Abstract
The role of chronic inflammation in the pathogenesis of type 2 diabetes mellitus and associated complications is now well established. Therapeutic interventions counteracting metabolic inflammation improve insulin secretion and action and glucose control and may prevent long-term complications. Thus, a number of anti-inflammatory drugs approved for the treatment of other inflammatory conditions are evaluated in patients with metabolic syndrome. Most advanced are clinical studies with IL-1 antagonists showing improved β-cell function and glycemia and prevention of cardiovascular diseases and heart failure. However, alternative anti-inflammatory treatments, alone or in combinations, may turn out to be more effective, depending on genetic predispositions, duration, and manifestation of the disease. Thus, there is a great need for comprehensive and well-designed clinical studies to implement anti-inflammatory drugs in the treatment of patients with metabolic syndrome and its associated conditions.
Essential Points
In patients with metabolic syndrome, activation of the innate immune system contributes to the development of type 2 diabetes and associated complications
Treatment of patients with type 2 diabetes with an IL-1 antagonist may improve insulin secretion and glycemia and prevent cardiovascular complications and heart failure and possibly other complications of diabetes
Other anti-inflammatory treatments, alone or in combinations, may be more effective, depending on genetic predispositions, duration, and manifestation of the disease
Anti-inflammatory treatments may improve simultaneously diabetes and associated inflammatory diseases such as rheumatoid arthritis, gout, or psoriasis
Over the past decades, major progress has been made in the understanding of the role of the immune system in the regulation of metabolism. It is now evident that inflammation contributes to the regulation of tissue adaptation to changes in metabolism in all situations, from physiology to pathology. Based on these pathogenetic findings, novel therapeutic opportunities arise, with the potential to palliate not only glycemia but also to prevent disease progression and beneficially target microvascular and macrovascular complications of diabetes. However, clinical translation is challenging, mainly due to the difficulties with implementing novel concepts. Therefore, the aim of this review is to generate interest in an emerging field and promote clinical translation. Accordingly, the main focus of this article is on clinically relevant aspects of metabolic inflammation.
Physiological Role of the Immune System in the Regulation of Metabolism
The endocrine system regulates the resorption, repartition, and metabolism of nutrients (commonly termed “metabolism”). The function of the immune system is to secure tissue integrity and repair. Although metabolism and immunity have distinct functions, both systems are required for maintaining and restoring homeostasis of an organism (1). Indeed, mounting an immune defense requires energy that needs to be directed to immune cells by endocrine signals. As an example, cortisol induces insulin resistance in muscle, liver, and fat, leading to increased circulating glucose concentrations that are consumed by immune cells during an infection. The availability of glucose will not only supply energy to immune cells, but also has a signaling function, delivering the message that sufficient energy and nutrients are available to mount a full immune response to a pathogen (2, 3). This intimacy between immunity and metabolism may explain why individuals are more prone to infection diseases in situations of a famine or food restriction. Indeed, it can be speculated that the limited calories available will then be used for basic vital functions, precluding the body to direct energy to the immune system.
Conversely, increasing evidence shows that the immune system contributes to the control of metabolism. Thereby, the NACHT, LRR, and PYD domain-containing protein 3 (NLRP3) inflammasome appears to be a sensor of metabolic changes, detecting increased glucose, fatty acid, and uric acid concentrations (4–9). The NLRP3 inflammasome is a multiprotein complex that consists of three subunits, the sensor molecules NLRP3 and apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC) as well as the effector protease caspase-1 (also termed IL-1–converting enzyme). Upon stimulation, ASC self-associates and forms so-called ASC specks, which then allows the caspase-1 to become active in an autocatalytic manner. Active caspase-1 processes pro–IL-1β and pro–IL-18 into their mature active forms. In nonactivated myeloid cells, the cellular levels of ASC and caspase-1 are stable, but NLRP3 seems to be a limiting factor, suggesting a biphasic model, in which NLPR3 gene transcription needs to be induced first [(10), “inflammasome priming”] before an activating stimulus can trigger ASC and caspase-1 recruitment, which eventually leads to maturation of IL-1β and IL-18. Several etiological agents are known activators of the NLRP3 inflammasome, thereby driving metabolic disorders. As such, glucose, fatty acids, and islet amyloid polypeptide activate the inflammasome in type 2 diabetes, which leads to pancreatic islet inflammation and subsequent β-cell failure and destruction (11–13). Furthermore, depending on food intake and dietary fiber content, the microbiota may have either stimulating effects on NLRP3 via bacterial components or inhibitory effects via metabolites such as short-chain fatty acids (14–16). In gout, deposition of uric acid crystals in joints and periarticular tissues engages the NLRP3 inflammasome, leading to increased IL-1β and IL-18 (4). In atherosclerosis, cholesterol crystals in the artery wall activate the NLRP3 inflammasome, contributing to the inflammatory state in this disease (17). Rodent models that lack components of the inflammasome or downstream targets thereof were used to mimic human disease and identified the NLRP3 inflammasome as a required component in many of these entities. Clinical translation using NLRP3 inhibitors is currently in phase 1 to 2. Furthermore, there is also inflammasome-independent cytokine activation in metabolic diseases. For example, IL-1α, which does not require cleavage by the NLRP3 inflammasome to be active, mediates inflammasome-independent vascular inflammation and is released via free fatty acid–mediated uncoupling of mitochondrial respiration (18). Downstream of the inflammasome IL-1β, one of the first described cytokines (19), and the IL-1–dependent IL-6 and IL-33 emerge as important physiological modulators of insulin secretion and glucose disposal (20–22) (Fig. 1). Indeed, food ingestion increases the number of peritoneal macrophages, which are stimulated by the gut flora and glucose to increase the release of IL-1β in mice (20) and humans (23). Thereafter, IL-1β participates in the postprandial stimulation of insulin secretion (20, 24, 25) by activating the highly expressed IL-1 receptor type 1 on pancreatic β-cells (26). Further, insulin stimulates macrophage-derived pro–IL-1β maturation via the NLRP3 inflammasome (20) (Fig. 2). This occurs via enhanced phosphorylation of AKT downstream of the insulin receptor, upregulation of hexokinase 2, and increased glycolysis and reactive oxygen species. Elevated glucose metabolism promotes serine and glutathione production, which is essential for IL-1β mRNA expression (27). Finally, both insulin and IL-1β regulate glucose disposal, by which IL-1β promotes the major blood glucose regulatory hormone insulin and in addition directly stimulates glucose uptake into the immune cell compartment. These interactions between metabolism and innate immunity suggest that the postprandial increase of IL-1β contributes to the regulation of insulin secretion as well as glucose disposal. Thereby, IL-1β alerts and fuels the immune system, possibly to prevent the potential dissemination of microorganisms contained in the food (20) and to contribute to acute islet compensation (24).
Figure 1.
Immune regulation of insulin secretion in physiology. Various cytokines signal to the pancreatic β-cells and promote insulin secretion. Release of IL-6 from exercising muscle promotes the release of the incretin hormone GLP-1 from enteroendocrine l-cells and pancreatic α-cells to stimulate insulin secretion. IL-33 induces innate lymphoid cells 2 cells to release CSF2 and IL-13, which promote retinoic acid secretion from dendritic cells and macrophages. IL-22 derived from natural killer cells and innate lymphoid cells improve β-cell function. Acute IL-1β derived from macrophages upon feeding promote insulin secretion via IL-1 receptor 1 (IL-1R1) in the β-cells.
Figure 2.
Food intake regulation of IL-1β secretion. Food intake promotes IL-1β production in macrophages via stimulation of glucose metabolism and gut flora products. Glucose enters macrophages via Glut1 and is metabolized via the rate limiting enzyme hexokinase 2. Increased glycolysis and reactive oxygen species (ROS) production induce the processing of pro–IL-1β to mature IL-1β via the NLRP3 inflammasome. Enhanced glucose metabolism increases serine and glutathione production, which is required for IL-1β mRNA expression. The gut flora further promote IL-1β gene expression. Insulin reinforces the proinflammatory state of macrophages via the insulin receptor and stimulation of pAKT (protein kinase B). IL-1β induces the counterregulatory IL-1 receptor antagonist (IL-1Ra).
Another example of an adaptive cross talk between immunity and metabolism is the role of IL-6 in the regulation of GLP-1. Indeed, IL-6 can be seen as the first described myokine, because it is produced and released by the contracting skeletal muscle during exercise (28). Interestingly, IL-6 stimulates GLP-1 secretion from intestinal l-cells and reprograms pancreatic α-cells to process proglucagon to GLP-1 via upregulation of prohormone convertase 1/3 (21, 29). Hence, IL-6 mediates a metabolic cross talk among insulin-sensitive tissues, l-cells, and pancreatic islets to adapt to changes in insulin demand. This interaction between incretin hormones and cytokines is bidirectional, because the GLP-1 analog exenatide increases plasma concentration of the anti-inflammatory IL-1 receptor antagonist IL-1Ra in patients with type 2 diabetes (30). An additional aspect of the metabolic function of IL-6 is its role in the regulation of visceral adipose tissue. Hence, it has recently been shown that exercise-mediated loss of visceral adipose tissue mass requires IL-6 receptor signaling (31). Beyond these specific examples, IL-6 displays multiple complex metabolic effects that have been reviewed previously (32–34).
In line with the adaptive mission of the immune system, cytokines and immune cells often regulate metabolic adaptations in tissues in response to altered energy needs. Supporting this notion, IL-33, another member of the IL-1 family, also emerges as a positive regulator of insulin secretion (22). Indeed, IL-33 is expressed by islet mesenchymal cells and is enhanced by a diabetic milieu characterized by increased levels of glucose, IL-1β, and saturated fatty acids. IL-33 promotes β-cell function through islet-resident group 2 innate lymphoid cells by stimulating macrophages and dendritic cells via IL-13 and colony-stimulating factor 2 (22). These findings illustrate an additional cross talk between immune cells and endocrine cells that contributes to the maintenance of insulin secretion.
A particularly interesting cytokine is IL-22, a member of the IL-10 family, which plays a specific role in the regulation of metabolism. IL-22 contributes to the preservation of the gut mucosal barrier and thereby prevents endotoxemia with subsequent chronic inflammation and insulin resistance (35). Furthermore, IL-22 protects β-cells from oxidative and endoplasmic reticulum stress and improves glycemia in animal models of diabetes (36).
One of the newest members of the IL-1 family is IL-37 (37). Unlike other IL-1–related cytokines, IL-37 broadly suppresses innate immunity via binding to the IL-18 receptor and subsequent recruitment of the orphan decoy IL-1R8 to suppress proinflammatory cytokines. Circulating IL-37 concentrations are low in healthy humans but are induced locally and also systemically in several diseases such as rheumatoid arthritis (38), potentially as a counterregulatory mechanism to limit damage. However, other diseases are also associated with reduced levels of IL-37, suggesting that a failure to mount an IL-37 response could drive disease progression. Importantly for metabolic syndrome, IL-37 levels in adipose tissue positively correlated with insulin secretion and lower inflammatory status in humans and mice (39). Employing transgenic mice that express human IL-37 or therapy with recombinant IL-37 revealed a generally reduced innate immune cell response and a beneficial role for IL-37 in various disease models, such as systemic endotoxemia (40), myocardial infarction (41), colitis (42), and aging-induced damage (43). This supports a therapeutic potential for IL-37 in inflammatory and autoimmune diseases.
Finally, intra- and peri-islet macrophages may participate in the increase of β-cell mass during obesity by promoting β-cell proliferation (44). Thereby, M2 macrophages may promote β-cell proliferation by upregulation of SMAD7 (45, 46).
These selected examples and additional studies showing interactions between immunity and metabolic dysfunction (see later in text) initiated a new field of research called immunometabolism.
Pathological Role of Inflammation in the Development of the Metabolic Syndrome and Its Complication
Historically, preceding the description of the physiological and adaptive role of inflammation in metabolism, the focus has been on its pathological role. First evidence of an activation of the innate system in patients with type 2 diabetes came from the observation of elevated circulating levels of acute-phase proteins, including serum amyloid A, sialic acid, C-reactive protein (CRP), haptoglobin, fibrinogen, plasminogen activator inhibitor, TNF-α, IL-1β, IL-6, and IL-1Ra (47–50). Additionally, elevated levels of IL-1β, IL-1Ra, IL-6, and CRP are predictive markers for the development of type 2 diabetes (48, 50–54). Of note, increased CRP levels are also a strong predictor of cardiovascular disease (47–50). Importantly, these inflammatory proteins are regulated in an IL-1–dependent way, and IL-1 antagonism reduces the concentrations of CRP, IL-6, and leukocytosis (55–58).
The interpretation of levels of circulating inflammatory factor concentrations requires some caution. IL-1β is one of the most potent cytokines, and only a few molecules suffice to induce an answer in target cells (59). Therefore, even very low concentrations may have strong effects. Such low concentrations are often not detectable by standard protein assays, also because a majority of circulating IL-1β is bound to proteins. Furthermore, most cytokines are produced locally and act in a paracrine manner. Therefore, circulating levels of inflammatory biomarkers do not necessarily reflect the degree of inflammation in individual tissues. Small organs such as pancreatic islets are less likely to contribute to the circulating levels of inflammatory markers. In contrast, spillover of the relatively large adipose tissue and liver inflammation may disproportionately contribute to the circulating levels of inflammatory factors. Thus, to conclude on the real contribution of cytokines such as IL-1β, IL-6, or TNF-α to insulin secretion, insulin resistance, or secondary complications of diabetes, carefully designed clinical studies with specific antagonists and well-defined end points are warranted (56, 60).
The first report on the role of tissue inflammation in the pathogenesis of insulin resistance is the seminal observation by Hotamisligil et al. (61) of increased production of TNF-α by adipose tissues during obesity and improved insulin sensitivity upon TNF-α antagonism. The cellular source of TNF-α in adipose tissue was originally thought to be the adipocytes themselves. However, in the meantime, macrophages and other immune cells have been described in adipose tissues, which may account for the release of TNF-α and other cytokines, including IL-1β, IL-6, and IL-33 (62–64). As of today, it is well established that tissue inflammation plays a critical role in insulin resistance. Its pathogenesis has been extensively reviewed elsewhere (64–67).
Inflammation appears to play a considerable role not only in defective insulin action but also in insulin secretion. Indeed, pancreatic islets of patients with type 2 diabetes display increased expression of cytokines and immune cell infiltration mainly consisting of proinflammatory macrophages (68, 69). This chronic inflammatory process is associated with fibrosis and amyloid deposits, as observed in islets of the majority of patients with type 2 diabetes. Metabolic stress induces inflammation not only in insulin target tissues but also in islets, although the underlying mechanisms are not completely identical. Thereby, elevated glucose concentrations increase metabolic activity in islets, leading to the production of IL-1β (5, 68). This is enhanced by endoplasmic reticulum stress due to increased insulin production, bacterial products (endotoxins), and free fatty acids, which all contribute to inflammasome activation (70–72). Further, as insulin and the amyloid-precursor islet amyloid polypeptide are cosecreted from β-cells (73), increased insulin secretion may also increase amyloid deposition, which in turn also contributes to NLRP3 inflammasome activation in macrophages (11). Initially, this inflammatory process may be beneficial, as described above, promoting β-cell proliferation and insulin production to compensate for insulin resistance (24, 74, 75). IL-1β induces various cytokines and chemokines promoting macrophages and other immune cells, which eventually leads to deleterious inflammation (69, 76, 77). Indeed, prolonged metabolic stress will decrease the protective islet endogenous production of IL-1Ra (78, 79) and induce IL-1β autostimulation (26), and islet amyloid polypeptide will activate the inflammasome and thus promote the synthesis of IL-1β (11, 80, 81). Further, IL-1β, IL-6, and TNF-α were shown to promote β-cell dedifferentiation in cultured human and mouse islets, including IL-1β–mediated suppression of Foxo1, and TNF-α antagonism in vivo partly restored the loss of β-cell identity genes (82). This suggests that one deleterious mechanism of prolonged exposure to low-grade inflammation in islets could be due to β-cell dedifferentiation (83). The final pathway that will impair β-cell secretory function probably involves cytokines released by immune cells (84, 85), in which IL-1β appears to have a critical role in impairing β-cell proliferation (79).
Importantly, from a therapeutic point of view, long-term complications of diabetes also appear to be driven by an inflammatory process. This has been proven for IL-1β in cardiovascular complications, in which the initial preclinical hypothesis (86) has been confirmed with the CANTOS study [(87); see later in text for details]. Accumulating evidence also supports a role for inflammation in the manifestation of nonalcoholic steatohepatitis (88, 89), nephropathy (90), polyneuropathy (91–95), retinopathy, and macular edema (96, 97).
Finally, several inflammatory diseases are associated with diabetes, including rheumatoid arthritis, gout, psoriasis, and Crohn’s disease (98, 99). Moreover, other features of the metabolic syndrome are at least partly due to a pathological activation of the innate system and can be improved by IL-1 antagonism. These include low testosterone levels in men (99), increased cortisol levels (100), and fatigue (101, 102).
Reconciliation of Physiology and Pathology
The observed physiological role of immunity in glucose homeostasis appears to be at odds with its deleterious effect and the glucose-lowering effects of anti-inflammatory drugs (see later in text). This can be explained by the duration (acute vs chronic), the magnitude of the inflammatory effect, diminished anti-inflammatory mechanisms, and the concomitantly induced inflammatory diseases. One example for the dual role of proinflammatory cytokines in metabolism is IL-1β, which, on one hand, is an insulin secretagogue during feeding (20) and, on the other hand, contributes to β-cell failure (84, 85). Therapeutic inhibition of acute IL-1β may act as a brake for insulin secretion and allow β-cells to rest (103, 104) and recover from chronic overstimulation in states of metabolic stress. Indeed, when insulin secretion is reduced by genetic means, endoplasmic reticulum stress is alleviated and in turn, β-cell proliferation increases (105). Alternatively, excessive IL-1β stimulation could lead to resistance to IL-1β signaling, which may be corrected by blockage of IL-1. Indeed, islets from patients with type 2 diabetes fail to respond to IL-1β with insulin secretion, whereas islet from healthy donors are reactive to IL-1β (24). A further reason for the establishment of chronicity of inflammation may be a defective activation of counterregulatory pathways in affected organs. Islet β-cells, for example, locally express the protective IL-1Ra. Although there is an increase of IL-1Ra during obesity and type 2 diabetes in the circulation (50, 106), probably to counteract the systemic low-grade inflammation in vain, there is diminished IL-1Ra expression in human islets of patients with type 2 diabetes or in human islets exposed to toxic concentrations of glucose or human amyloid polypeptide toxicity in vitro (26, 78, 107). Further, mice lacking β-cell–specific IL-1Ra expression show impaired insulin secretion and altered islet morphology (79). Chronic exposure to elevated IL-1β along with simultaneous reduction of IL-1Ra may alter transcriptional programming, which regulate β-cell identity, proliferation, and apoptosis. In vivo constitutive deletion of the protective IL-1Ra in mouse islets indeed leads to reduced expression of proliferation genes along with diminished β-cell function (79).
Clinical Translation
Retrospectively, the first hint for a link between inflammation and metabolism was published in 1876, when it was reported that high doses of sodium salicylate improve glycosuria in patients with diabetes (108). This paper, published in the Berliner Klinische Wochenschrift, concludes that the aim of this publication was “to stimulate research testing whether salicylic acid should have a place in the treatment of diabetes.” Although it took >100 years, it is the merit of Yuan et al. (109), who have followed up on this message and described the underlying mechanisms, which involve inhibition of the nuclear factor κB pathway. The validity of this concept has been shown in animal studies and together with Goldfine et al. (110) in clinical trials with humans. Treatment with salsalate improves glycemia and reduces markers of systemic inflammation (110–112). This was confirmed in two multicenter, placebo-controlled studies (113, 114) and in smaller studies at early stages of the disease (115, 116). Salsalate is a widely used drug, for which safety has been confirmed in these studies. The only limitations are a small increase in low-density lipoprotein cholesterol levels and a reversible rise in urinary albumin. Thus, salsalate may be an effective and cheap drug to improve glycemic control. However, due to lack of a patent, salsalate is not a financially attractive drug to introduce into the market for the treatment of diabetes, and it is unclear whether a commercial company will invest the financial resources needed to make it available for patients. Despite these rather frustrating economic aspects, these important clinical studies should at least serve as supporting evidence for a role of inflammation in diabetes and encourage further studies in this direction.
Despite convincing preclinical studies showing that TNF-α induces insulin resistance in rodents (61), the clinical translation of this finding has not (yet) occurred, mainly due to superficial interpretation of the initial clinic studies. Indeed, as reviewed previously in detail (98), these studies have serious limitations that should not have led to firm conclusions. These include sample size and duration [10 and 7 patients for 4 weeks and 2 days, respectively, for the first two human trials (117, 118)]. Later studies were also statistically underpowered and of too short duration to detect meaningful effects (119, 120). However, other clinical trials using TNF-α antagonists in obese subjects without diabetes (121) and in patients with rheumatoid arthritis (122–126), psoriasis (127), and Crohn disease (128) showed improved glycemia. Importantly, treatment of patients with rheumatoid arthritis or psoriasis with TNF-α antagonists reduces the incidence of type 2 diabetes (129, 130). Altogether, this argues in favor of relevant beneficial metabolic effects of TNF-α antagonists in humans. However, well-designed clinical studies assessing its precise role are warranted.
Since the initial observations assigning a role for IL-1β in the pathogenesis of type 2 diabetes (68), numerous observations and clinical studies have demonstrated its involvement in glucose metabolism [reviewed in Donath (98)]. An initial proof-of-concept clinical study randomly assigned 70 patients with type 2 diabetes to receive either anakinra (recombinant human IL-1Ra) or placebo (56). Anakinra not only improved glycemia but also improved β-cell secretory function over the 3-month treatment period, thereby pointing to a disease-modifying potential. Beyond this direct metabolic benefit, IL-1 antagonism reduced CRP levels, providing a rational for the CANTOS study (see later in text). The ability to improve defective insulin secretion was confirmed in several follow-up studies using anakinra (131, 132). Although anakinra is well tolerated, it often causes injection site reactions and requires daily application. Much more attractive to treat metabolic diseases are anti–IL-1β antibodies, with a half-life that allows injection once every 3 months. Each of these antibodies demonstrated beneficial effects in patients with type 2 diabetes, albeit the magnitude of the effects varied depending on baseline HbA1c levels and sample size (133–136).
As of today, the most informative study that investigates the role of inflammation in patients with a metabolic disease is the CANTOS study (87). This large cardiovascular outcome study involved >10,000 patients and demonstrated that treatment with canakinumab, an anti–IL-1β antibody, resulted in a lower number of cardiovascular events than placebo. A subanalyis focused on metabolic end points (137). However, it should be emphasized that the CANTOS study was not specifically devoted to diabetes; thus, diabetes was not a selection criterion, and in case of diabetes, lifestyle interventions and antidiabetic drugs were freely adapted. Furthermore, even patients with diabetes had an excellent glucose control with baseline HbA1c levels within or even below treatment target (see later in the text). Keeping these limitations in mind, the following can be learned from this study: (1) by selecting patients solely on the basis of elevated CRP levels (≥2 mg/L) and a history of myocardial infarction, the resulting study population consisted of 90% individuals with impaired glucose metabolism (40% diabetes and 50% prediabetes) and other features of the metabolic syndrome. This strongly argues for a link between inflammation and diabetes. (2) IL-1β antagonism significantly decreased HbA1c during the first 6 to 9 months of treatment with attenuation of the effect in the course of the study. This confirms findings of previous clinical studies mentioned previously and proves the role of IL-1β and inflammation in diabetes. The reason for the attenuation of the effect after 6 to 9 months is likely due to the design of the study, which allowed lifestyle interventions and adaptations of standard antidiabetic therapies. Indeed, in other cardiovascular outcome studies using classical antidiabetic drugs [dipeptidyl peptidase 4 (DPP4) or sodium-glucose–linked transporter 2 (SGLT2) inhibitors, the most widely used antidiabetic drugs today], similar patterns and magnitude of effects were observed (138). The magnitude of achievable effects depends on baseline HbA1c. Baseline HbA1c was 7.1%, which is far below the HbA1c level targeted by diabetes treatment of this patient population (<8%, American Diabetes Association guidelines). However, during the first months, the pure (without changes in antidiabetic drugs) anti–IL-1 effect was apparent in subjects with diabetes who had a higher baseline HbA1c of 7.5% (which is still quite low). Upon anti–IL-1β treatment, this group showed a statistically significant decreased HbA1c. In patients without diabetes, canakinumab decreased HbA1c for the whole duration of the study, albeit the effect was very mild, which is expected as patients were normoglycemic. (3) IL-1 antagonism prevented new onset of diabetes for 4 years. After this time, the number of patients, whom were further followed in the study, decreased by ∼50%, and the effect of canakinumab was not detected anymore. The reason for this loss of efficacy remains to be explained but could be related to the previously discussed patient selection or to the physiological role of IL-1β. Of note, none of the most widely used antidiabetic drugs (DPP4 inhibitor, SGLT2 inhibitor, insulin, and sulfonylurea) show diabetes prevention properties in contrast to the 4-year prevention by IL-1β blockade.
Following the CANTOS study, a meta-analysis of all 2921 reported cases with type 2 diabetes undergoing an IL-1 therapy (anakinra or anti–IL-1β antibody) has been performed (139). It demonstrated a highly substantial reduction in HbA1c (P < 0.00001), confirming a previous meta-analysis involving fewer patients (140). As mentioned previously, the magnitude of achievable decrease in HbA1c depends on baseline HbA1c. Because most patients had a relatively low HbA1c, no firm conclusion on the magnitude of the effect can be drawn. Interestingly, a meta-analysis also showed that anti–IL-1 therapy improves the level of C-peptide following meal intake (140).
The CANTOS study revealed several additional aspects of long-term IL-1β antagonism. Keeping in mind the multiple effects of IL-1β, the safety profile of canakinumab was surprisingly good. Nevertheless, canakinumab was associated with a higher incidence of fatal infections, which warrants caution in patients at risk. This has to be balanced with the additional favorable effects, including a decrease in cancer mortality and incident in lung cancer (141) and the confirmation of beneficial effects in arthritis, gout, and osteoarthritis.
Keeping in mind the unexpected protective effect of SGLT2 inhibitors on heart failure, a striking finding of the CANTOS study is that canakinumab reduced hospitalization for heart failure and the composite of hospitalization for heart failure or heart failure–related mortality (142). This was observed in particular in patients with higher body mass index, diabetes, hypertension, or prior coronary bypass surgery.
Concluding Remarks and Future Directions
Activation of the innate immune system is apparent at all stages of the development of diabetes and its complications. This includes impaired β-cell function (143), insulin resistance (64–67), cardiovascular diseases (87), heart failure (142), nonalcoholic steatohepatitis (88, 89), nephropathy (90), polyneuropathy (95–99), fatigue (105, 106), retinopathy, and macular edema (100, 101). Accordingly, IL-1β antagonism has been shown to prevent β-cell dysfunction and to improve glycemia (56, 78, 133–136), cardiovascular complications (87, 144), and heart failure (142), and it may counteract other complications of diabetes (Table 1) (4, 145, 146). Clearly, more studies are warranted to assess more precisely the magnitude of these effects. Furthermore, beyond IL-1β antagonism, other immunomodulatory drugs, either alone or in combination, may be more effective. However, the choice of the anti-inflammatory drugs to be tested should be based on precise pathophysiologic understanding of their role and not on an unspecific effect as with methotrexate, which does not decrease IL-1β and failed to prevent cardiovascular complications in patients with metabolic syndrome (147).
Table 1.
Benefits of Anti–IL-1β Treatment in Patients With Metabolic Syndrome
| β-Cell function ↑ | (56) |
| Glycemia ↓ | (56, 131–136, 139) |
| Cardiovascular complications ↓ | (87) |
| Heart failure ↓ | (142) |
| Gout ↓ | (4, 145, 146) |
| Arthritis ↓ | (136) |
| Safe: no hypoglycemia (cave: severe infections) | |
| Convenient (subcutaneous injection every 3 mo) | |
| Possible additional effects: treatment of nephropathy, retinopathy, nonalcoholic steatohepatitis, and polyneuropathy |
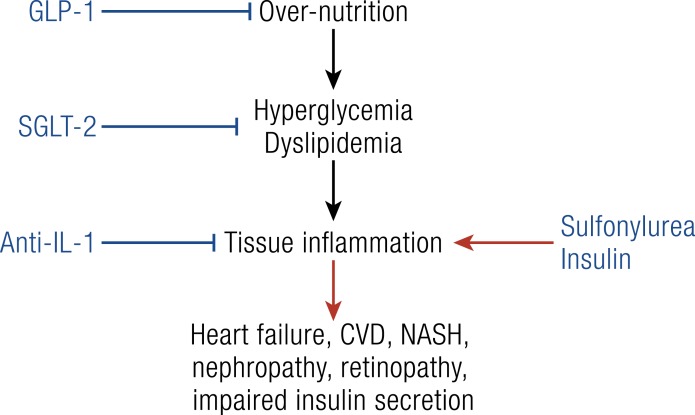
Until now, the only antidiabetic drugs demonstrating improvement in cardiovascular complications were GLP-1 analogs (148) and SGLT2 inhibitors in the EMPAREG study (149). With a similar design to the EMPAREG study (149), the CANTOS study showed that blocking IL-1β in patients with a metabolic syndrome has comparable effects, including prevention of cardiovascular diseases (87, 137) and heart failure (142). This is in contrast to the antidiabetic drugs insulin, sulfonylurea, and DPP4 inhibitors, which failed to show such preventive effects. This difference in outcomes can be explained by revisiting our understanding of the pathophysiologic role of insulin resistance, decreased insulin secretion, and glycosuria in the development of type 2 diabetes and its complication (Fig. 3). We suggest that once hyperglycemia due to overnutrition prevails, these three key features of type 2 diabetes are secondary protective mechanisms by preventing overloading of tissues with cell nutrients. If these mechanisms are overridden by increasing insulin production or action, damaging inflammation occurs, contributing to complications of diabetes. It follows that therapies of diabetes should prevent overfeeding (GLP-1 analogs), promote glycosuria (SGLT2 inhibitors), induce β-cell rest (IL-1β antagonists), and prevent inflammatory damages (IL-1β antagonists). In contrast, drugs increasing insulin action should be limited to patients with an absolute insulin deficiency. Beyond this glucocentric view, tissue inflammation in patients with metabolic syndrome is not only induced by glucose but also by cholesterol (17), fatty acids (71), uric acids (4), and bacterial products (150, 151).
Figure 3.
Development and treatment of complications of type 2 diabetes. Hyperglycemia and dyslipidemia due to overnutrition and genetic predisposition overload tissues with cell nutrients with subsequent tissue damage mediated by inflammatory processes. Overfeeding can be prevented with GLP-1 analogs, hyperglycemia can be reduced via glycosuria using SGLT2 inhibitors, and tissue damage can be alleviated via IL-1β antagonists. In contrast, increasing insulin signaling may enhance nutrient overload in tissues with subsequent induction of a damaging inflammation.
Pathological activation of the immune system plays a critical role in an increasing number of diseases (152), and some of them are associated with diabetes, such as rheumatoid arthritis, gout, and psoriasis. For all of these conditions, immunomodulatory treatments are approved and have substantially improved patient care. Somehow, the metabolic/endocrine field seems more reluctant to implement such anti-inflammatory treatments, despite increasing data supporting the beneficial role of these interventions. In particular, in cases of concomitant diseases, diabetes together with rheumatoid arthritis, gout, or psoriasis, a judicious choice of an anti-inflammatory treatment can improve both conditions with one drug. A convincing example is the recent study by Ruscitti et al. (136), showing that treatment of patients with diabetes and rheumatoid arthritis with an IL-1 antagonist decreased HbA1c by >1% for 6 months and simultaneously reduced rheumatoid disease activity.
We hope that this review will encourage clinician scientists and industrial partners to translate the large knowledge accumulated with animal and proof-of-concept clinical studies into the treatment of patients with a metabolic syndrome. Thereby, anti-inflammatory treatments, alone or in combination, have the potential to improve diabetes, its progression, and complications, as well as associated inflammatory diseases.
Acknowledgments
Financial Support: All authors are supported by the Swiss National Science Foundation.
Disclosure Summary: M.Y.D. is an inventor on patent WO-2004002512 A1. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- ASC
apoptosis-associated speck-like protein containing a caspase recruitment domain
- CRP
C-reactive protein
- DPP4
dipeptidyl peptidase 4
- NLRP3
NACHT
- LRR
and PYD domain-containing protein 3
- SGLT2
sodium-glucose–linked transporter 2
References and Notes
- 1. Donath MY. When metabolism met immunology. Nat Immunol. 2013;14(5):421–422. [DOI] [PubMed] [Google Scholar]
- 2. Bantug GR, Galluzzi L, Kroemer G, Hess C. The spectrum of T cell metabolism in health and disease. Nat Rev Immunol. 2018;18(1):19–34. [DOI] [PubMed] [Google Scholar]
- 3. Murphy MP, O’Neill LAJ. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell. 2018;174(4):780–784. [DOI] [PubMed] [Google Scholar]
- 4. Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. [DOI] [PubMed] [Google Scholar]
- 5. Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. [DOI] [PubMed] [Google Scholar]
- 6. Tschöp M, Thomas G. Fat fuels insulin resistance through Toll-like receptors. Nat Med. 2006;12(12):1359–1361. [DOI] [PubMed] [Google Scholar]
- 7. Stienstra R, Joosten LA, Koenen T, van Tits B, van Diepen JA, van den Berg SA, Rensen PC, Voshol PJ, Fantuzzi G, Hijmans A, Kersten S, Müller M, van den Berg WB, van Rooijen N, Wabitsch M, Kullberg BJ, van der Meer JW, Kanneganti T, Tack CJ, Netea MG. The inflammasome-mediated caspase-1 activation controls adipocyte differentiation and insulin sensitivity. Cell Metab. 2010;12(6):593–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. [DOI] [PubMed] [Google Scholar]
- 9. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183(2):787–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KH, Mok KH, Newsholme P, Nuñez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LA. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11(10):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17(2):179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wen H, Gris D, Lei Y, Jha S, Zhang L, Huang MT, Brickey WJ, Ting JP. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12(5):408–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6(1):6734. [DOI] [PubMed] [Google Scholar]
- 15. Feng Y, Wang Y, Wang P, Huang Y, Wang F. Short-Chain Fatty Acids Manifest Stimulative and Protective Effects on Intestinal Barrier Function Through the Inhibition of NLRP3 Inflammasome and Autophagy. Cell Physiol Biochem. 2018;49(1):190–205. [DOI] [PubMed] [Google Scholar]
- 16. Netea MG, Joosten LA. Inflammasome inhibition: putting out the fire. Cell Metab. 2015;21(4):513–514. [DOI] [PubMed] [Google Scholar]
- 17. Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr M, Espevik T, Lien E, Fitzgerald KA, Rock KL, Moore KJ, Wright SD, Hornung V, Latz E. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals [published correction appears in Nature. 2010;466:652]. Nature. 2010;464(7293):1357–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Freigang S, Ampenberger F, Weiss A, Kanneganti TD, Iwakura Y, Hersberger M, Kopf M. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1α and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14(10):1045–1053. [DOI] [PubMed] [Google Scholar]
- 19. Dinarello CA. Multiple biological properties of recombinant human interleukin 1 (beta). Immunobiology. 1986;172(3-5):301–315. [DOI] [PubMed] [Google Scholar]
- 20. Dror E, Dalmas E, Meier DT, Wueest S, Thévenet J, Thienel C, Timper K, Nordmann TM, Traub S, Schulze F, Item F, Vallois D, Pattou F, Kerr-Conte J, Lavallard V, Berney T, Thorens B, Konrad D, Böni-Schnetzler M, Donath MY. Postprandial macrophage-derived IL-1β stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat Immunol. 2017;18(3):283–292. [DOI] [PubMed] [Google Scholar]
- 21. Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, Eppler E, Bouzakri K, Wueest S, Muller YD, Hansen AM, Reinecke M, Konrad D, Gassmann M, Reimann F, Halban PA, Gromada J, Drucker DJ, Gribble FM, Ehses JA, Donath MY. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nat Med. 2011;17(11):1481–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dalmas E, Lehmann FM, Dror E, Wueest S, Thienel C, Borsigova M, Marc S, Traunecker E, Lucchini FC, Dapito D, Kallert SM, Guigas B, Pattou F, Kerr-Conte J, Maechler P, Girard J-P, Konrad D, Wolfrum C, Boni-Schnetzler M, Finke D, Donath MY. Interleukin-33-activated islet-resident innate lymphoid cells promote insulin secretion through myeloid cell retinoic acid production. Immunity. 2017;47:1774–1786. [DOI] [PubMed] [Google Scholar]
- 23. Traba J, Kwarteng-Siaw M, Okoli TC, Li J, Huffstutler RD, Bray A, Waclawiw MA, Han K, Pelletier M, Sauve AA, Siegel RM, Sack MN. Fasting and refeeding differentially regulate NLRP3 inflammasome activation in human subjects. J Clin Invest. 2015;125(12):4592–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hajmrle C, Smith N, Spigelman AF, Dai X, Senior L, Bautista A, Ferdaoussi M, MacDonald PE. Interleukin-1 signaling contributes to acute islet compensation. JCI Insight. 2016;1(4):e86055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Spinas GA, Hansen BS, Linde S, Kastern W, Mølvig J, Mandrup-Poulsen T, Dinarello CA, Nielsen JH, Nerup J. Interleukin 1 dose-dependently affects the biosynthesis of (pro)insulin in isolated rat islets of Langerhans. Diabetologia. 1987;30(7):474–480. [DOI] [PubMed] [Google Scholar]
- 26. Böni-Schnetzler M, Thorne J, Parnaud G, Marselli L, Ehses JA, Kerr-Conte J, Pattou F, Halban PA, Weir GC, Donath MY. Increased interleukin (IL)-1beta messenger ribonucleic acid expression in beta -cells of individuals with type 2 diabetes and regulation of IL-1beta in human islets by glucose and autostimulation. J Clin Endocrinol Metab. 2008;93(10):4065–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodriguez AE, Ducker GS, Billingham LK, Martinez CA, Mainolfi N, Suri V, Friedman A, Manfredi MG, Weinberg SE, Rabinowitz JD, Chandel NS. Serine metabolism supports macrophage IL-1β production. Cell Metab. 2019;29(4):1003–1011.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88(4):1379–1406. [DOI] [PubMed] [Google Scholar]
- 29. Traub S, Meier DT, Schulze F, Dror E, Nordmann TM, Goetz N, Koch N, Dalmas E, Stawiski M, Makshana V, Thorel F, Herrera PL, Böni-Schnetzler M, Donath MY. Pancreatic α cell-derived glucagon-related peptides are required for β cell adaptation and glucose homeostasis. Cell Reports. 2017;18(13):3192–3203. [DOI] [PubMed] [Google Scholar]
- 30. Dandona P, Ghanim H, Abuaysheh S, Green K, Dhindsa S, Makdissi A, Batra M, Kuhadiya ND, Chaudhuri A. Exenatide increases IL-1RA concentration and induces Nrf-2‒Keap-1‒regulated antioxidant enzymes: relevance to β-cell function. J Clin Endocrinol Metab. 2018;103(3):1180–1187. [DOI] [PubMed] [Google Scholar]
- 31. Wedell-Neergaard AS, Lang Lehrskov L, Christensen RH, Legaard GE, Dorph E, Larsen MK, Launbo N, Fagerlind SR, Seide SK, Nymand S, Ball M, Vinum N, Dahl CN, Henneberg M, Ried-Larsen M, Nybing JD, Christensen R, Rosenmeier JB, Karstoft K, Pedersen BK, Ellingsgaard H, Krogh-Madsen R. Exercise-induced changes in visceral adipose tissue mass are regulated by IL-6 signaling: a randomized controlled trial. Cell Metab. 2019;29(4):844–855. [DOI] [PubMed] [Google Scholar]
- 32. Mauer J, Denson JL, Brüning JC. Versatile functions for IL-6 in metabolism and cancer. Trends Immunol. 2015;36(2):92–101. [DOI] [PubMed] [Google Scholar]
- 33. Whitham M, Febbraio MA. The ever-expanding myokinome: discovery challenges and therapeutic implications. Nat Rev Drug Discov. 2016;15(10):719–729. [DOI] [PubMed] [Google Scholar]
- 34. Pedersen BK. Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest. 2017;47(8):600–611. [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Ota N, Manzanillo P, Kates L, Zavala-Solorio J, Eidenschenk C, Zhang J, Lesch J, Lee WP, Ross J, Diehl L, van Bruggen N, Kolumam G, Ouyang W. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514(7521):237–241. [DOI] [PubMed] [Google Scholar]
- 36. Hasnain SZ, Borg DJ, Harcourt BE, Tong H, Sheng YH, Ng CP, Das I, Wang R, Chen AC, Loudovaris T, Kay TW, Thomas HE, Whitehead JP, Forbes JM, Prins JB, McGuckin MA. Glycemic control in diabetes is restored by therapeutic manipulation of cytokines that regulate beta cell stress. Nat Med. 2014;20(12):1417–1426. [DOI] [PubMed] [Google Scholar]
- 37. Dinarello CA, Nold-Petry C, Nold M, Fujita M, Li S, Kim S, Bufler P. Suppression of innate inflammation and immunity by interleukin-37. Eur J Immunol. 2016;46(5):1067–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao PW, Jiang WG, Wang L, Jiang ZY, Shan YX, Jiang YF. Plasma levels of IL-37 and correlation with TNF-α, IL-17A, and disease activity during DMARD treatment of rheumatoid arthritis. PLoS One. 2014;9(5):e95346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ballak DB, van Diepen JA, Moschen AR, Jansen HJ, Hijmans A, Groenhof GJ, Leenders F, Bufler P, Boekschoten MV, Müller M, Kersten S, Li S, Kim S, Eini H, Lewis EC, Joosten LA, Tilg H, Netea MG, Tack CJ, Dinarello CA, Stienstra R. IL-37 protects against obesity-induced inflammation and insulin resistance [published correction appears in Nat Commun. 2015;6:6039]. Nat Commun. 2014;5(1):4711. [DOI] [PubMed] [Google Scholar]
- 40. Nold MF, Nold-Petry CA, Zepp JA, Palmer BE, Bufler P, Dinarello CA. IL-37 is a fundamental inhibitor of innate immunity. Nat Immunol. 2010;11(11):1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu B, Meng K, Ji Q, Cheng M, Yu K, Zhao X, Tony H, Liu Y, Zhou Y, Chang C, Zhong Y, Zhu Z, Zhang W, Mao X, Zeng Q. Interleukin-37 ameliorates myocardial ischaemia/reperfusion injury in mice. Clin Exp Immunol. 2014;176(3):438–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McNamee EN, Masterson JC, Jedlicka P, McManus M, Grenz A, Collins CB, Nold MF, Nold-Petry C, Bufler P, Dinarello CA, Rivera-Nieves J. Interleukin 37 expression protects mice from colitis. Proc Natl Acad Sci USA. 2011;108(40):16711–16716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Henry CJ, Casás-Selves M, Kim J, Zaberezhnyy V, Aghili L, Daniel AE, Jimenez L, Azam T, McNamee EN, Clambey ET, Klawitter J, Serkova NJ, Tan AC, Dinarello CA, DeGregori J. Aging-associated inflammation promotes selection for adaptive oncogenic events in B cell progenitors. J Clin Invest. 2015;125(12):4666–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ying W, Lee YS, Dong Y, Seidman JS, Yang M, Isaac R, Seo JB, Yang BH, Wollam J, Riopel M, McNelis J, Glass CK, Olefsky JM, Fu W. Expansion of islet-resident macrophages leads to inflammation affecting beta cell proliferation and function in obesity. Cell Metab. 2019;29(2):457–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xiao X, Gaffar I, Guo P, Wiersch J, Fischbach S, Peirish L, Song Z, El-Gohary Y, Prasadan K, Shiota C, Gittes GK. M2 macrophages promote beta-cell proliferation by up-regulation of SMAD7. Proc Natl Acad Sci USA. 2014;111(13):E1211–E1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cao X, Han ZB, Zhao H, Liu Q. Transplantation of mesenchymal stem cells recruits trophic macrophages to induce pancreatic beta cell regeneration in diabetic mice. Int J Biochem Cell Biol. 2014;53:372–379. [DOI] [PubMed] [Google Scholar]
- 47. Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40(11):1286–1292. [DOI] [PubMed] [Google Scholar]
- 48. Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52(3):812–817. [DOI] [PubMed] [Google Scholar]
- 49. Herder C, Illig T, Rathmann W, Martin S, Haastert B, Müller-Scholze S, Holle R, Thorand B, Koenig W, Wichmann HE, Kolb H; KORA Study Group. Inflammation and type 2 diabetes: results from KORA Augsburg. Gesundheitswesen. 2005;67(Suppl 1):S115–S121. [DOI] [PubMed] [Google Scholar]
- 50. Herder C, Brunner EJ, Rathmann W, Strassburger K, Tabák AG, Schloot NC, Witte DR. Elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the Whitehall II study. Diabetes Care. 2009;32(3):421–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. [DOI] [PubMed] [Google Scholar]
- 52. Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab. 2002;87(3):1184–1188. [DOI] [PubMed] [Google Scholar]
- 53. Carstensen M, Herder C, Kivimäki M, Jokela M, Roden M, Shipley MJ, Witte DR, Brunner EJ, Tabák AG. Accelerated increase in serum interleukin-1 receptor antagonist starts 6 years before diagnosis of type 2 diabetes: Whitehall II prospective cohort study. Diabetes. 2010;59(5):1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marculescu R, Endler G, Schillinger M, Iordanova N, Exner M, Hayden E, Huber K, Wagner O, Mannhalter C. Interleukin-1 receptor antagonist genotype is associated with coronary atherosclerosis in patients with type 2 diabetes. Diabetes. 2002;51(12):3582–3585. [DOI] [PubMed] [Google Scholar]
- 55. Dinarello CA. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N Engl J Med. 2000;343(10):732–734. [DOI] [PubMed] [Google Scholar]
- 56. Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–1526. [DOI] [PubMed] [Google Scholar]
- 57. Ehses JA, Lacraz G, Giroix MH, Schmidlin F, Coulaud J, Kassis N, Irminger JC, Kergoat M, Portha B, Homo-Delarche F, Donath MY. IL-1 antagonism reduces hyperglycemia and tissue inflammation in the type 2 diabetic GK rat. Proc Natl Acad Sci USA. 2009;106(33):13998–14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Donath MY, Weder C, Whitmore J, Bauer RJ, Der K, Scannon PJ, Dinarello C, Solinger AM. XOMA 052, an anti-IL-1beta antibody, in a double-blind, placebo-controlled, dose escalation study of the safety and pharmacokinetics in patients with type 2 diabets mellitus - a new approach to therapy. Diabetologia. 2008;51:S7. [Google Scholar]
- 59. Dinarello CA. Overview of the interleukin-1 family of ligands and receptors. Semin Immunol. 2013;25(6):389–393. [DOI] [PubMed] [Google Scholar]
- 60. Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE; TINSAL-T2D (Targeting Inflammation Using Salsalate in Type 2 Diabetes) Study Team. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152(6):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. [DOI] [PubMed] [Google Scholar]
- 62. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mathis D. Immunological goings-on in visceral adipose tissue. Cell Metab. 2013;17(6):851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lee YS, Wollam J, Olefsky JM. An integrated view of immunometabolism. Cell. 2018;172(1-2):22–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11(2):98–107. [DOI] [PubMed] [Google Scholar]
- 67. Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. [DOI] [PubMed] [Google Scholar]
- 68. Maedler K, Sergeev P, Ris F, Oberholzer J, Joller-Jemelka HI, Spinas GA, Kaiser N, Halban PA, Donath MY. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110(6):851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ehses JA, Perren A, Eppler E, Ribaux P, Pospisilik JA, Maor-Cahn R, Gueripel X, Ellingsgaard H, Schneider MK, Biollaz G, Fontana A, Reinecke M, Homo-Delarche F, Donath MY. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56(9):2356–2370. [DOI] [PubMed] [Google Scholar]
- 70. Oslowski CM, Hara T, O’Sullivan-Murphy B, Kanekura K, Lu S, Hara M, Ishigaki S, Zhu LJ, Hayashi E, Hui ST, Greiner D, Kaufman RJ, Bortell R, Urano F. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell Metab. 2012;16(2):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Böni-Schnetzler M, Boller S, Debray S, Bouzakri K, Meier DT, Prazak R, Kerr-Conte J, Pattou F, Ehses JA, Schuit FC, Donath MY. Free fatty acids induce a proinflammatory response in islets via the abundantly expressed interleukin-1 receptor I. Endocrinology. 2009;150(12):5218–5229. [DOI] [PubMed] [Google Scholar]
- 72. Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8):1279–1285. [DOI] [PubMed] [Google Scholar]
- 73. Kahn SE, D’Alessio DA, Schwartz MW, Fujimoto WY, Ensinck JW, Taborsky GJ Jr, Porte D Jr. Evidence of cosecretion of islet amyloid polypeptide and insulin by beta-cells. Diabetes. 1990;39(5):634–638. [DOI] [PubMed] [Google Scholar]
- 74. Maedler K, Schumann DM, Sauter N, Ellingsgaard H, Bosco D, Baertschiger R, Iwakura Y, Oberholzer J, Wollheim CB, Gauthier BR, Donath MY. Low concentration of interleukin-1beta induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets [published corrections appear in Diabetes. 2016;65(8):2462 and 2018;67(11):2479-2480]. Diabetes. 2006;55(10):2713–2722. [DOI] [PubMed] [Google Scholar]
- 75. Maedler K, Fontana A, Ris F, Sergeev P, Toso C, Oberholzer J, Lehmann R, Bachmann F, Tasinato A, Spinas GA, Halban PA, Donath MY. FLIP switches Fas-mediated glucose signaling in human pancreatic beta cells from apoptosis to cell replication. Proc Natl Acad Sci USA. 2002;99(12):8236–8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52(8):1686–1688. [DOI] [PubMed] [Google Scholar]
- 77. Butcher MJ, Hallinger D, Garcia E, Machida Y, Chakrabarti S, Nadler J, Galkina EV, Imai Y. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014;57(3):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Maedler K, Sergeev P, Ehses JA, Mathe Z, Bosco D, Berney T, Dayer JM, Reinecke M, Halban PA, Donath MY. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci USA. 2004;101(21):8138–8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Böni-Schnetzler M, Häuselmann SP, Dalmas E, Meier DT, Thienel C, Traub S, Schulze F, Steiger L, Dror E, Martin P, Herrera PL, Gabay C, Donath MY. β cell-specific deletion of the IL-1 receptor antagonist impairs β cell proliferation and insulin secretion. Cell Reports. 2018;22(7):1774–1786. [DOI] [PubMed] [Google Scholar]
- 80. Westwell-Roper CY, Ehses JA, Verchere CB. Resident macrophages mediate islet amyloid polypeptide-induced islet IL-1beta production and beta cell dysfunction. Diabetes. 2014;63(5):1698–1711. [DOI] [PubMed] [Google Scholar]
- 81. Westwell-Roper C, Dai DL, Soukhatcheva G, Potter KJ, van Rooijen N, Ehses JA, Verchere CB. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. 2011;187(5):2755–2765. [DOI] [PubMed] [Google Scholar]
- 82. Nordmann TM, Dror E, Schulze F, Traub S, Berishvili E, Barbieux C, Böni-Schnetzler M, Donath MY. The role of inflammation in β-cell dedifferentiation. Sci Rep. 2017;7(1):6285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012;150(6):1223–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986;232(4757):1545–1547. [DOI] [PubMed] [Google Scholar]
- 85. Mandrup-Poulsen T, Bendtzen K, Nerup J, Dinarello CA, Svenson M, Nielsen JH. Affinity-purified human interleukin I is cytotoxic to isolated islets of Langerhans. Diabetologia. 1986;29(1):63–67. [DOI] [PubMed] [Google Scholar]
- 86. Libby P, Warner SJ, Friedman GB. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988;81(2):487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 88. Febbraio MA, Reibe S, Shalapour S, Ooi GJ, Watt MJ, Karin M. Preclinical models for studying NASH-driven HCC: how useful are they? Cell Metab. 2019;29(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol. 2012;56(3):704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tesch GH. Diabetic nephropathy - is this an immune disorder? Clin Sci (Lond). 2017;131(16):2183–2199. [DOI] [PubMed] [Google Scholar]
- 91. Schlesinger S, Herder C, Kannenberg JM, Huth C, Carstensen-Kirberg M, Rathmann W, Bonhof GJ, Koenig W, Heier M, Peters A, Meisinger C, Roden M, Thorand B, Ziegler D. General and abdominal obesity and incident distal sensorimotor polyneuropathy: insights into inflammatory biomarkers as potential mediators in the KORA F4/FF4 cohort. Diabetes Care. 2019;42(2):240–247. [DOI] [PubMed] [Google Scholar]
- 92. Herder C, Kannenberg JM, Carstensen-Kirberg M, Strom A, Bönhof GJ, Rathmann W, Huth C, Koenig W, Heier M, Krumsiek J, Peters A, Meisinger C, Roden M, Thorand B, Ziegler D. A systemic inflammatory signature reflecting cross talk between innate and adaptive immunity is associated with incident polyneuropathy: KORA F4/FF4 Study. Diabetes. 2018;67(11):2434–2442. [DOI] [PubMed] [Google Scholar]
- 93. Herder C, Kannenberg JM, Huth C, Carstensen-Kirberg M, Rathmann W, Koenig W, Heier M, Püttgen S, Thorand B, Peters A, Roden M, Meisinger C, Ziegler D. Proinflammatory cytokines predict the incidence and progression of distal sensorimotor polyneuropathy: KORA F4/FF4 Study. Diabetes Care. 2017;40(4):569–576. [DOI] [PubMed] [Google Scholar]
- 94. Herder C, Bongaerts BW, Rathmann W, Heier M, Kowall B, Koenig W, Thorand B, Roden M, Meisinger C, Ziegler D. Association of subclinical inflammation with polyneuropathy in the older population: KORA F4 study. Diabetes Care. 2013;36(11):3663–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Herder C, Lankisch M, Ziegler D, Rathmann W, Koenig W, Illig T, Döring A, Thorand B, Holle R, Giani G, Martin S, Meisinger C. Subclinical inflammation and diabetic polyneuropathy: MONICA/KORA Survey F3 (Augsburg, Germany). Diabetes Care. 2009;32(4):680–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Mesquida M, Leszczynska A, Llorenç V, Adán A. Interleukin-6 blockade in ocular inflammatory diseases. Clin Exp Immunol. 2014;176(3):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Stahel M, Becker M, Graf N, Michels S. Systemic interleukin-1beta inhibition in proliferative diabetic retinopathy: a prospective open-label study using canakinumab. Retina. 2016;36(2):385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Donath MY. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov. 2014;13(6):465–476. [DOI] [PubMed] [Google Scholar]
- 99. Ebrahimi F, Urwyler SA, Straumann S, Doerpfeld S, Bernasconi L, Neyer P, Schuetz P, Mueller B, Donath MY, Christ-Crain M. IL-1 antagonism in men with metabolic syndrome and low testosterone: a randomized clinical trial. J Clin Endocrinol Metab. 2018;103(9):3466–3476. [DOI] [PubMed] [Google Scholar]
- 100. Urwyler SA, Schuetz P, Ebrahimi F, Donath MY, Christ-Crain M. Interleukin-1 antagonism decreases cortisol levels in obese individuals. J Clin Endocrinol Metab. 2017;102(5):1712–1718. [DOI] [PubMed] [Google Scholar]
- 101. Lehrskov LL, Dorph E, Widmer AM, Hepprich M, Siegenthaler J, Timper K, Donath MY. The role of IL-1 in postprandial fatigue. Mol Metab. 2018;12:107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cavelti-Weder C, Furrer R, Keller C, Babians-Brunner A, Solinger AM, Gast H, Fontana A, Donath MY, Penner IK. Inhibition of IL-1beta improves fatigue in type 2 diabetes. Diabetes Care. 2011;34(10):e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Greenwood RH, Mahler RF, Hales CN. Improvement in insulin secretion in diabetes after diazoxide. Lancet. 1976;1(7957):444–447. [DOI] [PubMed] [Google Scholar]
- 104. Brown RJ, Rother KI. Effects of beta-cell rest on beta-cell function: a review of clinical and preclinical data. Pediatr Diabetes. 2008;9(3 Pt 2):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Szabat M, Page MM, Panzhinskiy E, Skovsø S, Mojibian M, Fernandez-Tajes J, Bruin JE, Bround MJ, Lee JT, Xu EE, Taghizadeh F, O’Dwyer S, van de Bunt M, Moon KM, Sinha S, Han J, Fan Y, Lynn FC, Trucco M, Borchers CH, Foster LJ, Nislow C, Kieffer TJ, Johnson JD. Reduced insulin production relieves endoplasmic reticulum stress and induces β cell proliferation. Cell Metab. 2016;23(1):179–193. [DOI] [PubMed] [Google Scholar]
- 106. Grossmann V, Schmitt VH, Zeller T, Panova-Noeva M, Schulz A, Laubert-Reh D, Juenger C, Schnabel RB, Abt TG, Laskowski R, Wiltink J, Schulz E, Blankenberg S, Lackner KJ, Münzel T, Wild PS. Profile of the immune and inflammatory response in individuals with prediabetes and type 2 diabetes. Diabetes Care. 2015;38(7):1356–1364. [DOI] [PubMed] [Google Scholar]
- 107. Hui Q, Asadi A, Park YJ, Kieffer TJ, Ao Z, Warnock GL, Marzban L. Amyloid formation disrupts the balance between interleukin-1β and interleukin-1 receptor antagonist in human islets. Mol Metab. 2017;6(8):833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ebstein W. Zur therapie des diabetes mellitus, insbesondere über die Anwendung des Salicylsauren Natron bei demselben. Berliner Klinische Wochenschrift. 1876;13:337–340. [Google Scholar]
- 109. Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293(5535):1673–1677. [DOI] [PubMed] [Google Scholar]
- 110. Goldfine AB, Silver R, Aldhahi W, Cai D, Tatro E, Lee J, Shoelson SE. Use of salsalate to target inflammation in the treatment of insulin resistance and type 2 diabetes. Clin Transl Sci. 2008;1(1):36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Fleischman A, Shoelson SE, Bernier R, Goldfine AB. Salsalate improves glycemia and inflammatory parameters in obese young adults. Diabetes Care. 2008;31(2):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Koska J, Ortega E, Bunt JC, Gasser A, Impson J, Hanson RL, Forbes J, de Courten B, Krakoff J. The effect of salsalate on insulin action and glucose tolerance in obese non-diabetic patients: results of a randomised double-blind placebo-controlled study. Diabetologia. 2009;52(3):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Goldfine AB, Fonseca V, Jablonski KA, Pyle L, Staten MA, Shoelson SE; TINSAL-T2D (Targeting Inflammation Using Salsalate in Type 2 Diabetes) Study Team. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152(6):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Goldfine AB, Fonseca V, Jablonski KA, Chen YD, Tipton L, Staten MA, Shoelson SE; Targeting Inflammation Using Salsalate in Type 2 Diabetes Study Team. Salicylate (salsalate) in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2013;159(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Goldfine AB, Conlin PR, Halperin F, Koska J, Permana P, Schwenke D, Shoelson SE, Reaven PD. A randomised trial of salsalate for insulin resistance and cardiovascular risk factors in persons with abnormal glucose tolerance. Diabetologia. 2013;56(4):714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Faghihimani E, Aminorroaya A, Rezvanian H, Adibi P, Ismail-Beigi F, Amini M. Salsalate improves glycemic control in patients with newly diagnosed type 2 diabetes. Acta Diabetol. 2013;50(4):537–543. [DOI] [PubMed] [Google Scholar]
- 117. Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45(7):881–885. [DOI] [PubMed] [Google Scholar]
- 118. Paquot N, Castillo MJ, Lefèbvre PJ, Scheen AJ. No increased insulin sensitivity after a single intravenous administration of a recombinant human tumor necrosis factor receptor: Fc fusion protein in obese insulin-resistant patients. J Clin Endocrinol Metab. 2000;85(3):1316–1319. [DOI] [PubMed] [Google Scholar]
- 119. Dominguez H, Storgaard H, Rask-Madsen C, Steffen Hermann T, Ihlemann N, Baunbjerg Nielsen D, Spohr C, Kober L, Vaag A, Torp-Pedersen C. Metabolic and vascular effects of tumor necrosis factor-alpha blockade with etanercept in obese patients with type 2 diabetes. J Vasc Res. 2005;42(6):517–525. [DOI] [PubMed] [Google Scholar]
- 120. Bernstein LE, Berry J, Kim S, Canavan B, Grinspoon SK. Effects of etanercept in patients with the metabolic syndrome. Arch Intern Med. 2006;166(8):902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Stanley TL, Zanni MV, Johnsen S, Rasheed S, Makimura H, Lee H, Khor VK, Ahima RS, Grinspoon SK. TNF-alpha antagonism with etanercept decreases glucose and increases the proportion of high molecular weight adiponectin in obese subjects with features of the metabolic syndrome. J Clin Endocrinol Metab. 2011;96(1):E146–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yazdani-Biuki B, Stelzl H, Brezinschek HP, Hermann J, Mueller T, Krippl P, Graninger W, Wascher TC. Improvement of insulin sensitivity in insulin resistant subjects during prolonged treatment with the anti-TNF-alpha antibody infliximab. Eur J Clin Invest. 2004;34(9):641–642. [DOI] [PubMed] [Google Scholar]
- 123. Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64(5):765–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yazdani-Biuki B, Mueller T, Brezinschek HP, Hermann J, Graninger W, Wascher TC. Relapse of diabetes after interruption of chronic administration of anti-tumor necrosis factor-alpha antibody infliximab: a case observation. Diabetes Care. 2006;29(7):1712–1713. [DOI] [PubMed] [Google Scholar]
- 125. Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J, Llorca J. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(1):83–86. [PubMed] [Google Scholar]
- 126. Huvers FC, Popa C, Netea MG, van den Hoogen FH, Tack CJ. Improved insulin sensitivity by anti-TNFalpha antibody treatment in patients with rheumatic diseases. Ann Rheum Dis. 2007;66(4):558–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Marra M, Campanati A, Testa R, Sirolla C, Bonfigli AR, Franceschi C, Marchegiani F, Offidani A. Effect of etanercept on insulin sensitivity in nine patients with psoriasis. Int J Immunopathol Pharmacol. 2007;20(4):731–736. [DOI] [PubMed] [Google Scholar]
- 128. Timper K, Hruz P, Beglinger C, Donath MY. Infliximab in the treatment of Crohn disease and type 1 diabetes. Diabetes Care. 2013;36(7):e90–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305(24):2525–2531. [DOI] [PubMed] [Google Scholar]
- 130. Antohe JL, Bili A, Sartorius JA, Kirchner HL, Morris SJ, Dancea S, Wasko MC. Diabetes mellitus risk in rheumatoid arthritis: reduced incidence with anti-tumor necrosis factor α therapy. Arthritis Care Res (Hoboken). 2012;64(2):215–221. [DOI] [PubMed] [Google Scholar]
- 131. van Asseldonk EJ, Stienstra R, Koenen TB, Joosten LA, Netea MG, Tack CJ. Treatment with Anakinra improves disposition index but not insulin sensitivity in nondiabetic subjects with the metabolic syndrome: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2011;96(7):2119–2126. [DOI] [PubMed] [Google Scholar]
- 132. van Poppel PC, van Asseldonk EJ, Holst JJ, Vilsbøll T, Netea MG, Tack CJ. The interleukin-1 receptor antagonist anakinra improves first-phase insulin secretion and insulinogenic index in subjects with impaired glucose tolerance. Diabetes Obes Metab. 2014;16(12):1269–1273. [DOI] [PubMed] [Google Scholar]
- 133. Rissanen A, Howard CP, Botha J, Thuren T; Global Investigators. Effect of anti-IL-1β antibody (canakinumab) on insulin secretion rates in impaired glucose tolerance or type 2 diabetes: results of a randomized, placebo-controlled trial. Diabetes Obes Metab. 2012;14(12):1088–1096. [DOI] [PubMed] [Google Scholar]
- 134. Cavelti-Weder C, Babians-Brunner A, Keller C, Stahel MA, Kurz-Levin M, Zayed H, Solinger AM, Mandrup-Poulsen T, Dinarello CA, Donath MY. Effects of gevokizumab on glycemia and inflammatory markers in type 2 diabetes. Diabetes Care. 2012;35(8):1654–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Sloan-Lancaster J, Abu-Raddad E, Polzer J, Miller JW, Scherer JC, De Gaetano A, Berg JK, Landschulz WH. Double-blind, randomized study evaluating the glycemic and anti-inflammatory effects of subcutaneous LY2189102, a neutralizing IL-1β antibody, in patients with type 2 diabetes. Diabetes Care. 2013;36(8):2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ruscitti P, Alvaro S, Airò P, Battafarano N, Cantarini L, Paolo Cantore F, Carlino G, D’Abrosca V, Frassi M, Frediani B, Iacono D, Maggio R, Masedu F, Mulé R, Pantano I, Prevete I, Sinigaglia L, Valenti M, Viapiana O, Cipriani P, Giacomelli R. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): a multicentre, randomised, open, prospective, controlled, parallel-group trial [published online ahead of print 8 Oct 2018]. Lancet. doi: https://ssrn.com/abstract=3258674.
- 137. Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, Glynn RJ, Libby P, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71(21):2392–2401. [DOI] [PubMed] [Google Scholar]
- 138. Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf F, Peterson ED, Holman RR; TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232–242. [DOI] [PubMed] [Google Scholar]
- 139. Kataria Y, Ellervik C, Mandrup-Poulsen T. Treatment of type 2 diabetes by targeting interleukin-1 – a meta-analysis of 2921 patients. Semin Immunopathol. 2019;41(4):413–425. [DOI] [PubMed] [Google Scholar]
- 140. Huang J, Yang Y, Hu R, Chen L. Anti-interleukin-1 therapy has mild hypoglycaemic effect in type 2 diabetes. Diabetes Obes Metab. 2018;20(4):1024–1028. [DOI] [PubMed] [Google Scholar]
- 141. Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ; CANTOS Trial Group. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10105):1833–1842. [DOI] [PubMed] [Google Scholar]
- 142. Everett BM, Cornel J, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation. 2019;139(10):1289–1299. [DOI] [PubMed] [Google Scholar]
- 143. Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda). 2009;24:325–331. [DOI] [PubMed] [Google Scholar]
- 144. Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ; CANTOS Trial Group. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391(10118):319–328. [DOI] [PubMed] [Google Scholar]
- 145. So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9(2):R28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Vitale A, Cantarini L, Rigante D, Bardelli M, Galeazzi M. Anakinra treatment in patients with gout and type 2 diabetes. Clin Rheumatol. 2015;34(5):981–984. [DOI] [PubMed] [Google Scholar]
- 147. Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP, Glynn RJ; CIRT Investigators. Low-dose methotrexate for the prevention of atherosclerotic events. N Engl J Med. 2019;380(8):752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, Pocock S, Poulter NR, Ravn LS, Steinberg WM, Stockner M, Zinman B, Bergenstal RM, Buse JB; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. [DOI] [PubMed] [Google Scholar]
- 150. Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, Smirnova N, Bergé M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, Burcelin R. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3(9):559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. [DOI] [PubMed] [Google Scholar]
- 152. Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. [DOI] [PMC free article] [PubMed] [Google Scholar]