Abstract
Immune checkpoint inhibitors have shown anti-tumour activity in cancers such as melanoma, renal cell carcinoma, non-small-cell lung cancer, urothelial carcinoma, colorectal cancer, and Hodgkin's lymphoma. Though immune checkpoint inhibitors have revolutionized the treatment and prognosis of some advanced malignancies, they are also associated with a significant risk of immune-related adverse events. These adverse events can occur in any organ system, but gastrointestinal side effects are among the most commonly reported, with manifestations ranging from mild diarrhoea to severe colitis, sharing some features with inflammatory bowel disease. Anticipating a greater use of these drugs in the future, gastroenterologists should expect to be increasingly faced with gastrointestinal immune-related adverse events. Knowledge of these toxicities, as well as effective management algorithms, is essential to enable early diagnosis and treatment, decreasing morbidity and mortality. We reviewed the currently available literature on gastrointestinal toxicity induced by immune checkpoint inhibitors, namely the clinical features, diagnosis, and management.
Key Words: Immune checkpoint inhibitor, Immune-related adverse event, Colitis, Diarrhoea
Resumo
Os inibidores dos checkpoint imunológicos demonstraram atividade antitumoral em neoplasias como o melanoma, o carcinoma de células renais, o carcinoma pulmonar de não pequenas células, o carcinoma urotelial, o carcinoma colorretal e o linfoma de Hodgkin. Estes fármacos, embora tenham revolucionado o tratamento e o prognóstico de algumas neoplasias avançadas, também se associam a um risco considerável de efeitos adversos associados
ao sistema imunitário. Qualquer órgão pode ser afetado, mas os efeitos adversos gastrointestinais estão entre os mais comumente relatados. As manifestações gastrointestinais variam desde diarreia ligeira a colite grave, a qual pode evidenciar características idênticas às da doença inflamatória intestinal. Antecipando uma maior utilização destes fármacos no futuro, os gastroenterologistas devem esperar confrontar-se cada vez mais com este tipo de efeitos adversos. O conhecimento dessas toxicidades, bem como dos algoritmos de abordagem destes doentes é fundamental para um diagnóstico e tratamento precoces, diminuindo desta forma a morbilidade e a mortalidade. Os autores pretenderam rever a literatura mais atual sobre a toxicidade gastrointestinal induzida pelos inibidores dos checkpoint imunológicos, nomeadamente apresentação clínica, abordagem diagnóstica e terapêutica.
Palavras Chave: Inibidores dos “checkpoint” imunológicos ·, “Immune-related adverse event”, Colite, Diarreia
Introduction
Immunotherapy with monoclonal antibodies targeting cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and the programmed death-1 receptor (PD-1) and its ligand PD-L1 have joined the list of standard and effective anti-tumour therapy. Therefore, an increasing number of patients will be exposed to these drugs creating a new set of challenges for clinicians, who must develop a working knowledge of these agents, namely how to diagnose and effectively manage their toxicities [1, 2].
The physiological role of immune checkpoints, such as CTLA-4 and PD-1, is to regulate the degree of T cell activation, to prevent exaggerated immune responses or autoimmunity. CTLA-4 helps maintain peripheral immune tolerance and regulate adaptive immune responses by competing with CD28 to bind the B7 receptor on antigen-presenting cells, thus downregulating T-cell activation. CTLA-4 is constitutively expressed by regulatory T cells (Treg cells), an immunomodulatory subset of CD4+ effector T cells, which have an instrumental role in immunological tolerance. In addition to producing immunosuppressive cytokines, Treg cells might also affect activation of conventional CD4+ T cells and CD8+ T cells [3, 4, 5].
PD-1 is a cell surface co-inhibitory receptor, expressed on the surface of T cells, B cells, monocytes, natural killer T cells, and dendritic cells. In T cells, expression and localization of PD-1 to the cell surface requires activation through the T-cell receptor (TCR) and cytokine signaling. Binding of PD-L1 to PD-1 triggers the inhibition of the TCR signaling pathway and reduces the expression and production of cytokines and transcription factors that are associated with effector T-cell function. The PD-1 pathway might also promote Treg cell suppression and has been implicated as an important checkpoint in the activation and development of innate lymphoid cells, involved as significant effector cells at mucosal surfaces and especially the gut [3, 4, 5].
Given the central role of host immunity in the detection, recognition, and deletion of emergent neoplastic cells, targeting pathways that intrinsically inhibit T-cell activation was conceived as an attractive therapeutic strategy [5]. Nevertheless, a disruption in the functioning of immune checkpoint molecules can lead to imbalances in immunologic tolerance, which may manifest with autoimmune-like/inflammatory side-effects, designated immune-related adverse events (irAEs) [2].
In recent years, targeted immunotherapy against these immune checkpoints has been widely explored and is now approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of advanced melanoma, non-small cell lung carcinoma, renal cell carcinoma, colorectal carcinoma, Hodgkin's lymphoma, and urothelial carcinoma [1, 3, 5]. Approved immune checkpoint inhibitors (ICPIs) include five anti-PD-1 antibodies (nivolumab, pembrolizumab, atezolizumab, avelumab, and durvalumab) and one CTLA-4 inhibitor (ipilimumab) [3, 5].
Knowledge of toxicities associated with immunotherapy, as well as effective management algorithms for these toxicities, is pivotal in order to optimize clinical efficacy and safety. In this review, we describe gastrointestinal toxicity from checkpoints inhibitors targeting CTLA-4 and PD-1 and provide general recommendations for the appropriate management of these irAEs.
Epidemiology
Gastrointestinal toxicity is one of the most frequent and often the most severe of irAEs associated with anti-CTLA-4, leading to its discontinuation [6]. About one-third of patients treated with anti-CTLA-4 have irAEs of the gastrointestinal tract, including aphthous ulcers, esophagitis, gastritis, diarrhoea, and colitis. The frequency of colitis ranges from 8% to 22% [7]. In a large study including 676 patients, diarrhoea of any grade occurred in 27–31% of patients receiving ipilimumab [8]. In a phase III trial of 511 patients treated with ipilimumab for malignant melanoma, 26 (5%) were hospitalized for severe enterocolitis, 5 (1%) developed intestinal perforation, and 4 (0.8%) died as a result of complications [5]. In a study, enterocolitis was the most frequent irAE associated with ipilimumab use, occurring in 21% of patients [9]. In a multicenter study, the reported incidences of diarrhoea, colitis, and grade 3–4 colitis were, respectively, 54, 22, and 16% [10]. One study found that diarrhoea of any grade occurs in approximately 45% of patients on combination therapy, 34% of patients on ipilimumab monotherapy, and 21% of patients on nivolumab monotherapy; with grade 3–4 colitis occurring in 8% of patients on combination therapy, 8% of patients on ipilimumab monotherapy, and 1% of patients on nivolumab monotherapy [11]. PD-1 and PDL-1 inhibitors, either nivolumab or pembrolizumab, seem to be less toxic to the gastrointestinal tract than anti-CTLA-4 agents, with grade 3 to 4 irAEs occurring only in 1–2% of cases [1, 2, 3, 5]. Recent studies have suggested that patients exposed to nivolumab followed by ipilimumab, or the reverse sequence, who developed previous gastrointestinal toxicity with one of the drugs do not necessarily do so when subsequently treated with the other agent [1, 5]. It is not completely understood whether the effect on the frequency and severity of irAEs is dose dependent. A phase II trial demonstrated an association between the incidence and severity of irAEs and increasing ipilimumab dose [7].
Clinical Features
Side effects associated with ICPIs are frequently mild and transient. Diarrhoea is the most commonly reported gastrointestinal symptom [3, 12]. Watery, nonbloody diarrhoea occurs in 19% of patients with anti-PD-1/PD-L1 therapy and 33% receiving anti-CTLA-4 therapy. Diarrhoea and colitis, including severe forms, occur earlier and are more frequent with combined anti-CTLA-4 and anti-PD-1 agents than with either ipilimumab or anti-PD-1 [13]. Other presenting symptoms are abdominal pain, haematochezia, weight loss, fever, and vomiting [1, 7, 13]. Anti-CTLA-4-induced enterocolitis may be associated with mouth ulcers, anal lesions (fistulas, abscesses, fissures), and extra-intestinal manifestations [14]. The clinician should be alert to the possibility of colitis when bloody diarrhoea is present in conjunction with fever, abdominal pain, or mucus in the stool [12]. Colitis can mimic inflammatory bowel disease (IBD) [14]. Diarrhoea and colitis severity can be graded according to the Common Terminology Criteria for Adverse Events (Table 1) [13].
Table 1.
Common Terminology Criteria for Adverse Events (CTCAE) for diarrhoea and colitis
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|---|
| Diarrhoea | Increase of <4 stools per day over baseline; mild increase in ostomy output compared to baseline | Increase of 4–6 stools per day over baseline; moderate increase in ostomy output compared to baseline; limiting instrumental ADL | Increase of ≥7 stools per day over baseline; severe increase in ostomy output compared to baseline; hospitalization indicated; limiting self-care ADL | Life-threatening consequences; urgent intervention indicated | Death |
| Colitis | Asymptomatic; clinical or diagnostic observations only; intervention not indicated | Abdominal pain; mucus or blood in stool | Severe abdominal pain; peritoneal signs | Life-threatening consequences; urgent intervention indicated | Death |
ADL, activities of daily life. Adapted from the Cancer Therapy Evaluation Program, National Cancer Institute, Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
Time for colitis development after ICPI administration has been variable. Onset of gastrointestinal symptoms may occur at any time during 1–10 infusions of anti-CTLA-4 [1, 7]. Colitis may even occur several months after the last dose of ipilimumab, because the molecular effects on CTLA-4 inhibition may persist longer after clearance of the drug [7]. Colitis has been reported after the first dose of ipilimumab and more than 4 months after administration of the last dose [15]. A case series of 19 patients on anti-PD-1 therapy reported a median time of 3 months from drug initiation to symptom onset [16].
Less commonly, the upper gastrointestinal tract is involved and clinical manifestations include aphthous ulcers, esophagitis, and gastritis [13]. Nausea, vomiting, dysphagia, and epigastric pain may be present [1, 17]. These symptoms may develop later than those related to the lower gastrointestinal tract [18].
Diagnosis
The main biological abnormalities in 39 patients with anti-CTLA-4 induced enterocolitis were anaemia, increased serum C-reactive protein, and low serum albumin levels; faecal calprotectin, measured in 8 patients at the time of enterocolitis, was markedly increased [14]. The main differential diagnoses of ICPI-induced enterocolitis are gastrointestinal infections and tumour-related symptoms [1]. The initial diagnostics tests should include stool analyses for bacterial enteropathogens, parasites, and Clostridium difficile toxin [1, 12, 19]. In patients with disseminated melanoma, gastrointestinal metastases are not rare and should be excluded [1].
A definitive diagnosis of immunotherapy-induced enterocolitis must be based on the endoscopic and histopathological evaluation [1, 12, 19]. In the majority of patients, the sigmoid and the rectum are involved and, therefore, a flexible sigmoidoscopy is generally sufficient to make the diagnosis. However, in some patients, lesions affect only the proximal part of the colon or have a patchy distribution. A study of 39 patients with anti-CTLA-4 enterocolitis showed that in 38/39 patients (97%) there was involvement of the rectum and/or sigmoid, extensive colitis was observed in 23/35 patients (66%), and a patchy distribution in 18/33 patients (55%). In patients who underwent ileoscopy, ileitis was found in 20% [14]. The nature of intestinal inflammation caused by ICPIs shares several morphological features with those seen in IBD [5, 14, 16, 20]. Endoscopic findings can include oedema, erythema, loss of vascular pattern, erosions, and ulceration [1, 3, 5, 14]. In a systematic review of colitis associated with anti-CTLA-4 therapy, colonoscopy revealed diffuse inflammatory changes such as exudates, granularity, loss of vascular pattern, and ulcerations [7].
Clinical correlation and medication history are essential as in any other drug-related colitis, the histological features of colitis associated with ICPIs are non-specific and can mimic other forms of colitis such as infectious colitis, IBD, ischemic colitis, and other drug-related forms of colitis [3]. The vast majority of patients has features of an acute colitis, including either focal active colitis with patchy crypt abscesses or diffuse mucosal acute inflammation. In some cases, abnormalities consistent with chronic IBD such as granulomas, basal plasmocytosis, and crypt abnormalities have been reported [5, 14, 16]. Anti-CTLA-4 related colitis is characterized by neutrophilic inflammation with increased intraepithelial lymphocytes, crypt epithelial cell apoptosis, and few or no features of chronicity. Anti-PD-1-associated colitis typically follows one of two patterns: active colitis pattern with increased apoptosis (active inflammation, neutrophilic crypt micro-abscesses, increased crypt epithelial cell apoptosis, and presence of crypt atrophy/dropout) or a lymphocytic colitis pattern (increased intraepithelial lymphocytes in surface epithelium, surface epithelial injury, and expansion of the lamina propria) [12]. Pathology with immunohistochemical staining to rule out CMV infection is generally recommended in any immunocompromised patient with an enterocolitis [16].
Upper gastrointestinal symptoms (dysphagia, epigastric pain…) should prompt upper endoscopy [1, 7]. In a French and Belgian multicentric study, of the 22 patients with anti-CTLA-4-induced enterocolitis who underwent upper endoscopy, one had ulceration of the mid-oesophagus, 9 had gastritis, and 2 had erosive duodenitis. About half of the patients with anti-CTLA-4-induced enterocolitis have chronic, mild, patchy inflammation of the stomach and the duodenum [14]. The most commonly observed histological changes in the gastric mucosa were lamina propria expansion and intraepithelial neutrophils; the duodenal and ileal biopsies commonly showed lamina propria expansion by lymphoplasmocytic infiltrates and eosinophils, villous blunting, intraepithelial lymphocytes, and neutrophilic villitis [3].
Management
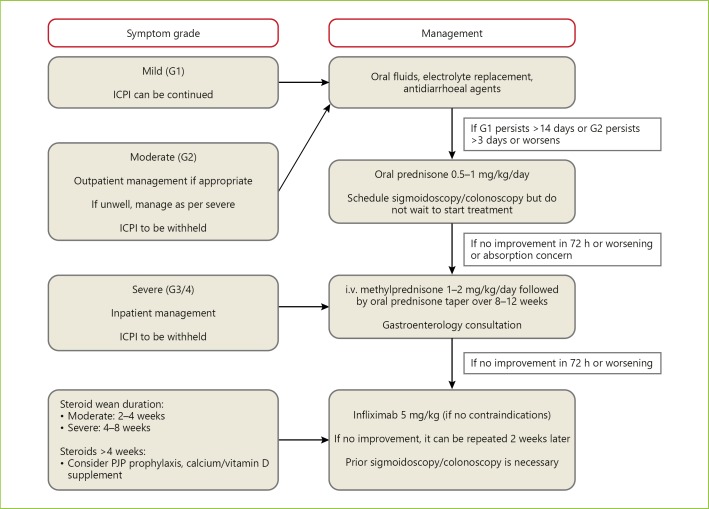
Patients on immunotherapy that develop mild diarrhoea can continue ICPI therapy (Fig. 1). Management of patients with non-severe diarrhoea is mainly supportive, with antidiarrhoeals, fluid, and electrolyte supplementation. Persistence of grade 2 diarrhoea (defined as four to six stools per day for more than 3 days), should prompt initiation of 0.5 to 1 mg/kg/day of oral corticosteroids and ICPIs should be withheld [2, 3, 5, 13, 19, 21, 22]. The use of budesonide seems attractive since it might avoid the systemic effects of corticosteroids, though its effect in this context seems limited. Loperamide should be withheld to avoid the risk of precipitating toxic dilatation. Thromboprophylaxis with low-molecular-weight heparin should be initiated since these patients are at increased risk of thromboembolism [5]. At this stage, it is advisable to consult gastroenterology specialists and confirm colitis by endoscopy and histology, although this approach should not delay initiation of corticosteroids. If symptoms do not improve with oral corticosteroids, patient hospitalization for intravenous (i.v.) corticosteroids should be considered. Patients with grade 3 and 4 colitis (≥7 stools per day, severe abdominal pain, or complications) require hospitalization and should receive treatment with systemic corticosteroids (1–2 mg/kg per day, i.v.). If response to i.v. corticosteroids is obtained within 3–5 days, they should be switched to the oral form and tapered over 8–12 weeks. Patients that do not improve with this therapy within 3–5 days or have a relapse requiring increase in the corticosteroid dosage during the course of steroid tapering should be switched to infliximab 5 mg/kg, unless contraindicated [3, 5, 13, 14, 19, 23]. Due its severe course, patients with immunotherapy-induced enterocolitis should be carefully monitored and a rapid escalation to infliximab should be advocated in patients who do not respond to corticosteroids [14, 23]. Almost all patients respond very well to a single dose of infliximab; in some cases, a second dose is needed 2 weeks later [14, 19]. When a suboptimal response is thought to be due to excessive enteral losses from inflamed gut, administering the drug sooner or at a higher dose (10 mg/kg) should be considered [5]. Vedolizumab is a humanized monoclonal IgG1 antibody against α4β7 integrin, approved for treatment of IBD. Some case reports suggest that vedolizumab may be an effective drug in steroid-refractory or steroid-dependent patients [24, 25]. A recent study with seven patients with mild to moderate, steroid-dependent or steroid-refractory ipilimumab-induced enterocolitis has revealed that six out of seven patients treated with vedolizumab achieve remission and no adverse events were reported [26]. Neither corticosteroids nor infliximab appear to influence response and survival of patients treated with ICPIs [1]. When a severe life-threatening enterocolitis develops, irresponsive to any medical treatment, which may result in perforation of the intestine, colectomy with ileostomy should be performed [1, 5]. Restarting the immunotherapy after successful management of the enterocolitis poses a high risk of relapse and should always be individualized. Usually, in grade 3 and 4 colitis, ICPI therapy is often permanently discontinued [1, 13, 19].
Fig. 1.
Management of gastrointestinal toxicity of immune checkpoint inhibitors.
Conclusion
The discovery of immune checkpoint proteins was a major breakthrough in cancer immunotherapy, which significantly impacted the management of patients with solid organ and hematopoietic malignancies. ICPIs are becoming an increasingly more common therapeutic approach in patients with advanced malignancy as a growing number of new ICPI agents are being subjected to clinical evaluation in numerous types of tumours; subsequently, the prevalence of ICPI-related adverse events may be expected to increase considerably in the near future. Gastroenterologists must be aware of these drugs, their gastrointestinal adverse event profiles, and how to manage them, taking the long-term cancer treatment strategy and the overall survival into account. Early recognition of the disease and close collaboration between oncologists, gastroenterologists, and surgeons is mandatory for optimal management of these patients.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28((suppl_4)):iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 2.Naidoo J, Page DB, Li BT, Connell LC, Schindler K, Lacouture ME, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol. 2015 Dec;26((12)):2375–91. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assarzadegan N, Montgomery E, Anders RA. Immune checkpoint inhibitor colitis: the flip side of the wonder drugs. Virchows Arch. 2018 Jan;472((1)):125–33. doi: 10.1007/s00428-017-2267-z. [DOI] [PubMed] [Google Scholar]
- 4.Boutros C, Tarhini A, Routier E, Lambotte O, Ladurie FL, Carbonnel F, et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016 Aug;13((8)):473–86. doi: 10.1038/nrclinonc.2016.58. [DOI] [PubMed] [Google Scholar]
- 5.Samaan MA, Pavlidis P, Papa S, Powell N, Irving PM. Gastrointestinal toxicity of immune checkpoint inhibitors: from mechanisms to management. Nat Rev Gastroenterol Hepatol. 2018 Apr;15((4)):222–34. doi: 10.1038/nrgastro.2018.14. [DOI] [PubMed] [Google Scholar]
- 6.Horvat TZ, Adel NG, Dang TO, Momtaz P, Postow MA, Callahan MK, et al. Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol. 2015 Oct;33((28)):3193–8. doi: 10.1200/JCO.2015.60.8448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A, De Felice KM, Loftus EV, Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015 Aug;42((4)):406–17. doi: 10.1111/apt.13281. [DOI] [PubMed] [Google Scholar]
- 8.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010 Aug;363((8)):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck KE, Blansfield JA, Tran KQ, Feldman AL, Hughes MS, Royal RE, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006 May;24((15)):2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013 Jul;24((7)):1813–21. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017 Oct;377((14)):1345–56. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puzanov I, Diab A, Abdallah K, Bingham CO, 3rd, Brogdon C, Dadu R, et al. Society for Immunotherapy of Cancer Toxicity Management Working Group Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J Immunother Cancer. 2017 Nov;5((1)):95. doi: 10.1186/s40425-017-0300-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marin-Acevedo JA, Harris DM, Burton MC. Immunotherapy-Induced Colitis: An Emerging Problem for the Hospitalist. J Hosp Med. 2018 Jun;13((6)):413–8. doi: 10.12788/jhm.2925. [DOI] [PubMed] [Google Scholar]
- 14.Marthey L, Mateus C, Mussini C, Nachury M, Nancey S, Grange F, et al. Cancer immunotherapy with antiCTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohn's Colitis. 2016 Apr;10((4)):395–401. doi: 10.1093/ecco-jcc/jjv227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lord JD, Hackman RC, Moklebust A, Thompson JA, Higano CS, Chielens D, et al. Refractory colitis following anti-CTLA4 antibody therapy: analysis of mucosal FOXP3+ T cells. Dig Dis Sci. 2010 May;55((5)):1396–405. doi: 10.1007/s10620-009-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez RS, Salaria SN, Bohannon CD, Huber AR, Feely MM, Shi C. PD-1 inhibitor gastroenterocolitis: case series and appraisal of ‘immunomodulatory gastroenterocolitis’. Histopathology. 2017 Mar;70((4)):558–67. doi: 10.1111/his.13118. [DOI] [PubMed] [Google Scholar]
- 17.Grover S, Rahma OE, Hashemi N, Lim RM. Gastrointestinal and Hepatic Toxicities of Checkpoint Inhibitors: algorithms for Management. Am Soc Clin Oncol Educ Book. 2018 May;((38)):13–9. doi: 10.1200/EDBK_100013. [DOI] [PubMed] [Google Scholar]
- 18.Onuki T, Morita E, Sakamoto N, Nagai Y, Sata M, Hagiwara K. Severe upper gastrointestinal disorders in pembrolizumab-treated non-small cell lung cancer patient. Respirol Case Rep. 2018 Aug;6((6)):e00334. doi: 10.1002/rcr2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rawa T, Reguła J. Management of gastrointestinal toxicity from nivolumab therapy. Oncol Clin Pract. 2017;5:225–9. [Google Scholar]
- 20.Kubo K, Kato M, Mabe K. Nivolumab-Associated Colitis Mimicking Ulcerative Colitis. Clin Gastroenterol Hepatol. 2017 Sep;15((9)):A35–6. doi: 10.1016/j.cgh.2017.03.026. [DOI] [PubMed] [Google Scholar]
- 21.Eigentler TK, Hassel JC, Berking C, Aberle J, Bachmann O, Grünwald V, et al. Diagnosis, monitoring and management of immune-related adverse drug reactions of anti-PD-1 antibody therapy. Cancer Treat Rev. 2016 Apr;45:7–18. doi: 10.1016/j.ctrv.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Kähler KC, Hassel JC, Heinzerling L, Loquai C, Mössner R, Ugurel S, et al. “Cutaneous Side Effects” Committee of the Work Group Dermatological Oncology (ADO) Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma. J Dtsch Dermatol Ges. 2016 Jul;14((7)):662–81. doi: 10.1111/ddg.13047. [DOI] [PubMed] [Google Scholar]
- 23.Arriola E, Wheater M, Karydis I, Thomas G, Ottensmeier C. Infliximab for IPILIMUMAB-Related Colitis-Letter. Clin Cancer Res. 2015 Dec;21((24)):5642–3. doi: 10.1158/1078-0432.CCR-15-2471. [DOI] [PubMed] [Google Scholar]
- 24.Diana P, Mankongpaisarnrung C, Atkins MB, Zeck JC, Charabaty A. Emerging Role of Vedolizumab in Managing Refractory Immune Checkpoint Inhibitor-Induced Enteritis. ACG Case Rep J. 2018 Feb;5:e17. doi: 10.14309/crj.2018.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh AH, Ferman M, Brown MP, Andrews JM. Vedolizumab: a novel treatment for ipilimumab-induced colitis. BMJ Case Rep. 2016 Aug;2016:bcr2016216641. doi: 10.1136/bcr-2016-216641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergqvist V, Hertervig E, Gedeon P, Kopljar M, Griph H, Kinhult S, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017 May;66((5)):581–92. doi: 10.1007/s00262-017-1962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]