Abstract
Clear cell renal cell carcinoma (ccRCC) is the most common type of kidney cancer, comprising approximately 75% of all kidney tumors. Recent the Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC) studies have significantly advanced the molecular characterization of RCC and facilitated the development of targeted therapies. Such advances have improved the median survival of patients with advanced disease from less than 10 months prior to 2004 to 30 months by 2011. However, approximately 30% of localized ccRCC patients will nevertheless develop recurrence or metastasis after surgical resection of their tumor. Therefore, it is critical to further analyze potential tumor-associated proteins and their profiles during disease progression. Over the past decade, tremendous effort has been focused on the study of molecular pathways, including genomics, transcriptomics, and proteomics in order to identify potential molecular biomarkers, as well as to facilitate early detection, monitor tumor progression and uncover potentially therapeutic targets. In this review, we focus on recent advances in the proteomic analysis of ccRCC, current strategies and challenges, and perspectives in the field. This insight will highlight the discovery of tumor-associated proteins, and their potential clinical impact on personalized precision-based care in ccRCC.
Keywords: Proteomics, Clear cell renal cell carcinoma (ccRCC), Protein profiling, Clinical application, Personalized medicine
1. Introduction
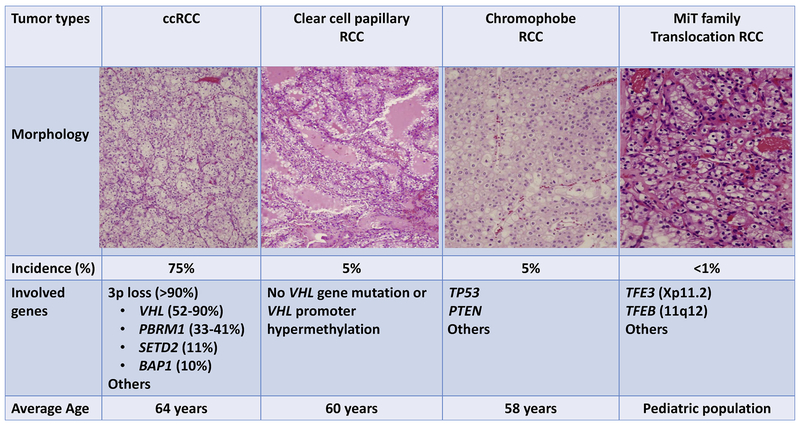
Renal cell carcinoma (RCC) is the third most common genitourinary cancer in the United States, and ranks among the top 10 malignant neoplasms in both men and women [1–4]. RCC is a heterogeneous group of cancers arising from epithelial cells of the renal tubules. Among RCCs, clear cell RCC (ccRCC) is the most common subtype, consisting of 75% of all kidney tumors [1–4]. Other major subtypes include papillary RCC (approximate 10% of RCC), chromophobe RCC (approximate 5% of RCC), collecting duct RCC (approximate 1% of RCC), translocation RCC (approximate 1% of RCC), and several other rare types [3–7]. In the past decade, genetic alterations of RCC have been extensively investigated, such as by The Cancer Genome Atlas (TCGA) [8,9], the International Cancer Genome Consortium (ICGC) and by other groups [10–13]. It is well-known now that specific genetic and epigenetic alterations are associated with distinct phenotypes of RCC [2,4,12]. Heterozygous germline or somatic alterations of the VHL gene on chromosome 3p are associated with the conventional clear cell phenotype [4,8,9]; alterations of TP53 and PTEN and monosomy of characteristic chromosomes have been found in chromophobe RCC [10], while trisomy and tetrasomy of chromosome 7, trisomy of chromosome 17 and loss of the Y chromosome are often seen in papillary RCC [9,13]. Furthermore, tumors with morphological clear cell features may not be conventional ccRCC (Fig. 1). For example, microphthalmia-associated transcription (MiT) family translocation tumors, which reveal “clear cell” morphology, are separated from conventional ccRCC by distinct molecular abnormalities of TFE3, TFEB, MITF, TSC1, and TSC2 [5,4–7,14]. Based on current molecular characteristics of RCC, the World Health Organization (WHO) and the International Society of Urological Pathology (ISUP) have also updated the criteria for the morphological classification of RCC [2,3].
Fig. 1.
Comparison of molecular characteristics and histomorphology of ccRCC with subtypes of kidney tumors with clear cell features.
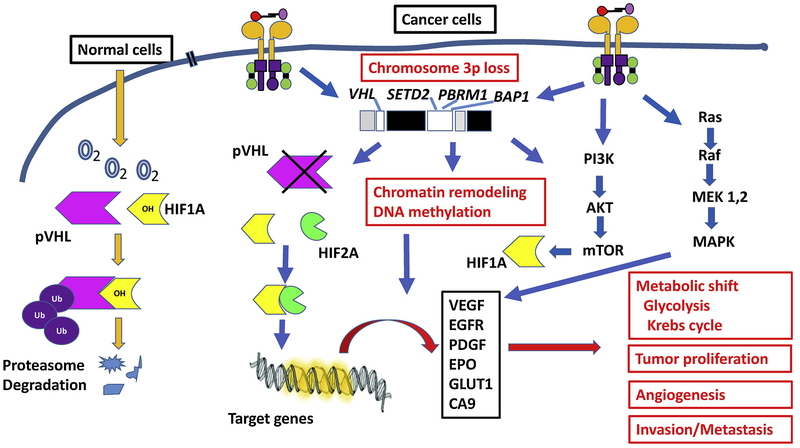
The loss of VHL gene on the chromosome 3p25–26 has been recognized as the oncogenic driving event in ccRCC [8,15–17]. In the VHL signaling pathway, VHL protein is the substrate of an E3 ligase complex that regulates ubiquitination of hypoxia-inducible factor 1α (HIF1 A) and HIF-2α (HIF2A) during proteasome-mediated degradation (Fig. 2) [18,19]. In a normoxic microenvironment, HIF prolyl hydroxylase (PHD) transfers hydroxyl groups to HIF which enables the VHL complex to target and degrade HIF. In hypoxia and cancer cells, PHD does not hydroxylate HIF, and the mutant or absent VHL complex cannot target and degrade HIF. The uncontrolled activation of HIF in turn results in dysregulation of angiogenesis, glycolysis and apoptosis [20–23]. Consequently, tumor cells have a high level of lipids and glycogens in the cytoplasm revealing “clear cell” morphology, and the tumors are rich in blood vessels.
Fig. 2.
Summary of major signaling pathways in ccRCC. The tumor demonstrates diverse genetic alterations, with primarily involving in chromosome 3p, including gene regulating cellular oxygen sensing (such as VHL), remodeling chromatin and DNA methylation (such as PBRM1, BAP1 and SETD2). Mutation of PI3K/AKT/MTOR and other signaling pathways are also involved in the progression of ccRCC.
In ccRCC, some 2–3% of tumors are familial/inherited cases, which are associated with certain autosomal dominant syndromes. The most notable one is von Hippel-Lindau (VHL) syndrome [8,11,15,17]. Patients with VHL syndrome have a germline abnormality (e.g. mutation or loss) of one of their VHL genes that results in the development of renal cysts and ccRCC. However, in sporadic cases, in addition to an acquired defect in the VHL gene, more complex molecular abnormalities have been identified [8,11,15,17]. For example, several genes on chromosome 3p adjacent to VHL are involved, including genes engaged in remodeling chromatin and DNA methylation (such as PBRM1, SETD2, and BAP1) [8,12,18,24]. Furthermore, the fact that PBRM1, SETD2, and BAP1 encode chromatin- and histone-regulating tumor suppressor proteins suggests epigenetic dysregulation as a convergent pathogenic event in the development and progression of ccRCC [12]. SETD2 plays a critical role in tumor cell lysine methylation of the histone H3 (H3K36me3), affecting metabolic pathways [18,24]. Whereas, PBRM1 is a key component of the PBAF SWI/SNF chromatin remodeling complex, and functions as a tumor suppressor by preventing amplification of HIF oncogenic signals, which may explain why its mutation in small renal masses is associated with tumor invasiveness [18,21,25,26]. BAP1 is a nuclear deubiquitinase and involved in the host cell factor 1 (HCF-1) pathway and cell proliferation [18,21,27]. All these molecular alterations are involved in the activation of AMP kinase and acetyl-CoA carboxylase, both of which contribute to a metabolic shift towards increased fatty acid synthesis [8,11,18,19]. Overall, a metabolic shift towards a ‘Warburg-like effect’ is noted, with down-regulation of AMPK complex and Krebs cycle genes and upregulation of pentose phosphate pathway genes. Thus, PBRM1, SETD2, BAP1 and other genetic mutations are considered as second, third or further downstream oncogenic drivers associated with tumor progression and aggressive clinical features. The PI3K/AKT/MTOR signaling pathway is also found to be involved in the progression of ccRCC [8,11,12]. Together, the involvement of the above pathways illustrates the complex genetic pathways identified in ccRCC development and progression. Rapid advances in the molecular and morphological characterization of ccRCC have also facilitated the development of targeted therapy in affected patients [18,19]. Altogether, these advances have improved the median survival of patients with advanced disease from less than 10 months prior to 2004 to 30 months in 2011, which may increase to more than 60 months by 2025 [12].
Clinically, ccRCC frequently occurs with few symptoms and/or laboratory abnormalities, and approximately 20–30% of patients present with advanced disease at the time of diagnosis. Furthermore, approximately 30% of ccRCC patients with localized disease will develop recurrence or metastasis after surgical resection of the tumor [2,3]. Clearly, the early detection of ccRCC and monitoring disease progression are critical steps for the improvement of patients’ survival. Although current body imaging techniques can detect a majority of renal masses when they are still small (termed small renal mass or SRM, <4.0 cm in size), the pathological features and biological behavior of these small tumors cannot be accurately characterized without percutaneous biopsy of the tumor [28–30]. 95% of SRM cases reveal slow growth kinetics and typically only grow a few mm per year [28]. Among SRMs, up to 20% of cases are benign, and only about 20% are found to be high-grade on surgically resected samples [28–30]. Indeed, active surveillance of SRM is a recommended option for select patients per the American Urological Association and National Comprehensive Cancer Network guidelines [30]. Meanwhile, many larger renal tumors have metastatic potential, especially at sizes over 4 cm, and they need to be separated from indolent cases and treated aggressively. A commonly used active surveillance approach consists of alternating between ultrasonography and computed tomography (CT) imaging or magnetic resonance imaging (MRI) every 3 months in the first year, every 6 months in the second year, and annually thereafter [28,30]. Based on data from the Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) registry [28], intervention should be considered for a SRM if it grows to over 3–4 cm in diameter, or develops a growth rate of 0.4–0.5 cm or greater per year. However, there are still no effective biomarkers to aid in the molecular characterization of SRM. Taken together, early detection, monitoring of tumor progression, and attempts at separating aggressive from indolent tumors should all be considered in order to avoid over-treatment and while treating those who truly harbor aggressive RCC. Importantly, biomarker screening tools for active surveillance of SRM need to be identified and developed.
Recently, tremendous efforts have been focused on the proteomic profiling of ccRCC for the identification of potential protein biomarkers [25,31–42]. Commonly used approaches in these studies compare protein profiles based upon TCGA data, or compare tumor tissue with normal tissue. These studies have identified many potential protein biomarkers. However, none of them have been validated or used for inclusion in clinical trials. In order to facilitate the discovery and validation of protein biomarkers, important questions, such as how to integrate molecular alterations with proteomic characterization of the tumor, how to address tumor heterogeneity in protein biomarker discovery, and how to capture dynamic protein expression and post translational modifications in proteomic analysis, still need to be answered. In this review, we focus our discussion on recent progress and contemporary challenges in the proteomic characterization of ccRCC. We also discuss the potential clinical implications of recent proteomic studies.
2. Challenges and opportunities
Several types of clinical samples have been analyzed, including serum [37–39], urine [40,41], and surgically resected tumor tissues [32–34,36,42]. These studies not only advanced our knowledge of the protein profile of ccRCC, but have also demonstrated challenges during the process. For example, serum and urine are well-known clinical specimens for the study of tumor-associated proteins in cancers, and they are relatively easy to obtain from patients. However, the tumor-associated proteins are usually present as low-abundance proteins, which may not be found or are difficult to detect in serum or urine samples. Furthermore, candidate protein biomarkers may be differentially expressed during tumor progression. Therefore, in addition to studying serum or urine, analysis of tumor tissues has several advantages, such as the high abundance and enrichment of tumor-associated proteins within tumor tissue, less contamination by serum proteins, and no loss of proteins in urine samples due to the biological barrier function of the renal glomeruli.
Tumor tissues can be obtained from patients by several techniques, such as fine needle aspiration (FNA) biopsy, and/or surgical resection of the tumor [28,29]. Among a variety of clinical approaches, surgically resected tumor tissue is preferred for proteomic analysis, since it can provide the highest concentration of tumor tissue for assessment of tumor-associated proteins. The obtained tumor tissues can be stored as fresh-frozen with or without OCT (Optimal Cutting Temperature) compound, formalin-fixed, and/or as paraffin-embedded samples [32–34,36,42,43].
Although it is recognized that proteomic analysis of tumor tissue can define protein signatures and identify potential protein biomarkers in ccRCC, several issues need to be considered during the proteomic analysis.
2.1. Inter- and intra-tumor heterogeneity
The concept of tumor heterogeneity was proposed several decades ago based on studies of clonal evolution of tumor cell populations [44]. Recent large-scale collaborative sequencing projects, such as TCGA and ICGC, have revealed extensive genetic diversity between (inter-tumor) and within (intra-tumor) neoplasms, including in the ccRCC [45–50]. To further understand the heterogeneity between primary tumors and associated metastatic sites, multiregional exome sequencing of the same primary tumor and subsequent metastases of four ccRCC patients was performed by Gerlinger M, et al [50]. In this study, they found that intra-tumor heterogeneity was present in every tumor. Although VHL mutation and 3p loss of heterozygosity were found to be universally present in all primary tumors and metastases, SETD2, PBRM1, MTOR, PIK3CA, PTEN and KDM5C mutations were present heterogeneously between the primary tumors and metastatic sites, representing inter-and intra-tumor heterogeneity. This study indicates that tumor heterogeneity can lead to an underestimation of the complexity of the tumor genomic and proteomic landscape, and may present major challenges to biomarker development. Inter- and Intra-tumor heterogeneity may explain the difficulties encountered in the discovery and validation of protein biomarkers.
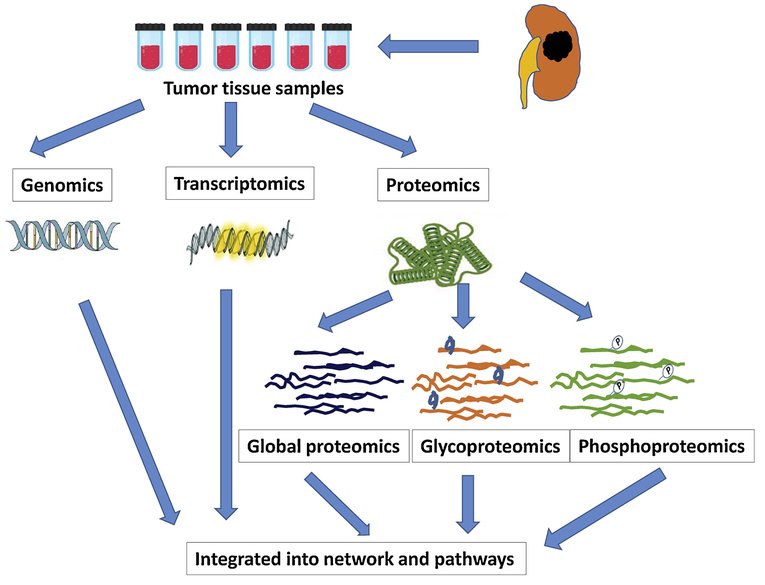
Current sequence-based proteomic profiling (as performed by tandem mass spectrometry) relies on a reference genome to provide identifiable protein sequences [51–54] (Fig. 3). Recent studies have begun to explore the proteogenome, in which the proteome is profiled against the reference genome and a customized genome specific to the sample under study [51,53,54]. When characterizing the genome and proteome from the same tumor, to limit the effects of heterogeneity, it is important to perform the analysis using the same tumor populations. One way to minimize heterogeneity is to use alternating serial slices from tumor tissue. That is, using a cryostat to slice through tumor tissue, and one slice to genomic analysis and the next slice to proteomic analysis, alternating so on through the tumor tissue. Another possibility is to cryopulverize the entire sample into homogenized powder, and aliquot it into several portions for genomics and proteomics profiling.
Fig. 3.
“Multi-omics” approach in the characterization of ccRCC, which includes genomics, transcriptomics and proteomics. Current sequence-based proteomic profiling (as performed by tandem mass spectrometry) relies on a reference genome to provide identifiable protein sequences. Recent studies have begun to explore the proteogenome, in which the proteome is profiled against the reference genome and a customized genome specific to the sample under study.
Furthermore, tumor tissue represents a mixture of tumor cells, stromal elements and other cellular components. Various amounts of inflammatory cells and tumor necrosis are also commonly present. When analyzing tumor tissue with proteomic approaches, tumor cells must be separated from stromal and necrotic cells to reduce the contamination of non-tumor components. When extracting tumor-associated proteins or peptides using a direct tissue lysis, the peripheral blood cells and inflammatory cells from blood and plasma may cause pre-analytic bias. A selective isolation of tumor cells from other cellular components should be considered to limit pre-analytic bias during the analytic process. Several approaches have been used to deal with the problem, including micro-dissection profiling, and laser capture microdissection (LCM) [55–57]. The approach of micro-dissecting tumor tissue uses microscopy to identify tumor areas, and provides samples from selective areas of the tumor. The technique is convenient and provides a relatively large quantity of tumor samples. The most accurate method for isolating tumor cells is LCM. Tumor cells can be isolated using LCM with high purity. However, the technique requires special instrumentation, and may cause cross-linking of cellular proteins due to a thermal-effect. The major drawback of the technique is that it only yields a limited quantity of tumor sample. Currently, one of these techniques is used by most proteomic studies.
Finally, in the study of multiregional exome sequencing of the primary tumor and metastasis, it was also demonstrated that a single tumor-biopsy core specimen only revealed a minority of genetic aberrations (including mutations, allelic imbalance, and ploidy) that were present in an entire tumor, indicating that a single core was not representative of the mutational landscape of the entire tumor bulk; and it may underestimate the mutational burden in the tumor [50]. Similarly, proteomic analyses of a single core or a few cores may not uncover the protein signature in the tumor. Thus, proposed diagnostic and/or prognostic protein biomarkers may not correctly predict outcomes if they are not representative of the entire tumor. Such findings suggest that a multiple coring strategies of the same tumor may be necessary for protein biomarker discovery and validation.
Taken together, inter- and intra-tumor heterogeneity have significant implications for discovery of protein biomarkers and the choice of biomarkers to guide clinical decision-making in cancer medicine. Despite some evidence of genetic and functional convergence, it will nevertheless be challenging to identify protein biomarkers to define phenotypically similar, yet genetically diverse, lesions to guide treatment.
2.2. Dynamic expression of cellular proteins in ccRCC
In the discovery of potential protein biomarkers, certain cellular proteins are expressed during different stages of the course of disease [58]. One mechanism which may relate to dynamic protein expression in the evaluation of tumor cells. Recent analysis of multiple tumor regions in the same ccRCCs enabled the construction of tumor phylogenies [50]. This phylogenetic modeling revealed a branched evolution of sub-clones of tumor cells, demonstrating that multiple sub-clones were evolving simultaneously within the same tumor. In the ‘trunk’ of the cancer cell evolutionary tree, common mutations found in all tumor cells are depicted, whereas, ‘branched’ mutations are found in some sub-clones of tumor cells but not others; these mutations may be regionally distributed across the tumor. During tumor progression, certain proteins are up-regulated and others are down-regulated. Spatial distribution of sub-clones and dynamic protein expression may contribute to difficulty in validation of candidates, causing failure of utility of potentially clinically qualified biomarkers in ccRCC. Thus, identification of tumor clonal architectures and common mutations located in the “trunk” as well as in “branches” of the phylogenetic tree may contribute to more robust biomarker discovery and validation.
In ccRCC, the phylogenetic model has demonstrated that the main oncogenic driving event is the VHL alteration. As we mentioned above, the loss of VHL expression leads to the accumulation of HIF 1 A and HIF2A [8,12,15–17] (Fig. 2). Both of these molecules are further involved in the regulation of many other intracellular signaling pathways, particularly involving metabolism [4,11,12,19,21,59]. HIF1 A and HIF2A are transcription factors, and regulate the expression of several hypoxia responsive genes, such as vascular endothelial growth factor (VEGF), platelet derived growth factor (PDGF), and glucose transporters GLUT1 and GLUT4. These studies have confirmed the dysregulation of the metabolic pathways and the Warburg shift in ccRCC [4,11,12,19,21,59]. Lack of degradation of HIF1 A and HIF2A also have different effects on promoting tumor growth: HIF1 A activates glycolytic genes; and leads to a downstream effect on the glucose and glutamine metabolic pathways, such as upregulation of glucose uptake and cellular biosynthesis of the glycolytic pentose phosphate [4,8,11,12,21,23]. Whereas, HIF2A promotes cancer cell growth via stimulation of the angiogenesis pathway [4,8,11,12,21,23]. However, elevated levels of both HIF1 A and HIF2A are only found in late stages of ccRCC [11,12,37,59]. It has also been reported that increased fatty acid synthesis is correlated with reduced transcription of AMP-activated kinase (AMPK) and increased levels of acetyl-CoA carboxylase (ACC), which corresponds to worse survival [11,12]. This discriminative or dynamic expression of cellular proteins leads to difficulty in the discovery of potential protein biomarkers.
Dynamic protein expression is also related to the differentiation of tumor cells. In poorly-differentiated tumors, tumor cells may reveal high-grade nuclear atypia, and often have an aggressive clinical course [2,5,12]. For example, approximately 5% of ccRCC contain a sarcomatoid component [2,27,48]. The biological nature of this component is not well understood. Jones et al. have studied the pattern of allelic loss between clear cell and sarcomatoid components of ccRCC [48]. In their study, they found that both components are derived from the same progenitor cell, however, different allelic losses were identified between clear cell and sarcomatoid components in the same tumor, indicating genetic divergence during the clonal evolution of the tumor such as mutations of TP53 and CDKN2A [8,11,12,23].
Taken together, molecular and metabolic pathway studies have demonstrated spatially separated sub-clones in the tumor during ccRCC development and progression. These sub-clones harbor distinct driver mutations and Warburg effect-related dynamic changes, such as down-regulation of gene expression in the tricarboxylic acid (TCA) cycle, and up-regulation of gene expression in the pentose phosphate pathway. This dynamic process and the differential expression of cellular proteins should be considered in proteomic analyses. Currently, the most commonly used approach is to analyze tumors of different pathology stages based upon TCGA data. However, better strategies are certainly needed to further define and account for dynamic protein expression in tumors to aid in the discovery and validation of potential protein biomarkers.
2.3. Post-translational modification (PTM) of proteins in ccRCC
Multi-omic studies have shown that ccRCC tumorigenesis involves complex molecular events, dynamic protein expression changes, PTMs, cellular and sub-cellular protein redistribution, and protein-protein interactions. Although the development of high resolution mass spectrometers has made it feasible to identify hundreds to thousands of potential protein candidates in one experiment, it is important to utilize complementary techniques to identify changes across biological hierarchy (abundance, isoforms, PTMs, etc.). Proteomic analysis and high-throughput proteomics can certainly be utilized for functional analysis, such as protein-protein interactions, protein-DNA interactions, and pathway analysis [11,12,31,33–37]. Several techniques have been routinely used for proteomics, such as label-free proteomic analysis [36], isobaric tagging reagent (iTRAQ), and tandem mass tags (TMT) followed by multidimensional LC and MS/MS analysis [37,38]. Advances in mass spectrometry technology, including development of the Orbitrap and data independent acquisition, now allow quantitative detection of 10,000 distinct proteins in a sample [51–54]. The advanced technologies have markedly improved the detection sensitivity, and allowed for the quantitative analysis of large-scale clinical samples. For example, several shotgun-proteomic analyses have detected thousands of proteins in ccRCC, and several of them have been found to play critical roles in the glycolysis and metabolic pathway [35,36,59].
In addition to protein profiling, analysis of post translational modification is also a critical step in cancer biology [60–66]. Post translational modification of proteins, including phosphorylation and glycosylation play important roles in regulating cellular functions. More than 50% of cellular proteins, including most secreted proteins, cell surface and intracellular proteins are glycoproteins [60,63,64]. Glycoproteins play critical roles in the regulation of cellular functions, including cell growth, differentiation and migration [60,63]; and many FDA approved clinical cancer biomarkers are glycoproteins, such as prostate specific antigen (PSA) in prostate cancer, alpha-fetoprotein (α-AFP) in hepatocellular carcinoma, and carbohydrate antigen 125 (CA125) in ovarian cancer [58,60,63]. Therefore, the study of the PTM profile is particularly important to understand kidney cancer biology and the identification of multiple candidate protein variants.
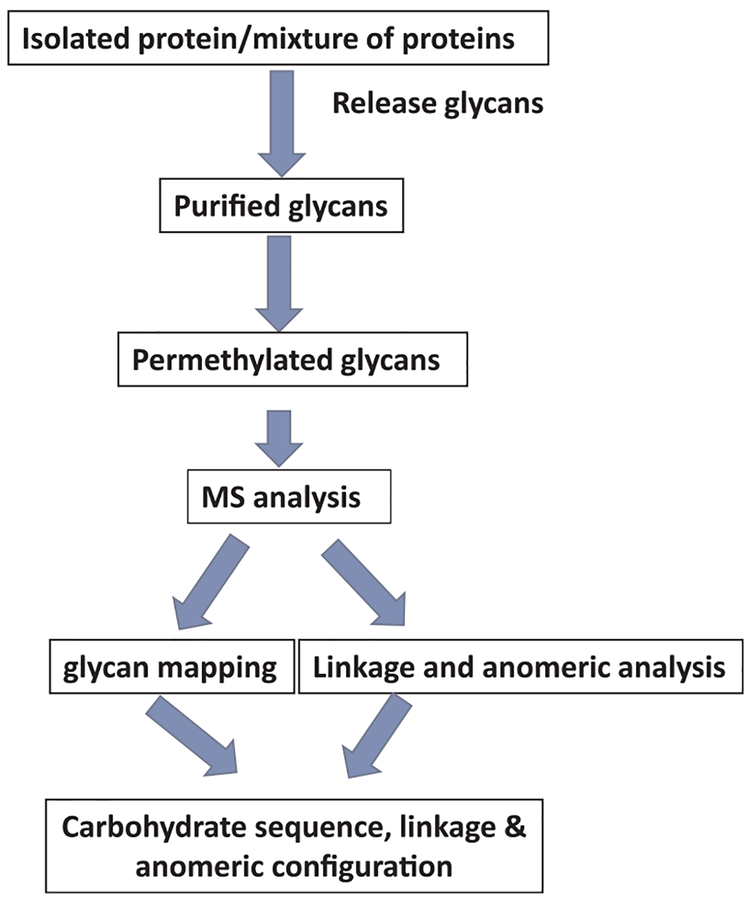
Recent advances in high throughput glycoproteomics allow for a way to evaluate thousands of glycoproteins in a single experiment [61,64]. These state-of-the-art technologies provide new platforms for the systematic study of glycoproteins and characterization of the complex biological pathways in cancers. It is well known that glycoproteins of cancer cells express aberrant glycosylation patterns, such as increased β1–6 branching, sialylation and/or fucosylation of N-linked glycans, and truncation of O-linked glycans [60,61]. One technique has been developed to profile the glycan on glycoproteins (Fig. 4) [61]. This strategy is based on mass spectrometry (MS) characterization of structures of glycans released from glycoproteins. The N-glycans are released from SDS-denatured glycoproteins by PNGase F digestion, whereas, the O-glycans are released by a reductive beta-elimination. Then, glycans are permethylated and analyzed using MS. Thus, it may be used to characterize the glycan on a selected subset of tumor-associated glycoproteins.
Fig. 4.
Strategy for profiling the glycan on a target glycoprotein or mixed glycoproteins. The approach is based on mass spectromety characterization of glycans released from glycoproteins. Then, glycans are permethylated and analyzed using glycan mapping, linkage and anomeric analysis.
Many important low abundant proteins are phosphoproteins. The phosphorylation sites of phosphoproteins occur on three major phosphoamino acids: phosphoserine (pSer), phosphotyrosine (pThr) and phosphotyrosine (pTyr), and regulated by the phosphorylation signaling pathways [62,65,66]. The commonly used methods for enrichment of phosphopeptides pSer and pThr are Fe3+/Ti4+-immobilized metal affinity chromatography (Fe3+/Ti4+-IMAC) and metal oxide (TiO2) affinity chromatography (MOAC), whereas, anti-phosphotyrosine antibodies [62] or Src homology 2 (SH2) domain affinity purification are used for phosphopeptides pTyr [65]. A recent study of kidney cancer by Peng X, et al, using a multistep immobilized metal ion affinity chromatography (IMAC) method, identified 8962 proteins from RCC, including 60,402 phophopeptides and 44,728 phosphosites from 6415 phosphoproteins [66]. In the study, phosphoproteome coverage of ccRCC and adjacent tissues was obtained through off-line high-pH reversed-phase fractionation, followed by Fe3+-IMAC phosphopeptide enrichment strategies, and the high-scan-speed mass spectrometer of LTQ Orbitrap Velos. Among 44,728 phosphosites, they also identified 10,266 phosphosites, which are not registered in the PhosphoSitePlus database. Chromosomal distribution analysis of these proteins demonstrated that a majority of these proteins are clustered on chromosome 2 and chromosome 1, and involved in the development of nephron tubule, kidney epithelium and kidney mesenchyme.
Taken together, these studies indicate the importance of proteomic techniques and post-translational modification analysis to identify protein-level changes at each biological pathway for candidate discovery, particularly for the analysis of low-abundance proteins, dynamic cellular proteins, and post-translationally modified proteins. In addition, technologies still need to be further improved in the areas of minimum sample amount, PTM site localization, accuracy, and sensitivity.
3. Perspectives and potential applications
Recent genetic characterization of ccRCC has identified several molecular markers as potential novel prognostic biomarkers, but none of them has been independently validated. Neither have these bio-markers been compared with each other to identify lead candidates for further development. The molecular signature, especially the protein signature of ccRCC is still far from fully understand. Proteomic analysis of tumor tissue can further define the protein profile, which may provide potential biomarkers for early detection, monitoring for progression (likelihood of cure or risk of progression and metastasis), and treatment outcome (probability of response to current targeted therapy and potential new therapeutic targets).
3.1. Discovery of diagnostic biomarkers for early detection
Similarly to other solid tumors, ccRCC development and progression are characterized by aberrant genetic and protein expression. Studies have shown that the development of kidney cancer is the result of accumulations of cellular and molecular aberrations, including epigenetic, transcriptomic, miRNA, proteomic, and metabolomic abnormalities [2,5,8,12]. These multi-omics studies attempt to discover diagnostic biomarkers for early detection, and also highlight not only the heterogeneity but also an underlying molecular commonality among different stages of this neoplasm. It is clear that ccRCCs have significant molecular heterogeneity and involve a large number of genetic and protein-level alterations, thus, a group of well-selected candidates may be necessary to be representative of all these tumors.
Tremendous efforts have been focused on the identification of potential biomarkers for early detection, and a literature search reveals many candidate biomarkers. However, these studies are based on small numbers of tumor samples and lack clinical validation. Studies using different stages of tumor samples have also demonstrated differential expression of several proteins, which were elevated in advanced stages of ccRCC [4,8,11,12,67], but none of them has been validated in early stage tumors or in large cohort of tumor samples. Importantly, although these studies have provided knowledge for the systematic analysis of proteins in ccRCC, none of these protein biomarkers has been validated for early detection, which currently relies on radiographic imaging, followed by tissue biopsy for tumor characterization. Further studies using carefully selected tumor samples and large-scale cohorts are necessary to identify reliable candidate biomarkers.
3.2. Detection of molecular changes (prognostic biomarkers) during tumor progression
It is well-known that familial von Hippel-Lindau disease-related ccRCC has an inactivated germline copy of VHL in all cells, with loss or alteration of the other VHL copy during tumorigenesis. Sporadic ccRCC has an acquired mutation of VHL in a majority of cases, and corresponding loss of heterozygosity at the VHL locus in more than 90% of cases, thus VHL loss appears to be the main event in ccRCC development [8]. In the TCGA study of ccRCC, in addition to VHL, the three other most commonly mutated tumor suppressor genes were PBRM1, BAP1 and SETD2, all on chromosome 3p21–3p25, where VHL is also located. Commonly, a universal alteration on the short arm of chromosome 3 in ccRCC is followed by simultaneous one-copy loss of these tumor suppressor genes. Taken together, these findings suggest potentially critical roles of the pathways subtended by these gene loci in the discovery of prognostic markers.
As we mentioned above, in advanced stage ccRCC, several abbreviated protein expression and cellular pathways have been identified, including decreased expression of AMPK and PTEN protein levels, increased acetyl-CoA carboxylase protein, upregulation of the pentose phosphate pathway and the glutamine transporter genes, and altered promoter methylation of miR-21 (also known as MIR21) and GRB10 [4,11,12,25–27,38,67–69]. However, whether these markers can be used as surrogate tests for monitoring tumor progression remains unknown. Further studies are still needed.
3.3. Identification of potential therapeutic targets
Metastatic ccRCC is known to be refractory to conventional chemotherapy [23,70–73]. 30% of patients with localized RCC, despite surgical resection of the tumor, eventually develop recurrence or metastasis, and about a quarter of patients still present with metastatic disease at the time of diagnosis [1–4]. In the past decade, the management of ccRCC has evolved to include systemic targeted therapies for stage IV patients, though randomized clinical trials support nephrectomy as a part of such treatment for patients with advanced disease but minimal metastatic burden [70–73]. Understanding the 3p tumor suppressor gene pathways, including VHL, PBRM1, SETD2 and BAP1, has provided the foundation for the development of a new generation of targeted therapies as well as immune checkpoint inhibitors.
Based on the TCGA study, several FDA-approved agents that target the VHL pathway have been approved for the treatment of patients with advanced RCC [12,18,19]. Currently approved targeted drugs for advanced ccRCC include: bevacizumab, sorafenib, sunitinib, pazopanib, axitinib, tivozanib, cabozantinib, lenvatinib, temsirolimus, and everolimus, and immunotherapies including interferon-α, IL-2, and nivolumab. The targeted agents involve at least 6 intracellular signaling pathways, i.e. inhibition of VEGFR, mTORC1, c-MET and FGFR, cytokines, and PD1/PDL1 immune checkpoint inhibition. Though targeted therapies and immunotherapy have rapidly evolved and improved overall survival, the complete response rate is still < 10% [73].
The TCGA study also indicated that some of the identified genes could be targeted for future therapeutic options, particularly the Warburg metabolic shift in cancer cells, as these metabolic shifts correlate with poor survival. To understand whether there are any common pathogenic pathways underlying the different ccRCC subtypes, the multi-omic study of ccRCC has revealed potential shared oncogenic pathways belying their histologic differences. Many studies have demonstrated that alterations of specific pathways including hypoxia, metabolism, NRF2-ARE, Hippo, immune checkpoint, and PI3K/AKT/mTOR correlate with patient survival [4,8,11,12,18,19,67]. Among these pathways, NRF2-ARE is particularly important because of its association with KEAP1 (Kelch-like ECH-associated protein) signaling pathway [74,75]. NRF2 is a transcription factor and regulates the expression of genes encoding antioxidants and xenobiotic detoxification enzymes. Elevated levels of NRF2 have been shown to relate to cancer cell survival and potential protection against chemotherapeutic agents [74]. The regulation of NRF2 expression is complex: in some cases, it is related to the mutation of KEAP1 gene and involved in oxidative stress responses [74,75]. Aberrant KEAP1 protein leads to the accumulation of NRF2 in the nucleus; where it can affect another gene, ARE (anti-oxidant response element) expression. MGAT5 (Mannosyl (alpha-1,6-)-glycoprotein beta-1,6,-N-accetyl-glucosaminyltransferase) gene also plays an important role by encoding mannosyl (alpha-1,6-)-glycoprotein beta-1,6-N-acetyl-glucosaminyltransferase, which regulates the synthesis of protein-bound oligosaccharides on the cell surface. In the mouse model, cell-cycle progression (p53 pathway) is dependent on Mgat5/N-glycan interaction. The alteration of cellular N-glycoproteins causes significant changes of cellular adhesion and migration [74]. These data indicate that oxidative stress and KEAP1 (including MGAT5) pathway may interact in the regulation of cell growth. The study of cellular proteins in different stages of the cell cycle may help elucidate the mechanism of ccRCC progression and thus facilitate further developments in targeted therapy. Mechanism-based selection of targeted therapy is a promising approach for future research and clinical trials.
In summary, ccRCC appears to represent a heterogeneous group of histologically similar neoplasms. Its development and progression are multistep processes characterized by aberrant genetic, epigenetic, and proteomic changes, which subsequently lead to phenotypic cellular transformation. In addition to the genetic characterization of ccRCC, it is also necessary to address the urgent need for protein biomarkers for early detection, accurate subclassification of ccRCC, prognostication, monitoring disease progression and selection of targeted treatment. Recent advances in proteomics technology evidently facilitate the pursuit of novel biomarkers in ccRCC. Although select candidate bio-markers have been studied and evaluated using clinical specimens, further improvement of the workflow and validation process in large scale cohorts remains necessary. Finally, in biomarker discovery and potential clinical application, it is important to use carefully selected clinical specimens in both the discovery process and subsequent validation phase, as successful biomarker discovery and application largely depends on the quality and availability of tumor specimens. This requires both a large number of carefully selected patient cohorts to determine the potential utility of the biomarkers as well as properly procured specimens. Clinical validation of potential biomarkers must be conducted in a way to maximally avoid false positive and/or false negative results. For each candidate biomarker, robust and reproducible assays need to be developed and used in the validation phase. The optimal goal of proteomics for ccRCC remains to improve clinical outcomes for patients affected by this often aggressive neoplasm.
Acknowledgements
This work is partially supported by Drs. Ji and Li Family Cancer Research Foundation (QKL). National Cancer Institute, the Clinical Proteomic Tumor Analysis Consortium (CPTAC, Grant U24CA210985) (DWC and HZ).
Footnotes
Conflict of interests
The authors declare no conflict of interest.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA Cancer J. Clin. 68 (1) (2018) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Moch H, Humphrey PA, Ulbright TM (Eds.), WHO Classification of Tumours of the Urinary System and Male Genital Organs, IARC, Lyon, 2016. [DOI] [PubMed] [Google Scholar]
- [3].Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, Hes O,Moch H, Montironi R, Tickoo SK, Zhou M, Argani P, ISUP renal tumor panel. The International Society of Urological Pathology (ISUP) vancouver classification of renal neoplasia, Am. J. Surg. Pathol. 37 (10) (2013) 1469–1489.24025519 [Google Scholar]
- [4].Hsieh JJ, Le V, Cao D, Cheng EH, Creighton CJ, Genomic classifications of renal cell carcinoma: a critical step towards the future application of personalized kidney cancer care with pan-omics precision, J. Pathol. 244 (5) (2018) 525–537. [DOI] [PubMed] [Google Scholar]
- [5].Delahunt B, Srigley JR, The evolving classification of renal cell neoplasia, Semin. Diagn. Pathol. 32 (2) (2015) 90–102. [DOI] [PubMed] [Google Scholar]
- [6].Argani P, Lal P, Hutchinson B, Lui MY, Reuter VE, Ladanyi M, Aberrant nuclear immunoreactivity for TFE3 in neoplasms with TFE3 gene fusions: a sensitive and specific immunohistochemical assay, Am. J. Surg. Pathol. 27 (2003) 750–761. [DOI] [PubMed] [Google Scholar]
- [7].Smith NE, Illei PB, Allaf M, Gonzalez N, Morris K, Hicks J, Demarzo A,Reuter VE, Amin MB, Epstein JI, Netto GJ, Argani P, T(6;11) renal cell carcinoma (RCC): expanded immunohistochemical profile emphasizing novel RCC markers and report of 10 new genetically confirmed cases, Am. J. Surg. Pathol. 38(5) (2014) 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].The Cancer Genome Atlas Research Network, Comprehensive molecular characterization of clear cell renal cell carcinoma, Nature 499 (7456) (2013) 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].The Cancer Genome Atlas Research Network, Comprehensive molecular characterization of papillary renal-cell carcinoma, N. Engl. J. Med. 374 (2) (2016) 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Davis CF, Ricketts CJ, Wang M, Yang L, Cherniack AD, Shen H, Buhay C,Kang H, Kim SC, Fahey CC, Hacker KE, Bhanot G, Gordenin DA, Chu A, Gunaratne PH, Biehl M, Seth S, Kaipparettu BA, Bristow CA, Donehower LA, Wallen EM, Smith AB, Tickoo SK, Tamboli P, Reuter V, Schmidt LS,Hsieh JJ, Choueiri TK, Hakimi AA, The Cancer Genome Atlas Research Network, Chin L, Meyerson M, Kucherlapati R, Park WY, Robertson AG,Laird PW, Henske EP, Kwiatkowski DJ, Park PJ, Morgan M, Shuch B,Muzny D, Wheeler DA, Linehan WM, Gibbs RA, Rathmell WK, Creighton CJ, The somatic genomic landscape of chromophobe renal cell carcinoma, Cancer Cell 26 (3) (2014) 319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sato Y, Yoshizato T, Shiraishi Y, et al. , Integrated molecular analysis of clear-cell renal cell carcinoma, Nat. Genet. 45 (8) (2013) 860–867. [DOI] [PubMed] [Google Scholar]
- [12].Hsieh JJ, Purdue MP, Signoretti S, Swanton C, Albiges L, Schmidinger M, Heng DY, Larkin J, Ficarra V, Renal cell carcinoma, Nat. Rev. Dis. Primers 3 (2017) 17009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li S, Shuch BM, Gerstein MB, Whole-genome analysis of papillary kidney cancer finds significant noncoding alterations, PLoS Genet. 13 (3) (2017) e1006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Argani P, MiT family translocation renal cell carcinoma, Semin. Diagn. Pathol. 32(2) (2015) 103–113. [DOI] [PubMed] [Google Scholar]
- [15].Linehan WM, Srinivasan R, Schmidt LS, The genetic basis of kidney cancer: a metabolic disease, Nat. Rev. Urol. 7 (2010) 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hakimi AA, Pham CG, Hsieh JJ, A clear picture of renal cell carcinoma, Nat. 45 (2013) 849–850. [DOI] [PubMed] [Google Scholar]
- [17].Kaelin WG, Von Hippel-Lindau disease, Annu. Rev. Pathol. 2 (2007) 145–173. [DOI] [PubMed] [Google Scholar]
- [18].Srinivasan R, Ricketts CJ, Linehan WM, New strategies in renal cell carcinoma: targeting the genetic and metabolic basis of disease, Clin. Cancer Res. 21 (2015) 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Linehan WM, Genetic basis of kidney cancer: role of genomics for the development of disease-based therapeutics, Genome Res. 22 (11) (2012) 2089–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Turajlic S, Larkin J, Swanton C, SnapShot: renal cell carcinoma, Cell 163 (6) (2015) e11556–1556. [DOI] [PubMed] [Google Scholar]
- [21].Tun HW, Marlow LA, von Roemeling CA, Cooper SJ, Kreinest P, Wu K, Luxon BA, Sinha M, Anastasiadis PZ, Copland JA, Pathway signature and cellular differentiation in clear cell renal cell carcinoma, PLoS One 5 (5) (2010) e10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Delahunt B, Srigley JR, Renal neoplasia: from morphologic to molecular diagnosis, Semin. Diagn. Pathol. 32 (2) (2015) 87–89. [DOI] [PubMed] [Google Scholar]
- [23].Jones J, Otu H, Spentzos D, Kolia S, Inan M, Beecken WD, Gene signatures of progression and metastasis in renal cell cancer, Clin. Cancer Res. 11 (2005) 5730–5739. [DOI] [PubMed] [Google Scholar]
- [24].Liu L, Guo R, Zhang X, Liang Y, Kong F, Wang J, Xu Z, Loss of SETD2, but not H3K36me3, correlates with aggressive clinicopathological features of clear cell renal cell carcinoma patients, Biosci. Trends 11 (May (2)) (2017) 214–220 23. [DOI] [PubMed] [Google Scholar]
- [25].Sun X, Zhang H, Luo L, Zhong K, Ma Y, Fan L, Fu D, Wan L, Comparative proteomic profiling identifies potential prognostic factors for human clear cell renal cell carcinoma, Oncol. Rep. 36 (6) (2016) 3131–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Varela I, Tarpey P, Raine K, et al. , Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma, Nature 469 (7331) (2011) 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Pena-Llopis S, Vega-Rubin-de-Celis S, Liao A, et al. , BAP1 loss defines a new class of renal cell carcinoma, Nat. Genet. 44 (7) (2012) 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Uzosike AC, Patel HD, Alam R, Schwen ZR, Gupta M, Gorin MA, Johnson MH, Gausepohl H, Riffon MF, Trock BJ, Chang P, Wagner AA, McKiernan JM, Allaf ME, Pierorazio PM, Growth kinetics of small renal masses on active surveillance: variability and results from the DISSRM registry, J. Urol. 17 (2017) 77614–77618 S0022–5347. [DOI] [PubMed] [Google Scholar]
- [29].Ball MW, Bezerra SM, Gorin MA, Cowan M, Pavlovich CP, Pierorazio PM, Netto GJ, Allaf ME, Grade heterogeneity in small renal masses: potential implications for renal mass biopsy, J. Urol. 193 (1) (2015) 36–40. [DOI] [PubMed] [Google Scholar]
- [30].Campbell S, Uzzo RG, Allaf ME, Bass EB, Cadeddu JA, Chang A, Clark PE, Davis BJ, Derweesh IH, Giambarresi L, Gervais DA, Hu SL, Lane BR,Leibovich BC, Pierorazio PM, Renal mass and localized renal cancer: AUA guideline, J. Urol. 198 (3) (2017) 520–529. [DOI] [PubMed] [Google Scholar]
- [31].Park YR, Chun JN, So I, Kim HJ, Baek S, Jeon JH, Shin SY, Data-driven analysis of TRP channels in cancer: linking variation in gene expression to clinical significance, Cancer Genomics Proteomics 13 (1) (2016) 83–90. [PubMed] [Google Scholar]
- [32].Morgan TM, Seeley EH, Fadare O, Caprioli RM, Clark PE, Imaging the clear cell renal cell carcinoma proteome, J. Urol. 189 (3) (2013) 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chinello C, L’imperio V, Stella M, Smith AJ, Bovo G, Grasso A, Grasso M,Raimondo F, Pitto M, Pagni F, Magni F, The proteomic landscape of renal tumors, Expert Rev. Proteomics 13 (12) (2016) 1103–1120. [DOI] [PubMed] [Google Scholar]
- [34].Song Y, Zhong L, Zhou J, Lu M, Xing T, Ma L, Shen J, Data-independent acquisition-based quantitative proteomic analysis reveals potential biomarkers of kidney cancer, Proteomics Clin. Appl. (2017) 1700066. [DOI] [PubMed] [Google Scholar]
- [35].Bouhamdani N, Joy A, Barnett D, Cormier K, Léger D, Chute IC, Lamarre S,Ouellette R, Turcotte S, Quantitative proteomics to study a small molecule targeting the loss of von hippel-Lindau in renal cell carcinomas, Int. J. Cancer 141 (August (4)) (2017) 778–790, 10.1002/ijc.30774 15, Epub 2017 May 25. [DOI] [PubMed] [Google Scholar]
- [36].Zhao Z, Wu F, Ding S, Sun L, Liu Z, Ding K, Lu J, Label-free quantitative proteomic analysis reveals potential biomarkers and pathways in renal cell carcinoma, Tumour Biol. 36 (2) (2015) 939–951. [DOI] [PubMed] [Google Scholar]
- [37].Zhang L, Jiang H, Xu G, Chu N, Xu N, Wen H, Gu B, Liu J, Mao S, Na R,Jing Y, Ding Q, Zhang Y, Wang L , iTRAQ-based quantitative proteomic analysis reveals potential early diagnostic markers of clear-cell Renal cell carcinoma, Biosci. Trends 10 (3) (2016) 210–219. [DOI] [PubMed] [Google Scholar]
- [38].Zhang Y, Cai Y, Yu H, Li H, iTRAQ-based quantitative proteomic analysis identified HSC71 as a novel serum biomarker for renal cell carcinoma, Biomed Res. Int. 2015 (2015) 802153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nuerrula Y, Rexiati M, Liu Q, Wang YJ, Differential expression and clinical significance of serum protein among patients with clear-cell renal cell carcinoma, Cancer Biomark. 15 (4) (2015) 485–491. [DOI] [PubMed] [Google Scholar]
- [40].Sandim V, Pereira Dde A, Kalume DE, Oliveira-Carvalho AL, Ornellas AA, Soares MR, Alves G, Zingali RB, Proteomic analysis reveals differentially secreted proteins in the urine from patients with clear cell renal cell carcinoma, Urol. Oncol. 34 (1) (2016) e11–e25 5. [DOI] [PubMed] [Google Scholar]
- [41].Papale M, Vocino G, Lucarelli G, Rutigliano M, Gigante M, Rocchetti MT,Pesce F, Sanguedolce F, Bufo P, Battaglia M, Stallone G, Grandaliano G,Carrieri G, Gesualdo L, Ranieri E, Urinary RKIP/p-RKIP is a potential diagnostic and prognostic marker of clear cell renal cell carcinoma, Oncotarget 8 (June (25)) (2017) 40412–40424 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Atrih A, Mudaliar MA, Zakikhani P, Lamont DJ, Huang JT, Bray SE,Barton G, Fleming S, Nabi G, Quantitative proteomics in resected renal cancer tissue for biomarker discovery and profiling, Br. J. Cancer 110 (6) (2014) 1622–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pietrowska M, Gawin M, Polańska J, Widłak P, Tissue fixed with formalin and processed without paraffin embedding is suitable for imaging of both peptides and lipids by MALDI-IMS, Proteomics 16 (11–12) (2016) 1670–1677. [DOI] [PubMed] [Google Scholar]
- [44].Nowell PC, The clonal evolution of tumor cell populations, Science 194 (1976) 23–28. [DOI] [PubMed] [Google Scholar]
- [45].Lawrence MS, Stojanov P, Polak P, et al. , Mutational heterogeneity in cancer and the search for new cancer-associated genes, Nature 499 (7457) (2013) 214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Scelo G, Riazalhosseini Y, Greger L, et al. , Variation in genomic landscape of clear cell renal cell carcinoma across Europe, Nat. Commun. 5 (2014) 5135. [DOI] [PubMed] [Google Scholar]
- [47].Gudbjartsson T, Jónasdóttir TJ, Thoroddsen A, et al. , A population-based familial aggregation analysis indicates genetic contribution in a majority of renal cell carcinomas, Int. J. Cancer 100 (4) (2002) 476–479. [DOI] [PubMed] [Google Scholar]
- [48].Jones TD, Eble JN, Wang M, MacLennan GT, Jain S, Cheng L, Clonal divergence and genetic heterogeneity in clear cell renal cell carcinomas with sarcomatoid transformation, Cancer 104 (2005) 1195–1203. [DOI] [PubMed] [Google Scholar]
- [49].Burrell RA, McGranahan N, Bartek J, Swanton C, The causes and consequences of genetic heterogeneity in cancer evolution, Nature 501 (September (7467)) (2013) 338–345 19. [DOI] [PubMed] [Google Scholar]
- [50].Gerlinger M, et al. , Intratumor heterogeneity and branched evolution revealed by multiregion sequencing, N. Engl. J. Med. 366 (2012) 883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Zhang B, Wang J, Wang X, Zhu J, Liu Q, Shi Z, Chambers MC, Zimmerman LJ, Shaddox KF, Kim S, Davies SR, Wang S, Wang P, Kinsinger CR, Rivers RC,Rodriguez H, Townsend RR, Ellis MJ, Carr SA, Tabb DL, Coffey RJ,Slebos RJ, Liebler DC, NCI CPTAC, Proteogenomic characterization of human colon and rectal cancer, Nature 513 (September (7518)) (2014) 382–387 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Thomas SN, Harlan R, Chen J, Aiyetan P, Liu Y, Sokoll LJ, Aebersold R, Chan DW, Zhang H, Multiplexed targeted mass spectrometry-based assays for the quantification of N-linked glycosite-containing peptides in serum, Anal. Chem. 87(21) (2015) 10830–10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mertins P, Mani DR, Ruggles KV, Gillette MA, Clauser KR, Wang P, Wang X, Qiao JW, Cao S, Petralia F, Kawaler E, Mundt F, Krug K, Tu Z, Lei JT,Gatza ML, Wilkerson M, Perou CM, Yellapantula V, Huang KL, Lin C,McLellan MD, Yan P, Davies SR, Townsend RR, Skates SJ, Wang J, Zhang B, Kinsinger CR, Mesri M, Rodriguez H, Ding L, Paulovich AG, Fenyö D,Ellis MJ, Carr SA, NCI CPTAC, Proteogenomics connects somatic mutations to signalling in breast cancer, Nature 534 (7605) (2016) 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang H, Liu T, Zhang Z, Payne SH, Zhang B, McDermott JE, Zhou JY,Petyuk VA, Chen L, Ray D, Sun S, Yang F, Chen L, Wang J, Shah P, Cha SW,Aiyetan P, Woo S, Tian Y, Gritsenko MA, Clauss TR, Choi C, Monroe ME,Thomas S, Nie S, Wu C, Moore RJ, Tabb DL Yu KH, Fenyö D, Bafna V,Wang Y, Rodriguez H, Boja ES, Hiltke T, Rivers RC, Sokoll L, Zhu H, Shih IM,Cope L, Pandey A, Zhang B, Snyder MP, Levine DA, Smith RD, Chan DW, Rodland KD, CPTAC investigators. Integrated proteogenomic characterization of human high-grade serous ovarian cancer, Cell 166 (3) (2016) 755–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hobeika L, Barati MT, Caster DJ, McLeish KR, Merchant ML, Characterization of glomerular extracellular matrix by proteomic analysis of laser-captured micro-dissected glomeruli, Kidney Int. 91 (2) (2017) 501–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Xu BJ, Shyr Y, Liang X, Ma LJ, Donnert EM, Roberts JD, Zhang X, Kon V, Brown NJ, Caprioli RM, Fogo AB, Proteomic patterns and prediction of glomerulosclerosis and its mechanisms, J. Am. Soc. Nephrol. 16 (October (10)) (2005) 2967–2975. [DOI] [PubMed] [Google Scholar]
- [57].Wittke S, Baxmann S, Fahlenkamp D, Kiessig ST, Tumor heterogeneity as a rationale for a multi-epitope approach in an autologous renal cell cancer tumor vaccine, Onco. Ther. 9 (2016) 523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Li QK, Gabrielson E, Zhang H, Application of glycoproteomics for the discovery of biomarkers in lung cancer, Proteomics Clin. Appl. 6 (2012) 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Unwin RD, Craven RA, Harnden P, Hanrahan S, Totty N, Knowles M,Eardley I, Selby PJ, Banks RE, Proteomic changes in renal cancer and co-ordinate demonstration of both the glycolytic and mitochondrial aspects of the Warburg effect, Proteomics 3 (8) (2003) 1620–1632. [DOI] [PubMed] [Google Scholar]
- [60].Drake PM, Cho W, Li B, Prakobphol A, Johansen E, Anderson NL, Regnier FE, Gibson BW, Fisher SJ, Sweetening the pot: adding glycosylation to the biomarker discovery equation, Clini. Chem. 56 (2010) 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Morelle W, Michalski JC, Analysis of protein glycosylation by mass spectrometry, Nat. Protoc. 2 (7) (2007) 1585–1602. [DOI] [PubMed] [Google Scholar]
- [62].Zhang H, Zha X, Tan Y, Hornbeck PV, Mastrangelo AJ, Alessi DR, Phosphoprotein analysis using antibodies broadly reactive against phosphorylated motifs, J. Biol Chem. 277 (42) (2002) 39379–39387. [DOI] [PubMed] [Google Scholar]
- [63].Helenius A, Aebi M, Intracellular functions of N-linked glycans, Science 291 (2001) 2364–2369. [DOI] [PubMed] [Google Scholar]
- [64].Zhang H, Li XJ, Martin DB, Aebersold R, Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry, Nat. Biotechnol. 21 (6) (2003) 660–666. [DOI] [PubMed] [Google Scholar]
- [65].Bian Y, Li L, Dong M, Liu X, Kaneko T, Cheng K, Liu H, Voss C, Cao X,Wang Y, Litchfield D, Ye M, Li SS, Zou H, Ultra-deep tyrosine phosphoproteomics enabled by a phosphotyrosine superbinder, Nat. Chem. Biol. 12 (11) (2016) 959–966. [DOI] [PubMed] [Google Scholar]
- [66].Peng X, Xu F, Liu S, Li S, Huang Q, Chang L, Wang L, Ma X, He F, Xu P, Identification of missing proteins in the phosphoproteome of kidney cancer, J. Proteome Res. (September) (2017) 12, 10.1021/acs.jproteome. 7b00332 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [67].Brugarolas J, Molecular genetics of clear-cell renal cell carcinoma, J. Clin. Oncol. 32 (June (18)) (2014) 1968–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Neely BA, Wilkins CE, Marlow LA, Malyarenko D, Kim Y, Ignatchenko A,Sasinowska H, Sasinowski M, Nyalwidhe JO, Kislinger T, Copland JA, Drake RR, Proteotranscriptomic analysis reveals stage specific changes in the molecular landscape of clear-cell renal cell carcinoma, PLoS One 11 (4) (2016) e0154074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Zheng J, Wang L, Peng Z, Yang Y, Feng D, He J, Low level of PDZ domain containing 1 (PDZK1) predicts poor clinical outcome in patients with clear cell renal cell carcinoma, EBioMedicine 15 (2017) 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S,Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M,Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM, TARGET Study Group, Sorafenib in advanced clear-cell renal-cell carcinoma, N. Engl. J. Med. 356 (January (2)) (2007) 125–134 11. [DOI] [PubMed] [Google Scholar]
- [71].Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O,Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA, Sunitinib versus interferon alfa in metastatic renal-cell carcinoma, N. Engl. J. Med. 356 (January (2)) (2007) 115–124 11. [DOI] [PubMed] [Google Scholar]
- [72].Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JR, Jr N Munshi, E.D. Crawford, Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer, N. Engl.J. Med. 345 (December (23)) (2001) 1655–1659 6. [DOI] [PubMed] [Google Scholar]
- [73].Motzer RJ, Tannir NM, McDermott DF, Arén Frontera O, Melichar B, Choueiri TK, Plimack ER, Barthélémy P, Porta C, George S, Powles T,Donskov F, Neiman V, Kollmannsberger CK, Salman P, Gurney H, Hawkins R,Ravaud A, Grimm MO, Bracarda S, Barrios CH, Tomita Y, Castellano D,Rini BI, Chen AC, Mekan S, McHenry MB, Wind-Rotolo M, Doan J, Sharma P, Hammers HJ, Escudier B, CheckMate 214 investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma, N. Engl. J. Med. 378 (April (14)) (2018) 1277–1290, 10.1056/NEJMoa1712126 5, Epub 2018 Mar 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Tian H, Zhang B, Di J, Jiang G, Chen F, Li H, Li L, Pei D, Zheng J, Keap1: one stone kills three birds Nrf2, IKKβ and Bcl-2/Bcl-xL, Cancer Lett. 325 (2012) 26–34. [DOI] [PubMed] [Google Scholar]
- [75].Li QK, Singh A, Biswal S, Askin F, Gabrielson E, KEAP1 gene mutations and NRF2 activation are common in pulmonary papillary adenocarcinoma, J. Hum. Genet. 56 (2011) 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]