Key Points
Question
What is the efficacy of phacoemulsification combined with goniosynechialysis compared with phacoemulsification without goniosynechialysis in lowering of intraocular pressure after 1 year?
Findings
In this randomized clinical trial of 78 patients with synechial primary angle-closure disease, lowering of intraocular pressure of approximately 7 to 8 mm Hg was achieved by both phacoemulsification alone and phacoemulsification with goniosynechialysis after 1 year.
Meaning
This randomized clinical trial was unable to show that goniosynechialysis with phacoemulsification provided additional lowering of the intraocular pressure compared with phacoemulsification without goniosynechialysis.
Abstract
Importance
The effectiveness of intraocular pressure (IOP) lowering phacoemulsification combined with goniosynechialysis (GSL) compared with phacoemulsification without GSL remains unknown.
Objective
To compare the IOP outcome after 1 year in patients with synechial primary angle-closure disease (PACD) and cataract who underwent phacoemulsification with intraocular lens implantation (PEI) alone compared with PEI with GSL (PEI-GSL).
Design, Setting, and Participants
A multicenter randomized clinical trial was conducted from September 29, 2011, to March 16, 2015; data analysis was performed from April 1, 2015, to March 4, 2019. Patients with PACD, defined as primary angle closure or primary angle-closure glaucoma, and at least 90° peripheral anterior synechiae (PAS) with cataract were included. Patients were randomized to undergo PEI alone or PEI-GSL. Patients were followed up for 1 year with standardized evaluations. Intention-to-treat analysis was performed.
Interventions
Phacoemulsification with intraocular lens implantation alone or with GSL.
Main Outcomes and Measures
Successful control of IOP at 12 months, defined as IOP 21 mm Hg or lower without use of topical IOP-lowering medications and a decrease in IOP of 20% or more from baseline IOP.
Results
Data from 78 patients (78 eyes) were analyzed. Of these, 37 patients were Chinese (47.4%) and 54 were women (69.2%); mean (SD) age was 67.7 (8.9) years. Mean deviation (SD) at baseline was −13.5 dB (9.4 dB). Forty patients were randomized to the PEI group and 38 to the PEI-GSL group. The mean (SD) IOP at baseline was 22.3 (8.5) mm Hg for the PEI group and 22.9 (5.3) mm Hg for the PEI-GSL group. At 1 year, the mean IOP was 14.3 (5.0) mm Hg for the PEI group and 15.9 (4.5) mm Hg for the PEI-GSL group. Successful control at 1 year occurred in 21 patients (52.5%) in the PEI group and 22 patients (57.9%) in the PEI-GSL group (mean difference, 5.4%; 95% CI, −18.0% to 28.2%; P = .63). In eyes that achieved successful control, mean IOP at 1 year was 12.5 (2.7) mm Hg (range, 7.0-19.0) for the PEI group and 13.6 (2.4) mm Hg (range, 9.0-18.0) for the PEI-GSL group. The number of medications at baseline and 1 year decreased from a mean of 2.2 (0.8) to 0.5 (0.9) in the PEI group and 1.9 (0.9) to 0.6 (1.2) in the PEI-GSL group (P < .001 for each), with a mean change difference of 0.4% (95% CI, −0.02% to 0.9%; P = .06). There were 3 postoperative complications (7.5%) in the PEI group and 3 (7.9%) in the PEI-GSL group. These included IOP spike (IOP≥30 mm Hg) (n = 3), excessive anterior chamber inflammation (n = 1), and posterior capsule opacification (n = 2).
Conclusions and Relevance
This randomized clinical trial was unable to show that PEI-GSL added additional IOP lowering compared with PEI alone in patients with PACD.
Trial Registration
ClinicalTrials.gov identifier: NCT02376725
This randomized clinical trial compares the intraocular pressure outcome after 1 year in patients with synechial primary angle-closure disease and cataract who underwent phacoemulsification combined with goniosynechialysis compared with phacoemulsification without goniosynechialysis.
Introduction
Primary angle-closure glaucoma (PACG) is estimated to affect 11.7 million people worldwide, with many of these individuals residing in East Asia1,2 where the disease is an important health problem. The defining qualities of an eye with PACG are a crowded anterior chamber with iridotrabecular meshwork contact, which frequently results in synechial closure of the angle leading to raised intraocular pressure (IOP) and subsequent progression to irreversible optic nerve damage.
Cataract surgery in eyes with angle closure is effective in reversing the angle-crowding mechanism, resulting in a decrease in iridotrabecular contact with the additional benefit of reducing IOP.3,4,5 However, studies have shown that the result of cataract surgery intended to open the anterior chamber angles and reduce the IOP varies depending on the amount of peripheral anterior synechiae (PAS).6,7 Other studies have shown that phacoemulsification (PEI) combined with goniosynechialysis (GSL) may be effective in releasing synechial closure; however, the additive efficacy of GSL in terms of IOP lowering is unclear.8,9 Goniosynechialysis involves the physical separation of PAS from the trabecular meshwork, using either a spatula or microforceps under direct visualization with a goniolens.
To our knowledge, there have been 2 randomized clinical trials comparing PEI with PEI-GSL.10,11 Both studies were limited by small numbers of participants and short times of follow-up. The purpose of this randomized clinical trial was to compare the IOP-lowering efficacy of PEI-GSL vs PEI alone during 1 year in patients with synechial primary angle-closure disease (PACD) and cataract. We hypothesized that PEI-GSL would result in significantly lower IOP at 1 year compared with PEI alone.
Methods
Study Design
A multicenter randomized clinical trial was conducted from September 29, 2011, to March 16, 2015; data analysis was performed from April 1, 2015, to March 4, 2019. This was a multicenter, international randomized clinical trial. There were 4 study sites: Singapore National Eye Centre, Singapore; Vietnam National Institute of Ophthalmology, Hanoi, Vietnam; Siriraj Hospital, Mahidol Thailand University, Thailand; and Queen Mary Hospital, Hong Kong. Consecutive patients with PACD who were attending glaucoma clinics were identified and, if eligible, were asked to participate in the study. Written informed consent was obtained and the study was conducted in accordance with the ethical principles defined by the Declaration of Helsinki12 and consistent with the Singapore Good Clinical Practice Guidelines and the applicable regulatory requirements. Ethics approval was obtained at each participating site. Patients were reimbursed for travel expenses. The trial protocol is available in Supplement 1.
Inclusion and exclusion criteria can be found in the eMethods in Supplement 2. Primary angle-closure disease was defined as eyes with primary angle closure (PAC) or PACG. Primary angle closure was diagnosed in the presence of angle closure (defined as eyes in which at least 180° of the posterior pigmented trabecular meshwork was not visible on gonioscopy in the primary position of gaze without indentation), with healthy optic discs and normal visual fields, but with elevated IOP (>21 mm Hg). Primary angle-closure glaucoma was defined as the presence of glaucomatous optic neuropathy with an associated visual field defect on automated perimetry (SITA-Standard 24-2 program; HFA II-750i, Carl Zeiss Meditec). All participants had 90° or more of PAS (not necessarily contiguous) as assessed by indentation gonioscopy. Peripheral anterior synechiae was defined as a region of iridotrabecular contact that could not be opened by indentation gonioscopy.
Randomization and Masking
Following patient consent, patients were randomized (1:1) using a random number generator. Group 1 underwent PEI alone and group 2 underwent PEI-GSL. Randomization was performed for patients rather than eyes. All personnel performing study procedures were masked to the randomization of patients. The masking code was broken after the study had been completed and analysis had taken place.
Surgical Technique
The anesthetic used was general or peribulbar. Paracentesis was performed followed by clear corneal incision, injection of sodium chondroitin sulfate, 4% with sodium hyaluronate, 3% (Viscoat; Alcon Laboratories Inc), and capsulorhexis. Hydrodissection was then performed and the crystalline lens was removed using phacoemulsification of the lens nucleus and aspiration of cortical lens matter. An injectable intraocular lens was inserted into the capsular bag.
For participants undergoing PEI-GSL, the goniosynechialysis procedure was performed after the anterior segment was refilled with viscoelastic (sodium chondroitin sulfate, 4%, with sodium hyaluronate, 3%). With a gonioscopy lens (with coupling agent) used to visualize areas of PAS, an iris repositor or similar instrument was used to break the PAS throughout the areas in which it existed. For areas of PAS not easily accessible via the main incision or sideport, 1 corneal incision was made at the superior or inferior nasal limbus through which the spatula was introduced. After this procedure was performed, the surgery continued as described above. All surgeries were performed by senior ophthalmic surgeons (R.H., T.D., J.L., N.K., S.A.P., and C.L.H.).
Outcome Measures
The main outcome criterion was control of IOP at 12 months. Complete success was defined as IOP of 21 mm Hg or lower without use of topical medications to reduce the IOP and a decrease in IOP of 20% or more from the baseline IOP. Qualified success was defined as an IOP of 21 mm Hg or lower with use of topical medications to reduce the IOP. Failure was defined as an IOP greater than 21 mm Hg with use of topical medications to reduce the IOP.
The secondary outcomes included intraoperative or postoperative complications, which were defined preoperatively. Other secondary outcomes were a change in the amount of PAS from baseline to 12 months (clock hours) as measured by gonioscopy and a change in the degree of angle opening as measured by gonioscopy.
Measurement of IOP
The investigators measuring the main outcomes (R.H., T.D., J.L., N.K., S.A.P., and C.L.H) were masked to the treatment. The protocol for measuring IOP followed the same guidelines used in the Collaborative Initial Glaucoma Treatment Study.13
Treatment Modification Protocol
The intervention protocol required that medication be initiated or changed whenever any of the following criteria were present: (1) inadequate reduction of the IOP, defined as an IOP greater than 21 mm Hg, as confirmed by 2 consecutive IOP measurements 1 to 14 days apart, excluding the first postoperative week, (2) deterioration of a visual field or optic disc appearance, and (3) adverse signs or symptoms severe enough to warrant a change in medication.
Postoperative Treatment
Patients in both study arms received the following postoperative medications, unless there were contraindications (eg, allergies): (1) guttae prednisolone acetate, 1%, for at least 3 weeks, (2) topical antibiotic medication for at least 3 weeks, and (3) acetazolamide, 250 mg, orally twice daily for the first 3 postoperative days. The frequency of the topical medication was left to the discretion of the examining consultant.
Sample Size Calculations
Based on previously published studies, the difference in IOP between the 2 groups at 1 year was estimated to be approximately 7 mm Hg in favor of PEI-GSL.14,15 Taking a conservative estimate of a difference of 4 mm Hg and an SD from the previously used sample populations of 5.5 mm Hg,14,15 35 participants were needed in each arm for the study to have an 88% power to detect a difference between the null (no difference in mean IOPs postoperatively) and alternative (4-mm Hg difference between the 2 postoperative means) hypotheses at the α significance level of .05. Assuming a dropout rate of 10%, 78 participants were needed to be recruited into this study. An independent data management institute was used to input the data.
Statistical Analysis
Data from 1 eye per patient were included in the final analysis; for patients in whom both eyes were treated, data from the right eye were used. The analysis was based on intention to treat, and the last-observation-carried-forward method was adopted for patients with missing data. The within-group differences between mean baseline IOP and IOP at 12 months after PEI or PEI-GSL treatment were compared using the paired t test for continuous variables. Independent 2-tailed t test was used to compare between the groups. Continuous data are expressed as mean (SD) and categorical data as percentages. Multivariate analysis to determine the predictors of failure (defined as IOP>21 mm Hg and a decrease in IOP of <20% from baseline IOP) was performed using Cox proportional hazards regression analysis with enter method. The level of significance was .05 for a 2-sided test. Statistical analysis was performed using SPSS for Windows, version 20.0 (IBM-SPSS Inc).
Results
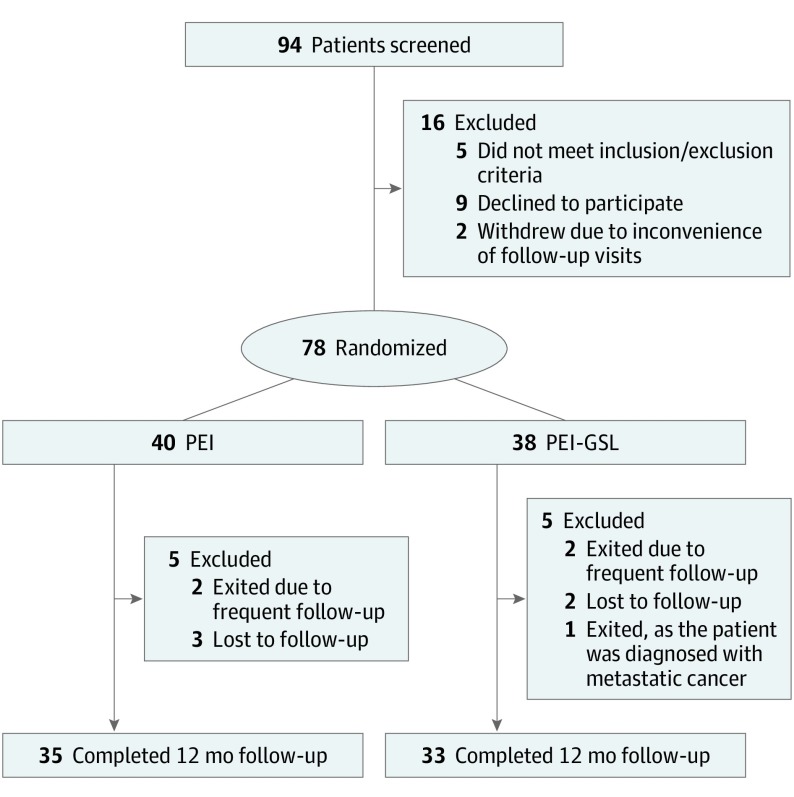
Between September 29, 2011, and March 16, 2015, a total of 94 patients were screened for the study. Of these, 9 patients declined participation after counseling and 5 did not meet the inclusion or exclusion criteria. This process resulted in a recruitment of 80 patients into the study; most were residents of Singapore (30) and Vietnam (30), followed by Hong Kong (10) and Thailand (10). Two patients withdrew from the study before randomization citing inconvenience of follow-up visits; they were not included in the final analysis. Of the 78 patients analyzed, 40 were randomized to PEI and 38 to PEI-GSL. At 12 months, 35 of 40 patients (87.5%) in the PEI group and 33 of 38 patients (86.8%) in the PEI-GSL group completed follow-up.
The screening, recruitment, and flow of randomization of participants are detailed in Figure 1. At baseline, there were no differences between the groups in any of the demographic or ocular characteristics recorded (Table 1), except for central corneal thickness (PEI, 531.5 μm vs PEI-GSL, 557.1 μm).
Figure 1. CONSORT Flow Diagram.
PEI-GSL indicates phacoemulsification with intraocular lens implantation combined with goniosynechialysis.
Table 1. Baseline Patient Characteristics.
| Characteristic | Mean (SD) | |
|---|---|---|
| PEI | PEI-GSL | |
| No. of eyes | 40 | 38 |
| Age, y | 67.3 (8.6) | 68.1 (9.2) |
| Sex, No. (%) | ||
| Men | 11 (27.5) | 13 (34.2) |
| Women | 29 (72.5) | 25 (65.8) |
| Mean BCVA | ||
| logMAR | 0.37 (0.26) | 0.37 (0.27) |
| Snellen | 20/50 | 20/50 |
| Baseline IOP, mm Hg | 22.3 (8.5) | 22.9 (5.3) |
| No. of medications | 2.2 (0.8) | 1.9 (0.9) |
| Mean Shaffer grade | 0.8 (0.7) | 0.8 (0.7) |
| PAS (clock hours) | 7.3 (2.8) | 7.8 (2.4) |
| Diagnosis | ||
| PAC | 11 | 11 |
| PACG | 29 | 27 |
| Axial length, mm | 22.65 (0.86) | 22.94 (0.90) |
| Anterior chamber depth, mm | 2.13 (0.37) | 2.19 (0.39) |
| Central corneal thickness, μm | 531.5 (36.9) | 557.1 (62.2) |
| Endothelial cell count, cells/mm2 | 2482.3 (280.7) | 2395.6 (339.3) |
| Mean deviation | −13.3 (8.2) | −13.8 (10.9) |
Abbreviations: BCVA, best-corrected visual acuity; IOP, intraocular pressure; PAC, primary angle closure; PACG, primary angle-closure glaucoma; PAS, peripheral anterior synechiae; PEI, phacoemulsification; PEI-GSL, PEI with goniosynechialysis.
Mean age (SD) for all 78 patients was 67.7 (8.9) and mean visual field mean deviation (SD) was −13.5 (19.1). There were 37 patients (47.4%) of Chinese race and 54 (69.2%) were women. The mean (SD) IOP at 1 year was 14.3 (5.0) mm Hg (range, 7.0-34.0) for the PEI group and 15.9 (4.5) mm Hg (range, 9.0-31.0) for the PEI-GSL group. Success at 1 year (defined as IOP≤21 mm Hg without topical IOP-lowering medications and a decrease in IOP of ≥20% from baseline IOP) occurred in 21 patients (52.5%) in the PEI group and 22 patients (57.9%) in the PEI-GSL group, the mean difference (95% CI) being 5.4% (−18.0% to 28.2%; P = .63). In eyes that achieved success, mean (SD) IOP at 1 year was 12.5 (2.7) mm Hg (range, 7.0-19.0 mm Hg) for the PEI group and 13.6 (2.4) mm Hg (range, 9.0-18.0 mm Hg) for the PEI-GSL group. The rates of complete success and qualified success are presented in Table 2. Qualified success was achieved in 38 patients (95.0%) in the PEI group and 35 patients (92.1%) in the PEI-GSL group (P = .60). At 12 months (Table 3), mean (SD) IOP decreased by 7.9 (9.4) mm Hg in the PEI group and 7.1 (6.6) mm Hg in the PEI-GSL group, with the difference between the groups being 0.9 mm Hg (95% CI, −2.8 to 4.6 mm Hg; P = .65) and the mean difference in the percentage change in IOP was −1.3% (95% CI, −15.4% to 12.8% P = .85). The mean (SD) number of medications at baseline and 1 year decreased from 2.2 (0.8) to 0.5 (0.9) in the PEI group and 1.9 (0.9) to 0.6 (1.2) in the PEI-GSL group (P < .001 for each). The mean (SD) percentage change in corneal endothelial cell count also decreased in each group from baseline to month 12% by 5.5% [10.1] in the PEI group and 9.0% [11.8] in the PEI-GSL group, but the between group difference was not significant (P = .27). However, comparing groups, there was no difference in the amount of change of number of medications or endothelial cell count between groups (Table 3).
Table 2. Complete or Qualified Success Rates at Final Follow-up at 12 Months.
| Outcome | No. (%) | Difference Between Success Rates, % (95% CI) | P Value | |
|---|---|---|---|---|
| PEI (n = 40) | PEI-GSL (n = 38) | |||
| Complete successa | 21 (52.5) | 22 (57.9) | 5.4 (−18.0 to 28.2) | .63 |
| Qualified successb | 38 (95.0) | 35 (92.1) | 2.9 (−10.6 to 17.1) | .60 |
Abbreviations: PEI, phacoemulsification; PEI-GSL, PEI with goniosynechialysis.
Defined as intraocular pressure (IOP) of 21 mm Hg or less without use of topical IOP-lowering medications and a decrease in IOP of 20% or more from baseline IOP.
Defined as IOP 21 mm Hg or less and a decrease in IOP of 20% or more from baseline IOP with use of topical IOP-lowering medications.
Table 3. Response of IOP, Medication Use, and Gonioscopy at Month 12.
| Response | Mean (SD) | Difference, Mean (95% CI) | P Value | |
|---|---|---|---|---|
| PEI (n = 40) | PEI-GSL (n = 38) | |||
| Month 12 IOP, mm Hg | 14.3 (5.0) | 15.9 (4.5) | −1.6 (−3.8 to 0.6) | .14 |
| Change in IOP from baseline, mm Hg | 7.9 (9.4) | 7.1 (6.6) | 0.9 (−2.8 to 4.6) | .65 |
| Change in IOP from baseline, % | 26.5 (37.0) | 27.8 (23.6) | −1.3 (−15.4 to 12.8) | .85 |
| No. of medication at month 12 | 0.5 (0.9) | 0.6 (1.2) | −0.1 (−0.6 to 0.4) | .63 |
| Change in No. of medications | 1.7 (1.0) | 1.3 (1.0) | 0.4 (−0.02 to 0.9) | .06 |
| Month 12 Shaffer grade | 2.09 (1.09) | 2.29 (1.01) | −0.21 (−0.78 to 0.36) | .47 |
| Change in Shaffer grade | 1.17 (1.18) | 1.54 (1.14) | −0.38 (−1.01 to 0.26) | .24 |
| PAS at month 12 | 4.30 (4.09) | 3.21 (3.56) | 1.09 (−0.66 to 2.85) | .22 |
| Change in PAS | 3.03 (3.90) | 4.53 (3.22) | −1.50 (−3.16 to 0.15) | .07 |
| Change in endothelial cell count, cells/mm2 | 142.8 (241.4) | 211.5 (276.4) | −68.6 (−216.2 to 78.9) | .35 |
| % Change in endothelial cell count | 5.5 (10.1) | 9.0 (11.8) | −3.5 (−9.7 to 2.8) | .27 |
Abbreviations: IOP, intraocular pressure; PAS, peripheral anterior synechiae; PEI, phacoemulsification; PEI-GSL, PEI with goniosynechialysis.
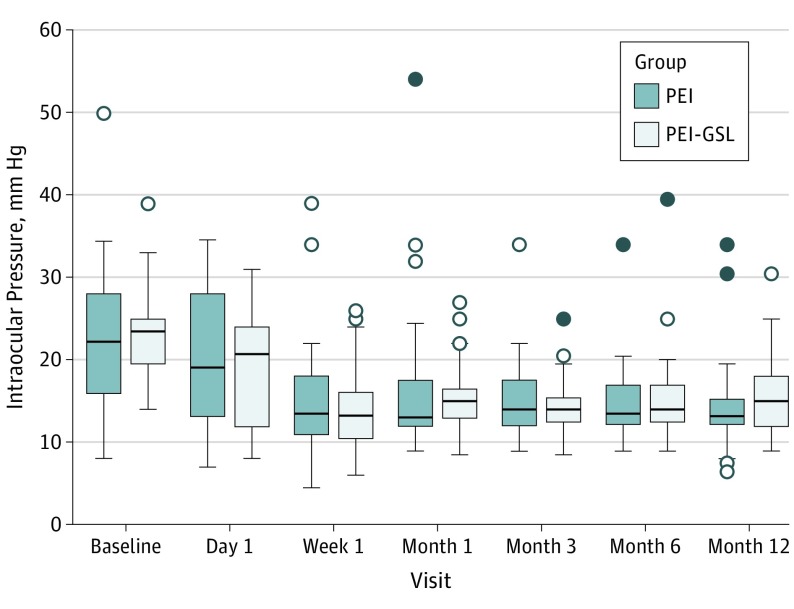
The box plots for IOP change over the 12-month period (Figure 2). Failure was noted in 2 patients (5.0%) in the PEI group and 3 patients (7.9%) in the PEI group. The mean (SD) IOP at 1 year was 32.3 (2.5) mm Hg for the PEI group and 26.3 (4.2) mm Hg for the PEI-GSL group.
Figure 2. Box Plots for Intraocular Pressure Measurements Over the 12 Months, Periods Based on the Intent-to-Treat Analysis.
The horizontal line in the middle of each box indicates the median. The top and bottom borders of the box mark the 75th and 25th percentiles, respectively. The whiskers above and below the box mark the 90th and 10th percentiles. The points beyond the whiskers are outliers beyond the 90th or 10th percentiles. PEI indicates phacoemulsification; PEI-GSL, PEI with goniosynechialysis.
In a multivariate Cox proportional hazards regression model for failure and adjusted for treatment group, site, PACD diagnosis, baseline IOP, and baseline PAS, we found that having a higher baseline IOP was less likely to be a hazardous event (hazard ratio, 0.93; 95% CI, 0.87-0.99; P = .02) (eTable 1 in the Supplement).
There was 1 intraoperative complication; mild zonulysis was noted during PEI-GSL in 1 patient and necessitated the insertion of a capsular tension ring. In terms of postoperative complications, there were 3 in the PEI group (7.5%) and 3 in the PEI-GSL group (7.9%). The most common postoperative complication was an IOP spike (IOP≥30 mm Hg) during week 1, which occurred in 3 patients (7.5%)—all in the PEI group (P = .24). The other complications, all in the PEI-GSL group, were excessive anterior chamber inflammation (1 patient [2.6%]) in the early postoperative period and posterior capsule opacification (2 of 38 [5.3%]). Best-corrected visual acuity improved significantly in both groups; mean difference from baseline for the PEI group was 0.11 (95% CI, 0.04-0.19; P = .003) and 0.14 (95% CI, 0.04-0.24; P = .006) for the PEI-GSL group. There was no difference in the month 12 BCVA between the groups (P = .92).
Analysis of data for the subgroup of patients who had previous acute PAC, showed no difference in success rates between those who had had PEI or PEI-GSL. Similar findings were true for those who had not had acute PAC. Analysis of data for the subgroup of patients who had had previous acute PAC (n=32), showed no difference in success rates between those who had had PEI (8 patients [34.8%]) or PEI-GSL (11 patients [47.8%]) (P = .37). Likewise, the success rate was similar in those who had not had acute PAC (n=46), in which success was achieved in 13 patients (76.5%) in the PEI group and in 11 patients (73.3%) in the PEI-GSL group (P = 0.84).
We next compared results from the 2 centers that provided the most participants: Singapore and Vietnam, each with 30 patients (15 patients in each group. The success rate for PEI-GSL was the same at both sites (9 of 15 patients [60.0%]). However, for patients who underwent PEI alone, in, Singapore only 3 of 15 patients (20.0%) achieved success compared with all 15 patients in the Vietnam group. Comparing groups, we found that the Vietnamese patients who underwent PEI alone had significant differences in the following characteristics compared with the Singapore patients: higher mean (SD) baseline IOP, 27.4 (3.7) mm Hg vs 18.0 (7.8), higher ratio of PACG:PAC (15:0 vs 6:9), and shallower anterior chamber depth, 1.85 (0.13) mm Hg vs 2.42 (0.30) (eTable 2 in the Supplement).
Discussion
We found that performing PEI or PEI-GSL in patients with synechial PACD and cataract resulted in a significant decrease in IOP and number of IOP-lowering medications used at 1 year compared with baseline, but that there was no significant difference between the groups in terms of success in IOP lowering. Both procedures had equally low postoperative complication rates. Although there is already good evidence in the literature that PEI alone decreases IOP in patients with PACD, this study found no additional benefit of performing GSL at the time of PEI. However, the results do not rule out the possibility that one approach may be superior to the other owing to the relatively small numbers involved in this analysis.
There have been 2 randomized clinical trials comparing PEI with PEI-GSL10,11 but both were limited by small sample sizes and short follow-up periods. Lee et al10 randomized 30 Korean patients with medically controlled PACG to PEI and PEI-GSL (15 in each group). Follow-up was only 2 months. Intraocular pressure decreased in both groups by 2.3 and 4.5 mm Hg, respectively, which was a clinically but not statistically significant reduction (P = .23). Shao et al11 compared 23 Chinese patients who underwent PEI-GSL with 20 who had PEI alone. At 6 months, the investigators found that all patients who had PEI-GSL achieved complete success (IOP<21 mm Hg without the use of medications) compared with 60% in the PEI group. This finding appears to be positive, but the statistical significance of the difference was not reported. The actual decrease in IOP between groups at 6 months was not statistically significant, although the reduction in the number of IOP-lowering medication was in favor of PEI-GSL.
There are 2 potential limitations of GSL. The first is that the trabecular meshwork that is behind the PAS may not be functioning normally and, therefore, mechanical relief of PAS may not result in lowering of the IOP. However, a study comparing outflow facility between PEI-GSL and PEI found significantly better outflow facility in the PEI-GSL group, which suggests that outflow via the trabecular meshwork may not be compromised in all cases.9,16 The second potential limitation of GSL is that the lowering of the IOP may not last long owing to reformation of PAS, although other case series on PEI-GSL showed that the IOP-lowering can be maintained over 3 years.17
Greater reduction in IOP with higher baseline IOP, which we found in our study, agrees with previous reports.18,19,20,21 As neither the type of surgical procedure (PEI and PEI-GSL) nor amount of baseline PAS was a significant predictor, our findings suggest that lowering of the IOP was due to the removal of the cataract. Our subgroup analysis revealed that, for the Singaporean cohort, PEI-GSL was significantly better than PEI in terms of success rates. However, the converse was true for the Vietnam group and, as these 2 groups of patients represented the majority of participants in the study, these contradictory results are likely the reason why there was no significant difference in success overall. One possible explanation for these conflicting findings was differences in the baseline characteristics between the 2 groups such as higher baseline IOP, higher proportion of patients with PACG and shallower ACD, all in the Vietnam group (eTable 2 in the Supplement) and the inference from this difference is that Vietnam group had more severe disease than the Singapore group. Examining the baseline IOP in the 2 cohorts (27.4 mm Hg for Vietnam and 16.8 mm Hg for Singapore) shows that, to be classified as achieving a successful outcome (ie, 20% reduction of IOP at 12 months compared with baseline), the Vietnam group would need an IOP of only 20 mm Hg at 1 year, whereas the Singapore cohort would need IOP levels of 13 mm Hg or below, without medications. This difference may explain why the PEI group achieved more success in the Vietnamese compared with Singapore cohort and hence skewed our results. This subgroup analysis was limited by small sample size; hence, any differences could be due to chance.
The amount of PAS at 12 months was different by only approximately 1 clock hour between groups, with the PEI-GSL group having slightly less PAS (Table 3). This finding implies 2 things, the first being that some PAS in the PEI-GSL group must have reformed because all areas of PAS were removed intraoperatively. Second, PEI alone was sufficient enough to break more than half of PAS present at baseline—an unexpected finding. This near equivalence in the reduction of PAS between the 2 groups at 1 year might explain why our IOP success results were not significantly different.
Limitations
Our study had some limitations. Differences in surgical techniques and patient profiles could have influenced our findings. We did not examine how much PAS was broken down immediately after the surgery; it is conceivable that PAS was not entirely relieved in some patients. The patients in the Vietnam and Singapore cohorts differed significantly in many ways, as mentioned above, which may have resulted in varying effects for PEI alone and PEI-GSL.
Conclusions
Given the large number of patients worldwide with PACD and the increased proportional burden of blindness that is associated with PACG, we believe our findings are of clinical significance. Although PEI is a relatively quick procedure that most cataract surgeons can perform safely, there was no clear advantage of performing PEI-GSL compared with PEI alone for synechial PACD with cataract. However, the subgroup analysis suggests that there may be some patients who, if correctly identified before surgery, may benefit from PEI-GSL. Future studies in this area seem warranted in order to identify these characteristics.
Protocol
eMethods. Detailed Methods
eTable 1. Cox Regression Analysis for Predictors of Failure
eTable 2. Baseline Patient Characteristics for Singapore and Vietnam Subjects Who Underwent Phacoemulsification Alone
Data Sharing Statement
References
- 1.Chan EW, Li X, Tham YC, et al. Glaucoma in Asia: regional prevalence variations and future projections. Br J Ophthalmol. 2016;100(1):78-85. doi: 10.1136/bjophthalmol-2014-306102 [DOI] [PubMed] [Google Scholar]
- 2.Tham YC, Li X, Wong TY, Quigley HA, Aung T, Cheng CY. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081-2090. doi: 10.1016/j.ophtha.2014.05.013 [DOI] [PubMed] [Google Scholar]
- 3.Thomas R, Walland M, Thomas A, Mengersen K. Lowering of intraocular pressure after phacoemulsification in primary open-angle and angle-closure glaucoma: a bayesian analysis. Asia Pac J Ophthalmol (Phila). 2016;5(1):79-84. doi: 10.1097/APO.0000000000000174 [DOI] [PubMed] [Google Scholar]
- 4.Moghimi S, Lin S. Role of phacoemulsification in angle closure glaucoma. Eye Sci. 2011;26(3):121-131. [DOI] [PubMed] [Google Scholar]
- 5.Masis Solano M, Lin SC. Cataract, phacoemulsification and intraocular pressure: is the anterior segment anatomy the missing piece of the puzzle? Prog Retin Eye Res. 2018;64:77-83. doi: 10.1016/j.preteyeres.2018.01.003 [DOI] [PubMed] [Google Scholar]
- 6.Lee YH, Yun YM, Kim SH, Lee EK, Lee JE, Kim CS. Factors that influence intraocular pressure after cataract surgery in primary glaucoma. Can J Ophthalmol. 2009;44(6):705-710. doi: 10.3129/i09-186 [DOI] [PubMed] [Google Scholar]
- 7.Shams PN, Foster PJ. Clinical outcomes after lens extraction for visually significant cataract in eyes with primary angle closure. J Glaucoma. 2012;21(8):545-550. doi: 10.1097/IJG.0b013e31821db1db [DOI] [PubMed] [Google Scholar]
- 8.Tun TA, Baskaran M, Perera SA, Htoon HM, Aung T, Husain R. Swept-source optical coherence tomography assessment of iris-trabecular contact after phacoemulsification with or without goniosynechialysis in eyes with primary angle closure glaucoma. Br J Ophthalmol. 2015;99(7):927-931. doi: 10.1136/bjophthalmol-2014-306223 [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues IA, Alaghband P, Beltran Agullo L, et al. Aqueous outflow facility after phacoemulsification with or without goniosynechialysis in primary angle closure: a randomised controlled study. Br J Ophthalmol. 2017;101(7):879-885. doi: 10.1136/bjophthalmol-2016-309556 [DOI] [PubMed] [Google Scholar]
- 10.Lee CK, Rho SS, Sung GJ, et al. Effect of goniosynechialysis during phacoemulsification on IOP in patients with medically well-controlled chronic angle-closure glaucoma. J Glaucoma. 2015;24(6):405-409. doi: 10.1097/IJG.0000000000000043 [DOI] [PubMed] [Google Scholar]
- 11.Shao T, Hong J, Xu J, Le Q, Wang J, Qian S. Anterior chamber angle assessment by anterior-segment optical coherence tomography after phacoemulsification with or without goniosynechialysis in patients with primary angle closure glaucoma. J Glaucoma. 2015;24(9):647-655. doi: 10.1097/IJG.0000000000000061 [DOI] [PubMed] [Google Scholar]
- 12.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 13.Musch DC, Gillespie BW, Niziol LM, Cashwell LF, Lichter PR; Collaborative Initial Glaucoma Treatment Study Group . Factors associated with intraocular pressure before and during 9 years of treatment in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2008;115(6):927-933. doi: 10.1016/j.ophtha.2007.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teekhasaenee C, Ritch R. Combined phacoemulsification and goniosynechialysis for uncontrolled chronic angle-closure glaucoma after acute angle-closure glaucoma. Ophthalmology. 1999;106(4):669-674. doi: 10.1016/S0161-6420(99)90149-5 [DOI] [PubMed] [Google Scholar]
- 15.Tham CC, Kwong YY, Leung DY, et al. Phacoemulsification versus combined phacotrabeculectomy in medically uncontrolled chronic angle closure glaucoma with cataracts. Ophthalmology. 2009;116(4):725-731, 731.e1-731.e3. doi: 10.1016/j.ophtha.2008.12.054 [DOI] [PubMed] [Google Scholar]
- 16.Tanihara H, Nishiwaki K, Nagata M. Surgical results and complications of goniosynechialysis. Graefes Arch Clin Exp Ophthalmol. 1992;230(4):309-313. doi: 10.1007/BF00165936 [DOI] [PubMed] [Google Scholar]
- 17.Kameda T, Inoue T, Inatani M, Tanihara H; Japanese Phaco-Goniosynechialysis Multicenter Study Group . Long-term efficacy of goniosynechialysis combined with phacoemulsification for primary angle closure. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):825-830. doi: 10.1007/s00417-012-2091-8 [DOI] [PubMed] [Google Scholar]
- 18.Sengupta S, Venkatesh R, Krishnamurthy P, et al. Intraocular pressure reduction after phacoemulsification versus manual small-incision cataract surgery: a randomized controlled trial. Ophthalmology. 2016;123(8):1695-1703. doi: 10.1016/j.ophtha.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 19.Bilak S, Simsek A, Capkin M, Guler M, Bilgin B. Biometric and intraocular pressure change after cataract surgery. Optom Vis Sci. 2015;92(4):464-470. doi: 10.1097/OPX.0000000000000553 [DOI] [PubMed] [Google Scholar]
- 20.Huang G, Gonzalez E, Peng PH, et al. Anterior chamber depth, iridocorneal angle width, and intraocular pressure changes after phacoemulsification: narrow vs open iridocorneal angles. Arch Ophthalmol. 2011;129(10):1283-1290. doi: 10.1001/archophthalmol.2011.272 [DOI] [PubMed] [Google Scholar]
- 21.Yang HS, Lee J, Choi S. Ocular biometric parameters associated with intraocular pressure reduction after cataract surgery in normal eyes. Am J Ophthalmol. 2013;156(1):89-94.e1. doi: 10.1016/j.ajo.2013.02.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol
eMethods. Detailed Methods
eTable 1. Cox Regression Analysis for Predictors of Failure
eTable 2. Baseline Patient Characteristics for Singapore and Vietnam Subjects Who Underwent Phacoemulsification Alone
Data Sharing Statement