Abstract
Aims
There is a need for predictive and surrogate response biomarkers to support treatment with antiangiogenic vascular endothelial growth factor (VEGF) inhibitors. We aimed to identify a minimally‐invasive biomarker predicting benefit from cediranib pretreatment or early during treatment in patients with recurrent or metastatic cervical cancer.
Methods
Blood samples were collected before treatment, during treatment and upon disease progression where appropriate from patients enrolled in CIRCCa, a randomised phase II trial of carboplatin and paclitaxel with or without cediranib. Plasma concentrations of VEGF‐A, VEGF‐receptor 2, Ang1 and Tie2 were measured using multiplex enzyme‐linked immunosorbent assay. Pretreatment and temporal changes of the biomarkers were investigated using proportional hazard regression and unsupervised clustering analysis.
Results
Samples (n = 556) from 52 patients were analysed. VEGF‐receptor 2 (P = .0006) and Tie2 (P = .04) were downregulated following cediranib, while VEGF‐A (P = .0025) was upregulated. High Eastern Cooperative Oncology Group performance status (P = .02, hazard ratio [HR] = 2.15, 95% confidence interval [CI] 1.13–4.09) and low pretreatment Tie2 concentrations (P = .003, HR = 0.57, 95%CI 0.39–0.83) were independent prognostic factors associated with reduced progression‐free survival. Two patterns of changes in VEGF‐A following cediranib were identified. Patients with elevated VEGF‐A in the first 3 treatment cycles, regardless of magnitude, had reduced progression‐free survival in the placebo arm but improved survival with the addition of cediranib (P = .019, HR = 0.13, 95% CI 0.02–0.71).
Conclusion
Patterns of early elevation in plasma VEGF‐A should be studied further as a potential biomarker to predict treatment benefit from cediranib.
Keywords: angiogenesis, cediranib, cervical cancer, CIRCCa trial, response biomarker, vascular endothelial growth factor
What is already known about this subject
Adding antiangiogenic therapies to conventional chemotherapy improves outcomes in recurrent/metastatic cervical cancer.
Biomarkers are in need to guide optimal use of antiangiogenic therapies.
What this study adds
The pattern of early changes in vascular endothelial growth factor‐A during cediranib treatment predicts benefit on patient survival.
Vascular endothelial growth factor‐A should be used as a surrogate response biomarker to guide optimal use of cediranib.
1. INTRODUCTION
Cervical cancer is the fourth most common cancer in women worldwide,1 and in the UK approximately 3200 women receive the diagnosis each year. The incidence of cervical cancer has decreased by almost a quarter in the UK since the 1990s, although a slight increase (4%) was observed in the last decade.2 Overall 5‐year survival rates are ~65%: there is a dramatic difference with disease stage with 95% year survival in stage 0–1 patients and only ~5% for stage 4 patients.2 Treatment options for patients who develop metastatic disease or relapse within the irradiated pelvis are limited.
High tumour angiogenesis3 and high vascular endothelial growth factor (VEGF) expression4, 5 are adverse prognostic factors for cervical cancer patients. The GOG240 trial showed a survival benefit for adding bevacizumab to paclitaxel‐topotecan or paclitaxel‐cisplatin chemotherapy6 with a recent meta‐analysis of 19 trials showing paclitaxel‐cisplatin‐bevacizumab had the highest probability of efficacy.7 The UK CIRCCa trial8 showed that in patients with metastatic or relapsed cervical cancer the addition of the VEGF pathway inhibitor cediranib to cisplatin and paclitaxel improved progression‐free survival (PFS; P = .032, hazard ratio [HR] = 0.58). Adding antiangiogenic therapies to conventional chemotherapy is associated with increased toxicity8 and cost9 and not all patients benefit. Thus, there is a need to identify biomarkers that predict or monitor the benefit conferred by VEGF inhibitors.
Several candidate biomarkers have been proposed previously. For instance, early phase clinical trial evaluation of cediranib detected pharmacodynamic changes in VEGF receptor 2 (VEGF‐R2) concentrations over the first 4 weeks of treatment.10 This finding was corroborated in CIRCCa,8 which included an assessment of changes in VEGF‐R2 concentration 28 days after treatment as a secondary endpoint of the trial. The trajectory of VEGF‐R2, however, has not been investigated. We and others have reported on the clinical significance of angiopoietin pathway components (Ang1, Ang2 and Tie2) in patients receiving the VEGF inhibitor bevacizumab treatment for ovarian and colorectal cancer.11, 12, 13 On the basis of these previous studies, VEGF‐R2, VEGF‐A, Ang1 and Tie2 were selected for evaluation in this study. The primary aim was to characterise the trajectories of these angiogenesis‐associated plasma biomarkers during treatment and to determine the clinical significance of the biomarkers in patients receiving cediranib–cytotoxic therapy combinations for cervical cancer.
2. METHODS
2.1. Clinical trial protocol
Supplementary Figure 1 shows the CONSORT diagram for the translational study. Blood samples were taken from each patient twice before treatment, on days 1, 8 and 15 of the first cycle of chemotherapy, on days 1 and 8 of the second cycle of chemotherapy, at the beginning of each following cycle of chemotherapy and every 2 months after chemotherapy. The samples were collected in lithium–heparin tubes. Blood samples were processed to obtain plasma using an established Standard Operating Procedure and plasma aliquoted and stored at –80°C. Anonymised samples were shipped in batches to the central sample bank managed by the Translational Radiobiology Group, Division of Cancer Sciences at the University of Manchester, UK where they were stored at –80°C.
2.2. Outcome measures
The primary outcome of interest was progression‐free survival (PFS), defined as the interval from the date of randomisation to the date of disease progression or death, whichever occurred first. Patients who were alive without disease progression at the end of the study were censored at the date of their last assessment. Disease progression was defined clinically or by Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.14, 15 Secondary endpoints included overall survival (OS), defined as the interval from the date of randomisation to the date of death.
2.3. Enzyme‐linked immunosorbent assay
Multiplex enzyme‐linked immunosorbent assays (ELISAs) were used to measure the concentrations of the circulating biomarkers Ang1, Tie2, VEGF‐A and VEGF‐R2 in patient plasma samples. The ELISAs were performed using SearchLight chemiluminescent arrays and a SearchLight Plus charged couple device imaging system (Aushon BioSystems, Billerica, MA, USA). VEGF‐R2, VEGF‐A, Ang1 and Tie2 assays were performed as a 2‐plex. All assays were performed in the Clinical and Experimental Pharmacology Group laboratories, Cancer Research UK Manchester Institute in a Good Clinical Practice compliant facility. In‐house validation experiments for the analytes used in the assays are described elsewhere.11, 16
2.4. Data analysis
Time‐dependent changes in concentrations of each circulating biomarker, measured as log2 ratios relative to pretreatment concentrations, were plotted against linear time as well as the percentage time that elapsed between the start of treatment and the date of progression or censoring (%PFS; time elapsed divided by PFS interval). The concept of percentage time is a method designed to address variation in patient survival.12 Missing data were interpolated for plotting.
The pretreatment prognostic significance of each candidate biomarker was assessed by including the biomarker as a sole covariate in a proportional hazards model for survival, while its predictive significance was assessed by including an additional term for treatment‐biomarker interaction in the model. In each case, we tested for the corresponding null hypothesis of no effect via a Wald test. Assumption of proportionality was verified based on Schoenfeld residuals.17 A plot of the Martingale residuals from each marker specific analysis was examined for evidence of nonlinearity in the biomarker–hazard relationship.18 The covariate was subjected to appropriate transformation, such as log2 transformation, or dichotomisation by its median if the above assumptions were violated. Biomarkers with P‐values <.2 in the univariate analysis were selected for subsequent multivariate proportional hazard regression analysis. A backward stepwise method was applied in the multivariate analysis to identify the optimum subset of biomarkers that were associated with survival. Due to the limited number of patients, interactions of biomarkers were not explored except for Ang1 and Tie2, for which an interaction was identified in an earlier study.11
Acute changes of biomarkers were defined as the average of biomarker levels from day 15, first treatment cycle to day 1, third treatment cycle. This approach smooths out the large variation of biomarkers observed in the first 2 cycles of treatment, which typically introduce artefact in cross‐sectional analysis. The acute changes were examined for their prognostic and predictive significance for PFS and OS, using the proportional hazard regression analysis described above. There was no progression event during the first 2 cycles of treatment; therefore, no time‐dependent bias was introduced.
An unsupervised hierarchical clustering analysis was applied to the longitudinal data of biomarkers from patients treated with cediranib to explore heterogeneity within biomarkers. Based on correlation, the method identifies patients with similar biomarker changes during treatment without predefined criteria or cut‐offs.
The analyses were carried out following the REMARK guideline19 and were implemented using Matlab R2017a20 and R 3.4.1.21
2.5. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY,22 and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18.23
3. RESULTS
3.1. Descriptive analysis
Sixty‐nine patients with recurrent or metastatic cervical cancer were recruited into the CIRCCa trial, of whom 61 consented to give blood samples for translational study and 52 were included in this longitudinal biomarker study (Supplementary Figure 1). Nine patients were discarded from the biomarker study because insufficient longitudinal samples were available. The demographic characteristics of the 52 patients are shown in Table 1.
Table 1.
Patient characteristics
| Control (placebo) | Research (cediranib) | |
|---|---|---|
| Patient number | 27 | 25 |
| Age (y) | 44 (25–64) | 42 (24–77) |
| ECOG performance status | ||
| 0 | 14 (19%) | 14 (27%) |
| 1 | 13 (33%) | 11 (21%) |
| Disease site | ||
| Local relapse only | 0 (0%) | 6 (12%) |
| Extra‐pelvic metastases only | 9 (17%) | 6 (12%) |
| Local relapse and extrapelvic metastases | 18 (35%) | 13 (25%) |
| Disease‐free survival after primary therapy | ||
| ≤12 months | 12 (23%) | 14 (27%) |
| >12 months | 15 (29%) | 11 (21%) |
| Histology | ||
| Squamous | 19 (37%) | 17 (33%) |
| Adenocarcinoma | 7 (13%) | 5 (10%) |
| Mixed | 1 (2%) | 2 (4%) |
| Other | 0 (0%) | 1 (2%) |
| Degree of differentiation | ||
| Well | 4 (8%) | 1 (2%) |
| Moderate | 10 (19%) | 10 (19%) |
| Poor | 9 (17%) | 10 (19%) |
| Unknown | 4 (8%) | 4 (8%) |
| Previous lines of treatment | ||
| 0 | 3 (6%) | 2 (4%) |
| 1 | 24 (46%) | 23 (44%) |
| Metastatic sites | ||
| Local | 8 (15%) | 7 (13%) |
| Lung | 8 (15%) | 6 (12%) |
| Liver | 4 (8%) | 3 (6%) |
| Para‐aortic | 10 (19%) | 7 (13%) |
ECOG, Eastern Cooperative Oncology Group
The number of samples collected for each patient ranged from 5 to 15, with a mean ± standard deviation of 11.0 ± 2.9 samples from the 27 patients in the control arm and 10.5 ± 2.7 samples from the 25 patients in the research arm. In total, 2224 biomarker data points were available for analysis. The variation of biomarker concentrations prior to treatment are illustrated in Supplementary Figure 2, where Ang1 demonstrated largest pretreatment variation. During treatment, the plasma concentrations of biomarkers are summarised in Supplementary Table 1 and Figure 1, and the relative changes of biomarkers were plotted in Figure 2.
Figure 1.
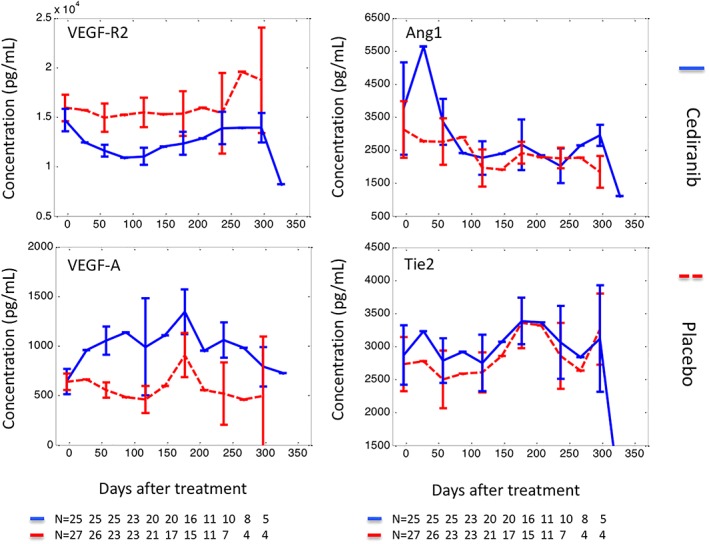
Concentrations of circulating biomarkers during treatment. Biomarker concentrations, measured before treatment, during treatment and up to the point of diagnosis of progressive disease, were plotted against treatment time. The data are presented as median ± standard error, with solid lines representing biomarkers measured in the cediranib arm and dashed lines biomarkers measured in the control arm
Figure 2.
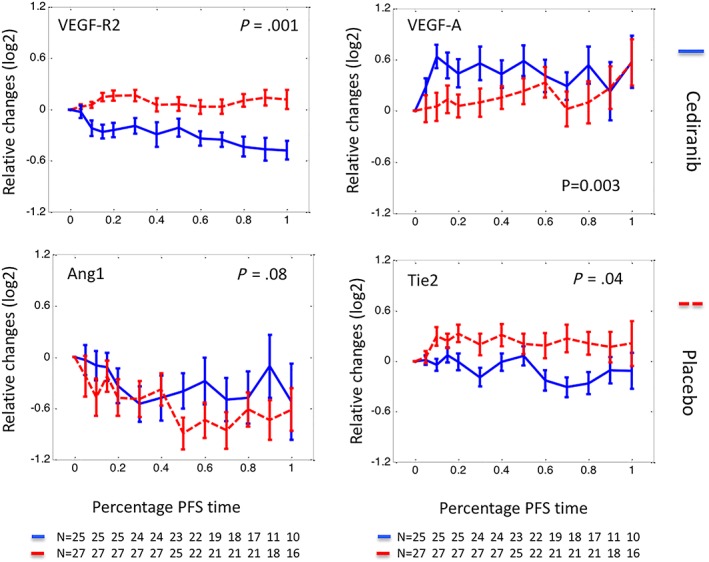
Relative changes of circulating biomarkers normalised by progression‐free survival (PFS). Relative changes of biomarkers were plotted against the percentage of PFS interval (%PFS). Here relative changes of a biomarker at a given time were defined as a log2 transformed ratio between biomarker concentration measured at the time and that measured prior to‐treatment. Plotting in relative changes reduced the pretreatment biomarker variation between different arms, most notably in Ang1 and Tie2. The %PFS interval was defined as elapsed treatment time divided by the length of PFS. A %PFS of 0 represents the start of treatment and 100 represents the diagnosis of PD or censoring. Using a %PFS scale allows biomarkers from patients with different PFS intervals to be compared, especially at the later phase of treatment. The data are presented as median ± standard error, with solid lines representing biomarkers measured in the cediranib arm and dashed lines biomarkers measured in the control arm
3.2. The clinical significance of pretreatment plasma biomarker concentrations
Prognostic and predictive significance of biomarkers for PFS and OS were investigated based on Cox proportional hazard regression analysis. Pretreatment univariate analysis (Supplementary Table 2) and subsequent multivariate analyses (Table 2) are reported. High Eastern Cooperative Oncology Group performance status (P = .02, HR = 2.15, 95% confidence interval [CI] 1.13–4.09) and low plasma Tie2 (P = .003, HR = 0.57, 95%CI 0.39–0.83) were adverse prognostic factors for PFS (Table 2). VEGF‐A had a borderline predictive significance (P = .06, HR = 0.90, 95% CI 0.81–1.01, Table 2), indicating that patients with higher pretreatment concentrations of VEGF‐A had reduced PFS when treated with standard chemotherapy, whereas those treated with the cediranib combination were not affected. Prior to treatment, no biomarker had prognostic or predictive significance for OS (data not shown). The interaction of Ang1 and Tie2 had no predictive significance (P = .63).
Table 2.
Pretreatment multivariate analysis of prognostic and predictive significance
| Biomarker name | P‐value | Hazard ratio | 95% confidence interval of hazard ratio |
|---|---|---|---|
| Prognostic model | |||
| ECOG PS | .02 | 2.15 | 1.13–4.09 |
| Tie2 * | .003 | 0.57 | 0.39–0.83 |
| Predictive model | |||
| ECOG PS | .03 | 2.09 | 1.07–4.07 |
| Tie2 * | .01 | 0.62 | 0.43–0.90 |
| VEGF‐A ** | .01 | 1.11 | 1.02–1.20 |
| Treatment | .71 | 1.23 | 0.42–3.60 |
| VEGF‐A: treatment *** | .06 | 0.90 | 0.81–1.01 |
log2 transformed due to non‐linearity.
VEGF‐A divided by 100.
interaction between a biomarker and treatment, representing the predictive significance of the biomarker.
ECOG PS, Eastern Cooperative Oncology Group performance status; VEGF, vascular endothelial growth factor
3.3. Correlation analysis
There were weak or no correlations between the pretreatment plasma concentrations of circulating biomarkers (Supplementary Table 3). The strongest correlations were VEGF‐A/Ang1 (r = 0.34) and VEGF‐A/VEGF‐R2 (r = 0.25). During the first 3 cycles of treatment, a considerable reduction in correlation between VEGF‐A and VEGF‐R2 was observed in the cediranib arm but not in the placebo arm (reduction of 0.5 vs 0.04 respectively). The lack of correlation between Ang1 and Tie2 remained unchanged after the first 3 cycles of treatment in the cediranib arm.
3.4. Biomarker trajectories during treatment
As illustrated in Figure 2, the concentration of circulating VEGF‐R2 reduced significantly following cediranib (P = .001) but increased in the placebo arm, representing the expected pharmacodynamic changes of VEGF‐R2. Most of the reduction occurred during the first cycle of treatment although median VEGF‐R2 concentrations reached the nadir at the end of cycle 3 (day 65). Acute changes of the biomarkers (average changes in the first 3 cycles of treatment, see methods) were examined and changes of VEGF‐R2 predicted PFS (P = .04, HR = 8.91, Supplementary Table 4). However, while elevated VEGF‐R2 was associated with improved PFS in the placebo arm, it did not predict PFS in the cediranib arm. Acute changes of other biomarkers were not associated with PFS or OS.
The median plasma concentration of VEGF‐A elevated following cediranib (P = .003, Figure 2) but unsupervised clustering analysis revealed that this was heterogeneous. Significant and prolonged elevation occurred in approximately 2/3 of patients, whereas moderate and transient increases were seen in the other patients, resembling the VEGF‐A trajectories seen in the placebo arm (Figure 3). To understand the observed heterogeneity, VEGF‐R2 of the same clusters were plotted where little difference was found (Figure 3), indicating that the observed VEGF‐A heterogeneity was not caused by VEGF‐R2.
Figure 3.
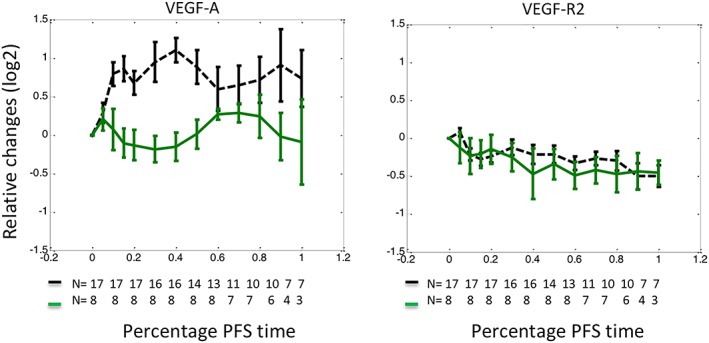
Heterogeneity of vascular endothelial growth factor (VEGF)‐A response to cediranib. An unsupervised clustering analysis dichotomised patients treated with cediranib into 2 cohorts with distinct patterns of VEGF‐A changes. Patients represented by the green solid curve demonstrated moderate changes resembling those treated with standard chemotherapy. In contrast, patients illustrated with the black dashed curve demonstrated significant elevation following cediranib. In these figures, relative changes of biomarkers were plotted against the percentage of progression‐free survival interval (%PFS). Relative changes of a biomarker at a given time were defined as a log2 transformed ratio between biomarker concentration measured at the time and that measured prior to‐treatment. The %PFS interval was defined as elapsed treatment time divided by the length of PFS. VEGF‐receptor 2 (R2) demonstrated little difference on the same clusters of patients, indicating that the difference in VEGF‐A elevation was not caused by VEGF‐R2. The data are presented as median ± standard error
The VEGF‐A heterogeneity seen in the cediranib arm can be translated into rules that dichotomise patients in both treatment arms. Based on a linear regression of VEGF‐A plasma concentrations measured in the first–third cycles of treatment, patients with positive slopes and positive average concentrations were considered to have elevating VEGF‐A trajectories, whereas the others were considered to have stable/reducing VEGF‐A trajectories. The prognostic and predictive significance of the rules were examined with identified prognostic and predictive factors taken into account. While patients with elevated VEGF‐A demonstrated reduced PFS in the placebo arm, PFS was significantly improved for these patients in the cediranib arm (P = .02, HR = 0.13, 95%CI 0.02–0.71, Table 3). PFS stratified by trial arms and VEGF‐A elevation are plotted in Figure 4. A similar trend was observed for OS but was not significant (P = .06, data not shown due to no significant factors being found). Examples of individual patients with elevated or stable/reduced VEGF‐A concentrations can be found in Figure 5, where patients with the longest PFS in the cediranib arm are illustrated.
Table 3.
Patterns of vascular endothelial growth factor (VEGF)‐A changes following treatment predicts benefit from cediranib
| Biomarker name | P‐value | Hazard ratio | 95% CI |
|---|---|---|---|
| ECOG PS | 0.04 | 2.05 | 1.05–3.98 |
| Tie2 * | 0.01 | 0.61 | 0.42–0.89 |
| VEGF‐A pretreatment ** | 0.01 | 1.13 | 1.04–1.23 |
| Treatment | 0.07 | 5.70 | 0.88–37.14 |
| VEGF‐A pretreatment: Treatment *** | 0.01 | 0.83 | 0.71–0.96 |
| VEGF‐A elevation | 0.08 | 2.43 | 0.91–6.51 |
| VEGF‐A elevation: Treatment *** | 0.02 | 0.13 | 0.02–0.71 |
log2 transformed due to non‐linearity.
VEGF‐A divided by 100.
interaction between a biomarker and treatment, representing the predictive significance of the biomarker.
ECOG PS, Eastern Cooperative Oncology Group performance status
Figure 4.
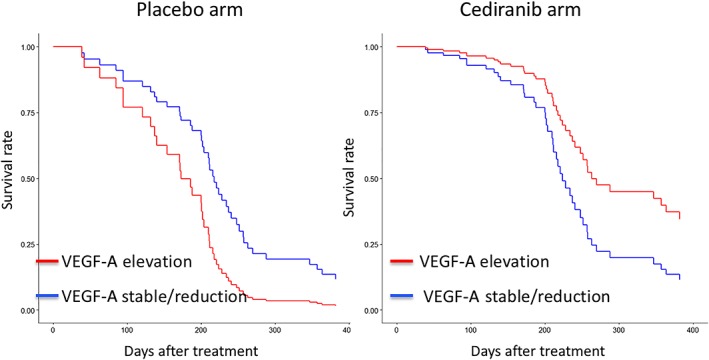
Vascular endothelial growth factor (VEGF)‐A changes predicts benefit from cediranib: survival curves. Progression‐free survival (PFS) of patients with or without elevated VEGF‐A were plotted based on multivariate Cox proportional hazard model. In the placebo arm, patients with elevated VEGF‐A have reduced PFS than those without, whereas in the cediranib arm patients with elevated VEGF‐A demonstrate improved PFS
Figure 5.
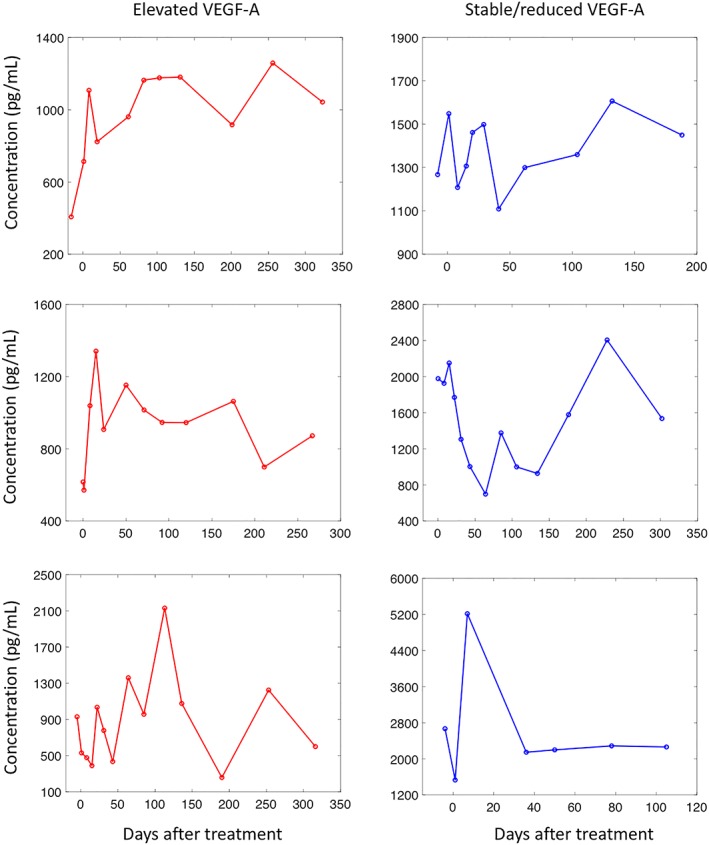
Vascular endothelial growth factor (VEGF)‐A changes predicts benefit from cediranib: Individual examples. VEGF‐A demonstrated 2 distinct patterns of trajectories following cediranib and the patterns can be used to predict treatment benefit from cediranib. Patients with elevated VEGF‐A concentrations in the first 3 cycles of treatment have significantly improved progression‐free survival (PFS) over those with decreased or stable VEGF‐A concentration. Examples of the former were given in the left panel, where patients demonstrated longest PFS (1014, 1042 and 731 respectively), while example of the latter can be found in the right panel, where patients had moderate PFS (270, 364 and 237 respectively)
Circulating Ang1 was downregulated in both arms demonstrating only limited difference. Circulating Tie2 was downregulated in the cediranib arm and moderately upregulated in the placebo arm (P = .04, Figure 2).
4. DISCUSSION
In this study, circulating biomarkers were selected based on their clinical significance in published studies of antiangiogenic therapy and were investigated for their association with PFS and OS of cervical cancer patients treated with cediranib. One of the primary objectives of this study was to identify biomarkers that would predict the treatment benefits associated with cediranib either prior to treatment or at an early stage during treatment. Pretreatment plasma concentrations of VEGF‐A and acute changes of VEGF‐R2 demonstrated predictive significance in multivariate Cox proportional hazard regression analysis. However, further investigation indicated that they only predict survival for patients in the placebo arm but not for those in the cediranib arm. Thus, this finding is in keeping with literature that describes the poor prognostic effect of plasma VEGF‐A,24 with cediranib able to overcome this association presumably through inhibition of the angiogenic effects of VEGF‐A.
Analysis of the biomarkers at pretreatment revealed a correlative relationship similar to that observed in ovarian cancer patients.12 While the correlation between Ang1 and Tie2 increased following bevacizumab in ovarian cancer patients, they were largely unchanged in cervical cancer patients treated with cediranib. At the same time, a reduction in correlation was observed between VEGF‐A and VEGF‐R2 in patients treated with cediranib, but not in those treated with bevacizumab. The difference may be attributed to the different mechanism of VEGF inhibition with bevacizumab and cediranib. When the ligand of the VEGF pathway was inhibited, the plasma concentrations of its receptors were not affected. Whereas, if the receptors of the VEGF pathway were inhibited, plasma VEGF‐A concentration increased, demonstrating an attempt by the tumour to escape antiangiogenic treatment via greater release of VEGF‐A. We also observed that the changes of VEGF‐A concentrations following cediranib are heterogeneous, and the patients with elevated VEGF‐A had significantly improved PFS. Based on the hypothesis that tumours will only activate the negative feedback loop to release more VEGF‐A when the VEGF pathway was fully inhibited, elevation of VEGF‐A can be considered as a response biomarker for cediranib representing the likelihood of patients to benefit from cediranib. Interestingly, it was also discovered that the magnitude of VEGF‐A elevation had little association with treatment benefit from cediranib. It is the pattern of VEGF‐A changes that bestow value in clinic. A similar observation on VEGF‐A was reported in non‐small cell lung cancer patients treated with cediranib.25
The concept of VEGF‐A elevation as a response biomarker for cediranib can be translated as follows for its use in the clinic: benefit from cediranib is expected if a patient demonstrates (i) a positive concentration–time relationship and (ii) a positive average change on VEGF‐A concentration up to day 1 of the third cycles of treatment. Making use of a series of post‐treatment data, such a definition will help remove any artefact from random noise inherent in cross‐sectional analysis.
Pretreatment concentrations of Tie2 and Ang1 predicted benefit from bevacizumab in ovarian cancer patients.11 In this study, however, the predictive significance of the product of Ang1 and Tie2 was not validated here for cediranib in cervix cancer patients. Instead, high pretreatment concentrations of Tie2 were prognostic for improved PFS. This result is in contrast with what observed in metastatic colorectal cancer24 and muscle‐invasive bladder cancer patients,26 where low pretreatment concentrations of Tie2 were associated with improved PFS. On the other hand, it was observed that high tissue concentrations of Tie2‐expressing monocytes, defined by co‐expression of CD14 and Tie2, were associated with prolonged survival in hilar cholangiocarcinoma.27
4.1. Conclusion
Patterns of change in plasma VEGF‐A concentration following treatment predicted benefit from cediranib in cervical cancer patients and should be explored further as a biomarker for selecting patients for antiangiogenic therapies.
COMPETING INTERESTS
G.C.J. receives research funding and has attended advisory boards for AstraZeneca. The other authors have no competing interests to declare.
CONTRIBUTORS
All authors contributed to paper writing. C.Z. analysed the dataset and draft the paper; S.T., J.T. and K.S. performed ELISAs; G.C.J. contributed to data analysis and interpretation of results; P.S., J.P., S.D., K.C., E.M. and D.R. are responsible for the CIRCCa clinical trial; C.D. and C.W. designed and supervised the study.
Supporting information
Table S1. Biomarker plasma concentrations prior to and during treatment
Table S2. Pretreatment univariate prognostic and predictive models for progression‐free survival and overall survival
Table S3. Correlations of biomarkers prior to and during treatment
Table S3a: Correlation at pretreatment.
Table S3b: Correlation after 3 cycles of treatment in the cediranib arm
Table S3c: Correlation after 3 cycles of treatment in the placebo arm
Table S4. Prognostic and predictive significance of acute changes in biomarkers
Figure S1. Study design
Figure S2. Pretreatment variation of plasma biomarkers
ACKNOWLEDGEMENTS
Helen Valentine managed the CIRCCa sample collection.
This work was supported by funding for the CIRCCa trial from Cancer Research UK (C1256/A11416) and an investigator sponsored study collaboration with AstraZeneca. Additional support was received from CRUK funding to the Cancer Research Manchester Centre (C5759/A25254), to the CRUK Manchester Institute (C5759/A27412), and CRUK Manchester Experimental Cancer Medicines Centre (A25146). C.W. is supported by the NIHR Manchester Biomedical Research Centre.
Zhou C, Taylor S, Tugwood J, et al. Dynamics of circulating vascular endothelial growth factor‐A predict benefit from antiangiogenic cediranib in metastatic or recurrent cervical cancer patients. Br J Clin Pharmacol. 2019;85:1781–1789. 10.1111/bcp.13965
The authors confirm that the PI for this paper is Prof. Catharine West and that she had direct clinical responsibility for patients.
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Research UK cervical cancer statistics . Available from: http://www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/cervical‐cancer. 2018.
- 3. Cooper RA, Wilks DP, Logue JP, et al. High tumor angiogenesis is associated with poorer survival in carcinoma of the cervix treated with radiotherapy. Clin Cancer Res. 1998;4(11):2795–2800. [PubMed] [Google Scholar]
- 4. Loncaster JA, Cooper RA, Logue JP, Davidson SE, Hunter RD, West CM. Vascular endothelial growth factor (VEGF) expression is a prognostic factor for radiotherapy outcome in advanced carcinoma of the cervix. Br J Cancer. 2000;83(5):620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gadducci A, Guerrieri ME, Greco C. Tissue biomarkers as prognostic variables of cervical cancer. Crit Rev Oncol Hematol. 2013;86(2):104–129. [DOI] [PubMed] [Google Scholar]
- 6. Tewari KS, Sill MW, Long HJ 3rd, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370(8):734–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rosen VM, Guerra I, McCormack M, et al. Systematic review and network meta‐analysis of bevacizumab plus first‐line Topotecan‐paclitaxel or cisplatin‐paclitaxel versus non‐bevacizumab‐containing therapies in persistent, recurrent, or metastatic cervical cancer. Int J Gynecol Cancer. 2017;27(6):1237–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Symonds RP, Gourley C, Davidson S, et al. Cediranib combined with carboplatin and paclitaxel in patients with metastatic or recurrent cervical cancer (CIRCCa): a randomised, double‐blind, placebo‐controlled phase 2 trial. Lancet Oncol. 2015;16(15):1515–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Minion LE, Bai J, Monk BJ, et al. A Markov model to evaluate cost‐effectiveness of antiangiogenesis therapy using bevacizumab in advanced cervical cancer. Gynecol Oncol. 2015;137(3):490–496. [DOI] [PubMed] [Google Scholar]
- 10. Drevs J, Siegert P, Medinger M, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25(21):3045–3054. [DOI] [PubMed] [Google Scholar]
- 11. Backen A, Renehan AG, Clamp AR, et al. The combination of circulating Ang1 and Tie2 levels predicts progression‐free survival advantage in bevacizumab‐treated patients with ovarian cancer. Clin Cancer Res. 2014;20(17):4549–4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhou C, Clamp A, Backen A, et al. Systematic analysis of circulating soluble angiogenesis‐associated proteins in ICON7 identifies Tie2 as a biomarker of vascular progression on bevacizumab. Br J Cancer. 2016;115(2):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jayson G, Zhou C, Horsley L, et al. Plasma Tie2 is a tumour vascular response biomarker for VEGF inhibitors. Nat Commun. 2018. Accepted;9(1):4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. RECIST . 2010. Available from: http://www.recist.com/index.html.
- 15. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92(3):205–216. [DOI] [PubMed] [Google Scholar]
- 16. Backen AC, Cummings J, Mitchell C, Jayson G, Ward TH, Dive C. 'Fit‐for‐purpose' validation of SearchLight multiplex ELISAs of angiogenesis for clinical trial use. J Immunol Methods. 2009;342(1–2):106–114. [DOI] [PubMed] [Google Scholar]
- 17. Gill R, Schumacher M. A simple test of the proportional hazards assumption. Biometrika. 1987;74(2):289–300. [Google Scholar]
- 18. Therneau TM, Grambsch PM, Fleming TR. Martingale‐based residuals for survival models. Biometrika. 1990;77(1):147–160. [Google Scholar]
- 19. Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (REMARK): explanation and elaboration. PLoS Med. 2012;9(5):e1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. MathWorks T . MATLAB release 2017a. The MathWorks, Inc., Natick, Massachusetts, United States; 2017.
- 21. R Core team: a language and environment for statistical computing. 3.4.1 ed: R Foundation for Statistical Computing, Vienna, Austria.; 2013.
- 22. Harding SD, Sharman JL, Faccenda E, et al. NC‐IUPHAR. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46:D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alexander SPH, Fabbro D, Kelly E, et al. The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol. 2017;174:S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spencer SK, Pommier AJ, Morgan SR, et al. Prognostic/predictive value of 207 serum factors in colorectal cancer treated with cediranib and/or chemotherapy. Br J Cancer. 2013;109(11):2765–2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Addison CL, Ding K, Seymour L, et al. Analysis of serum protein levels of angiogenic factors and their soluble receptors as markers of response to cediranib in the NCIC CTG BR.24 clinical trial. Lung Cancer. 2015;90(2):288–295. [DOI] [PubMed] [Google Scholar]
- 26. Szarvas T, Jager T, Laszlo V, et al. Circulating angiostatin, bFGF, and Tie2/TEK levels and their prognostic impact in bladder cancer. Urology. 2012;80(3):737. e13e18. [DOI] [PubMed] [Google Scholar]
- 27. Atanasov G, Hau HM, Dietel C, et al. Prognostic significance of TIE2‐expressing monocytes in hilar cholangiocarcinoma. J Surg Oncol. 2016;114(1):91–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Biomarker plasma concentrations prior to and during treatment
Table S2. Pretreatment univariate prognostic and predictive models for progression‐free survival and overall survival
Table S3. Correlations of biomarkers prior to and during treatment
Table S3a: Correlation at pretreatment.
Table S3b: Correlation after 3 cycles of treatment in the cediranib arm
Table S3c: Correlation after 3 cycles of treatment in the placebo arm
Table S4. Prognostic and predictive significance of acute changes in biomarkers
Figure S1. Study design
Figure S2. Pretreatment variation of plasma biomarkers


