Abstract
Mehdi Adjeroud, Mohsen Kayal, Christophe Peignon, Matthieu Juncker, Suzanne C. Mills, Ricardo Beldade, and Pascal Dumas (2018) Outbreaks of the coral predator Acanthaster spp., the crown-of-thorns seastar (COTS), cause major coral declines across the Indo-Pacific. However, the processes surrounding the initiation and propagation of COTS outbreaks are still unclear. We observed COTS outbreak abundances on several mid-shelf and inner-barrier reefs in the southern section of the New Caledonian lagoon, a multi-location initiation event that we expected to precede a broader region-wide disturbance. However, reef monitoring over 3 years revealed the highly localized and ephemeral character of these outbreaks. Outbreaks that were observed at four reef locations at the beginning of the survey simply faded away, without any specific management actions such as culling efforts. We also found no distinct reef biotope on which COTS outbreaks originated, although mid-shelf and inner-shelf barrier reefs seem to be favoured. New Caledonia has been exempted from to widespread regional COTS outbreaks, which is surprising given its proximity to the Australian Great Barrier Reef - a reef complex particularly vulnerable to COTS - and Vanuatu - where large-scale outbreaks were recorded during the same period. The availability of coral prey is probably not a limiting factor for the propagation of COTS outbreaks on New Caledonian reefs, given the high abundance and diversity of coral assemblages. Our findings reveal that localized and ephemeral COTS outbreaks can be naturally contained and do not necessarily result in widespread disturbances.
Keywords: Coral reefs, Acanthaster, Predator outbreaks, Monitoring, Spatio-temporal variation, New Caledonia
BACKGROUND
Populations of the coral predator Acanthaster spp., the crown-of-thorns seastar (COTS), often oscillate between extended periods at low density, with individuals scarcely distributed among large reef areas, and episodes of unsustainably high densities commonly termed ‘outbreaks’ (Vine 1970; Endean 1973; Moran et al. 1992; Pratchett et al. 2014 2017). These outbreaks exacerbate reef decline and are responsible for widespread coral mortality in many regions (De’ath et al. 2012; Kayal et al. 2012; Leray et al. 2012; Vercelloni et al. 2017). While COTS populations are natural components of coral reef ecosystems in the Indo- Pacific Ocean, historical observations and recent research suggest changing marine environments are increasing the frequency and intensity of outbreaks, particularly as a consequence of degrading water quality and altered food webs (Sweatman 2008; Fabricius et al. 2010; Brodie and Waterhouse 2012; Uthicke et al. 2015; Kamya et al. 2016). Since the 1960s, extensive coral mortality caused by COTS outbreaks across the Indo- Pacific has raised public awareness and scientific interest (Chesher 1969; Vine 1970; Endean 1973; Pratchett et al. 2014 2017; Kayal and Kayal 2017). These events were also opportunities to improve scientific knowledge on COTS biology and ecology (Houk and Raubani 2010; Sigl et al. 2016), and helped understand the processes driving outbreak dynamics (Kayal et al. 2012; Wooldridge and Brodie 2015; Uthicke et al. 2016), their demise (Mills 2012), and the development of control measures (Rivera-Posada et al. 2014; Moutardier et al. 2015; Boström-Einarsson and Rivera-Posada 2016; Buck et al. 2016). However, the primary processes surrounding the initiation of COTS outbreaks are still unclear (Pratchett et al. 2014). Identifying where COTS outbreaks originate and which reef environments promote their propagation would help coral reef management across the Indo-Pacific.
So far, coral reefs in New Caledonia have been spared from widespread regional COTS outbreaks. This is surprising, given their geographical proximity and ecological similarity to reefs in Australia’s Great Barrier Reef and in the Vanuatu region, two reef systems highly susceptible to COTS outbreaks (Osborne et al. 2011; De’ath et al. 2012; Dumas et al. 2016; Vercelloni et al. 2017). Indeed, New Caledonia’s barrier reef is the second longest continuous coral reef system in the world and is situated less than 1,500 km east of the Great Barrier Reef and ~500 km south of Vanuatu (Andréfouët et al. 2009). Each reef’s size and proximity to the other two in this three-reef system makes it likely that COTS outbreaks would spread within and possible even between the reefs (Moran et al. 1992; Uthicke et al. 2016; Harrison et al. 2017). However, unlike the Great Barrier Reef and Vanuatu, where COTS outbreaks are recurrent drivers of widespread coral decline (Osborne et al. 2011; De’ath et al. 2012; Dumas et al. 2016; Vercelloni et al. 2017), high densities of COTS have only been reported on two occasions on a restricted number of reefs in New Caledonia. In 1983, densities of up to 3.3 ind. per 100 m-2 were recorded at Ilot Maître (Fig. 1), a mid-shelf reef situated ~2 km south-west of the main city, Nouméa (Conand 1983 1984). A second event was reported in 2000 on the same reef, where coral cover declined from ~50% to 4% (Sulu et al. 2002), as well as other locations in New Caledonia’s southwestern and southeastern lagoon, where culling campaigns were undertaken locally (~1,300 seastars removed from Récif Tabu and ~1,000 from Ilot Maître reefs; Fig. 1). Both of these sporadic outbreaks remained confined to the southern lagoon despite the highly connected nature of the reef system (Andréfouët et al. 2009). No further COTS outbreaks were reported in the southwestern lagoon of New Caledonia until 2012. It is highly unlikely that such outbreaks would have gone unnoticed, given the frequent surveys on reef communities conducted by local researchers coupled with the elevated number of reef users in this densely populated area (David et al. 2010; Grenz et al. 2010).
In early 2012, high COTS densities were reported in New Caledonia’s southwestern lagoon by divers and fishermen, as well as by observations performed during routine research monitoring (Quod and Malfait 2016). At this date, no other elevated densities of COTS were reported elsewhere around New Caledonia. In June 2012, we conducted a survey at 19 stations covering all reef habitats, from fringing reefs along the coast of the island to the outer reefs beyond the lagoon, to examine spatial variation in COTS abundance and determine specific reef biotopes more susceptible to outbreaks. These stations were re-surveyed four times until March 2015 to monitor changes in the spatial distribution of COTS abundance and to detect a potential spread of outbreaks. This survey constituted a unique opportunity to investigate the early dynamics of COTS outbreaks in New Caledonia’s reef system, a unique Natural World Heritage (UNESCO) that so far has been exempted from widespread COTS damage.
MATERIALS AND METHODS
Study area
The study area was located in the south- western portion of the main island (‘Grande Terre’) of New Caledonia (Fig. 1) and comprised reefs around Nouméa, the most populated and industrialized city in the country. The southwestern reef complex is composed of four distinct habitats (or ‘reef types’): coastal fringing reefs, mid- shelf reefs, inner-shelf barrier reefs, and outer- reef slopes (Andréfouët et al. 2009; Adjeroud et al. 2010). The southwestern lagoon is primarily exposed to southeasterly trade winds that govern the general direction of surface currents (Jouon et al. 2006). Oligotrophic oceanic waters enter the lagoon via the open southern shelf, flow through the lagoon, and then exit via the passes on the western shelf (Jouon et al. 2006). As hydrodynamic circulation in the study area is generally very active, run-off mainly influences water quality and sediment composition of fringing reefs, particularly those within bays with high water residence time (Le Borgne et al. 2010; Ouillon et al. 2010). Conversely, most mid-shelf and barrier reefs are under oceanic influences (Ouillon et al. 2010).
Sampling
COTS abundances (Acanthaster cf. solaris; Haszprunar et al. 2017) were surveyed at 19 stations, representing all major reef habitats of the southwestern lagoon: coastal fringing reefs, mid- shelf reefs, inner-shelf barrier reefs, and outer-reef slopes (Fig. 1). Sampling was performed using the standardized swim-time method (Chesher 1969; Faure 1989). This commonly used method has the advantage of being easy to implement, thus allowing both effective surveys of multiple reefs and comparisons with previous studies. COTS were counted, during the day (between 09.00 and 12.00 am), by three observers during six replicate 20 min swims (each observer conducting two replicates), covering a total reef area of approximately 10,000 m2 (1 ha) at each station (this area was estimated at the first station that we prospected, Récif Larégnère, and was representative of all stations thereafter). Surveys were carried out by free diving when water depth was ≤ 3 m, and by scuba at deeper stations (stations 18 and 19; Table S1). The swim-transects were carried out parallel to the coast, with 5 m between observers to avoid overlap between replicate swims. As in many previous studies since the 1970s (Vine 1970; Endean 1973; Faure 1989), abundances above the threshold of 40 ind. per 20 min swim were considered indicative of an outbreak, whereas scarce abundances are 1-5 ind. per 20 min swim, and abundances < 1 ind. per 20 min swim represent healthy/non affected stations. COTS abundances were surveyed at all stations in June 2012, June 2013, October 2013, November 2014, and March 2015. The start and end positions of the swim transects were recorded with a GPS to allow for resurveys within the same area. No COTS control efforts, such as culling campaigns, were conducted at any of the study sites during the entire duration of the survey.
In June 2012, the size structure of COTS populations was estimated at the two stations where COTS abundance was higher than the threshold of 40 ind. per 20 min swim (Ilot Maître, and Récif M’Béré). At each of these two stations, the first 30 individuals encountered after the 20 min swim and on the return journey were recorded by scuba (to increase the detectability of small and cryptic individuals), and assigned to one of these four size classes: < 10 cm (i.e., considered as juveniles), 11-20 cm, 21-35 cm, and > 35 cm in diameter, respectively. Additionally, the impacts of COTS aggregations on coral assemblages were estimated at the 19 stations in June 2012, June 2013 and March 2015, by recording the number of coral colonies presenting feeding scars characteristic of recent (< ~3 weeks) COTS predation during the 20 min swims (see Kayal et al. 2012 for further details on the methodology).
Fig. 1.
Fig. 1. Map of the study site showing the location of the 19 sampling stations in New Caledonia’s southern lagoon - including all major reef habitats - where abundance of crown-of-thorns seastar (COTS) populations were monitored. Stations where abundance was higher than the outbreak threshold of 40 ind. per 20 min swim are indicated in red. Stations in yellow are those where 1 to 5 ind. per 20 min swim were recorded, and in green, stations where abundances were always < 1 ind. per 20 min swim. Stations 1 and 2 are located on coastal fringing reefs, stations 3 to 13 on mid-shelf reefs, stations 14 to 17 on the inner-shelf barrier reefs, and stations 18 and 19 on the outer-reef slopes.
Statistical analyses
Spatio-temporal variation in the abundance of COTS and impacted coral colonies was investigated using the non-parametric Kruskal- Wallis test (KW), since data were not normally distributed and variance remained non-homogeneous even after transformation. In complement, the Mann-Whitney U-test (MW) was performed a posteriori for each pairwise comparison (pairs of survey periods for each station, and pairs of stations for each survey period). We used the Bonferroni correction for multiple tests to avoid Type 1 error.
RESULTS
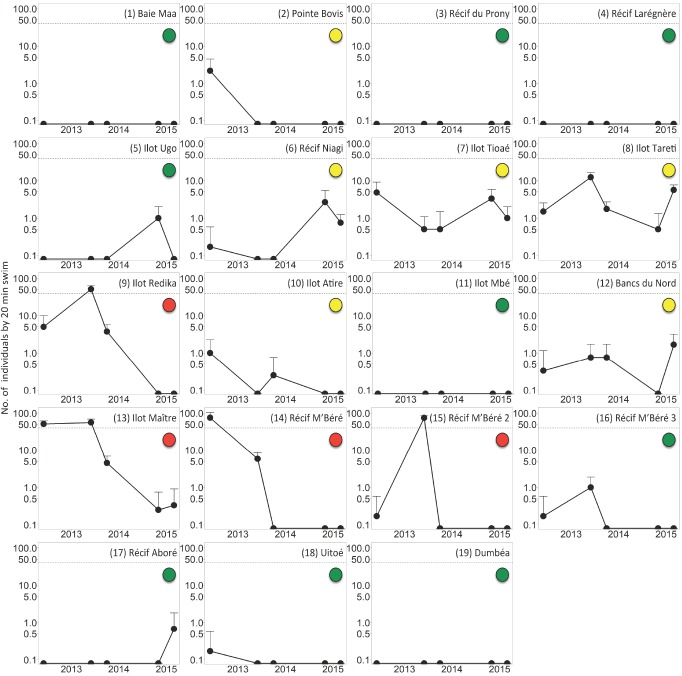
Significant spatial variability was recorded for COTS abundance at each survey period (KW and MW tests, p < 0.05; Tables S2 and S3). Temporal variability was also significant at stations where COTS were recorded, except at stations 7 (Ilot Tioaé), 10 (Ilot Atire), 12 (Bancs du Nord) and 18 (Uitoé; KW and MW tests, p < 0.05; Table S4). COTS observations were common during the survey, yet populations with abundances above the outbreak threshold of 40 ind. per 20 min swim only affected 4 out of the 19 stations (Fig. 1). COTS outbreaks were initially (June 2012) restricted to the two reefs Ilot Maître (station 13: 51.0 ± 11.1 ind. per 20 min swim, mean ± standard deviation) and Récif M’Béré (station 14: 75.1 ± 30.8), a mid- shelf reef situated in the proximity of Nouméa and an inner-shelf barrier reef proximal to Dumbéa Pass, respectively. One year later, an additional outbreak was detected at Ilot Redika (station 9: 51.1 ± 14.5), a mid-shelf reef situated ~30 km southeast of Nouméa, whereas peak COTS abundance initially observed at Récif M’Béré was then detected at Récif M’Béré 2 (station 15: 74.8 ± 11.5) situated 2 km southeast along the continuous inner-shelf barrier reef (Fig. 2). By October 2013, COTS abundances had fallen below the outbreak threshold of 40 ind. per 20 min swim on all reefs and remained so afterwards.
The vast majority of COTS recorded at station Ilot Maître and Récif M’Béré were between 21- 35 cm in diameter (70.0 and 83.3%, respectively), while the other individuals were greater than 35 cm in diameter (30.0 and 16.7%, respectively). No juveniles (< 10 cm in diameter) or small adults (10- 20 cm diameter) were recorded at these stations.
In agreement with our results of COTS abundance, significant spatial variation was recorded for the abundance of feeding scars (KW and MW tests, p < 0.05; Tables S5 and S6). At stations where feeding scars were recorded, a significant temporal variability was recorded, except at stations 2 (Pointe Bovis), 6 (Récif Niagi), 12 (Bancs du Nord), 16 (Récif M’Béré 3), and 18 (Uitoé; KW and MW tests, p < 0.05; Table S7). Feeding scars resulting from recent COTS predation on corals were particularly abundant at the early stages of the outbreaks in June 2012 at Ilot Maître (station 13: 53.3 ± 11.9 scars per 20 min swim, mean ± standard deviation) and M’Béré (station 14: 133.3 ± 21.6; Fig. 3). Lower abundances of < 20 feeding scars per 20 min swim were observed otherwise.
Fig. 2.
Fig. 2. Spatio-temporal dynamics of crown-of-thorns seastar (COTS) abundance in New Caledonia’s southern lagoon. Points represent the mean abundance (log-scale), and error bars the standard error. Horizontal dotted lines indicate the outbreak threshold of 40 ind. per 20 min swim. Color code for stations is as in figure 1.
Fig. 3.
Fig. 3. Recent impacts of crown-of-thorns seastar (COTS) populations on coral assemblages. Variation in the number of coral colonies presenting white feeding scars representative of COTS predation (white colonies recently preyed upon) among stations. Error bars represent the standard error. Color code for stations is as in figure 1.
DISCUSSION
Despite their significant role in coral reef dynamics and health, the processes surrounding the initiation of COTS outbreaks are still elusive (Moran et al. 1992; Pratchett et al. 2014 2017; Miller et al. 2015). Identifying where COTS outbreaks originate and how they propagate can help determine the environmental triggers of these disturbances, detect pre- or early- outbreak populations, and enforce control measures to prevent coral loss. However, the starting locations of COTS outbreaks are often unknown, as outbreaks are usually only noticed once concentrations of adult seastars are already depleting coral communities on large spatial scales (Kayal et al. 2012; Miller et al. 2015; Roche et al. 2015). Our study demonstrates that multiple or recurrent localized outbreaks of COTS do not necessarily result in widespread devastation of coral assemblages, as demonstrated by the overall high and stable coral cover recorded annually between 2003 and 2013 at various monitoring stations around New Caledonia (Quod and Malfait 2016). Our survey in the southern lagoon of New Caledonia discerned four distinct reef locations where COTS outbreaks were found. Ilot Maître, a mid-shelf reef ~2 km proximal to the main city Nouméa was already identified during the two previous outbreaks in the early 1980s and 2000s (Conand 1983; Sulu et al. 2002), and seems to constitute an historical hotspot of COTS outbreaks. Ilot Redika is another mid-shelf reef situated 28 km southeast of Ilot Maître, whereas the Récif M’Béré reef complex (including stations 14, 15 and 16; Fig. 1) constitutes a continuous inner-shelf barrier reef situated 20 km north-west of Ilot Maître. Therefore, there was not one distinct reef biotope on which COTS outbreaks originated, although mid-shelf and inner-shelf barrier reefs seem to be favoured.
Our understanding of COTS biology in relation to its environment is only in its infancy, and the reasons why some reefs constitute nurseries favourable to the development of dense COTS populations and others do not remain elusive (Pratchett et al. 2014 2017; Hall et al. 2017). Recent studies have demonstrated how differences in the coral taxa diet available to seastars influence COTS reproduction and early survival, with potentially regulatory effects on population outbreaks (Buck et al. 2016; Caballes et al. 2016; Dumas et al. 2016; Johansson et al. 2016). Temperature has also been identified as a major regulatory factor in COTS reproduction, and shorter warm-water seasons may influence the ability of seastars to produce population outbreaks at higher latitudes, such as in New Caledonia (Conand 1984; Pratchett et al. 2014). Furthermore, diverse reef organisms have been identified as potential predators of COTS at different stages of its life cycle and these may mitigate outbreaking populations (Cowan et al. 2017, Pratchett et al. 2017). In the southwestern lagoon of New Caledonia, the high diversity and abundance of lethrinids, triggerfishes, and pufferfishes (Mallet et al. 2016), known predators of adult COTS, may also explain the lack of large-scale outbreaks. However, which of these processes, in isolation or combination, determine the apparent resistance of New Caledonian reefs to widespread COTS outbreaks remains an open question that should be addressed in the near future.
COTS outbreaks may result from one recruitment event in a single reef location (Pratchett 2005). On the other hand, as COTS aggregations can migrate for several years and travel over long distances across continuous reefs (Kayal et al. 2012), outbreaks may also result from the accumulation of seastars from consecutive cohorts (Pratchett 2005). Yet, whether outbreaking populations in New Caledonia emerge from a single reef location or propagate from multiple sources is unclear. The lack of empirical data on pre- or early- outbreak COTS dynamics has limited our understanding of key demographic processes that produce outbreaks. However, our survey in the southern lagoon of New Caledonia carried out on early-outbreak COTS enables us to hypothesize how outbreaks propagate. The outbreaks of COTS populations in New Caledonia’s southwestern lagoon were observed on reefs separated by extended (> 20 km) stretches of sand substrate interrupted by patchy reef structures that were unaffected by high COTS densities (Fig. 1), confirming the relatively limited ability of COTS to move between such isolated reef structures (Sigl and Laforsch 2016; Sigl et al. 2016). This suggests that the recent episode of COTS outbreaks in New Caledonia is more likely to have resulted from recruitment of seastars across multiple sites rather than a single adult population migrating. Although limited (Kayal et al. 2017), our data on the size-structure of COTS revealed similarly- sized and relatively large COTS at both Ilot Maître and Récif M’Béré in June 2012, suggesting that outbreaks probably initiated quickly from one spawning/recruitment event at multiple locations. This contrasts with the situation on the west coast of Okinawa, Japan, where multiple, successive recruitment events of < 1 cm diameter COTS juveniles maintain chronically high population densities for decades (Nakamura et al. 2014).
COTS aggregations were restricted in space in New Caledonia’s lagoon despite the absence of control efforts, revealing the highly confined and ephemeral nature of the outbreaks. This outcome was unexpected, given that the relatively continuous and healthy reef system of New Caledonia provides an extensive feeding ground for COTS populations (Adjeroud et al. 2010; Quod and Malfait 2016), which at high densities form migrating consumer fronts that can devastate coral communities over long distances across uninterrupted reefs (Kayal et al. 2012; Silliman et al. 2013). Two major hypotheses have been proposed to explain the processes that bring active COTS outbreaks to an end. Starvation due to food limitation often becomes obvious on geographically isolated systems where the live coral stock can be rapidly depleted (Kayal et al. 2012; Suzuki et al. 2012). When availability in prey corals is not a limiting factor for the maintenance of dense COTS populations, epidemics of infectious pathogens eventually decimate the seastars (Zann et al. 1990; Pratchett 1999; but see Mills 2012). Death by starvation is unlikely in New Caledonia, given the ample availability of live coral at the scale of the southwestern lagoon, with diverse and abundant coral assemblages - including Acropora spp., the favorite prey of COTS (Pratchett 2007; Kayal et al. 2011) - on unaffected reefs in the vicinity of the outbreak populations (sometimes < 500 m apart; M. Adjeroud pers. observation; Quod and Malfait 2016). The epizootic hypothesis is difficult to assess, as the diseased and dying period can be very short and dead seastars disappear within 2-3 days (Pratchett et al. 2014). This difficulty in documenting the causal explanation for the end of outbreaks following epidemics of infectious pathogens is further exacerbated in large systems of small interconnected reefs, such as the southwestern lagoon of New Caledonia.
The temporal synchrony of the COTS outbreaks recorded in New Caledonia, the Great Barrier Reef and Vanuatu suggests that large scale environmental conditions, such as chlorophyll and nutrient concentrations and seawater temperature, likely promoted these outbreaks (Houk and Raubani 2010; Pratchett et al. 2017). The degree of larval connectivity between New Caledonia and the reefs prone to recurrent COTS outbreaks in the nearby region is unknown (Osborne et al. 2011; De’ath et al. 2012; Dumas et al. 2016), but given the potential of COTS larvae to disperse over long geographical distances (Uthicke et al. 2016; Harrison et al. 2017), this remains a compelling hypothesis that should be tested to explain these regional outbreaks.
CONCLUSIONS
This is the third time in 30 years that localized COTS population outbreaks have emerged in New Caledonia’s southern lagoon without producing a region-wide outbreak. Our study demonstrates that multiple seastar outbreaks can be naturally self-contained and do not necessarily result in widespread devastation of coral reefs. We identified four reefs as localities in which COTS outbreaks emerged, among which the Ilot Maître is an historical hotspot for outbreaks. We advocate special attention to the reefs identified as potential sources of COTS outbreaks in the long-term monitoring of New Caledonia’s reef system, a UNESCO Natural World Heritage. The considerable small-scale patchiness and the brevity of these COTS aggregations have important implications for the conservation of New Caledonian reefs, as it makes the detection of affected areas, necessary for the implementation of effective actions, particularly difficult in this vast and highly connected reef system. But on the other hand, such patchy distribution may increase the success of removal actions of quickly identified patches of Acanthaster spp. aggregations.
Supplementary materials
Characteristics of the 19 sampling stations established in the southwest lagoon of New Caledonia. *: Stations at which abundances higher than the outbreak threshold of 40 ind. per 20 min swim were recorded.
Temporal variability of Acanthaster cf. solaris abundance at each station. Mean number of individuals per 20 min swim, with Standard Error in parentheses.
Results of Kruskal-Wallis tests that examined the spatial variability of Acanthaster cf. solaris abundance in each survey period. Mann-Whitney post-hoc multiple comparison tests were performed to determine which pairs of stations showed significant difference (p < 0.05).
Results of Kruskal-Wallis tests that examine the temporal variability of Acanthaster cf. solaris abundance at each station. Mann-Whitney post-hoc multiple comparison tests were performed to determine which pairs of dates showed significant difference (p < 0.05).
Temporal variability of the number of coral colonies presenting white feeding scars (representative of COTS predation) at each station. Mean number of white coral colonies, with Standard Error in parentheses.
Results of Kruskal-Wallis tests that examine the spatial variability of the number of coral colonies presenting white feeding scars representative COTS predation for each survey period. Mann-Whitney post-hoc multiple comparison tests were performed to determine which pairs of stations showed significant difference (p < 0.05).
Results of Kruskal-Wallis tests that examine the temporal variability of the number of coral colonies presenting white feeding scars representative of COTS predation for each station. Mann-Withney post-hoc multiple comparison tests were performed to determine which pairs of dates showed significant difference (p < 0.05).
Acknowledgments
Acknowledgments: We thank Jean De Dinechin, A. Renaud (IRD Nouméa) and G. Lasne (Biocenose Marine SARL) for assistance with the field survey, and P. Naudin, M. Clarque, and S. Tereua for logistic help. The 2012 survey was supported by a grant from OEIL, Nouméa. Surveys of 2013, 2014 and 2015 were conducted within the framework of the research programmes DYNACANTH (funded by the LabEx Corail) and OREANET (funded by the Fonds Pacifique).
Footnotes
Authors’ contributions: MA and MJ designed the study. MA, MK, CP, MJ and PD performed the field work. MA, MK, SCM, RB and PD analyzed the data, and wrote the manuscript. All authors participated in revising the manuscript. All authors read and approved the final manuscript.
Competing interests: MA, MK, CP, MJ, SCM, RB and PD declare that they have no conflict of interest.
Availability of data and materials: The key datasets of the manuscript are presented as additional files (Tables S2 and S5 in Electronic Supplementary Material).
Consent for publication: Not applicable.
Ethics approval consent to participate: Not applicable.
References
- Adjeroud M, Fernandez J M, Carroll A G, Harrison P L, Penin L. Spatial patterns and recruitment processes of coral assemblages among contrasting environmental conditions in the southwestern lagoon of New Caledonia. Mar Pollut Bull. 61:375–386. doi: 10.1016/j.marpolbul.2010.06.015. [DOI] [PubMed] [Google Scholar]
- Andréfouët S, Cabioch G, Flamand B, Pelletier B. A reappraisal of the diversity of geomorphological and genetic processes of New Caledonian coral reefs: a synthesis from optical remote sensing, coring and acoustic multibeam observations. Coral Reefs. 28:691–707. [Google Scholar]
- Boström-Einarsson L, Rivera-Posada J. Controlling outbreaks of the coral-eating crown-of-thorns starfish using a single injection of common household vinegar. Coral Reefs. 35:223–228. [Google Scholar]
- Brodie J, Waterhouse J. A critical review of environmental management of the 'not so Great' Barrier Reef. Estuar Coast Shelf Sci. 104:1–22. [Google Scholar]
- Buck Ace, Gardiner N M, Boström-Einarsson L. Citric acid injections: An accessible and efficient method for controlling outbreaks of the Crown-of-Thorns starfish Acanthaster cf. solaris. Diversity; 2016. 8 [Google Scholar]
- Caballes C F, Pratchett M S, Kerr A M, Rivera-Posada J A. The role of maternal nutrition on oocyte size and quality, with respect to early larval development in the coral-eating starfish, Acanthaster planci. PLoS ONE; 2016. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesher R H. Destruction of the Pacific corals by the sea star Acanthaster planci. Science. 165:280–283. doi: 10.1126/science.165.3890.280. [DOI] [PubMed] [Google Scholar]
- Conand C. Abondance, cycle sexuel et relations biométriques de l'étoile Acanthaster planci en NouvelleCalédonie. Nouméa, New Caledonia; Océanographie): 1983. [Google Scholar]
- Conand C. Distribution, reproductive cycle and morphometric relationships of Acanthaster planci (Echinodermata: Asteroidea) in New Caledonia, western tropical Pacific. Proc Fifth Int Echinoderm Conf. 0:499–506. [Google Scholar]
- Cowan Z L, Pratchett M, Messmer V. Known predators of crown-of-thorns starfish (Acanthaster spp.) and their role in mitigating, if not preventing, population outbreaks. Diversity; 2017. 9 [Google Scholar]
- David G, Leopold M, Dumas P S, Ferraris J, Herrenschmidt J B, Fontenelle G. Integrated coastal zone management perspectives to ensure the sustainability of coral reefs in New Caledonia. Mar Pollut Bull. 61:323–334. doi: 10.1016/j.marpolbul.2010.06.020. [DOI] [PubMed] [Google Scholar]
- De'ath G, Fabricius K E, Sweatman H, Puotinen M. The 27-year decline of coral cover on the Great Barrier Reef and its causes. Proc Natl Acad Sci. 109:17995–17999. doi: 10.1073/pnas.1208909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas P, Moutardier P, Ham J, Kaku R, Gereva S, Lefèvre J, Adjeroud M. Timing within the reproduction cycle modulates the efficiency of village-based crown-of-thorns starfish removal. Biol Conserv. 204:237–246. [Google Scholar]
- Endean R. Population explosion of Acanthaster planci and associate destruction of hermatypic coral in the IndoWest Pacific region. Academic Press. 0:389–438. [Google Scholar]
- Fabricius K E, Okaji K, De ' Three lines of evidence to link outbreaks of the crown-of-thorns seastar Acanthaster planci to the release of larval food limitation. Coral Reefs. 29:593–605. [Google Scholar]
- Faure G. Degradation of coral reefs at Moorea Island (French Polynesia) by Acanthaster planci. J Coast Res. 5:295–305. [Google Scholar]
- Grenz C, Borgne Le, Fichez R, Torréton R. Tropical lagoon multidisciplinary investigations: An overview of the PNEC New Caledonia pilot site. Mar Pollut Bull. 61:267–268. doi: 10.1016/j.marpolbul.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Hall M R, Kocot K M, Baughman K W, Fernandez-Valverde S L, Gauthier Mea, Hatleberg W L, Krishnan A, Mcdougall C, Motti C A, Shoguchi E, Wang T, Xiang X, Zhao M, Bose U, Shinzato C, Hisata K, Fujie M, Kanda M, Cummins S F, Satoh N, Degnan S M, Degnan B M. The crown-ofthorns starfish genome as a guide for biocontrol of this coral reef pest. Nature. 544:231–234. doi: 10.1038/nature22033. [DOI] [PubMed] [Google Scholar]
- Harrison H B, Pratchett M S, Messmer V, Saenz-Agudelo P, Berumen M L. Microsatellites reveal genetic homogeneity among outbreak populations of Crown-ofThorns Starfish (Acanthaster cf. solaris) on Australia's Great Barrier Reef. Diversity; 2017. 9 [Google Scholar]
- Haszprunar G, Vogler C, Wörheide G. Persistent gaps of knowledge for naming and distinguishing multiple species of Crown-of-Thorns Seastar in the Acanthaster planci species complex. Diversity; 2017. 9 [Google Scholar]
- Houk P, Raubani J. Acanthaster planci outbreaks in Vanuatu coincide with ocean productivity, furthering trends throughout the Pacific Ocean. J Oceanogr. 66:435–438. [Google Scholar]
- Johansson C L, Francis D S, Uthicke S. Food preferences of juvenile corallivorous crown-of-thorns (Acanthaster planci) sea stars. Mar Biol. 163:1–7. [Google Scholar]
- Jouon A, Douillet P, Ouillon S, Fraunié P. Calculations of hydrodynamic time parameters in a semi-opened coastal zone using a 3D hydrodynamic model. Cont Shelf Res. 26:1395–1415. [Google Scholar]
- Kamya P Z, Byrne M, Graba-Landry A, Dworjanyn S A. Near-future ocean acidification enhances the feeding rate and development of the herbivorous juveniles of the crown-of-thorns starfish, Acanthaster planci. Coral Reefs. 35:1241–1251. [Google Scholar]
- Kayal M, Kayal E. Colonies of the fire coral Millepora platyphylla constitute scleractinian survival oases during Acanthaster outbreaks in French Polynesia. Mar Biodivers. 47:255–258. [Google Scholar]
- Kayal M, Bosserelle P, Adjeroud M. Biais associated to the detectability of the coral-eating pest crown-of-thorns seastar (Acanthaster planci) and implications for reef management. R Soc Open Sci; 2017. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayal M, Lenihan H S, Pau C, Penin L, Adjeroud M. Associational refuges among corals mediate impacts of a crown-of-thorns starfish Acanthaster planci outbreak. Coral Reefs. 30:827–837. [Google Scholar]
- Kayal M, Vercelloni J, De Loma Lison, Bosserelle T, Chancerelle P, Geoffroy Y, Stievenart S, Michonneau C, Penin F, Planes L, Adjeroud S. Predator crownof-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PloS ONE; 2012. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgne Le, Douillet R, Fichez P, Torréton R. Hydrography and plankton temporal variabilities at different time scales in the southwest lagoon of New Caledonia: a review. Mar Pollut Bull. 61:297–308. doi: 10.1016/j.marpolbul.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Leray M, Beraud M, Anker A, Chancerelle Y, Mills S C. Acanthaster planci outbreak: Decline in coral health, coral size structure modification and consequences for obligate decapod assemblages. PLoS ONE; 2012. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet D, Vigliola L, Wantiez L, Pelletier D. Diurnal temporal patterns of the diversity and the abundance of reef fishes in a branching coral patch in New Caledonia. Austral Ecol. 41:733–744. [Google Scholar]
- Miller I, Sweatman H, Cheal A, Emslie M, Johns K, Jonker M, Osborne K. Origins and implications of a primary crown-of-thorns starfish outbreak in the southern Great Barrier Reef. J Mar Biol. 2015:1–10. [Google Scholar]
- Mills S C. Density-dependent prophylaxis in the coraleating crown-of-thorns seastar, Acanthaster planci. Coral Reefs. 31:603–612. [Google Scholar]
- Moran P J, Baker V J, Christie C A, Miller-Smith B A, De'ath G, Bass D K, Miller I, Thompson A A. Pattern of outbreaks of crown-of-thorns starfish (Acanthaster planci L.) along the Great Barrier Reef since 1966. Aust J Mar Freshwater Res. 43:555–569. [Google Scholar]
- Moutardier G, Gereva S, Mills S C, Adjeroud M, Beldade R, Ham J, Kaku R, Dumas P. Lime juice and vinegar injections as a cheap and natural alternative to control COTS outbreaks. PLoS ONE; 2015. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Okaji K, Higa Y, Yamakawa E, Mitarai S. Spatial and temporal population dynamics of the crown-ofthorns starfish, Acanthaster planci, over a 24-year period along the central west coast of Okinawa Island. Mar Biol. 161:2521–2530. [Google Scholar]
- Osborne K, Dolman A M, Burgess S C, Johns K A. Disturbance and the dynamics of coral cover on the Great Barrier Reef. PLoS ONE; 1995. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouillon S, Douillet P, Lefebvre J P, Gendre Le, Jouon R, Bonneton A, Fernandez P, Chevillon J M, Magand C, Lefèvre O, Hir Le, Laganier P, Dumas R, Marchesiello F, Madani Bel, Andréfouët A, Panché S, Fichez J Y. Circulation and suspended sediment transport in a coral reef lagoon: the south-west lagoon of New Caledonia. Mar Pollut Bull. 61:269–296. doi: 10.1016/j.marpolbul.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Pratchett M S. An infectious disease in crown-of-thorns starfish on the Great Barrier Reef. Coral Reefs; 1999. 18 [Google Scholar]
- Pratchett M S. Dynamics of an outbreak population of Acanthaster planci at Lizard Island, Northern Great Barrier Reef (1995-1999) Coral Reefs. 24:453–462. [Google Scholar]
- Pratchett M S. Feeding preferences of Acanthaster planci (Echinodermata: Asteroidea) under controlled conditions of food availability. Pac Sci. 61:113–120. [Google Scholar]
- Pratchett M S, Caballes C F, Rivera-Posada J A, Sweatman Hpa. Limits to understanding and managing outbreaks of crown-of-thorns starfish (Acanthaster spp. Oceanogr Mar Biol. 52:133–200. [Google Scholar]
- Pratchett M S, Caballes C F, Wilmes J C, Matthews S, Mellin C, Sweatman Hpa, Nadler L E, Brodie J, Thompson C A, Hoey J, Bos A R, Byrne M, Messmer V, Fortunato Sav, Chen Ccm, Buck Ace, Babcock R C, Uthicke S. Thirty years of research on Crown-of-Thorns starfish; 1986. 9 [Google Scholar]
- Quod J P, Malfait G. Etat des récifs coralliens et des écosystèmes associés des Outre-mer français en. 2015.
- Rivera-Posada J, Pratchett M S, Aguilar C, Grand A, Caballes C F. Bile salts and the single-shot lethal injection method for killing crown-of-thorns sea stars (Acanthaster planci) 102:383–390. [Google Scholar]
- Roche R C, Pratchett M S, Carr P, Turner J R, Wagner D, Head C, Sheppard Crc. Localized outbreaks of Acanthaster planci at an isolated and unpopulated reef atoll in the Chagos Archipelago. Mar Biol. 162:1695–1704. [Google Scholar]
- Sigl R, Laforsch C. The influence of water currents on movement patterns on sand in the crown-of-thorns seastar (Acanthaster cf. solaris) Diversity; 2016. 8 [Google Scholar]
- Sigl R, Steibl S, Laforsch C. The role of vision for navigation in the crown-of-thorns seastar. Acanthaster planci. Sci Rep; 2016. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silliman B R, Mccoy M W, Angelini C, Holt R D, Griffin J N, Van De Koppel J. Consumer fronts, global change, and runaway collapse in ecosystems. Ann Rev Ecol Evol Syst. 44:503–538. [Google Scholar]
- Sulu R, Cumming R, Wantiez L, Kumar L, Mulipola A, Lober M, Sauni S, Poulasi T, Pakoa K. Status of coral reefs in the Southwest Pacific: Fiji. 0:181–201. [Google Scholar]
- Suzuki G, Kai S, Yamashita H. Mass stranding of crownof-thorns starfish. Coral Reefs; 2012. 31 [Google Scholar]
- Sweatman H. No-take reserves protect coral reefs from predatory starfish. Curr Biol. 18:598–599. doi: 10.1016/j.cub.2008.05.033. [DOI] [PubMed] [Google Scholar]
- Uthicke S, Logan M, Liddy M, Francis D, Hardy N, Lamare M. Climate change as an unexpected co-factor promoting coral-eating seastar. Acanthaster planci) outbreaks. Sci Rep; 2015. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthicke S, Doyle J, Duggan S, Yasuda N, Mckinnon A D. Outbreak of coral-eating crown-of-thorns creates continuous cloud of larvae over 320 km of the Great Barrier Reef. Sci Rep; 2016. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercelloni J, Caley M J, Mengersen K. Crown-of-thorns starfish undermine the resilience of coral populations on the Great Barrier Reef. Glob Ecol Biogeogr. 26:846–853. [Google Scholar]
- Vine P J. Field and laboratory observations of the Crownof-Thorns Starfish, Acanthatser planci. Nature. 228:341–342. doi: 10.1038/228341a0. [DOI] [PubMed] [Google Scholar]
- Wooldridge S A, Brodie J E. Environmental triggers for primary outbreaks of crown-of-thorns starfish on the Great Barrier Reef. Mar Pollut Bull. 101:805–815. doi: 10.1016/j.marpolbul.2015.08.049. [DOI] [PubMed] [Google Scholar]
- Zann L, Brodie J, Vuki V. History and population dynamics of the COTS starfish Acanthaster planci (L.) in the Suva area. Coral Reefs. 9:135–144. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the 19 sampling stations established in the southwest lagoon of New Caledonia. *: Stations at which abundances higher than the outbreak threshold of 40 ind. per 20 min swim were recorded.
Temporal variability of Acanthaster cf. solaris abundance at each station. Mean number of individuals per 20 min swim, with Standard Error in parentheses.
Results of Kruskal-Wallis tests that examined the spatial variability of Acanthaster cf. solaris abundance in each survey period. Mann-Whitney post-hoc multiple comparison tests were performed to determine which pairs of stations showed significant difference (p < 0.05).
Results of Kruskal-Wallis tests that examine the temporal variability of Acanthaster cf. solaris abundance at each station. Mann-Whitney post-hoc multiple comparison tests were performed to determine which pairs of dates showed significant difference (p < 0.05).
Temporal variability of the number of coral colonies presenting white feeding scars (representative of COTS predation) at each station. Mean number of white coral colonies, with Standard Error in parentheses.
Results of Kruskal-Wallis tests that examine the spatial variability of the number of coral colonies presenting white feeding scars representative COTS predation for each survey period. Mann-Whitney post-hoc multiple comparison tests were performed to determine which pairs of stations showed significant difference (p < 0.05).
Results of Kruskal-Wallis tests that examine the temporal variability of the number of coral colonies presenting white feeding scars representative of COTS predation for each station. Mann-Withney post-hoc multiple comparison tests were performed to determine which pairs of dates showed significant difference (p < 0.05).