Key Points
Question
Does the number-needed-to-biopsy for cutaneous melanoma differ among clinicians according to the medical literature?
Findings
In this systematic review of 46 articles on the number-needed-to-biopsy metric for melanoma diagnosis, the number ranged from 2.2 to 287.
Meaning
The number needed to biopsy appeared to vary significantly across geographic regions and according to prevalence of disease and clinician characteristics, including level of training, age, and use of dermoscopy; standardization of NNB and its reporting may be warranted.
Abstract
Importance
To date, no concerted effort has been made to date to evaluate the literature on number-needed-to-biopsy (NNB) metrics, particularly to account for the differences in clinician type and melanoma prevalence in certain geographic locations.
Objective
To review and synthesize worldwide data for NNB for the diagnosis of cutaneous melanoma.
Data Source
MEDLINE, Embase, and PubMed databases were searched for English-language articles published worldwide from January 1, 2000, to November 28, 2018.
Study Selection
A total of 46 studies were included that addressed NNB for at least 3681 clinicians worldwide and included 455 496 biopsied tumors and 29 257 melanomas; primary care practitioner (PCP) data were only available from Australia.
Data Extraction and Synthesis
Articles were screened for eligibility, and possible overlapping data sets were resolved. Data extracted included clinician specialization, use of dermoscopy, geographic region and location-specific health care system, study design, number of benign tumors, number of melanomas, and NNB. The review followed the PRISMA guidelines.
Main Outcome and Measures
The NNB for the diagnosis of cutaneous melanoma.
Results
A total of 46 studies were included that addressed NNB for at least 3681 clinicians worldwide and included 455 496 biopsied tumors and 29 257 melanomas; primary care practitioner (PCP) data were only available from Australia. The reported NNB ranged from 2.2 to 287, and the weighted mean NNB for all included publications was 15.6. The exclusion of publications structured as all biopsied tumors, owing to variable data characterization, resulted in reported NNB ranging from 2.2 to 30.5, with a global weighted mean NNB of 14.8 for all clinicians, 7.5 for all dermatologists, 14.6 for Australian PCPs, and 13.2 for all US-based dermatological practitioners, including dermatologists and advanced practice professionals. The summary effect size (ES) demonstrates that a mean 4% of biopsies demonstrated melanoma for study stratum A (all biopsied skin tumors, ES, 0.04; 95% CI, 0.03-0.05), and a mean 12% of biopsies demonstrated melanoma for study strata B (melanocytic tumors on pathology review, ES, 0.12; 95% CI, 0.10-0.14) and C (clinical concern for melanoma, ES; 0.12; 95% CI, 0.09-0.14).
Conclusions and Relevance
The existing NNB for cutaneous melanoma appeared to vary widely worldwide, lacking standardization in the metric and its reporting, and according to clinician characteristics as well; the NNB of US-based clinicians may warrant further exploration.
This systematic review and meta-analysis evaluates the worldwide variations in the number-needed-to-biopsy (NNB) metric, lack of related data on US-based dermatologists, and nonstandardized processes for reporting NNB for diagnosing cutaneous melanoma.
Introduction
The number needed to biopsy (NNB) quantifies biopsies that result in benign diagnoses in the course of identifying cutaneous melanoma. Biopsy of benign lesions not only has fiscal implications in an era of increasing insurance deductibles and co-insurance rates, but it also contributes to patient morbidity. Patients may experience anxiety while waiting for the laboratory report as well as morbidity from the biopsy procedure and resulting scar.
In assessments of the accuracy and cost-effectiveness of cutaneous melanoma diagnostic tools, 2 metrics are frequently identified in the literature: diagnostic accuracy and NNB. Diagnostic accuracy refers to the ability of a test to distinguish a specific condition (eg, melanoma) from normalcy (eg, benign nevus). The NNB, a metric similar in purpose to the number needed to treat, is an indicator of diagnostic sensitivity (or willingness to assume risk of missing a subtle melanoma) and melanoma prevalence in a patient population (eg, a higher melanoma prevalence patient population results in a lower NNB). Although an imperfect metric, NNB allows for a clinically understandable measure of the number of benign (ie, false-positive) biopsies performed.
To our knowledge, no concerted effort has been made to date to evaluate the literature on NNB metrics, particularly to account for the differences in clinician type and melanoma prevalence in certain geographic locations. We conducted a systematic review of articles published worldwide between January 1, 2000, and November 28, 2018, including information on NNB variance based on clinician type (when specified), to provide a perspective on the strengths and weaknesses of data categories. We also elaborated on the design for future studies of NNB in the setting of cutaneous melanoma.
Methods
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline. The following bibliographic databases were searched from January 1, 2000, through November 28, 2018: MEDLINE, Embase, and PubMed. The results were limited to publications in the English language. All search strategies were developed and tested by a medical librarian, with input from our lead author (K.C.N.). The search strategies contained a combination of controlled vocabulary (eg, MeSH or Emtree) and keyword terms to identify articles concerning a diagnosis of melanoma, nevi, seborrheic keratosis, basal and squamous cell carcinoma, and skin neoplasms in general. The sensitivity of preliminary search strategies was tested by their ability to retrieve a pool of known, relevant citations. The precision of the strategies was strengthened through author feedback on preliminary results (eMethods in the Supplement). An EndNote library was created for managing the retrieved records and for de-duplication.
The inclusion criteria were publication date between January 1, 2000, and November 28, 2018, to account for variability in pathologic interpretation, patterns of dermoscopy use, and changes in melanoma prevalence over time; clearly cited and internally consistent tumor counts for benign neoplasms and melanoma, or clearly cited author-calculated NNB for melanoma; availability of the full text article in the English language; and inclusion of at least 1 melanoma in the data set. Clinicians of all medical specialties were included, as were available data from advanced practice professionals (ie, nurse practitioners and physician assistants), although specific data pertaining to primary care practitioners (PCPs) were only identified for Australian PCPs. In addition, the data cohorts were reviewed for each publication, and subsequent publications that used overlapping data over the same period were excluded.
We identified 770 records through database searches and 5 records through other sources. After removal of 125 duplicate records, one of us (K.C.N.) analyzed the remaining 650 records and summarily excluded 551 of these records for not meeting the inclusion criteria. Ninety-nine full-text articles were assessed for eligibility, and of these 99 articles, 47 were excluded after a single-reviewer evaluation (K.C.N.). The remaining 52 publications received a dual-reviewer assessment (K.C.N. and C.C.L.), resulting in the exclusion of an additional 6 articles. Ultimately, we selected 46 publications for inclusion (eFigure 1 in the Supplement).
Selected studies were evaluated by multiple reviewers (K.C.N., C.C.L., and S.C.C.) to find the total numbers of tumors and melanomas included and to compare them with author-reported NNB, if provided. If the author-reported NNB did not align with the provided tumor counts (mainly owing to the number of tumors that may be “concerning for melanoma” not being clearly specified), we included total tumor counts in Table 1.1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47
Table 1. Studies Selected for Inclusion in Systematic Review, 2000-2018.
| Study | Diagnosing Specialists | Dermoscopy | Years Included | Location | Design Strata | No. of Clinicians | Tumors, Total No. | Melanomas, Total No. | NNBa |
|---|---|---|---|---|---|---|---|---|---|
| Duff et al,1 2001 | Plastic surgeons | Yes | 1993-1998 | Frenchay Hospital, Bristol, United Kingdom | All biopsied tumors | 2 | 2372 | 586 | 4.0 |
| Westbrook et al,2 2006 | Dermatologists | Not referenced | 2003 | Royal Hallamshire Hospital, Sheffield, United Kingdom | All biopsied tumors | Not referenced | 145 | 21 | 6.9 |
| Rosendahl et al,3 2011 | PCPs | Yes | Not referenced | University of Queensland, Brisbane, Australia | All biopsied tumors | 1 | 463 | 29 | 16.0 |
| Wilkinson et al,4 2006 | PCPs | Yes | 2005 | Skin Alert Clinics, Northern Territory, Australia | All biopsied tumors | 20 | 3317 | 116 | 28.6 |
| Har-shai et al,5 2001 | Plastic surgeons | Not referenced | 1997-1998 | Linn Clinic of Plastic Surgery, Israel | All biopsied tumors | 4 | 835 | 28 | 29.8 |
| Scrace et al,6 2009 | PCPs | Yes | 2001-2008 | Royal Flying Doctor Service, Australia | All biopsied tumors | 6 | 269 | 7 | 38.4 |
| Byrnes et al,7 2007 | PCPs | Yes | 2005-2006 | Queensland Innovative Practices, Queensland, Australia | All biopsied tumors | 23 | 1220 | 31 | 39.4 |
| Matteucci et al,8 2011 | Plastic surgeons | Not referenced | 2006 | Castle Hill Hospital, Cottingham, United Kingdom | All biopsied tumors | Not referenced | 1187 | 24 | 49.5 |
| Heal et al,9 2006 | PCPs | Not referenced | 2004-2005 | Queensland (North), Australia | All biopsied tumors | 16 | 1205 | 20 | 60.3 |
| Youl et al,10 2007 | PCPs | Not referenced | Not referenced | Queensland, Australia | All biopsied tumors | 16 | 2544 | 42 | 60.6 |
| Green et al,11 2004 | Dermatologists | Mixed | 2002 | University of Miami, United States | All biopsied tumors | 22 | 11 072 | 156 | 71.0 |
| Youl et al,12 2007 | PCPs | Mixed | 2005 | Queensland (Southeast), Australia | All biopsied tumors | 154 | 11 116 | 152 | 73.1 |
| Rolfe,13 2012 | Dermatologists | Not referenced | 2009-2010 | Queensland public hospital, Australia | All biopsied tumors | Not referenced | 5039 | 55 | 91.6 |
| Moffatt et al,14 2006 | PCPs | Not referenced | 2003 | Queensland skin cancer clinic, Australia | All biopsied tumors | Not referenced | 287 | 1 | 287.0 |
| Ahnlide et al,15 2014 | Dermatologists | Yes | 2009-2012 | Helsingborg Hospital, Lund, Sweden | All melanocytic tumors + SKs on path review | 10 | 1717 | 252 | 6.8 |
| English et al,16 2004 | PCPs | Not referenced | 1998-2000 | Perth, Australia | All melanocytic tumors + SKs on pathologic review | 468 | 4699 | 160 | 29.4 |
| Hansen et al,17 2009 | PCPs | Not referenced | 2005-2007 | Skin Cancer Clinics, Australia | All melanocytic tumors + SKs on pathologic review | 57 | 10612 | 348 | 30.5 |
| Esdaile et al,18 2014 | Dermatologists | Yes | 2006, 2011 | Churchill Hospital, Oxford, United Kingdom | All melanocytic tumors on pathologic review | Not referenced | 1380 | 454 | 3.0 |
| Moloney et al,19 2014 | Dermatologists | Yes | 2006-2009 | Sydney Melanoma Diagnostic Center, Australia | All melanocytic tumors on pathologic review | 4 | 441 | 82 | 5.4 |
| Argenziano et al,20 2014 | Dermatologists | Yes | 2011 | Italian Pigmented Lesion Clinics, Italy | All melanocytic tumors on pathologic review | 22 | 5402 | 866 | 6.2 |
| Sidhu et al,21 2012 | Dermatologists | Yes | 2005-2009 | Welsh Institute of Dermatology; Swansea University, United Kingdom | All melanocytic tumors on pathologic review | 9 | 4691 | 750 | 6.3 |
| Tschandl et al,22 2013 | Dermatologists | Mixed | 1998-2008 | Medical University of Vienna, Vienna | All melanocytic tumors on pathologic review | Not referenced | 9100 | 1365 | 6.7 |
| Carli et al,23 2004 | Dermatologists | Mixed | 1997-2001 | Department of Dermatology, Florence, Italy | All melanocytic tumors on pathologic review | 6 | 3053 | 319 | 9.6 |
| Soares et al,24 2009 | Dermatologists | Mixed | 2005 | Mayo Clinic, Scottsdale, Arizona, United States | All melanocytic tumors on pathologic review | Not referenced | 1547 | 147 | 10.5 |
| Lott et al,25 2018 | Not referenced | Not referenced | 2007-2012 | Kaiser Permanente, Washington, United States | All melanocytic tumors on pathologic review | Not referenced | 18 715 | 1609 | 11.6 |
| Argenziano et al,26 2012 | Multidisciplinary | Mixed | 1998-2007 | International | All melanocytic tumors on pathologic review | 21 | 300 215 | 17 172 | 17.5 |
| Goodson et al,27 2010 | Dermatologists | Yes | 1999-2004 | University of Utah, Salt Lake City, United States | All melanocytic tumors on pathologic review | Not referenced | 548 | 28 | 19.6 |
| Koelink et al,28 2014 | PCPs | Mixed | 2010-2011 | Northeastern Netherlands | Clinical concern for melanoma | 55 | 22 | 10 | 2.2 |
| Rauniyar et al,29 2003 | Not referenced | Not referenced | 2000 | Kathmandu Valley, Nepal | Clinical concern for melanoma | Not referenced | 21 | 9 | 2.3 |
| Chia et al,30 2008 | Dermatologists | Not referenced | 2005-2006 | Sydney, Australia | Clinical concern for melanoma | 35 | 686 | 195 | 3.5 |
| Brown et al,31 2016 | Multidisciplinary | Not referenced | 2015 | Royal Devon and Exeter NHS (National Health Service) Foundation Trust, United Kingdom | Clinical concern for melanoma | Not referenced | 775 | 186 | 4.2 |
| Carli et al,32 2003 | Dermatologists | Yes | 1998 | University of Florence, Italy | Clinical concern for melanoma | 2 | 13 | 3 | 4.3 |
| Verykiou et al,33 2012 | Dermatologists | Not referenced | 2009, 2011 | Royal Victoria Infirmary, Newcastle upon Tyne, United Kingdom | Clinical concern for melanoma | Not referenced | 1313 | 288 | 4.6 |
| Aitken et al,34 2006 | PCPs | Not referenced | 1998-2001 | Queensland, Australia | Clinical concern for melanoma | Not referenced | 161 | 33 | 4.9 |
| Carli et al,35 2003 | Dermatologists | Mixed | 1997-1999 | Italian Pigmented Lesion Clinics, Italy | Clinical concern for melanoma | 6 | 114 | 16 | 7.1 |
| Rosendahl et al,36 2012 | PCPs | Mixed | 2008-2012 | Skin Cancer Audit Database, Australia | Clinical concern for melanoma | 193 | 21 900 | 2367 | 9.3 |
| Terushkin et al,37 2010 | Dermatologists | Mixed | 2004-2005 | NYU (New York University) Langone, New York, United States | Clinical concern for melanoma | 2 | 438 | 42 | 10.4 |
| Lynch et al,38 2013 | Dermatologists | Yes | Not referenced | St John's Institute of Dermatology, London, United Kingdom | Clinical concern for melanoma | Not referenced | 121 | 10 | 12.1 |
| Wilson et al,39 2012 | Dermatologists (6 MDs, 2 APPs) | Mixed | 2008-2009 | Wake Forest University Medical Center, Winston-Salem, North Carolina, United States | Clinical concern for melanoma | 8 | 410 | 28 | 14.6 |
| van der Rhee et al,40 2010 | Dermatologists | Yes | Not referenced | Leiden University Medical Center, Leiden, Netherlands | Clinical concern for melanoma | 17 | 209 | 14 | 14.9 |
| Soltani et al,41 2015 | Dermatologists | Not referenced | 2013 | University of Utah, Salt Lake City, United States | Clinical concern for melanoma | 43 | 2643 | 165 | 16.0 |
| Waldman et al,42 2012 | Dermatologists and PCPs | Not referenced | 2003-2004 | Schleswig-Holstein region, Germany | Clinical concern for melanoma | 1789 | 7408 | 452 | 16.4 |
| Baade et al,43 2008 | PCPs | Mixed | 2005 | Queensland (Southeast), Australia | Clinical concern for melanoma | 154 | 2977 | 152 | 19.6 |
| Nault et al,44 2015 | Dermatologists (13 MDs; 5 APPs) | Mixed | 2010 | University of Wisconsin, Madison, United States | Clinical concern for melanoma | 18 | 492 | 23 | 21.4 |
| Anderson et al,45 2018 | Dermatologists (15 MDs; 15 APPs) | Not referenced | 2011-2015 | University of Pittsburgh Medical Center, Pittsburgh, Pennsylvania, United States | Clinical concern for melanoma | 30 | 4039 | 149 | 27.1 |
| English et al,46 2003 | PCPs | Not referenced | 1998-2000 | Queensland, Australia | Clinical concern for melanoma | 468 | 8563 | 295 | 29.0 |
| Totals | NA | NA | NA | NA | NA | 3681 | 455 496 | 29 257 | 15.6 |
| Totals excluding studies in design stratum A, all biopsied tumors | NA | NA | NA | NA | NA | 3417 | 414 425 | 27 989 | 14.8 |
Abbreviations: APP, advanced practice professional; NA, not applicable; NNB, number needed to biopsy; PCP, primary care practitioner; SK, seborrheic keratoses.
The NNB, a metric similar in purpose to the number needed to treat, is an indicator of diagnostic sensitivity (or willingness to assume risk of missing a subtle melanoma) and melanoma prevalence in a patient population (eg, a higher melanoma prevalence patient population results in a lower NNB).
All extracted data of the selected studies were imported into Stata, version 15 (StataCorp LLC). The proportion of melanoma diagnoses was calculated by dividing the number of melanomas by the number of biopsies performed (1/NNB). For the meta-analysis, the 1/NNB data were stratified by study design and presented in forest plot format (Figure 1).1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27 Heterogeneity was quantified using I2, and effect sizes were expressed with 95% CI.
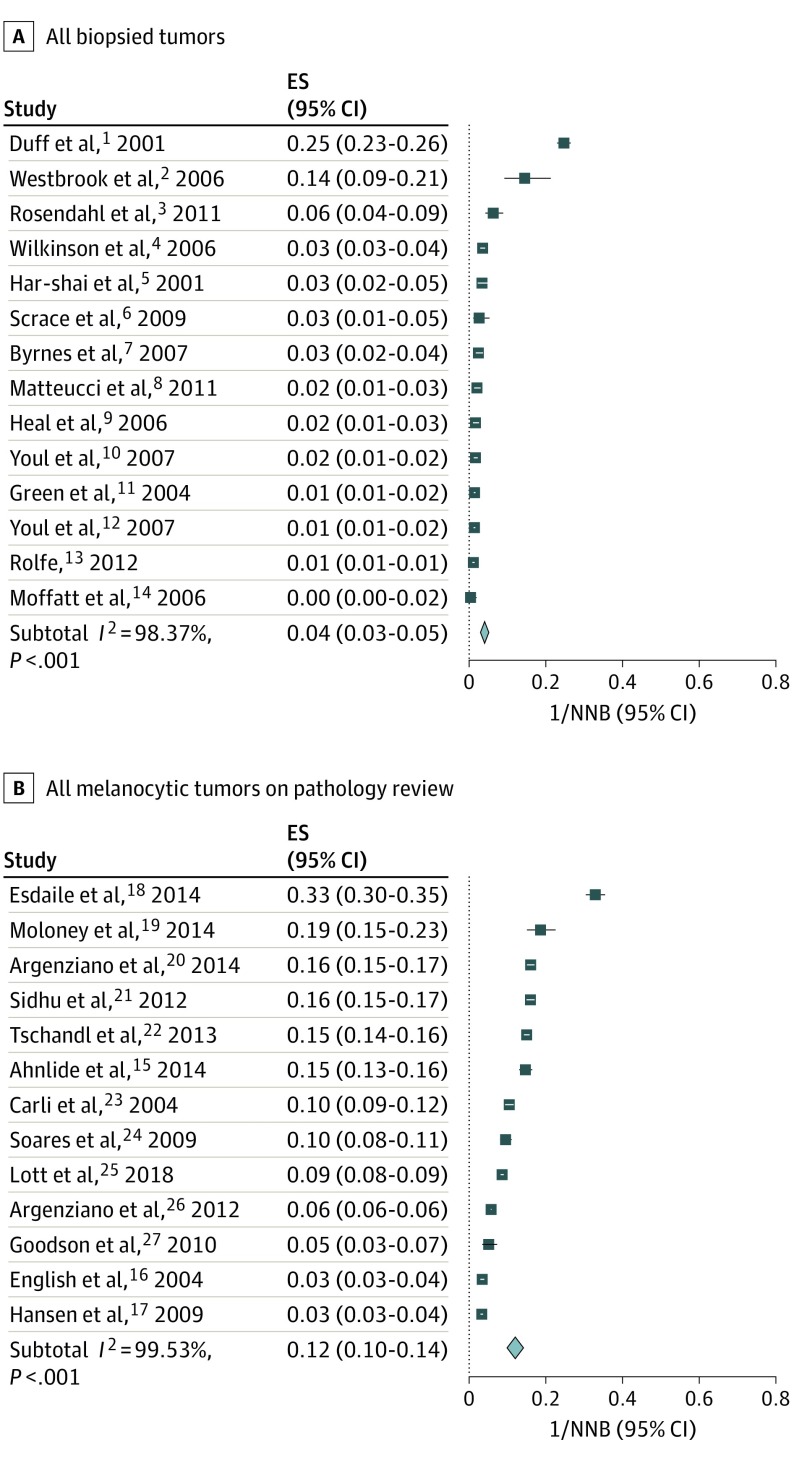
Figure 1. Proportions of Melanoma Diagnoses in All Biopsied Tumors (Stratum A Studies) and Melanocytic Tumors (Stratum B Studies).
Data represent the number of melanoma diagnoses as a proportion of the number needed to biopsy (1/NNB). Design strata A and B demonstrate statistically significant heterogeneity (I2>98.37%); thus, combining studies within individual study design stratum should be approached with caution. The summary effect size (ES) for each stratum demonstrates that a mean 4% of biopsies demonstrated melanoma for study stratum A (ES, 0.04; 95% CI, 0.03-0.05), and a mean 12% of biopsies demonstrated melanoma for study stratum B (ES, 0.12; 95% CI, 0.10-0.14). The horizontal lines adjacent to each square indicate CIs. The diamonds represent the meta-analyzed effect size for the individual study strata.
Results
The 46 publications selected for inclusion addressed the NNB of at least 3681 clinicians and 455 496 biopsied tumors. Most of the included articles were retrospective medical record reviews, with most categorized as level 3 Quality of Evidence on SORT (Strength of Recommendation Taxonomy).47
The data in Table 11,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 and Figure 11,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27 and Figure 228,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46 represent 3 study design strata: all biopsied tumors (stratum A), all melanocytic tumors on pathologic review (stratum B), and clinical concern for melanoma (stratum C). Inclusion or exclusion of seborrheic keratosis varied across all 3 design strata or types, as did the specificity of histopathologic diagnostic parameters.
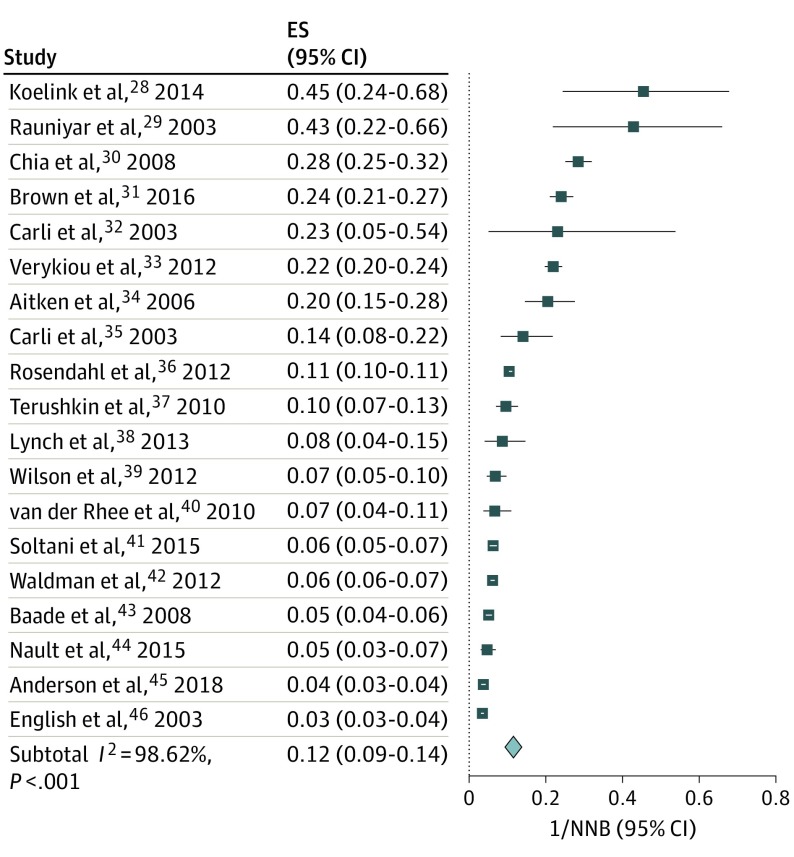
Figure 2. Proportion of Clinical Concern for Melanoma in Stratum C Studies.
Data represent the number of melanoma diagnoses as a proportion of the number needed to biopsy (1/NNB). Design stratum C demonstrates statistically significant heterogeneity (I2>98.37%); thus, combining studies within individual study design stratum should be approached with caution. The summary effect size (ES) demonstrates that a mean 12% of biopsies demonstrated melanoma for study stratum C (ES, 0.12; 95% CI, 0.09-0.14). The horizontal lines adjacent to each square indicate CIs. The diamond represents the meta-analyzed effect size for the individual study stratum.
To facilitate estimations of variance (Figure 1 and Figure 2), the data were structured as proportions, or number of melanomas per biopsies performed (1/NNB). All strata demonstrated statistically significant heterogeneity (I2 = 98.37% to 99.53%; P < .001); thus combining studies within individual study strata should be approached with caution. The summary effect size (ES) for each stratum demonstrates that a mean 12% of biopsies demonstrated melanoma for study stratum A (ES, 0.04; 95% CI, 0.03-0.05), and a mean 12% of biopsies demonstrated melanoma for study strata B (ES, 0.12; 95% CI, 0.10-0.14) and C (ES, 0.12; 95% CI, 0.09-0.14).
Analysis of all 3 strata demonstrated NNB ranging from 2.2 to 287 (Table 1; eFigure 2 in the Supplement), with data representing at least 3681 clinicians (as not all studies explicitly referenced the number of clinicians) and with 29 257 cutaneous melanomas and 455 496 biopsied tumors. Analysis of all selected worldwide publications and clinicians included a weighted mean NNB of 15.6 (455 496 tumors divided by 29 257 melanomas) (Table 2). Exclusion of publications structured as stratum A (all biopsied tumors), owing to variable data characterization, resulted in author-reported NNB that ranged from 2.2 to 30.5, with a weighted mean NNB of 14.8 for all worldwide clinicians, 7.5 for all worldwide dermatologists, 14.6 for Australian PCPs, and 13.2 for all US-based clinicians. Source cutaneous melanoma and biopsied tumor data were used to calculate these reported weighted means. When the Australian article by Rosendahl et al36 was excluded, because most of the included Australian PCPs reported a high level of dermoscopic proficiency, the weighted mean NNB of the Australian PCPs increased to 27.3.
Table 2. Summary Data of Systematic Review, 2000-2018.
| Data Categorization | Location | NNB, Weighted Mean |
|---|---|---|
| All publications | Worldwide | 15.6 |
| All publications, excluding all biopsied tumor data structure | ||
| Dermatologists | Worldwide | 7.5 |
| Primary care practitioners | Australia | 14.6 |
| Dermatologists and APPs | United States | 13.2 |
Abbreviations: APP, advanced practice professional; NNB, number needed to biopsy.
Discussion
Identified publications addressing NNB for cutaneous melanoma appeared to document statistically significant heterogeneity and widely variable NNB. A direct comparison, particularly between US and Australian publications, was complicated by varying study design, clinician specialization, use of dermoscopy, and location-specific melanoma prevalence. Even single-institution publications demonstrated clinician-specific NNB variance.45 This variance, assuming equivalent cutaneous melanoma prevalence within the institution’s general dermatological patient population, highlights a potential association between clinician-specific factors and NNB, and thus clinician-directed educational opportunities may improve NNB.
In the field of dermatology, NNB varies from physician to physician, with an even greater variance reported when nondermatologist clinicians (physicians and advanced practice professionals) are considered.45 Additional clinician factors, such as older age,11 completion of dermatology residency training,36,44,45 and skillful use of dermoscopy3,32 and total body photography,27 have been associated with lower NNB. Patient factors, such as melanoma prevalence per geographic location and in the clinician’s patient cohort,48 melanoma subtype, and patient anxiety,43 may also be associated with changing NNB, but patient factors are seldom included as companion data to country-specific articles on NNB.
Additional database challenges were present in this NNB metric review. The degree of clinician concern for melanoma was not always precisely represented in the laboratory report (with potential concern for either biasing the pathologist or seeking to avoid denial of insurance coverage for removal of a clinically benign skin tumor), such as categorizing tumors that did not truly worry the clinician as concerning for melanoma. For borderline melanocytic neoplasms (eg, nevus with severe atypia or dysplasia vs melanoma in situ), interobserver variability in pathologic interpretation49 may result in dissimilar NNB. What may be most challenging to overcome was the lack of representation of false-negatives (ie, missed melanomas) in most of the included articles; these data would be of value in cost and cost-effectiveness analyses.
The published NNB studies were structured according to 1 of 3 distinct design strata: (A) all biopsied tumors, (B) all melanocytic tumors on pathologic review, and (C) clinical concern for melanoma. Stratum A had the advantage of representing a real-world picture of clinical practice and presented greater diversity in clinician specialization, with the inclusion of more PCPs. However, all PCP data we identified in this systematic review originated from Australia, which had higher melanoma prevalence and a well-established culture of PCP-based melanoma screening34 with dermoscopic proficiency36; thus, stratum A may not align with the PCP-based NNB in the United States, which was not represented in the data set. The lack of information on the degree of clinician concern for melanoma was also a substantial challenge with this data set, as tumors that were not concerning for melanoma were likely included in the total tumor counts, thus potentially falsely elevating the clinicians’ NNB. Two other concerns with stratum A included the interobserver pathologic interpretation variance and discordant diagnostic groupings.
Stratum B design included all tumors that were interpreted as melanocytic on pathologic review, with variable inclusion and exclusion of seborrheic keratosis, the most common lesion mimicking cutaneous melanoma. This strata excluded most nonmelanocytic tumors from evaluation, but to the detriment of excluding nonmelanocytic tumors that may have also simulated melanoma, such as pigmented basal cell carcinomas.
Stratum C design included all lesions for which the clinician indicated a clinical concern for melanoma; however, the number of articles with this structure was relatively low, representing only 5 US-based publications with data for 101 clinicians. Thus, the true range of NNB for US-based dermatologists when evaluating a lesion concerning for melanoma was likely not precisely represented in this data grouping. In addition, the practice of routinely listing rule out melanoma as the clinical impression on a pathologic order, in an effort to avoid insurance denial for the removal of clinically and dermoscopically innocuous melanocytic neoplasms, may disproportionately skew the data in a US-based clinician cohort.
The absence of identified US-based PCP data was notable and represented an undefined portion of the melanoma screening biopsies performed. Primary care practitioners address dermatological concerns with variable expertise and, often out of necessity, in geographic regions with low-level access to dermatological services. Clinics that publicly profess their dermatological expertise but are staffed by physicians who lack dermatology residency training and board certification are increasing in number. The NNB for cutaneous melanoma of these clinicians is entirely absent in the literature we reviewed.
The wide range of NNB, paucity of US-based clinician data, and varying study designs reported in the included publications highlight the potential advantage of developing consensus-led, structured clinical impression and pathologic diagnostic data to standardize NNB reporting across electronic health records and health care systems. Performance metrics, when paired with opportunities for clinical practice improvement, have the potential to optimize patient comfort and satisfaction, improve clinicians’ self-efficacy regarding noninvasive diagnosis, improve busy clinicians’ allocations of time and effort (for both dermatologists and pathologists), and reduce health care costs.
The number of studies that had to be excluded highlights the need for minimal metrics for meaningful NNB comparison across publications, including explicit reference to the number of participating clinicians, clinician specialization and training, reported use of dermoscopy by clinicians, specific criteria for data inclusion or exclusion, methods of data extraction and validation, and reporting of total tumor counts to support NNB metric calculations. Removal of benign skin lesions is entirely expected, given that perfect diagnostic accuracy in cutaneous melanoma has not been demonstrated even by pigmented lesion experts,50 and such removal may be appropriate to avoid missing a clinically subtle melanoma. However, these data indicate an opportunity for education-driven and technology-supported melanoma screening programs that may mitigate the concern for patient harms by optimizing NNB when indicated.51
Limitations
This systematic review, despite the careful structuring and multireviewer evaluations of identified articles, carries the inherent limitations of retrospective analyses: specifically, publications appropriate for study inclusion were not identified through the search process. The paucity of data on US-based clinicians and varying study designs also limited our consolidated data analysis and highlighted the need to develop consensus-led, structured clinical impression and pathologic diagnostic data to standardize NNB reporting across electronic health records and health care systems. Given the statistically significant heterogeneity of the data we reviewed, representing patient populations with variable melanoma prevalence and clinicians with diverse training and experience, the interpretation of any summary metrics should be approached with extreme caution.
Conclusions
The reported NNB for cutaneous melanoma varies widely across clinicians, geography, and patient populations. Variations in study designs compromise the ability to compare publications across design types. Standardization of NNB reporting offers an opportunity to improve the quality of published reports on this important but imperfect metric, which quantifies the volume of biopsies that result in benign diagnoses in the course of identifying cutaneous melanoma.
eMethods. MEDLINE Search Strategy
eFigure 1. PRISMA 2009 Flow Diagram
eFigure 2. Systematic Review of Publications Addressing Number Needed to Biopsy for Melanoma
References
- 1.Duff CGM, Melsom D, Rigby HS, Kenealy JM, Townsend PL. A 6 year prospective analysis of the diagnosis of malignant melanoma in a pigmented-lesion clinic: even the experts miss malignant melanomas, but not often. Br J Plast Surg. 2001;54(4):317-321. doi: 10.1054/bjps.2000.3561 [DOI] [PubMed] [Google Scholar]
- 2.Westbrook RHG, Goyal N, Gawkrodger DJ. Diagnostic accuracy for skin cancer: comparison of general practitioner with dermatologist and dermatopathologist. J Dermatolog Treat. 2006;17(1):57-58. doi: 10.1080/09546630500442864 [DOI] [PubMed] [Google Scholar]
- 3.Rosendahl C, Tschandl P, Cameron A, Kittler H. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64(6):1068-1073. doi: 10.1016/j.jaad.2010.03.039 [DOI] [PubMed] [Google Scholar]
- 4.Wilkinson D, Askew DA, Dixon A. Skin cancer clinics in Australia: workload profile and performance indicators from an analysis of billing data. Med J Aust. 2006;184(4):162-164. [DOI] [PubMed] [Google Scholar]
- 5.Har-Shai Y, Hai N, Taran A, et al. Sensitivity and positive predictive values of presurgical clinical diagnosis of excised benign and malignant skin tumors: a prospective study of 835 lesions in 778 patients. Plast Reconstr Surg. 2001;108(7):1982-1989. doi: 10.1097/00006534-200112000-00022 [DOI] [PubMed] [Google Scholar]
- 6.Scrace M, Margolis SA. The Royal Flying Doctor Service primary care skin cancer clinic: a pilot program for remote Australia. Rural Remote Health. 2009;9(1):1048. doi: 10.22605/RRH1048 [DOI] [PubMed] [Google Scholar]
- 7.Byrnes P, Ackermann E, Williams ID, Mitchell GK, Askew D. Management of skin cancer in Australia–a comparison of general practice and skin cancer clinics. Aust Fam Physician. 2007;36(12):1073-1075. [PubMed] [Google Scholar]
- 8.Matteucci P, Pinder R, Magdum A, Stanley P. Accuracy in skin lesion diagnosis and the exclusion of malignancy. J Plast Reconstr Aesthet Surg. 2011;64(11):1460-1465. doi: 10.1016/j.bjps.2011.06.017 [DOI] [PubMed] [Google Scholar]
- 9.Heal C, Buettner P, Raasch B, Browning S. Minor skin excisions in general practice in North Queensland. Aust Fam Physician. 2006;35(10):825-828. [PubMed] [Google Scholar]
- 10.Youl PHR, Raasch BA, Janda M, Aitken JF. The effect of an educational programme to improve the skills of general practitioners in diagnosing melanocytic/pigmented lesions. Clin Exp Dermatol. 2007;32(4):365-370. doi: 10.1111/j.1365-2230.2007.02414.x [DOI] [PubMed] [Google Scholar]
- 11.Green AR, Elgart GW, Ma F, Federman DG, Kirsner RS. Documenting dermatology practice: ratio of cutaneous tumors biopsied that are malignant. Dermatol Surg. 2004;30(9):1208-1209. [DOI] [PubMed] [Google Scholar]
- 12.Youl PHB, Baade PD, Janda M, Del Mar CB, Whiteman DC, Aitken JF. Diagnosing skin cancer in primary care: how do mainstream general practitioners compare with primary care skin cancer clinic doctors? Med J Aust. 2007;187(4):215-220. [DOI] [PubMed] [Google Scholar]
- 13.Rolfe HM. Accuracy in skin cancer diagnosis: a retrospective study of an Australian public hospital dermatology department. Australas J Dermatol. 2012;53(2):112-117. doi: 10.1111/j.1440-0960.2011.00855.x [DOI] [PubMed] [Google Scholar]
- 14.Moffatt CRMG, Green AC, Whiteman DC. Diagnostic accuracy in skin cancer clinics: the Australian experience. Int J Dermatol. 2006;45(6):656-660. doi: 10.1111/j.1365-4632.2006.02772.x [DOI] [PubMed] [Google Scholar]
- 15.Ahnlide I, Nielsen K, Bjellerup M. Diagnosis of pigmented skin tumours in a dermatological setting: different aspects of the number needed to excise as a measure of efficiency. Acta Derm Venereol. 2014;94(6):683-686. doi: 10.2340/00015555-1831 [DOI] [PubMed] [Google Scholar]
- 16.English DRDM, Del Mar C, Burton RC. Factors influencing the number needed to excise: excision rates of pigmented lesions by general practitioners. Med J Aust. 2004;180(1):16-19. [DOI] [PubMed] [Google Scholar]
- 17.Hansen C, Wilkinson D, Hansen M, Argenziano G. How good are skin cancer clinics at melanoma detection? Number needed to treat variability across a national clinic group in Australia. J Am Acad Dermatol. 2009;61(4):599-604. doi: 10.1016/j.jaad.2009.04.021 [DOI] [PubMed] [Google Scholar]
- 18.Esdaile B, Mahmud I, Palmer A, Bowling J. Diagnosing melanoma: how do we assess how good we are? Clin Exp Dermatol. 2014;39(2):129-134. doi: 10.1111/ced.12223 [DOI] [PubMed] [Google Scholar]
- 19.Moloney FJ, Guitera P, Coates E, et al. Detection of primary melanoma in individuals at extreme high risk: a prospective 5-year follow-up study. JAMA Dermatol. 2014;150(8):819-827. doi: 10.1001/jamadermatol.2014.514 [DOI] [PubMed] [Google Scholar]
- 20.Argenziano G, Moscarella E, Annetta A, et al. Melanoma detection in Italian pigmented lesion clinics. G Ital Dermatol Venereol. 2014;149(2):161-166. [PubMed] [Google Scholar]
- 21.Sidhu S, Bodger O, Williams N, Roberts DL. The number of benign moles excised for each malignant melanoma: the number needed to treat. Clin Exp Dermatol. 2012;37(1):6-9. doi: 10.1111/j.1365-2230.2011.04148.x [DOI] [PubMed] [Google Scholar]
- 22.Tschandl P, Pehamberger H, Kittler H. Trends in the diagnosis of melanoma at a university center over time. J Dtsch Dermatol Ges. 2013;11(3):251-256. [DOI] [PubMed] [Google Scholar]
- 23.Carli P, De Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the ‘dermoscopy era’: a retrospective study 1997-2001. Br J Dermatol. 2004;150(4):687-692. doi: 10.1111/j.0007-0963.2004.05860.x [DOI] [PubMed] [Google Scholar]
- 24.Soares TFL, Laman SD, Yiannias JA, et al. Factors leading to the biopsy of 1547 pigmented lesions at Mayo Clinic, Scottsdale, Arizona, in 2005. Int J Dermatol. 2009;48(10):1053-1056. doi: 10.1111/j.1365-4632.2009.04137.x [DOI] [PubMed] [Google Scholar]
- 25.Lott JP, Boudreau DM, Barnhill RL, et al. Population-based analysis of histologically confirmed melanocytic proliferations using natural language processing. JAMA Dermatol. 2018;154(1):24-29. doi: 10.1001/jamadermatol.2017.4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Argenziano G, Cerroni L, Zalaudek I, et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67(1):54-59. doi: 10.1016/j.jaad.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 27.Goodson AG, Florell SR, Hyde M, Bowen GM, Grossman D. Comparative analysis of total body and dermatoscopic photographic monitoring of nevi in similar patient populations at risk for cutaneous melanoma. Dermatol Surg. 2010;36(7):1087-1098. doi: 10.1111/j.1524-4725.2010.01589.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koelink CJ, Vermeulen KM, Kollen BJ, et al. Diagnostic accuracy and cost-effectiveness of dermoscopy in primary care: a cluster randomized clinical trial. J Eur Acad Dermatol Venereol. 2014;28(11):1442-1449. doi: 10.1111/jdv.12306 [DOI] [PubMed] [Google Scholar]
- 29.Rauniyar SKA, Agarwal A. Histomorphologic pattern of skin lesions in Kathmandu Valley: a retrospective study. Nepal Med Coll J. 2003;5(1):22-24. [PubMed] [Google Scholar]
- 30.Chia ALS, Simonova G, Dutta B, Lim A, Shumack S. Melanoma diagnosis: Australian dermatologists’ number needed to treat. Australas J Dermatol. 2008;49(1):12-15. doi: 10.1111/j.1440-0960.2007.00410.x [DOI] [PubMed] [Google Scholar]
- 31.Brown AM, McGrath EJ. Diagnostic accuracy in pigmented lesions: further supportive evidence for consultant-led dermatology skin cancer services. Br J Dermatol. 2016;175(suppl 1):126-127. doi: 10.1111/bjd.14573 [DOI] [Google Scholar]
- 32.Carli P, Mannone F, De Giorgi V, Nardini P, Chiarugi A, Giannotti B. The problem of false-positive diagnosis in melanoma screening: the impact of dermoscopy. Melanoma Res. 2003;13(2):179-182. doi: 10.1097/00008390-200304000-00011 [DOI] [PubMed] [Google Scholar]
- 33.Verykiou S, Langtry JAA, Rajan N. Cutaneous malignant melanoma: does increased number of biopsies increase melanoma detection? Br J Dermatol. 2012;167:95-96. doi: 10.1111/j.1365-2133.2012.10986.x [DOI] [Google Scholar]
- 34.Aitken JFJ, Janda M, Elwood M, Youl PH, Ring IT, Lowe JB. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54(1):105-114. doi: 10.1016/j.jaad.2005.08.072 [DOI] [PubMed] [Google Scholar]
- 35.Carli P, De Giorgi V, Betti R, et al. Relationship between cause of referral and diagnostic outcome in pigmented lesion clinics: a multicentre survey of the Italian Multidisciplinary Group on Melanoma (GIPMe). Melanoma Res. 2003;13(2):207-211. doi: 10.1097/00008390-200304000-00017 [DOI] [PubMed] [Google Scholar]
- 36.Rosendahl C, Williams G, Eley D, et al. The impact of subspecialization and dermatoscopy use on accuracy of melanoma diagnosis among primary care doctors in Australia. J Am Acad Dermatol. 2012;67(5):846-852. doi: 10.1016/j.jaad.2011.12.030 [DOI] [PubMed] [Google Scholar]
- 37.Terushkin V, Warycha M, Levy M, Kopf AW, Cohen DE, Polsky D. Analysis of the benign to malignant ratio of lesions biopsied by a general dermatologist before and after the adoption of dermoscopy. Arch Dermatol. 2010;146(3):343-344. [DOI] [PubMed] [Google Scholar]
- 38.Lynch MS, Syed A, Lacy K. Analysis of factors leading to the diagnosis of malignant melanoma in a tertiary centre high-risk melanoma screening clinic. J Dtsch Dermatol Ges. 2013;11(7):38. [Google Scholar]
- 39.Wilson RLY, Yentzer BA, Isom SP, Feldman SR, Fleischer AB Jr. How good are US dermatologists at discriminating skin cancers? A number-needed-to-treat analysis. J Dermatolog Treat. 2012;23(1):65-69. doi: 10.3109/09546634.2010.512951 [DOI] [PubMed] [Google Scholar]
- 40.van der Rhee JIB, Bergman W, Kukutsch NA. The impact of dermoscopy on the management of pigmented lesions in everyday clinical practice of general dermatologists: a prospective study. Br J Dermatol. 2010;162(3):563-567. doi: 10.1111/j.1365-2133.2009.09551.x [DOI] [PubMed] [Google Scholar]
- 41.Soltani-Arabshahi R, Sweeney C, Jones B, Florell SR, Hu N, Grossman D. Predictive value of biopsy specimens suspicious for melanoma: support for 6-mm criterion in the ABCD rule. J Am Acad Dermatol. 2015;72(3):412-418. doi: 10.1016/j.jaad.2014.11.030 [DOI] [PubMed] [Google Scholar]
- 42.Waldmann A, Nolte S, Geller AC, et al. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch Dermatol. 2012;148(8):903-910. doi: 10.1001/archdermatol.2012.893 [DOI] [PubMed] [Google Scholar]
- 43.Baade PD, Youl PH, Janda M, Whiteman DC, Del Mar CB, Aitken JF. Factors associated with the number of lesions excised for each skin cancer: a study of primary care physicians in Queensland, Australia. Arch Dermatol. 2008;144(11):1468-1476. doi: 10.1001/archderm.144.11.1468 [DOI] [PubMed] [Google Scholar]
- 44.Nault A, Zhang C, Kim K, Saha S, Bennett DD, Xu YG. Biopsy use in skin cancer diagnosis: comparing dermatology physicians and advanced practice professionals. JAMA Dermatol. 2015;151(8):899-902. doi: 10.1001/jamadermatol.2015.0173 [DOI] [PubMed] [Google Scholar]
- 45.Anderson AM, Matsumoto M, Saul MI, Secrest AM, Ferris LK. Accuracy of skin cancer diagnosis by physician assistants compared with dermatologists in a large health care system. JAMA Dermatol. 2018;154(5):569-573. doi: 10.1001/jamadermatol.2018.0212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.English DRB, Burton RC, del Mar CB, Donovan RJ, Ireland PD, Emery G. Evaluation of aid to diagnosis of pigmented skin lesions in general practice: controlled trial randomised by practice. BMJ. 2003;327(7411):375. doi: 10.1136/bmj.327.7411.375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ebell MH, Siwek J, Weiss BD, et al. Strength of recommendation taxonomy (SORT): a patient-centered approach to grading evidence in the medical literature. Am Fam Physician. 2004;69(3):548-556. [PubMed] [Google Scholar]
- 48.Marchetti MA, Dusza SW, Halpern AC. A closer inspection of the number needed to biopsy. JAMA Dermatol. 2016;152(8):952-953. doi: 10.1001/jamadermatol.2016.0936 [DOI] [PubMed] [Google Scholar]
- 49.Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi: 10.1136/bmj.j2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carli P, Nardini P, Crocetti E, De Giorgi V, Giannotti B. Frequency and characteristics of melanomas missed at a pigmented lesion clinic: a registry-based study. Melanoma Res. 2004;14(5):403-407. doi: 10.1097/00008390-200410000-00011 [DOI] [PubMed] [Google Scholar]
- 51.Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122(20):3152-3156. doi: 10.1002/cncr.30177 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. MEDLINE Search Strategy
eFigure 1. PRISMA 2009 Flow Diagram
eFigure 2. Systematic Review of Publications Addressing Number Needed to Biopsy for Melanoma