restricted expression pattern and is found only in vertebrates13. The single HIF in invertebrates resembles HIF1α more closely than HIF2α and modulates the lifespan in Caenorhabditis elegans14. HIF1α has a role in mammalian development and in adult physiology as a regulator of intermediary metabolism, notably the control of glycolysis under low O2 levels, and an activator of genes involved in the regulation of glucose metabolism13. Conversely, HIF2α is preferentially expressed in endothelial cells of the lung and epithelial cells of the intestine and other tissues15 and has a number of functions in physiology and disease16. HIF1α and HIF2α both regulate the expression of Glut1 (encoding glucose transporter 1 (GLUT1; also known as SLC2A1)), Vegfa (encoding vascular endothelial growth factor A (VEGFA)) and many other target genes. Both HIF1α and HIF2α bind to the same partner HIF1β and response elements; however, some selectivity in target gene activation might exist between the two HIFαs that depends in part on the chromatin context, which influences gene expression in different cell types13,17,18. Both HIFs can be stabilized and activated in cancer cells, where they induce expression of genes such as VEGF19,20, which promotes angiogenesis in solid tumours, and either directly or indirectly activate genes involved in cell proliferation, epithelial-to-mesenchymal transition, apoptosis and metastasis or tumour invasion18. To inhibit angiogenesis and tumour growth, antibodies targeting VEGFA were developed for use in cancer therapy21. Inhibitors of HIF2α have been developed and are in clinical trials to evaluate their use in treating kidney cancers associated with VHL mutations (where HIF2α is overexpressed because of the genetic loss of VHL expression)22–24.
Hypoxia and HIF1α in metabolic diseases
Hypoxia occurs within the expanding adipose tissue of people with obesity and in animal models of obesity25,26. This hypoxia is largely due to the increased size of adipocytes, decreased adipose tissue vascularization and increased fatty acid metabolism that consume oxygen27. Additionally, during the early stages of advancing obesity caused by a high-fat diet (HFD), adipocyte respiration is uncoupled, resulting in increased oxygen consumption and adipocyte hypoxia28. The elevated uncoupling consumes oxygen, in part because saturated fatty acids activate the inner mitochondrial membrane ADP/ATP translocase 2 (ANT2; also known as SLC25A5). In vitro studies using 3T3-L1 adipocytes and human subcutaneous abdominal adipocytes show that insulin resistance is also aggravated by HIFα activation during hypoxia29. These studies suggest that HIF1α and HIF2α in adipocytes would be stabilized and thus accumulate during hypoxia, resulting in the activation of HIFα target genes.
HIF1α in adipose tissue
HIF1α activation protects against obesity and insulin resistance.
A number of studies suggest that HIF1α either promotes or inhibits metabolic diseases (TABLE 1). The first clue that HIF1α in adipocytes influences obesity and associated metabolic diseases was the observation that mice overexpressing HIF1α had elevated obesity and insulin resistance associated with increased inflammation and fibrosis30,31. However, another group found that mice in which HIF1α expression is inhibited in adipose tissue, owing to transgenic expression of a dominant negative protein that inhibits HIF1α signalling, were more obese and insulin resistant after an HFD than wild-type mice after an HFD32. These mice also had larger lipid droplets in brown adipose tissue (BAT) that probably resulted from decreased expression of mitochondrial biogenesis-related genes. In addition, another study did not find an effect on mitochondrial biogenesis-related genes in brown adipocytes, as knockdown of HIF1α expression actually decreased expression of glycolytic enzymes33. Another group produced transgenic mice with constitutive expression of both HIF1α and HIF2α in adipocytes by tissue-specific knockout of PHD2 (REF.34). When these mice were fed an HFD, they were more insulin sensitive with less body weight than the corresponding wild-type mice expressing PHD2 in adipose tissue. The BAT depot in these transgenic mice was also expanded as revealed by increased UCP1 expression34. Taken together, these studies suggest that HIF1α stimulates the thermogenic functions of BAT by controlling mitochondrial biogenesis and glycolysis, implying that activation of HIF1α in adipose tissue could be of benefit for the treatment of obesity and insulin resistance. Additionally, studies suggesting that HIF1α protects against obesity and insulin resistance examined mice with forced or transgenic overexpression of HIF1α; thus, the data must be carefully interpreted relative to physiological importance in wild-type mice.
Table 1 |.
Summary of the effects of HIF1α, HIF2α and HIF1β on metabolic disease
| HIF | Tissue | HIF signalling | Phenotype | Refs |
|---|---|---|---|---|
| HIF1α | Adipose | Activation | Increased obesity and insulin resistance | 30 |
| HIF1α | Adipose | Inhibition with dominant negative HIF1α | Increased obesity and insulin resistance | 32 |
| HIF1α | Adipose | Inhibition | Decreased obesity and insulin resistance | 31,36,38,40,41 |
| HIF1α | Adipose | Inhibition | Decreased insulin resistance and unchanged obesity | 28 |
| HIF1α | Macrophage | Inhibition | No phenotype | 51 |
| HIF1α | Pancreatic β-cell | Inhibition | Increased β-cell dysfunction and glucose intolerance | 55 |
| HIF1α | Pancreatic β-cell | Activation | Increased β-cell dysfunction and glucose intolerance | 58,59 |
| HIF2α | Liver | Activation | Increased hepatic steatosis and fibrosis | 63,65,74,76 |
| HIF2α | Liver | Activation | Decreased glucose intolerance, gluconeogenesis and glucagon response | 82 |
| HIF2α | Liver | Inhibition | Decreased non-alcoholic steatohepatitis | 80 |
| HIF2α | Intestine | Inhibition | Decreased obesity, insulin resistance and hepatic steatosis | 88 |
| HIF2α | Adipose | Inhibition | Slightly increased insulin resistance | 28 |
| HIF1β | Pancreatic β-cell | Inhibition | Increased β-cell dysfunction and glucose intolerance | 55 |
| HIF1β | Liver | Inhibition | Increased glucose intolerance | 69 |
HIF, hypoxia-inducible factor.
HIF1α inhibition ameliorates obesity and insulin resistance.
Although some studies have suggested that HIF1α activation is beneficial for diet-induced metabolic diseases, other studies have found that HIF1α in adipose tissue potentiates obesity and insulin resistance instead of alleviating these conditions. It is well established that levels of HIF1α are elevated in the adipose tissues of obese mice. Two potential mechanisms could account for this increase: hypoxia due to mitochondrial consumption of oxygen and increased insulin signalling. Saturated fatty acids in mouse adipose tissue increased expression of ANT2, resulting in an elevation in adipocyte oxygen consumption via the uncoupling of mitochondrial respiration28. This uncoupling leads to cellular hypoxia, which triggers the stabilization of HIF1α expression. Other studies have shown that insulin also increased HIF1α protein expression and HIF1α signalling in adipocytes; however, the mechanism remains unknown35. The elevated levels of HIF1α might potentiate insulin signalling in adipocytes to promote conversion of glucose into fatty acids and triglycerides, resulting in obesity.
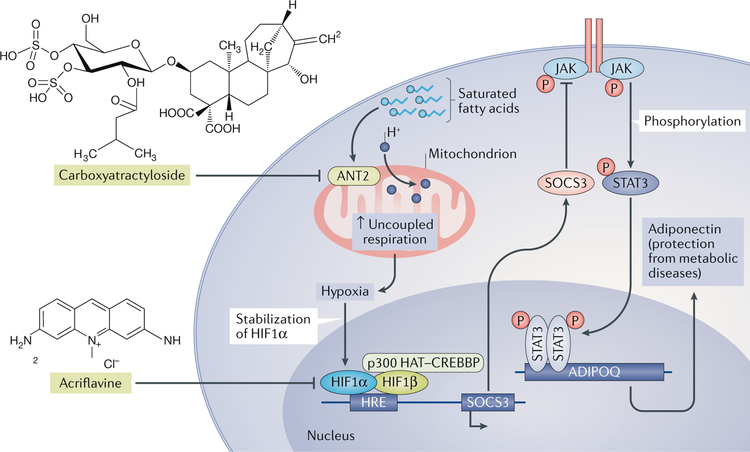
To investigate the role of adipose hypoxia and HIF in obesity and insulin resistance, two independent studies characterized mice lacking the expression of HIF1α and HIF1β36 and HIF1β alone37 in adipose tissue. Mouse lines lacking HIF1α and HIF1β expression exhibited similar metabolic phenotypes, including reduced fat formation, protection from HFD-induced obesity and decreased insulin resistance, suggesting a role for HIF1α and its dimerization partner HIF1β in the pathogenesis of obesity and insulin resistance36. Another group also observed that lack of HIF1α expression in adipocytes renders mice resistant to HFD-induced obesity, which correlated with increased fatty acid β-oxidation in white adipose tissue38. Others found a similar phenotype of decreased insulin resistance when either HIF1α or both HIF1α and HIF2α expression was disrupted in adipose tissue28. Furthermore, acriflavine (a molecule that inhibits heterodimerization of HIF1β)39 reduced insulin resistance in obese mice fed an HFD40. Similarly, another selective HIF1α inhibitor, PX-478, alleviates the HFD-induced glucose intolerance, insulin resistance and obesity that were attributed to inhibition of adipose tissue fibrosis and inflammation41. Mice lacking HIF1α expression in adipose tissue had decreased inflammation, whereas mice with disrupted HIF2α expression in adipocytes had elevated inflammation and insulin resistance, indicating opposing roles for these two HIFα proteins in adipocytes28. However, in adipocytes of obese mice fed an HFD, where HIF1α is the predominantly expressed isoform, adipocyte-specific HIF1 inhibition protects the mice from metabolic disorders36.
Mechanisms that increase obesity and insulin resistance.
HIF1α signalling in adipocytes affects obesity and insulin resistance by several potential mechanisms. In adipose tissue, HIF1α regulates the gene encoding suppressor of cytokine signalling 3 (SOCS3)40. Following the activation of the Socs3 gene by HIF1α, SOCS3 inhibits Janus kinase (JAK), which phosphorylates signal transducer and activator of transcription 3 (STAT3) and thus inhibits the expression of adiponectin42 (FIG. 2). Therefore, when hypoxia occurs during the expansion of adipose tissue, the accumulation of HIF1α results in decreased adiponectin production from adipocytes and increased insulin resistance40. In addition, homocysteine (a sulfur-containing amino acid derived from the metabolism of methionine) treatment triggers HIF1α activation in adipocytes43. Homocysteine markedly induces endoplasmic reticulum stress, inflammation and subsequent insulin resistance in adipose tissue44,45. Adipocyte HIF1α regulates lysophosphatidylcholine metabolism, as revealed by the identification of a novel HIF1α target gene encoding phospholipase A2 group 16. Adipocyte-specific HIF1α knockout abrogated the homocysteine-induced activation of NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3; also known as NALP3) inflammasome (a multiprotein complex that detects pathogenetic stressors and activates inflammatory responses) activation and insulin resistance through the phospholipase A2 group 16-lysophosphatidylcholine pathway. Thus, the adipocyte HIF1α-lysophosphatidylcholine axis is necessary for homocysteine-induced insulin resistance43.
Fig. 2 |. Hypoxia-inducible factor 1α in adipose tissue.
Adipose tissues become hypoxic because of saturated fatty acids binding to ADP/ATP translocase 2 (ANT2) in mitochondria, which increases uncoupled respiration. This uncoupling causes the stabilization of hypoxia-inducible factor 1α (HIF1α). Inhibition of ANT2 by carboxyatractyloside lowers saturated fatty acid-induced hypoxia. HIF1α induces expression of the suppressor of cytokine signalling 3 (SOCS3) and, by activating Janus kinase (JAK), SOCS3 phosphorylates and activates signal transducer and activator of transcription 3 (STAT3), which inhibits the expression of adiponectin (encoded by ADIPOQ). Acriflavine inhibits the dimerization of HIF1α and HIF1β, resulting in non-transcriptional activation of target genes. H+, proton; HRE, HIF regulating element; P, phosphate; p300 HAT-CREBBP, histone acetyltransferase p300-cAMP-response element binding protein (CREB)-binding protein.
Another mechanism by which adipose HIF1α could influence metabolic disease is through the modulation of inflammation, as the increased inflammation associated with adipocytes in obese mice contributes to obesity and insulin resistance46,47. In macrophages, PHD2 serves as a control for the metabolic shift from anaerobic glycolysis to oxidative phosphorylation; inhibition of PHD2 and the resultant increase in HIF1α reverse the metabolic phenotype of anaerobic glycolysis48. Even at the early stages of diet-induced obesity in mouse models, there is increased inflammation associated with infiltration of adipose tissue by macrophages. M1 macrophages release pro-inflammatory cytokines that can damage tissue and inhibit cell proliferation whereas M2 macrophages release anti-inflammatory cytokines that promote proliferation of nearby cells and tissue repair. M1–M2 polarization is a tight process that interconverts M1 and M2 macrophages by a number of mechanisms that involve the tissues in which the macrophages infiltrate and the associated tissue microenvironments49.
HIF1α can promote classic M1 macrophage activation, regulate phosphofructokinase and modulate the expression of inflammation-related genes50. These findings suggest that hypoxia is linked to M1 macrophage polarization and inflammation, thus indicating that HIF1α expression in macrophages might trigger adverse physiological responses, resulting in not only obesity but also insulin resistance, through the modulation of macrophage metabolic reprogramming and inflammation. However, macrophage-specific hHif1α-knockout mice showed no indication that HIF1α expressed by macrophage in adipose tissue has an important role in the early stages of obesity51. However, adipose-specific knockout of HIF1α results in less adipose tissue macrophage infiltration than wild-type mice, decreased inflammation and amelioration of diet-induced obesity28,36.
Although HIF1α from macrophages does not appear to have a major influence on the early stages of adipose inflammation and obesity, others have found that macrophage-specific Hif1α-knockout mice have decreased insulin resistance after 18 weeks of HFD treatment52. HIF1α stimulates glucose uptake by increasing GLUT1 expression and glycolysis (by induction of glycolytic enzymes), which promotes the utilization of glucose in lipid synthesis53. HIF1α also suppresses deacetylation of peroxisome proliferator-activated receptor-γ (PPARγ) co-activator 1α (PGC1α) and expression of genes involved in fatty acid β-oxidation in white adipocytes mainly through repression of Sirt2, encoding sirtuin 2 (an NAD-dependent deacetylase)38. These studies might partially explain the obesity phenotype of transgenic mice overexpressing HIFα30,31 and the lean phenotype in mice lacking HIF1α or HIF1β expression in adipose tissue28,36,37.
Although obesity is associated with increased HIF1α expression and activated downstream signalling, in normal-weight mice, levels of basal HIF1α are almost undetectable. Acute or transient exposure of differentiating adipocytes to hypoxia reprogrammes cells for increased triglyceride accumulation, decreased fatty acid β-oxidation and pyruvate dehydrogenase activity and increased insulin sensitivity as revealed by rapid glucose uptake54. AMP-activated protein kinase (AMPK; also known as PRKAA2) was activated along with increased levels of mRNAs encoding GLUT1, PPARγ, PGC1α and sterol regulatory element-binding protein (SREBP). Repeated exposure to short-term hypoxia further increased glucose uptake in adipocytes. Although this study was performed in cultured adipocytes, the results suggest that acute and chronic hypoxia might have opposing effects on the activation of HIF1α and HIF2α.
HIF1α in pancreatic β-cells
HIF1α expression in pancreatic β-cells has been linked to metabolic diseases. For instance, in pancreatic β-cells from patients with type 2 diabetes mellitus (T2DM), the level of HIF1β mRNA was reduced by 90% compared with non-diabetic controls55. Mechanistic studies have revealed that short interfering RNA-mediated knockdown of HIF1β in mouse β-cell-derived MIN6 cells impairs glucose-stimulated insulin release and changes gene expression patterns, which is similar to what is seen in pancreatic islets from patients with T2DM55. Additionally, mice lacking the expression of HIF1β in β-cells had abnormal glucose tolerance, impaired insulin secretion and altered gene expression patterns compared with wild-type mice. However, this study was performed with the HIFα heterodimerization partner HIF1β; thus, it is not clear which HIFα subunit is responsible for the phenotype, as knockdown of HIF1α, HIF2α and even a mechanistically unrelated bHLH-PAS superfamily member AHR in MIN6 cells each independently decreased insulin secretion slightly55. Furthermore, β-cell-specific HIF1α disruption exacerbates β-cell dysfunction and glucose intolerance by downregulating glycolysis and electron-transport-chain-related gene expression, leading to decreased ATP generation56. Additionally, HIF1α is also a protective factor for islet cell transplantation57. By contrast, other studies have shown that overexpression of HIF1α and HIF2α in conjunction with VHL disruption worsens β-cell function and glucose homeostasis58,59. These studies suggest that hypoxia and HIF signalling might have a vital role in the function of pancreatic β-cells.
HIF1α in liver
HIF1α influences liver disease through the regulation of genes involved in glucose and lipid metabolism. Altered expression of these genes could occur under conditions of hypoxia, possibly induced by increased mitochondrial metabolism as in adipocytes. HIF1α regulates genes encoding GLUT1 (REF.60) and 3-phosphoinositide-dependent protein kinase 1 (PDK1; also known as PDPK1)61, which are involved in glucose transport and fructose production, respectively, and could influence the development and progression of non-alcoholic fatty liver disease (NAFLD)62. Metabolic diseases such as obesity, NAFLD, T2DM and atherosclerosis are all linked to altered lipid and glucose metabolism. Early evidence for a role of HIFs in metabolic diseases was provided by hepatocyte-specific disruption of VHL63, PHD2 (REF.64) or PHD3 (REF.65), which triggered the O2-independent overexpression of both HIF1α and HIF2α and induced hepatic steatosis. Chronic ethanol administration was shown to activate hepatic HIF1α, and overexpression of hepatocyte HIF1α aggravated ethanol-induced hepatic steatosis66. Hepatocyte-specific HIF1α disruption ameliorated chronic ethanol-induced hepatic steatosis and inflammation66. By contrast, another group reported that hepatocyte-specific HIF1α disruption exacerbated hepatic steatosis upon chronic ethanol administration67. The same group also found that hepatocyte-specific HIF1α disruption aggravated high-fat and sucrose-diet-induced glucose intolerance68. However, others did not observe metabolic phenotypes in mice with hepatocyte-specific HIF1α disruption69.
In addition, digoxin has been shown to protect mice from liver inflammation and cellular damage caused by non-alcoholic steatohepatitis (NASH)70. This protective effect is because digoxin inhibits the interaction between pyruvate kinase PKM and histones and downregulates HIF1α signalling. Thus, the possibility exists that the effect of digoxin on NASH could be the result of HIF1α inhibition as other studies have reported that hepatic HIF1α activation promotes inflammation66 and further suggests that downregulation or inhibition of HIF1α in the liver could be a therapeutic strategy for the treatment of metabolic diseases.
Liver disease, such as NASH, is accompanied by increased fibrosis. The HIF1α inhibitor 3-(5-hydroxymethyl-2-furyl)-1-benzylindazole (YC-1) ameliorated liver fibrosis in part by downregulating SOCS1 and SOCS3, which resulted in the inhibition of nuclear factor-κB (NF-κB) activation and STAT3 phosphorylation71. This pathway is similar to that uncovered in adipose tissue that results in adiponectin expression40.
HIF1α is also involved in the control of cholesterol synthesis in the liver. HIF1α directly activates insulin-induced gene 2 protein (INSIG2), which is located in the endoplasmic reticulum membrane and subsequently inhibits the rate-limiting cholesterol synthesis enzyme 3-hydroxy-3-methylglutaryl-coA reductase (HMGCR)72. This observation indicates a potential beneficial role for hepatic HIF1α activation, which triggers the degradation of HMGCR under conditions of lipid overload.
HIF2α in metabolic disease
HIF2α in the liver
Although the role of HIF2α in cancer has been extensively studied, its function in metabolism and metabolic disease has received more attention in recent years. Several studies have shown that HIF2α either promotes or inhibits metabolic disease (TABLE 1). Furthermore, a study published in 20l7 revealed that HIF2α has a role in the control of glucose and fatty acid metabolism in the liver, as summarized in FIG. 3 (as discussed in this review73). The first study to report a more definitive connection between HIF2α and lipid metabolism showed that Vhl and Hif1α-double-knockout mice, which have constitutively stabilized HIF2α, and not HIF1α, exhibit severe hepatic steatosis with decreased fatty acid β-oxidation74. However, administration of the PHD inhibitor FG-4497 decreased serum levels of cholesterol and de novo lipid synthesis and protected mice from hepatic steatosis and atherosclerosis75. In addition, increased hepatic hypoxia and HIF2α (but not HIF1α) expression, which was assessed using temporal VHL disruption with a cre-ERT2 system, caused hepatic steatosis by regulating hepatic fatty acid uptake, synthesis and catabolism76. Acute activation of HIF2α in the liver upregulated the expression of genes involved in fatty acid synthesis, including fatty acid synthase (FASN), which is controlled by SREBP1C and fatty acid uptake (via CD36); the latter is a plasma membrane transporter responsible for the import of fatty acids into cells. HIF2α activation is also correlated with downregulation of PPARα and enzymes encoded by its target gene, including peroxisomal acyl-coA oxidase 1 (ACOX1), which is involved in fatty acid β-oxidation76. The mechanism by which HIF2α controls SREBP1C and PPARα in the liver has not been determined. However, in hepatocytes, HIF2α represses PPARα and exacerbates acetaminophen-induced hepatotoxicity77,78. Furthermore, HIF2α directly regulates angiopoietin-related protein 3 (ANGPTL3)76, an endogenous lipoprotein lipase inhibitor and an important mediator of lipid homeostasis79. Additionally, HIF2α activation increases liver inflammation and fibrosis, although the mechanism is still unclear76.
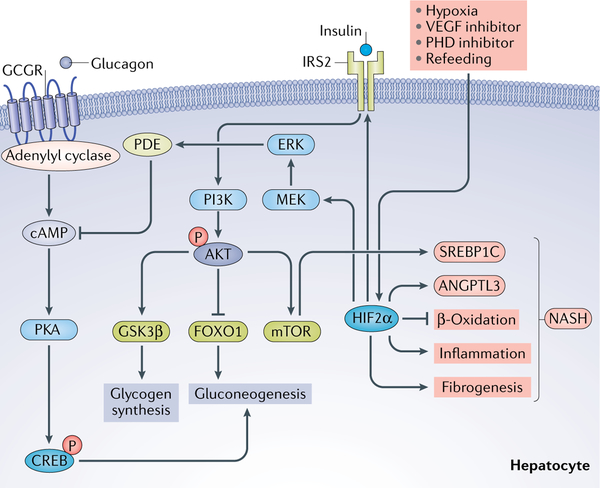
Fig. 3 |. Hypoxia-inducible factor 2α in liver glucose metabolism.
Liver glucose metabolism and transport are activated by insulin signalling through insulin receptor substrate 2 (IRS2), which activates phosphoinositide 3-kinase (PI3K) to phosphorylate AKT. This phosphorylation results in the activation of glycogen synthase kinase 3β (GSK3β), stimulating glycogen synthesis and mechanistic target of rapamycin (mTOR), which activates fatty acid synthesis through sterol regulatory element-binding protein 1C (SREBP1C) and inhibits forkhead box protein O1 (FOXO1), which controls gluconeogenesis. Hypoxia-inducible factor 2α (HIF2α) levels can be increased in the liver by hypoxia, vascular endothelial growth factor (VEGF) inhibition, prolyl hydroxylase domain (PHD) inhibition and refeeding. Glucagon exerts the opposite effects on glucose than insulin by binding to glucagon-like protein receptor 1 (GCGR), which increases cAMP through adenylyl cyclase. An increase in cAMP leads to the activation of protein kinase A (PKA) and phosphorylation of cAMP-responsive element-binding protein (CREB), which controls hepatic gluconeogenesis. Chronic activation of HIF2α also leads to increased inflammation and fibrosis and decreased fatty acid β-oxidation, which suggest that chronic activation would have detrimental consequences to liver physiology, such as non-alcoholic fatty liver disease and non-alcoholic steatohepatitis (NASH). ANGPTL3, angiopoietin-related protein 3; ERK, extracellular-signal-regulated kinase; MEK, mitogen-activated protein kinase kinase (also known as MAP2K); P, phosphate; PDE, phosphodiesterase.
Clinical biopsy samples from patients with NAFLD showed an overexpression of HIF2α; a mouse model of NASH (with NASH induced by feeding mice a diet deficient in methionine and choline) further supported this result80. Disruption of HIF2α expression ameliorated liver fibrosis and inflammation via downregulation of hepatocyte production of histidine-rich glycoprotein, which potentiates M1 macrophage migration and polarization leading to increased hepatic inflammation81, suggesting a potentially harmful outcome from overexpression of HIF2α in the liver.
Most studies investigating the relationship between HIF and NAFLD have focused on evaluating the effects of HIF1α and HIF2α in the liver. By contrast, activation of HIF2α in the liver ameliorates hyperglycaemia through an insulin-dependent pathway with increased levels of insulin receptor substrate 2 (IRS2) or through the insulin-independent pathway through repression of glucagon action16,73,82–84. Evidence that HIF2α regulates lipid and glucose metabolism was further revealed in hepatic Phd3 (also known as Egln3)-null mice83. Acute disruption of Phd3 stabilized HIF2α expression and further upregulated Irs2 expression, which increased insulin-stimulated AKT activation and forkhead box protein O1 (FOXO1)-dependent suppression of gluconeogenesis. Physiological liver hypoxia and VEGF inhibition through vascular regression are two stimuli that can activate the HIF2α-IRS2 pathway to modulate glucose metabolism (FIG. 3). Furthermore, hepatic Phd3-null mice exhibited decreased β-oxidation and increased insulin sensitivity. In contrast to Phd1 and Phd2-knockout mice, hepatic Phd3-null mice specifically stabilized HIF2α, which was not associated with increased hepatic toxicity83. Additionally, HIF2α attenuates postprandial glucagon signalling through the extracellular-signal-regulated kinase (ERK; also known as MAPK)-dependent increase in phosphodiesterase-mediated hydrolysis of intracellular cAMP, resulting in decreased protein kinase A (PKA)-mediated activation of CREB82. This decreased activation leads to the suppression of the gluconeogenic target genes encoding the enzymes phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6 phosphatase (G6Pase).
These studies imply that pharmacological inhibition of hepatic HIF2α might not be a suitable target for NAFLD therapy owing to the risk of increased hepatic glucose production and T2DM. However, transcription factor signalling pathways in the intestine are also involved in the development of metabolic disease, including NAFLD85–88. These studies demonstrate the complexity of the role of HIF2α in obesity, insulin resistance, NAFLD and other metabolic diseases involving dysfunction of glucose and lipid metabolism.
Another aspect of HIF2α stabilization and activation is modulation of lipid metabolism in liver and adipose tissue. Peroxisomes, which carry out fatty acid β-oxidation of long-chain and very-long-chain fatty acids, are dependent on oxygen, thus indicating a potential role for oxygen-sensing HIFs in the control of metabolism by this organelle. In Vhl-Hif1a-null mice that constitutively express HIF2α, liver HIF2α activation leads to a decrease in peroxisomes expressing the gene encoding next to BRCA1 gene 1 protein (NBR1) through pexophagy, the selective autophagy of peroxisomes89. The mechanism and functional importance of induced pexophagy in liver and other tissues requires further investigation90. Perhaps under low oxygen, peroxisome numbers are reduced, leading to lower oxygen consumption and accumulation of very-long-chain fatty acids because of increased HIF2α signalling.
Upon global Pdh2 gene disruption, which stabilizes both HIF1α and HIF2α, mice fed either normal chow or an HFD display less adipose tissue and less adipose inflammation91. In adipose tissue, loss of HIF2α signalling exacerbates adipose dysfunction and impairs thermogenesis, coinciding with decreased UCP1 expression (a marker for brown adipocytes)92. Administration of VEGFA reversed the obese phenotype and adipose inflammation in the absence of HIF2α. Overexpression of VEGFA, a gene target of the HIF2α in adipose, in mice fed an HFD to induce obesity triggers adipose browning, improved metabolism and reduced adipose inflammation93.
HIF2α in macrophages
Intermittent fasting improves metabolism and obesity by inducing VEGF overexpression in adipose tissue, which coincides with the activation of anti-inflammatory M2 macrophages in adipose tissue94. The anti-inflammatory M2 macrophages increased the browning of white adipose tissue95. A VEGF-M2 axis was suggested to promote adipose browning94, indicating that upstream HIF induction of VEFG might influence adipose browning and thermogenesis. These data suggest that HIF1α and HIF2α have opposing effects in adipose tissues (as discussed previously) and knockout or chemical inhibition of HIF1α in adipose tissue reduces inflammation and obesity36–38. Determining the role of adipose hypoxia and HIF1α in this process and rectifying the differences in other studies showing that hypoxia and increased levels of HIF1α in adipose tissues exacerbate obesity require additional experimentation.
HIF2α could also influence the M1–M2 macrophage transition and cooperate with HIF1α via controlling the balance of inducible nitric oxide synthase (iNOS; also known as NOS2) and arginase 1 (ARG1)96. Owing to the tight association between metabolism and inflammation47, compared with inflammation-prone HIF1α, HIF2α exerts more anti-inflammatory activity that inhibits macrophage activation by inhibiting mitochondrial reactive oxygen species (ROS)97. Furthermore, HIF1α and HIF2α influence ROS production via modulation of different targets98. HIF1α upregulates cytochrome NADPH oxidase 2 (NOX2; also known as CYBB), which increases ROS production99; HIF2α upregulates mitochondrial superoxide dismutase (SOD2) and then suppresses ROS production100. Taken together, HIF1α and HIF2α have different roles in macrophage polarization, ROS production and inflammation.
Iron transport and metabolic diseases
HIF2α has a major role in the control of iron transport in the intestine101–103. The incidence of iron deficiency is increased in children and adults with obesity, suggesting that intestinal HIF2α signalling, through its control of iron metabolism, might influence obesity102–106. As an explanation for the findings of these epidemiological studies, HIF2α expression could be suppressed in the intestine, leading to iron deficiency, which causes or potentiates obesity. Alternatively, iron-deficiency-induced activation of intestinal HIF2α could affect obesity, which is dependent on the modulation of another pathway. Expression of HIF2α and its target genes encoding divalent metal transporter 1 (DMT1; also known as NRAMP2) and duodenal cytochrome b (DCYTB; also known as CYBRD1) are elevated in the ileum of people with obesity compared with individuals who are not obese88. The mRNA levels of Dmt1 and Dcytb1 are positively correlated with BMI and levels of the liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST), which are markers of liver damage associated with NAFLD. Although this finding suggests that HIF2α is activated in the intestine in obesity and influences obesity-associated phenotypes, the question arises whether hypoxia is the cause or the result of obesity.
HIF2α in intestine and metabolic disease
To determine the mechanism by which hypoxia in the intestine affects metabolic disease, a diet-induced obesity mouse model was used with genetically manipulated mice and pharmacological inhibition to explore the role of HIFα in metabolic disease. Transgenic mice expressing an HIF1α oxygen-dependent degradation domain linked to a luciferase reporter107 were fed an HFD to induce obesity and showed increased hypoxia88. To understand the role of HIF1α and HIF2α in the intestine in metabolic disease and identify the precise mechanism responsible, metabolomic profiling of mice with intestine-specific knockout of HIF1α and HIF2α or activation of both HIFα subunits by intestine-specific disruption of Vhl was undertaken. As summarized in FIG. 4, HIF2α, but not HIF1α, signalling in the intestine was activated during obesity. Intestine-specific HIF2α ablation substantially ameliorated HFD-induced hepatic steatosis. HIF2α expression and signalling were directly correlated with obesity in humans88, thus indicating the potential for the translation of the mouse studies to metabolic disease in humans.
Fig. 4 |. Hypoxia-inducible factor 2α in metabolic disease.
Under conditions of obesity, the small intestine becomes hypoxic, leading to the accumulation of hypoxia-inducible factor 2α (HIF2α) in epithelial cells. HIF2α activates the gene encoding sialidase 3 (NEU3), which hydrolyses gangliosides (located in the plasma membrane) to form ceramides. Increased levels of ceramides cause obesity as a result of decreased adipose browning, increased steatosis owing to upregulation of fatty acid synthesis and increased insulin resistance. Inhibition of HIF2α by PT2385 or inhibition of NEU3 by N-acetyl-2,3-didehydro-N-acetyl-neuraminic acid (DANA) or naringin decreases serum levels of ceramides, reduces obesity and fatty liver and increases insulin sensitivity. HRE, HIF regulating element.
However, there is the question of whether this pathway can be pharmacologically targeted. A family of ligands were developed that inhibit HIF2α heterodimerization with HIF1β, resulting in loss of DNA-binding activity and HIF signalling108. Notably, a specific HIF2α inhibitor, PT2385 ((S)-3-((2,2-difluoro-1-hydroxy-7-(methylsulfonyl)-2,3-dihydro-1H-inden-4-yl)oxy)-5-fluorobenzonitrile), is in clinical trials for the treatment of renal cancer22–24. Oral administration of PT2385 to obese mice selectively inhibits HIF2α in the intestine and prevents the development of metabolic disorders in mice fed an HFD88. Importantly, PT2385 administration to obese mice markedly decreases all adverse phenotypes associated with obesity, including insulin resistance and NAFLD. This work suggests that HIF2α in the intestine is a target for the treatment of metabolic disease.
Neu3, encoding sialidase 3 (NEU3), a key enzyme in the ceramide salvage pathway that produces ceramide109, is an HIF2α target gene in the intestine. Two inhibitors of NEU3, N-acetyl-2,3-didehydro-N-acetyl-neuraminic acid (DANA) and the flavonoid naringin, reduced the metabolic abnormalities associated with HFD treatment. Although having low systemic bioavailability, these compounds effectively inhibited NEU3 in the intestine and reduced the severity of HFD-induced metabolic disorders88. PT2385 administration to mice shifted the metabolism of bile acids via hepatic cholesterol 7α-monooxygenase (CYP7A1), the key enzyme in bile acid biosynthesis110. Thus, this selective HIF2α antagonist shows great potential in the treatment of metabolic disease.
These studies established a novel HIF2α-NEU3-ceramide pathway that promotes the development of metabolic disease. The different metabolic phenotypes found in wild-type and intestine-specific Hif2α-knockout mice were positively correlated with serum levels of ceramide. High serum levels of ceramides were associated with increased risk in all adverse metabolic phenotypes, including obesity, insulin resistance and NAFLD88. The role of ceramides in metabolic disease has been established in a number of earlier studies111–114. The induction and activation of Neu3 by HIF2α in small intestinal epithelial cells resulted in hypoxia, which increases serum levels of ceramides in HFD-fed intestine-specific Hif2α-knockout mice, or when wild-type mice treated with PT2385 or the NEU3 inhibitors (DANA and naringin) had decreased serum levels of ceramides by ~30%88. Restoration of ceramides to intestine-specific Hif2α-knockout mice fed an HFD, by injection of C16:0 ceramide, reversed the improved metabolic abnormalities88. No gastrointestinal toxicities (such as diarrhoea or inflammation) were found, as revealed by similar faecal levels of neutrophil gelatinase-associated lipocalin (LCN2) in the drug-treated mice versus controls or in mice genetically deficient in intestinal HIF2α signalling.
Additionally, HIF2α is also involved in the control of iron absorption103. However, mice lacking expression of HIF2α in the intestine show no signs of anaemia, suggesting that targeting HIF2α in the gut is a safe and effective treatment for metabolic disease. Other mechanisms underlie the control of ceramide synthesis in the intestine, such as the farnesoid X receptor (FXR; also known as NR1H4), which is highly expressed in the liver and intestine and controls bile acid synthesis and transport and the enterohepatic circulation of bile acids87,115. FXR in the intestinal epithelial cells activates the gene encoding sphingomyelin phosphodiesterase 3 (SMPD3) that catalyses the hydrolysis of sphingomyelin to form ceramide and phosphocholine116,117. FXR is constitutively activated by bile acids in the ileum, leading to increased serum levels of ceramides through the induction of Smpd3; inhibition of intestinal FXR decreases the incidence of HFD-induced metabolic diseases86,87,117,118. Thus, both intestinal HIF2α and FXR contribute to diet-induced obesity and related disorders, and both can be targeted for the treatment of metabolic disease.
HIF1β in metabolic diseases
Conditional knockout of HIF1β in mice was produced119 and used to determine the role of HIF signalling in metabolic disease. However, results from mice in which HIF1β is disrupted in various tissues can be difficult to interpret as HIF1β is the obligate heterodimeric partner of HIF1α, HIF2α, AHR and other members of the bHLH-PAS superfamily10. In comparison with hepatic-specific Hif1a-null and Hif1b-null mice, hepatic-specific HiF1b-null mice have increased fasting plasma levels of glucose, glucose tolerance and postprandial triglycerides, which are associated with increased expression of G6Pase, carbohydrate-responsive element-binding protein (ChREBP), FASN and acyl-CoA desaturase (SCD)69,120. Hif1a-null and Hif2a-null mice showed the same metabolic parameters (such as fasting plasma levels of glucose and glucose tolerance) as wild-type mice, thus indicating that HIF1β, the obligate partner of HIFα, has an impact on metabolism, possibly through its interactions with another bHLH-PAS transcription factor69. For example, expression of fibroblast growth factor 21 (FGF21), which contributes to energy homeostasis during fasting, is repressed by activation of AHR, another partner of HIF1β121. These studies indicate that HIF1β has individual functions in metabolic disorders and that understanding the relationship of HIF1α and HIF2α with their heterodimeric partner molecule HIF1β requires additional studies.
HIF inhibitors and therapeutic effects
As HIF has critical functions in cancer, inhibitors of HIF were developed and exhibited therapeutic potential122. These inhibitors show a great potential in cancer treatment, but owing to the potential for important roles of HIFs in metabolic disorders, they might have broader therapeutic effects. Although some small molecules were reported to be an inhibitor of HIF, other inhibitors function indirectly. For example, acriflavine, PX-478, 3-(2-(4-adamantan-1-yl-phenoxy)-acetylamino)-4-hydroxybenzoic acid methyl ester (LW6), 3,4-dimethoxy-N-((2,2-dimethyl-2H-chromen-6-yl) methyl)-N-phenylbenzenesulfonamide (KCN1), YC-1 and PT2385 are the most widely used experimental inhibitors. Acriflavine was demonstrated to protect from HFD-induced obesity and insulin resistance dependent on the adipose HIF1α-SOCS3-STAT3-adiponectin pathway40. PX-478 treatment selectively inhibits adipose HIF1α, leading to improvement in metabolic dysfunctions, partially through reduced adipose fibrosis41. LW6 is an adamantyl-based derivative, and this compound indirectly inhibits HIF1α via the mitochondrial malate dehydrogenase (MDH2) protein123,124. LW6 administration can decrease activated human T cell proliferation without affecting cell survival by inhibiting the tricarboxylic acid cycle125. KCN1 is a direct inhibitor of HIF1α by downregulating HIF1α target gene expression, and it could be used to treat metabolic disorders126,127. YC-1 is an HIF1α inhibitor that is widely used in experimental studies. In lung cancer cells, YC-1 inhibited the HIF1α-induced reprogramming of glucose metabolism from mitochondrial oxidative phosphorylation to anaerobic glycolysis and lactic acid fermentation128. YC-1 can also modulate lipolysis, but in a cell-type-specific manner. In RAW 264.7 macrophage cells, YC-1 increased lipid droplet and oxidized LDL foam cell formation through cGMP-dependent protein kinase129, whereas in adipocytes, YC-1 induced lipolysis130. Another study revealed that YC-1 is a non-competitive inhibitor of p-glycoprotein (multidrug resistance protein 1 (MDR1; also known as ABCB1)) also act via the cGMP-dependent protein kinase ERK131. These studies suggest the potential for a broader therapeutic use for the HIF inhibitor YC-1.
PT2385 is an HIF2α inhibitor effective in treating renal cell carcinoma23. Furthermore, PT2385 administration decreases intestinal and serum levels of ceramides, resulting in metabolic improvements, a finding consistent with studies in the intestinal Hif2α-knockout mice88. As the effects of either activating or inhibiting HIF1α and HIF2α in different tissues can affect metabolic diseases to different degrees, more studies are needed to focus on tissue-specific targeting of the two HIFα proteins to achieve favourable metabolic end points.
Conclusion
Since the discovery in 1992 (REF.1) that a transcription factor controls the cellular adaption to low levels of oxygen, there have been many studies showing the unique mechanism by which HIFα proteins are stabilized by the oxygen-dependent PHDs and FIH1 enzymes132. The major function of HIF that has received the most attention is its role in the control of angiogenesis during mammalian development and in the growth of tumours, largely by the induction of the gene encoding VEGF. However, evidence has emerged that modulation of HIF1α and HIF2α signalling could be of potential benefit for metabolic diseases, which is beyond their known roles in cancer treatment.
These new functions for HIF were discovered primarily through the analysis of Hif1α, Hif2α and Hif1β-conditional-knockout mice and through limited pharmacological studies in which HIFs were chemically inhibited. Notably, targeted inhibition of HIF1α in adipose tissue and HIF2α in intestine restored to normal many adverse phenotypes of metabolic disease found caused by feeding mice the high-fat Western diet (including obesity, T2DM and NAFLD). In addition, the direct inhibition of the HIF2α target gene encoding NEU3, involved in ceramide production, could also be explored as a potential therapeutic target. Because HIF2α expression and signalling and NEU3 are conserved between mice and humans, it is probable that the studies in mice would translate to humans. Indeed, intestinal HIF2α expression and activity is associated with human obesity88, and increased levels of ceramides, which were recently called the ‘new cholesterol’114, are correlated with metabolic diseases in humans and promote obesity, T2DM and NAFLD in mice87,113.
Key points.
Obesity triggers hypoxia in adipose tissue and the small intestine, which stabilizes and activates hypoxia-inducible factor (HIF)1α and HIF2α signalling, resulting in adverse metabolic effects, including insulin resistance and non-alcoholic fatty liver disease.
Induction of HIF1α in adipocytes, through a suppressor of cytokine signalling 3 (SOCS3)-signal transducer and activator of transcription 3 (STAT3) axis, leads to the upregulation of inflammation and downregulation of adiponectin expression, resulting in insulin resistance.
Activation of HIF2α in the small intestine increases expression of sialidase 3, resulting in an elevation of small intestinal and serum levels of ceramides that in turn potentiate obesity-associated metabolic diseases.
Genetic or chemical inhibition of HIF1α and HIF2α signalling in adipose tissue and the small intestine ameliorates obesity-associated metabolic diseases, indicating that they could be targeted for treatment of metabolic disorders.
Acknowledgements
The authors acknowledge the support of the National Cancer Institute Intramural Research Program, the NIH, the National Key Research and Development Program of China (2016YFC0903100), the National Natural Science Foundation of the People’s Republic of China (81522007, 81470554 and 31401011) and the Fundamental Research Funds for the Central Universities: Clinical Medicine Plus X-Young Scholars Project of Peking University (PKU2018LCXQ013).
Footnotes
Competing interests
The authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Semenza GL & Wang GL A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell. Biol 12, 5447–5454 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang GL & Semenza GL Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem 268, 21513–21518 (1993). [PubMed] [Google Scholar]
- 3.Hoffman EC et al. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 252, 954–958 (1991). [DOI] [PubMed] [Google Scholar]
- 4.Ravenna L, Salvatori L & Russo MA HIF3α: the little we know. FEBS J. 283, 993–1003 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Haase VH HIF-prolyl hydroxylases as therapeutic targets in erythropoiesis and iron metabolism. Hemodial. Int 21 (Suppl. 1), S110–S124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisy K & Peet DJ Turn me on: regulating HIF transcriptional activity. Cell Death Differ. 15, 642–649 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Maxwell PH et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 (1999). [DOI] [PubMed] [Google Scholar]
- 8.Ivan M et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Jaakkola P et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Labrecque MP, Prefontaine GG & Beischlag TV The aryl hydrocarbon receptor nuclear translocator (ARNT) family of proteins: transcriptional modifiers with multi-functional protein interfaces. Curr. Mol. Med 13, 1047–1065 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Nebert DW Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog. Lipid Res 67, 38–57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bersten DC, Sullivan AE, Peet DJ & Whitelaw ML bHLH-PAS proteins in cancer. Nat. Rev. Cancer 13, 827–841 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Shao Z, Zhai Z, Shen C & Powell-Coffman JA The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans. PLOS One 4, e6348 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian H, Hammer RE, Matsumoto AM, Russell DW & McKnight SL The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 12, 3320–3324 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramakrishnan SK & Shah YM Role of intestinal HIF-2α in health and disease. Annu. Rev. Physiol 78, 301–325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keith B, Johnson RS & Simon MC HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12, 9–22 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dengler VL, Galbraith M & Espinosa JM Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol 49, 1–15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Cox SR, Morita T & Kourembanas S Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5’ enhancer. Circ. Res 77, 638–643 (1995). [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P VEGF as a key mediator of angiogenesis in cancer. Oncology 69 (Suppl. 3), 4–10 (2005). [DOI] [PubMed] [Google Scholar]
- 21.Arjaans M et al. VEGF pathway targeting agents, vessel normalization and tumor drug uptake: from bench to bedside. Oncotarget 7, 21247–21258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wallace EM et al. A small-molecule antagonist of HIF2α Is efficacious in preclinical models of renal cell carcinoma. Cancer Res. 76, 5491–5500 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Chen W et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 539, 112–117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Saez O, Gajate Borau P, Alonso-Gordoa T, Molina-Cerrillo J & Grande E Targeting HIF-2α in clear cell renal cell carcinoma: A promising therapeutic strategy. Crit. Rev. Oncol. Hematol 111, 117–123 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Ye J, Gao Z, Yin J & He Q Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab 293, E1118–1128 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Trayhurn P, Wang B & Wood IS Hypoxia in adipose tissue: a basis for the dysregulation of tissue function in obesity? Br. J. Nutr 100, 227–235 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Pasarica M et al. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee YS et al. Increased adipocyte O2 consumption triggers HIF-1α, causing inflammation and insulin resistance in obesity. Cell 157, 1339–1352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Regazzetti C et al. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes 58, 95–103 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halberg N et al. Hypoxia-inducible factor 1α induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol 29, 4467–4483 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jun JC et al. Adipose HIF-1α causes obesity by suppressing brown adipose tissue thermogenesis. J. Mol. Med. (Berl.) 95, 287–297 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X et al. Adipose tissue-specific inhibition of hypoxia-inducible factor 1α induces obesity and glucose intolerance by impeding energy expenditure in mice. J. Biol. Chem 285, 32869–32877 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basse AL et al. Regulation of glycolysis in brown adipocytes by HIF-1α. Sci. Rep 7, 4052 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsuura H et al. Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation 127, 2078–2087 (2013). [DOI] [PubMed] [Google Scholar]
- 35.He Q et al. Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am. J. Physiol. Endocrinol. Metab 300, E877–885 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang C et al. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 60, 2484–2495 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee KY, Gesta S, Boucher J, Wang XL & Kahn CR The differential role of Hif1βArnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab. 14, 491–503 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan J et al. Dietary obesity-associated Hif1α activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev. 26, 259–270 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee K et al. Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization. Proc. Natl Acad. Sci. USA 106, 17910–17915 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Jiang C et al. Hypoxia-inducible factor 1α regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. J. Biol. Chem 288, 3844–3857 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun K, Halberg N, Khan M, Magalang UJ & Scherer PE Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol. Cell. Biol 33, 904–917 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kanatani Y et al. Effects of pioglitazone on suppressor of cytokine signaling 3 expression: potential mechanisms for its effects on insulin sensitivity and adiponectin expression. Diabetes 56, 795–803 (2007). [DOI] [PubMed] [Google Scholar]
- 43.Zhang SY et al. Adipocyte-derived lysophosphatidylcholine activates adipocyte and adipose tissue macrophage nod-Like receptor protein 3 inflammasomes mediating homocysteine-induced insulin resistance. EBioMed 31, 202–216 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Y et al. Homocysteine upregulates resistin production from adipocytes in vivo and in vitro. Diabetes 57, 817–827 (2008). [DOI] [PubMed] [Google Scholar]
- 45.Pang Y et al. Intermedin restores hyperhomocysteinemia-induced macrophage polarization and improves insulin resistance in mice. J. Biol. Chem 291, 12336–12345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saltiel AR & Olefsky JM Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Invest 127, 1–4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reilly SM & Saltiel AR Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol 13, 633–643 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Guentsch A et al. PHD2 Is a regulator for glycolytic reprogramming in macrophages. Mol. Cell. Biol 37, e00236–00216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang N, Liang H & Zen K Molecular mechanisms that influence the macrophage m1–m2 polarization balance. Front. Immunol 5, 614 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Han S et al. Liver X receptor agonist therapy prevents diffuse alveolar hemorrhage in murine lupus by repolarizing macrophages. Front. Immunol 9, 135 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boutens L et al. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses. Diabetologia 61, 942–953 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takikawa A et al. HIF-1α in myeloid cells promotes adipose tissue remodeling toward insulin resistance. Diabetes 65, 3649–3659 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Krishnan J et al. Activation of a HIF1α-PPARγ axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab. 9, 512–524 (2009). [DOI] [PubMed] [Google Scholar]
- 54.Lu H, Gao Z, Zhao Z, Weng J & Ye J Transient hypoxia reprograms differentiating adipocytes for enhanced insulin sensitivity and triglyceride accumulation. Int. J. Obes. (Lond.) 40, 121–128 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gunton JE et al. Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122, 337–349 (2005). [DOI] [PubMed] [Google Scholar]
- 56.Cheng K et al. Hypoxia-inducible factor-1α regulates βcell function in mouse and human islets. J. Clin. Invest 120, 2171–2183 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stokes RA et al. Hypoxia-inducible factor-1α (HIF-1α) potentiates β-cell survival after islet transplantation of human and mouse islets. Cell Transplant. 22, 253–266 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zehetner J et al. PVHL is a regulator of glucose metabolism and insulin secretion in pancreatic β cells. Genes Dev. 22, 3135–3146 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cantley J et al. Deletion of the von hippel-lindau gene in pancreatic β cells impairs glucose homeostasis in mice. J. Clin. Invest 119, 125–135 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zelzer E et al. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1α/ARNT. EMBO J. 17, 5085–5094 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JW, Tchernyshyov I, Semenza GL & Dang CV HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 (2006). [DOI] [PubMed] [Google Scholar]
- 62.Gorden DL et al. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J. Lipid Res 56, 722–736 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kucejova B et al. Uncoupling hypoxia signaling from oxygen sensing in the liver results in hypoketotic hypoglycemic death. Oncogene 30, 2147–2160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo B et al. Hepatic PHD2/HIF-1α axis is involved in postexercise systemic energy homeostasis. FASEB J. 10.1096/fj.201701139R (2018). [DOI] [PubMed] [Google Scholar]
- 65.Minamishima YA et al. A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol. Cell. Biol 29, 5729–5741 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nath B et al. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology 53, 1526–1537 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nishiyama Y et al. HIF-1α induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J. Hepatol 56, 441–447 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Ochiai D et al. Disruption of HIF-1α in hepatocytes impairs glucose metabolism in diet-induced obesity mice. Biochem. Biophys. Res. Commun 415, 445–449 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scott CH et al. Hepatic aryl hydrocarbon receptor nuclear translocator (ARNT) regulates metabolism in mice. PLOS One 12, e0186543 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ouyang X et al. Digoxin suppresses pyruvate kinase M2-promoted HIF-1α transactivation in steatohepatitis. Cell Metab. 27, 339–350.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee TY, Leu YL & Wen CK Modulation of HIF-1α and STAT3 signaling contributes to anti-angiogenic effect of YC-1 in mice with liver fibrosis. Oncotarget 8, 86206–86216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hwang S et al. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J. Biol. Chem 292, 9382–9393 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramakrishnan SK & Shah YM A central role for hypoxia-inducible factor (HIF)-2α in hepatic glucose homeostasis. Nutr. Healthy Aging 4, 207–216 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rankin EB et al. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol. Cell. Biol 29, 4527–4538 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rahtu-Korpela L et al. Hypoxia-inducible factor prolyl 4-hydroxylase-2 inhibition protects against development of atherosclerosis. Arterioscler. Thromb. Vasc. Biol 36, 608–617 (2016). [DOI] [PubMed] [Google Scholar]
- 76.Qu A et al. Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology 54, 472–483 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li D et al. Hepatic hypoxia-inducible factors inhibit PPARα expression to exacerbate acetaminophen induced oxidative stress and hepatotoxicity. Free Radic. Biol. Med 110, 102–116 (2017). [DOI] [PubMed] [Google Scholar]
- 78.Patterson AD, Shah YM, Matsubara T, Krausz KW & Gonzalez FJ Peroxisome proliferator-activated receptor α induction of uncoupling protein 2 protects against acetaminophen-induced liver toxicity. Hepatology 56, 281–290 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kersten S Angiopoietin-like 3 in lipoprotein metabolism. Nat. Rev. Endocrinol 13, 731–739 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Morello E et al. Hypoxia-inducible factor 2α drives nonalcoholic fatty liver progression by triggering hepatocyte release of histidine-rich glycoprotein. Hepatology 67, 2196–2214 (2018). [DOI] [PubMed] [Google Scholar]
- 81.Bartneck M et al. Histidine-rich glycoprotein promotes macrophage activation and inflammation in chronic liver disease. Hepatology 63, 1310–1324 (2016). [DOI] [PubMed] [Google Scholar]
- 82.Ramakrishnan SK et al. HIF2α Is an essential molecular brake for postprandial hepatic glucagon response independent of insulin signaling. Cell Metab. 23, 505–516 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taniguchi CM et al. Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat. Med 19, 1325–1330 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wei K et al. A liver Hif-2α-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat. Med 19, 1331–1337 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Singh V et al. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. CellMetab. 22, 983–996 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang C et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest 125, 386–402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez FJ, Jiang C & Patterson AD An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 51, 845–859 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie C et al. Activation of intestinal hypoxia-inducible factor 2α during obesity contributes to hepatic steatosis. Nat. Med 23, 1298–1308 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walter KM et al. Hif-2α promotes degradation of mammalian peroxisomes by selective autophagy. Cell Metab. 20, 882–897 (2014). [DOI] [PubMed] [Google Scholar]
- 90.Schonenberger MJ, Krek W & Kovacs WJ EPAS1/HIF-2α is a driver of mammalian pexophagy. Autophagy 11, 967–969 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rahtu-Korpela L et al. HIF prolyl 4-hydroxylase-2 inhibition improves glucose and lipid metabolism and protects against obesity and metabolic dysfunction. Diabetes 63, 3324–3333 (2014). [DOI] [PubMed] [Google Scholar]
- 92.Garcia-Martin R et al. Adipocyte-specific hypoxia-inducible factor 2α deficiency exacerbates obesity-induced brown adipose tissue dysfunction and metabolic dysregulation. Mol. Cell. Biol 36, 376–393 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park J et al. VEGF-A-expressing adipose tissue shows rapid beiging and enhanced survival after transplantation and confers IL-4-Iidependent metabolic improvements. Diabetes 66, 1479–1490 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim KH et al. Intermittent fasting promotes adipose thermogenesis and metabolic homeostasis via VEGF-mediated alternative activation of macrophage. Cell Res. 27, 1309–1326 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiu Y et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takeda N et al. Differential activation and antagonistic function of HIF-α isoforms in macrophages are essential for NO homeostasis. Genes Dev. 24, 491–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dehn S et al. HIF-2α in resting macrophages tempers mitochondrial reactive oxygen species to selectively repress MARCO-dependent phagocytosis. J. Immunol 197, 3639–3649 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Semenza GL & Prabhakar NR The role of hypoxia-inducible factors in carotid body (patho) physiology. J. Physiol 10.1113/JP275696 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yuan G et al. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J. Cell. Physiol 226, 2925–2933 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nanduri J et al. Intermittent hypoxia degrades HIF-2α via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc. Natl Acad. Sci. USA 106, 1199–1204 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mastrogiannaki M et al. HIF-2α, but not HIF-1α, promotes iron absorption in mice. J. Clin. Invest 119, 1159–1166 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pinhas-Hamiel O et al. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int. J. Obes. Relat. Metab. Disord 27, 416–418 (2003). [DOI] [PubMed] [Google Scholar]
- 103.Shah YM, Matsubara T, Ito S, Yim SH & Gonzalez FJ Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 9, 152–164 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yanoff LB et al. Inflammation and iron deficiency in the hypoferremia of obesity. Int. J. Obes. (Lond.) 31, 1412–1419 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cepeda-Lopez AC et al. Sharply higher rates of iron deficiency in obese Mexican women and children are predicted by obesity-related inflammation rather than by differences in dietary iron intake. Am. J. Clin. Nutr 93, 975–983 (2011). [DOI] [PubMed] [Google Scholar]
- 106.Simcox JA & McClain DA Iron and diabetes risk. Cell Metab. 17, 329–341 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Safran M et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: assessment of an oral agent that stimulates erythropoietin production. Proc. Natl Acad. Sci. USA 103, 105–110 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Scheuermann TH et al. Allosteric inhibition of hypoxia inducible factor-2 with small molecules. Nat. Chem. Biol 9, 271–276 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Miyagi T, Wada T, Yamaguchi K, Hata K & Shiozaki K Plasma membrane-associated sialidase as a crucial regulator of transmembrane signalling. J. Biochem 144, 279–285 (2008). [DOI] [PubMed] [Google Scholar]
- 110.Xie C et al. Metabolic profiling of the novel HIF2α inhibitor PT2385 in vivo and in vitro. Drug Metab. Dispos 46, 336–345 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chavez JA & Summers SA A ceramide-centric view of insulin resistance. Cell Metab. 15, 585–594 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Chaurasia B et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 24, 820–834 (2016). [DOI] [PubMed] [Google Scholar]
- 113.Summers SA & Goodpaster BH CrossTalk proposal: Intramyocellular ceramide accumulation does modulate insulin resistance. J. Physiol 594, 3167–3170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Summers SA Could ceramides become the new cholesterol? Cell Metab. 27, 276–280 (2018). [DOI] [PubMed] [Google Scholar]
- 115.Matsubara T, Li F & Gonzalez FJ FXR signaling in the enterohepatic system. Mol. Cell Endocrinol 368, 17–29 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Khavandgar Z & Murshed M Sphingolipid metabolism and its role in the skeletal tissues. Cell. Mol. Life Sci 72, 959–969 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li F et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun 4, 2384 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Xie C et al. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66, 613–626 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tomita S, Sinal CJ, Yim SH & Gonzalez FJ Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1α. Mol. Endocrinol 14, 1674–1681 (2000). [DOI] [PubMed] [Google Scholar]
- 120.Wang XL et al. Ablation of ARNT/HIF1β in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 9, 428–439 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Girer NG, Murray IA, Omiecinski CJ & Perdew GH Hepatic aryl hydrocarbon receptor attenuates fibroblast growth factor 21 expression. J. Biol. Chem 291, 15378–15387 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bhattarai D, Xu X & Lee K Hypoxia-inducible factor-1 (HIF-1) inhibitors from the last decade: a “structure-activity relationship” perspective. Med. Res. Rev 38, 1404–1442 (2017). [DOI] [PubMed] [Google Scholar]
- 123.Lee K et al. Identification of malate dehydrogenase 2 as a target protein of the HIF-1 inhibitor LW6 using chemical probes. Angew. Chem. Int. Ed Engl 52, 10286–10289 (2013). [DOI] [PubMed] [Google Scholar]
- 124.Ban HS et al. A novel malate dehydrogenase 2 inhibitor suppresses hypoxia-inducible factor-1 by regulating mitochondrial respiration. PLOS One 11, e0162568 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Eleftheriadis T, Pissas G, Antoniadi G, Liakopoulos V & Stefanidis I Malate dehydrogenase-2 inhibitor LW6 promotes metabolic adaptations and reduces proliferation and apoptosis in activated human T cells. Exp. Ther. Med 10, 1959–1966 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yin S et al. Arylsulfonamide KCN1 inhibits in vivo glioma growth and interferes with HIF signaling by disrupting HIF-1α interaction with cofactors p300/CBP Clin. Cancer Res. 18, 6623–6633 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang W et al. KCN1, a novel synthetic sulfonamide anticancer agent: in vitro and in vivo anti-pancreatic cancer activities and preclinical pharmacology. PLOS One 7, e44883 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhao T et al. HIF-1-mediated metabolic reprogramming reduces ROS levels and facilitates the metastatic colonization of cancers in lungs. Sci. Rep 4, 3793 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tsui L, Chang SF, Huang HP, Fong TH & Wang IJ YC-1 induces lipid droplet formation in RAW 264.7 macrophages. J. Biomed. Sci 23, 2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chin CH et al. YC-1, a potent antithrombotic agent, induces lipolysis through the PKA pathway in rat visceral fat cells. Eur. J. Pharmacol 689, 1–7 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Hung CC & Liou HH YC-1, a novel potential anticancer agent, inhibit multidrug-resistant protein via cGMP-dependent pathway. Invest. New Drugs 29, 1337–1346 (2011). [DOI] [PubMed] [Google Scholar]
- 132.Pugh CW Modulation of the hypoxic response. Adv. Exp. Med. Biol 903, 259–271 (2016). [DOI] [PubMed] [Google Scholar]