Abstract
This study examines the insurance coverage and out-of-pocket costs to Medicare beneficiaries for the angiotensin receptor–neprilysin inhibitor sacubitril/valsartan.
Sacubitril/valsartan, an angiotensin receptor–neprilysin inhibitor (ARNI), is the first new drug to show mortality benefit for heart failure with reduced ejection fraction (HFrEF) in more than a decade. Based on a 20% reduction in cardiovascular death in patients who received it compared with those who received enalapril in the Prospective Comparison of ARNI With ACEI (Angiotensin-Converting Enzyme Inhibitors) to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial,1 sacubitril/valsartan received expedited US Food and Drug Administration approval in 2015 and a class I American Heart Association/American College of Cardiology/Heart Failure Society of America guideline recommendation in 2016.2 However, clinical adoption remains slow, with less than 3% of patients with HFrEF receiving the drug as of 2016.3 Since more than 80% of deaths from heart failure occur in people older than 65 years,4 we examined Medicare Part D plans nationwide to explore whether high cost sharing or a lack of coverage could be barriers to the adoption of sacubitril/valsartan under Medicare Part D.
Methods
We examined Medicare formulary and pricing files for all Part D plans for the first quarter of 2018 (excluding special needs plans, which may have specialized formularies). We analyzed coverage and 30-day cost-sharing requirements, determining a mean across all plans by county and state, for a patient with HFrEF who received guideline-directed therapy with carvedilol, furosemide, and either sacubitril/valsartan or valsartan (and no other drugs). Annual out-of-pocket costs were projected according to the 4 phases of a standard 2018 Part D plan: (1) an initial $405 deductible; (2) a standard coverage period until total drug costs reach $3750; (3) a coverage gap with 35% brand-name and 44% generic cost sharing until out-of-pocket costs reach $5000; and (4) catastrophic coverage with 5% cost sharing thereafter.5 An institutional review board waiver was provided by the University of Hawaii Office of Research Compliance. Informed consent was not required because the study used public formulary data rather than patient claims. Analyses were conducted using SAS version 9.4 (SAS Institute).
Results
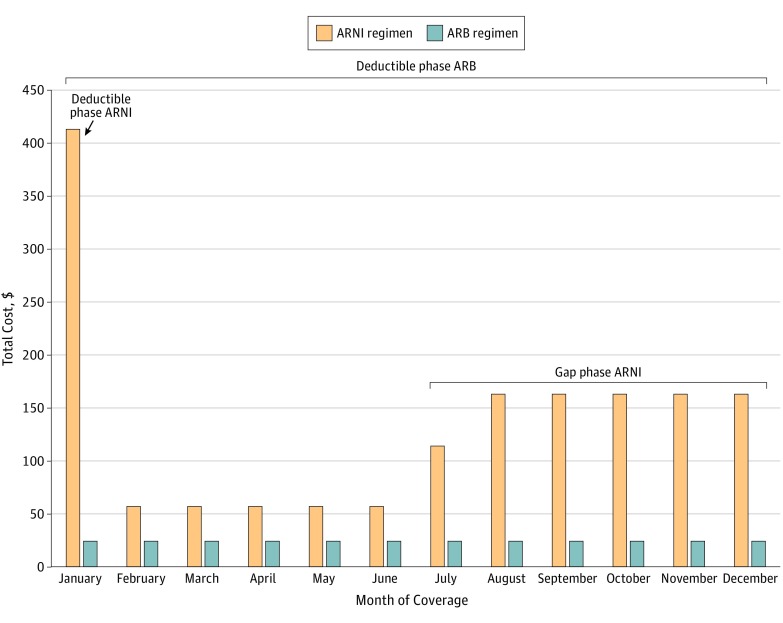
We analyzed 2818 Part D plans nationwide. In early 2018, 100% of plans covered sacubitril/valsartan (with 1069 of 2818 [38.0%] requiring prior authorization). Mean (SD) cost-sharing for a 30-day supply of an ARNI during the coverage period was $57 ($9), compared with a range of $2 to $5 for other examined drugs (Table). Under a standard 2018 plan, beneficiaries receiving an ARNI, carvedilol, and furosemide would pay their full $405 deductible in January and hit the coverage gap in July, after which their monthly cost would increase to $163 (Figure). Their projected annual out-of-pocket costs would be $1685, of which $1632 of $1685 (96.9%) would be attributable to the ARNI. By comparison, beneficiaries receiving an angiotensin II receptor blocker (ARB), carvedilol, and furosemide would have projected annual costs of $291 and would not meet their deductible before the end of the year.
Table. Part D Plan Coverage and Cost Sharing for Sacubitril/Valsartan and Other Heart Failure Drugs.
| Drug Type or Regimen | Single Drug | Plans Providing Coverage, No. (%) | Prior Authorization, No. (%)a | Monthly Cost, Mean (SD), $a | Projected Annual Costb | ||||
|---|---|---|---|---|---|---|---|---|---|
| Total Cost | Beneficiary Cost | Total Cost, $ | Beneficiary Cost, $ (% of Total Cost) | Plan Cost, $ (% of Total Cost) | Pharmaceutical Discount, $ (% of Total Cost) | ||||
| Angiotensin receptor-neprilysin inhibitor | Sacubitril/ valsartan | 2818 (100) | 1069 (38.0) | 465 (8) | 57 (9) | 5576 | 1626 (29.2) | 2662 (47.7) | 1288 (23.1) |
| Angiotensin II receptor blocker | Valsartan | 2811 (99.8) | 0 | 15 (4) | 5 (1) | 185 | 185 (100)c | 0 | 0 |
| β-Blocker | Carvedilol | 2818 (100) | 0 | 6 (3) | 2 (0) | 76 | 76 (100)c | 0 | 0 |
| Diuretic | Furosemide | 2818 (100) | 0 | 2 (1) | 2 (0) | 30 | 30 (100)c | 0 | 0 |
| HFrEF regimen | |||||||||
| Angiotensin II receptor blocker, β-blocker, and diuretic | Valsartan, carvedilol, and furosemide | 2811 (99.8) | 0 | 23 (8) | 9 (1) | 291 | 291 (100)c | 0 | 0 |
| Angiotensin receptor-neprilysin inhibitor, β-blocker, and diuretic | Sacubitril/ valsartan, carvedilol, and furosemide | 2818 (100) | 1069 (38.0) | 473 (12) | 61 (10) | 5682 | 1685 (29.7) | 2681 (47.2) | 1316 (23.2) |
Abbreviation: HFrEF, heart failure with reduced ejection fraction.
We analyzed coverage and 30-day cost-sharing requirements, with means determined across all plans by county and state for 2818 Medicare Part D plans nationwide. The mean monthly cost was calculated during the covered phase (ie, after the deductible is met and before the coverage gap).
We projected total annual costs under a standard 2018 Part D plan, which included an initial $405 deductible, a coverage phase up to $3750 in total drug costs, a coverage gap until beneficiaries exceeded $5000 in out-of-pocket costs, and a catastrophic coverage phase with 5% cost sharing thereafter (with a minimum cost sharing of $3.35 for generic drugs and $8.35 for brand-name drugs).
For beneficiaries receiving valsartan, carvedilol, furosemide, or the combination of valsartan, carvedilol, and furosemide and no other drugs, projected annual costs were low enough to fall entirely within the $405 deductible phase in which beneficiaries pay 100% of drug costs.
Figure. Projected Out-of-Pocket Costs by Month for a Medicare Part D Beneficiary Receiving a Diuretic, β-Blocker, and an Angiotensin Receptor-Neprilysin Inhibitor (ARNI) or Angiotensin II Receptor Blocker (ARB).
Examined drugs were sacubitril/valsartan (an ARNI), valsartan (an ARB), carvedilol (a β-blocker), and furosemide (a diuretic drug). Patients receiving an ARNI would meet their $405 deductible in January and enter the coverage gap phase in July. In contrast, patients receiving an ARB would not meet their deductible before the end of the year.
Discussion
In 2018, sacubitril/valsartan was covered by all Part D plans, mostly without prior authorization. However, affordability is a potential barrier to adoption. Health care professionals should be aware that Medicare patients prescribed an ARNI as part of a HFrEF regimen could face annual costs of $1685 (nearly $1400 more than those prescribed an ARB), with monthly costs exceeding $160 during the coverage gap. Even with new legislation capping out-of-pocket costs at 25% during the coverage gap, cost sharing for an ARNI would exceed $100 per month.5 This is concerning because high out-of-pocket costs have been associated with poorer adherence and worse health outcomes among patients with cardiovascular disease.6 Regular conversations with patients about out-of-pocket costs may be needed to facilitate early identification of and intervention for cost-associated discontinuation of therapy.
Limitations
Annual cost sharing was projected based on the use of only sacubitril/valsartan and other HFrEF drugs. Actual out-of-pocket costs would reflect beneficiaries’ use of other prescription drugs.
Conclusions
Sacubitril/valsartan was well covered by Part D plans. However, high out-of-pocket costs may impede adoption by Medicare beneficiaries.
References
- 1.McMurray JJV, Packer M, Desai AS, et al. ; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. ; WRITING COMMITTEE MEMBERS . 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure, a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2016;134(13):e282-e293. doi: 10.1161/CIR.0000000000000435 [DOI] [PubMed] [Google Scholar]
- 3.Sangaralingham LR, Sangaralingham SJ, Shah ND, Yao X, Dunlay SM. Adoption of sacubitril/valsartan for the management of patients with heart failure. Circ Heart Fail. 2018;11(2):e004302. doi: 10.1161/CIRCHEARTFAILURE.117.004302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8(1):30-41. doi: 10.1038/nrcardio.2010.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaiser Family Foundation An overview of the Medicare Part D prescription drug benefit. https://www.kff.org/medicare/fact-sheet/an-overview-of-the-medicare-part-d-prescription-drug-benefit/. Published October 12, 2018. Accessed May 9, 2019.
- 6.Choudhry NK, Avorn J, Glynn RJ, et al. ; Post-Myocardial Infarction Free Rx Event and Economic Evaluation (MI FREEE) Trial . Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365(22):2088-2097. doi: 10.1056/NEJMsa1107913 [DOI] [PubMed] [Google Scholar]