Key Points
Question
Is elevated systolic blood pressure a risk factor for major valvular heart disease?
Findings
In this mendelian randomization study of 329 237 individuals, genetically associated 20–mm Hg increments of elevated systolic blood pressure appeared to be associated with a higher risk of aortic stenosis, aortic regurgitation, and mitral regurgitation.
Meaning
Lifetime exposure to elevated systolic blood pressure may be associated with an increased risk of major valvular heart disease, suggesting that blood pressure lowering might be a useful strategy for prevention of this condition.
Abstract
Importance
Modifiable risk factors for valvular heart disease remain largely unknown, which limits prevention and treatment.
Objective
To assess the association between systolic blood pressure (BP) and major valvular heart disease.
Design, Setting, and Participants
A UK Biobank population-based cohort of 502 602 men and women aged 40 to 96 years at baseline was evaluated through mendelian randomization using individual participant data. Inclusion criteria were valid genetic data and BP measurements. The participants were recruited between 2006 and 2010; data analysis was performed from June 2018 to January 2019.
Exposures
Systolic BP was measured during clinical assessment and instruments for the genetic effect of high BP were identified from variants that were independently (linkage disequilibrium threshold of r2<0.1) associated with systolic BP with minor allele frequency greater than 0.01. A total of 130 single-nucleotide polymorphisms that have been shown to be associated with systolic BP in a genome-wide association meta-analysis involving 1 million participants of European ancestry were selected.
Main Outcomes and Measures
Incident aortic stenosis, aortic regurgitation, and mitral regurgitation, individually and combined. Cases were largely based on hospital records linked to the UK Biobank with International Classification of Diseases and Health Related Problems, Tenth Revision codes.
Results
Of the 502 602 individuals screened, 329 237 participants (177 741 [53.99%] women; mean [SD] age, 56.93 [7.99] years) had valid genetic data and BP measurements; of this cohort, 3570 individuals (1.08%) had a diagnosis of valvular heart disease (aortic stenosis, 1491 [0.45%]; aortic regurgitation, 634 [0.19%]; and mitral regurgitation, 1736 [0.53%]). Each genetically associated 20–mm Hg increment in systolic BP was associated with an increased risk of aortic stenosis (odds ratio [OR], 3.26; 95% CI, 1.50-7.10), aortic regurgitation (OR, 2.59; 95% CI, 0.75-8.92), and mitral regurgitation (OR, 2.19; 95% CI, 1.07-4.47), with no evidence for heterogeneity by type of valvular heart disease (P = .90). Sensitivity analyses confirmed the robustness of the association.
Conclusions and Relevance
Lifetime exposure to elevated systolic BP appears to be associated with an increased risk of major valvular heart disease.
This population-based study uses mendelian randomization to evaluate whether there is an association between elevated systolic blood pressure and valvular heart disease.
Introduction
In the past century, a substantial change in the spectrum of valvular heart disease (VHD) has occurred. Age-related valve degeneration replaced rheumatic valvular disease as the predominant cause of valve dysfunction in high-income countries, and it typically presents as aortic stenosis or mitral regurgitation.1,2 In particular, the prevalence of aortic stenosis has been rapidly increasing owing to population aging, with poor patient outcomes and high health care costs associated with the only effective treatment available: valve repair or replacement.3,4 Therefore, there is an unmet need to identify modifiable risk factors for VHD to develop effective prevention and treatment strategies.
Given the shared pathways between several cardiovascular conditions, it has been suggested that elevated blood pressure (BP) increases the risk of VHD as it does with stroke or ischemic heart disease.5 Initial supportive evidence for this hypothesis came from cross-sectional studies showing a positive association between elevated systolic BP and risk of aortic stenosis6,7,8 and aortic regurgitation.9 These findings were recently confirmed and extended in large-scale cohort studies, which showed that long-term exposure to elevated systolic BP was associated with an increased risk of aortic stenosis,10,11 aortic regurgitation,10 and mitral regurgitation.12 However, those nonrandomized studies were unable to rule out residual confounding.
In the absence of randomized clinical trials of BP-lowering treatment on the risk of incident VHD, we aimed to test the hypothesis that systolic BP is associated with the risk of aortic stenosis, aortic regurgitation, and mitral regurgitation, using a mendelian randomization study design. This approach uses the naturally occurring randomized exposure of individuals to genetic variants that are highly associated with lifetime systolic BP. Mendelian randomization is similar to random allocation of intervention in randomized clinical trials and can thus overcome the problems of reverse causation and confounding that are typical of nonrandomized observational studies.13
Methods
Study Population
The UK Biobank is a large prospective cohort study of 502 602 men and women aged 40 to 96 years who were recruited between 2006 and 2010 from 22 assessment centers across the United Kingdom. Details of the study design and cohort profile have been published elsewhere.14,15,16 Data analysis for the present study was conducted from June 2018 to January 2019. The protocol of the present mendelian randomization study was approved by the UK Biobank. The UK Biobank has ethical approval from the Northwest Multi-Center Research Ethics Committee, and written informed consent was obtained from all participants.
Of 487 381 participants who were genotyped in the UK Biobank, we included 419 056 white, British descent participants with valid baseline blood measurements. Racial/ethnic background information was requested through a touchscreen questionnaire during the UK Biobank initial assessment center visit (UKB data-field 21000). Participants did not receive financial compensation.
The analysis was restricted to homologous racial/ethnic background because of possible significant different allele frequencies in racial/ethnic groups that can induce bias in genetic analysis. eFigure 1 in the Supplement shows participants’ selection, including initial inclusion and exclusion criteria and genetic data quality check. We used the last updated genetic (imputed data set, version 3) and baseline data (March 16, 2018).
Exposure and Outcomes
Systolic BP was measured twice, using automated measurement (Omron Digital monitor; OmronHealthcare Inc). There was significant variation in the mean (SD) systolic BP between the first and second measurements (mean first, 139.8 [19.6] mm Hg; mean second, 135.9 [18.6] mm Hg; mean difference: 3.9 [9.1] mm Hg; P < .001 for difference). We therefore analyzed systolic BP as the mean of the 2 measurements.
Outcomes were occurrence of the following VHDs individually and as a composite: aortic stenosis, aortic regurgitation, and mitral regurgitation. We used binary classification of valvular disease (present or not present) that was identified from electronic hospital records that were linked to the UK Biobank, as well as self-reports collected in the assessment center verbal interview at cohort entry. The diagnostic codes related to congenital and rheumatic valve causes were excluded. The following diagnostic codes were used for definition of outcomes: aortic stenosis (International Diagnostic Classification of Diseases and Health Related Problems, Tenth Revision [ICD-10]: I35.2; I35.0; UK Biobank self-report code: 1490), aortic regurgitation (ICD-10: I35.1; UK Biobank self-report code: 1587), and mitral regurgitation (ICD-10: I34.0; UK Biobank self-report code: 1585).
Other Measurements
Standing height and weight measured at the initial assessment visit were used to calculate body mass index (calculated as weight in kilograms divided by height in meters squared).2 Values were set as missing if either of these readings were omitted. Age was derived based on date of birth when participants attended the initial assessment center. Self-reported smoking status was categorized as never, previous, current, and prefer not to answer. Self-reported alcohol intake was classified as never, occasionally, 1 to 3 times a month, 1 to 2 times a week, 3 to 4 times a week, daily, and prefer not to answer. Participants reported their racial/ethnic background at the first assessment and we classified them as white, British, or Irish ethnic background vs mixed or nonwhite background.
Genotype Data
The genetic variants and single-nucleotide polymorphisms (SNPs) used in the present study were extracted from the UK Biobank imputation data set.17 Genotype data were imputed with IMPUTE4 using the Haplotype Reference Consortium and the UK10K + 1000 Genomes panel18 to identify approximately 96 million variants for 487 381 participants. From 419 056 self-reported white, British-descent individuals with valid genetic and BP measurements, we excluded those without a genetic racial/ethnic grouping as white (n = 46 071), missingness rate (variant call rate) higher than 1% (n = 42 773), mismatch between reported and genetic sex (n = 257), sex chromosome aneuploidy (n = 311), and recommended genomic analysis exclusions (n = 407). In total, 329 237 white British individuals were included in the final analysis (eFigure 1 in the Supplement).
Instrumental Variable for Systolic BP
We built genetic instruments for systolic BP using SNPs with minor allele frequency greater than 0.01 that were independently (linkage disequilibrium r2<0.1) associated with systolic BP (at P < 5 × 10−8) in the European population genome-wide association studies. We selected 130 SNPs that have been shown to be associated with systolic BP in a genome-wide association meta-analysis, including more than 1 million participants of European ancestry (eTable 1 in the Supplement).19 All 130 SNPs had imputation quality above 0.9 and collectively explained 0.48% of the variance in the mean of 2 systolic BP measurements in the present analysis. PLINK, version 2,20 was used for genotype data management and extraction of variants from the UK Biobank version 3 imputed data set.
The SNPs were used to develop a genetic risk score (GRS) for systolic BP. First, each variant was recoded additively (0, 1, and 2) according to the number of systolic BP–increasing alleles. Then, after harmonization in a positive direction (increased systolic BP), each variant was weighed according to the regression coefficient obtained from a previous genome-wide association meta-analysis to give more weight to SNPs with stronger effects.19 A weighted GRS was constructed using the following formula:
| (β1 × SNP1[+]β2 × SNP2[+⋯]βn × SNPn), |
where βi was the regression coefficient associated with SNPi and obtained from the previous genome-wide association meta-analysis. The weighted score was then rescaled to indicate the number of trait-increasing alleles: rescaled GRS = (weighted score × number of selected SNPs)/sum of regression coefficients. An unweighted GRS was constructed to further assess the effect between the weighting method and the estimations.
Statistical Analysis
Instrumental variable analysis was performed using an adjusted, 2-stage predictor substitution method that used the GRS as an instrument variable.21 In this analysis, a serial regression method was done by performing 2 regression models consecutively. In the first stage, measured systolic BP was regressed on the GRS as an instrumental variable using a multiple linear regression model. The predicted probability and residuals from this model were saved for the next stage. In the second stage, binary outcomes of valvular diseases were regressed on the predicted probability from the first stage, which served as an independent variable (this predicted value was a proxy for unconfounded estimate of systolic BP), in a multiadjusted, binary logistic regression model with robust SEs. All statistical analyses were performed using Stata Statistical Software, release 14 (StataCorp LP). PASS software, version 15 (NCSS) was used to calculate statistical power (eTable 2 and eMethods in the Supplement). Statistical significance was determined using 2-tailed testing.
Mendelian Randomization Assumptions
We used 1-sample mendelian randomization, which makes the following general assumptions: (1) the GRS that is used as an instrument is reliably associated with the measured systolic BP, (2) the GRS is only associated with the outcomes through the exposure of interest (ie, no pleiotropic effects), and (3) the GRS is not associated with any confounding factors.22 We further assumed that the association between the GRS and systolic BP was linear and there was no modification of the association.
Assessment of Instrumental Variable Assumptions
We estimated the F statistic from the first-stage regression of the systolic BP per 20-mm Hg increase on the GRS to check the strength of the instrument.23
A genetic instrumental variable is referred to as pleiotropic when it is in association with other confounders or exposure variables through a separate causal pathway.24 To test this assumption, we conducted a sensitivity analysis, using the mendelian randomization Egger intercept test. In this method, zero intercept indicates that the pleiotropic effect is, on average, null.25 The MendelianRandomization package for R, version 3.4.3 (R Foundation) was used to implement the mendelian randomization Egger methods for multiple genetic variants.26
We assessed the association between the GRS and possible confounders using the Pearson correlation coefficient test and scatterplot. In addition, to address population stratification and genetic relatedness, all of the models were adjusted for the first 10 genetic principal components and relatedness of up to the third degree using kinship coefficients (>0.044) calculated using the KING toolset.27
Sensitivity Analyses
To test the validity of the instrumental variable, we performed a positive control analysis, with stroke, heart failure (HF) and coronary heart disease (CHD) as the outcomes. In addition, we conducted a negative control analysis using chronic obstructive pulmonary disease, expecting a null finding. To further test for a possible association with pleiotropy, we reconstructed a GRS excluding SNPs associated with any type of well-known cardiovascular risk factors or disease. We performed a sensitivity analysis to check that the association between elevated systolic BP and VHD is not an epiphenomenon of the known association with CHD and HF. To investigate the validity of the outcome definition and potential association between disease severity and estimations, we performed the following 2 analyses. First, we conducted stratified analyses by cases determined using ICD-10 codes only vs cases defined with ICD-10 codes or self-report. Second, we provided additional analysis by restricting cases to those with valve replacement surgery serving as a proxy for VHD severity. We then further adjusted the final models for BP-lowering treatment to assess the potential association of BP-lowering medications with the estimations.
Results
Descriptive Statistics and Observational Findings
Baseline characteristics of the 329 237 participants of white British ancestry with valid genetic data and BP measurements overall and by sex are listed in Table 1. Of these, 177 741 were women (53.99%); mean (SD) age was 56.93 (7.99) years. A total of 3570 participants (1.08%) had a diagnosis of VHD. We identified 1491 cases (0.45%) of aortic stenosis, 634 cases (0.19%) of aortic regurgitation, and 1736 cases (0.53%) of mitral regurgitation. In addition, eTable 3 in the Supplement reports the correlation between systolic BP GRS, measured systolic BP, and potential confounders. We did not find an observable correlation between GRS and well-known confounders.
Table 1. Summary of Characteristics of 329 237 Participants of White British Ancestry Included in the Final Analysis.
| Characteristic | No. (%) | ||
|---|---|---|---|
| All (N = 329 237) | Men (n = 151 496) | Women (n = 177 741) | |
| Age, mean (SD), y | 56.93 (7.99) | 57.16 (8.09) | 56.73 (7.91) |
| Sex | |||
| Men | 151 496 (46.01) | ||
| Women | 177 741 (53.99) | ||
| BMI, mean (SD) | 27.38 (4.71) | 27.83 (4.22) | 27.00 (5.07) |
| Smoking status | |||
| Never | 178 969 (54.36) | 73 775 (48.70) | 105 194 (59.18) |
| Previous | 116 116 (35.27) | 59 424 (39.22) | 56 692 (31.90) |
| Current | 33 009 (10.03) | 17 765 (11.73) | 15 244 (8.58) |
| Prefer not to answer | 1143 (0.35) | 532 (0.35) | 611 (0.34) |
| Alcohol intake frequency | |||
| Never | 21 463 (6.52) | 7326 (4.84) | 14 137 (7.95) |
| Special occasion only | 34 888 (10.60) | 9943 (6.56) | 24 945 (14.03) |
| 1-3 times/mo | 36 402 (11.06) | 13 185 (8.70) | 23 217 (13.06) |
| 1-2 times/wk | 86 741 (26.35) | 39 735 (26.23) | 47 006 (26.45) |
| 3-4 times/wk | 79 339 (24.10) | 41 075 (27.11) | 38 264 (21.53) |
| Daily or almost daily | 70 188 (21.32) | 40 126 (26.49) | 30 062 (16.91) |
| Prefer not to answer | 216 (0.07) | 106 (0.07) | 110 (0.06) |
| Systolic BP, mean (SD), mm Hg | 138.24 (18.59) | 141.32 (17.38) | 135.62 (19.17) |
| Aortic valve stenosis | 1491 (0.45) | 988 (0.65) | 503 (0.28) |
| Aortic valve regurgitation | 634 (0.19) | 411 (0.27) | 223 (0.13) |
| Mitral valve regurgitation | 1736 (0.53) | 1039 (0.69) | 697 (0.39) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure.
Mendelian Randomization Findings
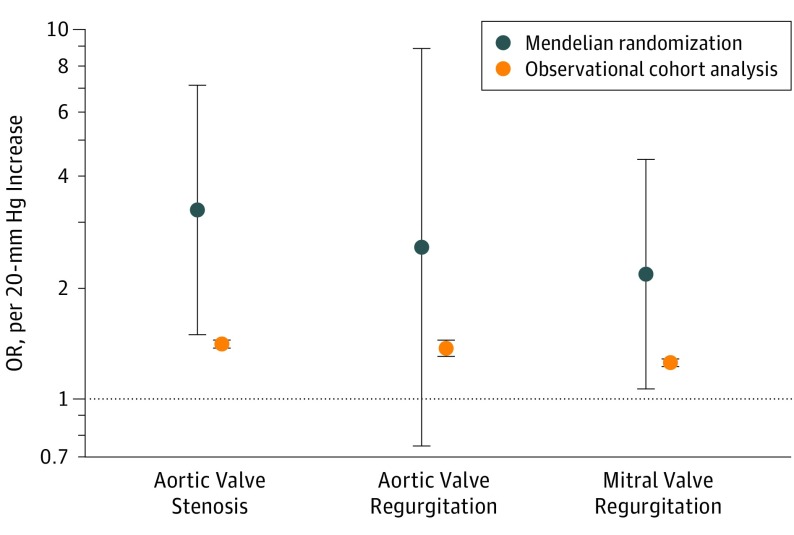
Table 2 provides the mendelian randomization estimates for the associations between systolic BP and VHD. We found that each genetically associated, 20-mm Hg higher systolic BP was associated with approximately 3-fold higher odds of aortic stenosis in an age- and sex-adjusted model (odds ratio [OR], 3.29; 95% CI, 1.52-7.12; P = .002). Adjustment for additional variables minimally changed the results. We found a similar, but statistically less robust, association between systolic BP and aortic regurgitation both in the age-and sex-adjusted model (OR, 2.56; 95% CI, 0.73-8.85; P = .13) and in the fully adjusted model (OR, 2.59; 95% CI, 0.75-8.92; P = .13). A similar pattern of association was observed for mitral regurgitation (age- and sex-adjusted model: OR, 2.22; 95% CI, 1.09 to 4.52; P = .02; fully adjusted model: OR, 2.19; 95% CI, 1.07-4.47; P = .03). The observed associations were broadly consistent across individual valvular diseases (P = .90 for heterogeneity) and the odds for any of the 3 valvular diseases under study increased almost 3-fold per 20-mm Hg increase in systolic BP (OR, 2.85; 95% CI, 1.69-4.78; P < .001). Figure 1 shows that the direction of associations was similar for both systolic BP exposures: an increasing risk of valvular disease per 20 mm Hg higher systolic BP based on clinically measured readings (aortic stenosis, hazard ratio [HR], 1.41; 95% CI, 1.38-1.45; P < .001; aortic regurgitation, HR, 1.38; 95% CI, 1.31-1.45; P < .001; and mitral regurgitation, HR, 1.26; 95% CI, 1.23-1.29; P < .001), and based on estimations from the genotype data in the present mendelian randomization analysis (aortic stenosis, OR, 3.26; 95% CI, 1.50-7.10; P = .002; aortic regurgitation, OR, 2.59; 95% CI, 0.75-8.92; P = .13; and mitral regurgitation, OR, 2.19; 95% CI, 1.07-4.47; P = .03]), although the magnitude of risks using the mendelian randomization approach was stronger than those based on observational clinical data.
Table 2. Associations Between Systolic Blood Pressure (per 20 mm Hg) and 3 Major Outcomes of Valvular Disease Using Mendelian Randomization Approach.
| Valve Disease | Age and Sex Adjusted | Fully Adjusteda | |||
|---|---|---|---|---|---|
| No. of Cases | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Aortic valve stenosis | 1491 | 3.29 (1.52-7.12) | .002 | 3.26 (1.50-7.10) | .002 |
| Aortic valve regurgitation | 634 | 2.56 (0.73-8.85) | .13 | 2.59 (0.75-8.92) | .13 |
| Mitral valve regurgitation | 1736 | 2.22 (1.09-4.52) | .02 | 2.19 (1.07-4.47) | .03 |
| All cases | 3570 | 2.86 (1.70-4.80) | <.001 | 2.85 (1.69-4.78) | <.001 |
Abbreviation: OR, odds ratio.
Adjusted for age, sex, body mass index, UK Biobank assessment center, genotype measurement batch, alcohol intake frequency, smoking status, genetic kinship to other participants, and 10 genetic principal components.
Figure 1. Observational Hazard Ratio Derived From Previous Large-Scale Cohort Studies vs Odds Ratio Derived From the Present 1-Sample Mendelian Randomization for the Association Between Systolic Blood Pressure per 20–mm Hg and Valvular Heart Disease Outcomes.
Mendelian randomization was adjusted for age, sex, body mass index, UK Biobank assessment center, genotype measurement batch, alcohol intake frequency, smoking status, genetic kinship to other participants, and 10 genetic principal components. Dark blue circles represent point estimation and vertical lines represent 95% CIs. Odds ratio indicates hazard ratio in observational cohort studies and odds ratio in mendelian randomization estimation.
Evidence of Instrumental Variable Robustness
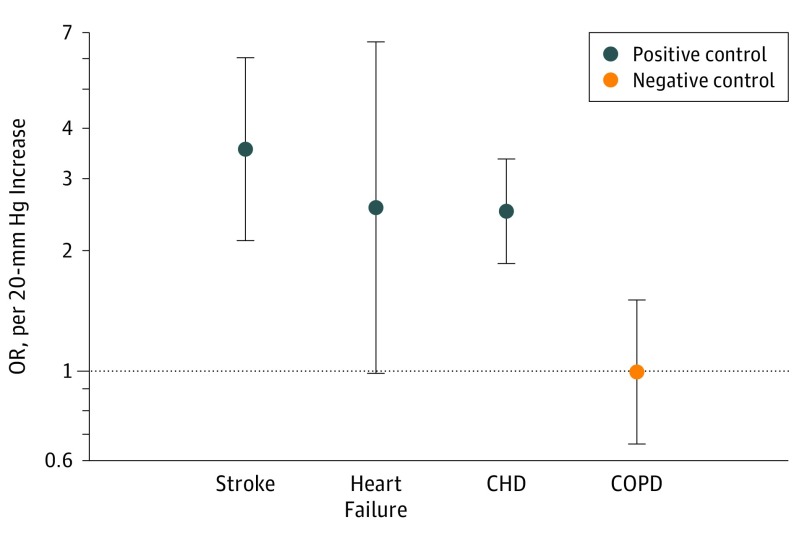
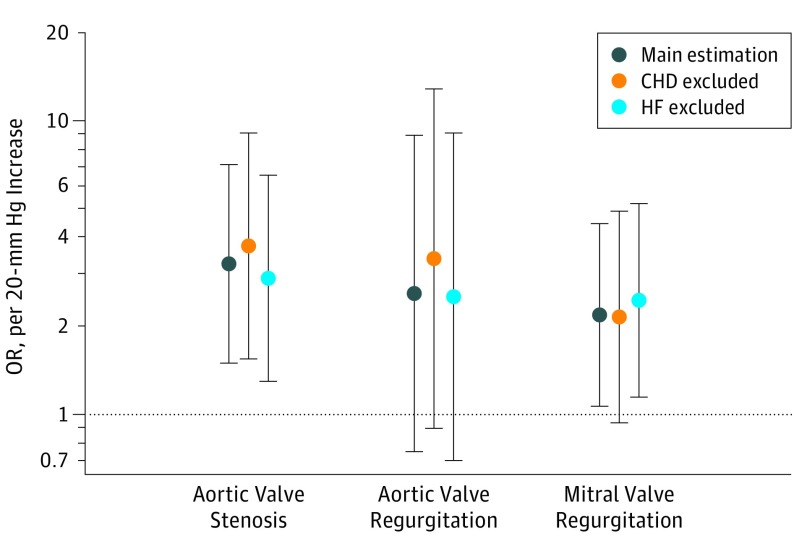
The F statistic (F = 1574) from the first-stage regression of the measured systolic BP on the GRS supported that our GRS was a strong instrument. Estimates using the unweighted GRS also produced similar estimates to the main analyses (eTable 4 in the Supplement). We found a strong linear correlation between the GRS and measured systolic BP (regression coefficient, 0.199; P < .001) (eFigure 2 in the Supplement). The mendelian randomization–Egger intercept test showed that, on average, there was no significant pleiotropic effect (intercept, 0.001; SE, 0.017; P = .94) (eFigure 3 in the Supplement). eResults 1 in the Supplement compares the estimations using an alternative case definition. The results showed that the estimations are similar and robust to case definitions based on ICD-10 vs ICD-10 plus self-report for aortic stenosis (OR, 3.28 [95% CI, 1.46-7.36] vs 3.26 [95% CI, 1.50-7.10]); for aortic regurgitation (OR, 2.60 [95% CI, 0.71-9.49] vs 2.59 [95% CI, 0.75-8.92]); and for mitral regurgitation (OR, 1.85 [95% CI, 0.87-3.94)]vs 2.19 ([95% CI, 0.07-4.47]). In addition, we restricted the outcome definition to patients who have had aortic valve replacement or mitral valve replacement surgery and the results were consistent with the main findings for aortic stenosis (OR, 4.31 [95% CI, 1.35-13.75]), aortic regurgitation (OR, 2.15 [95% CI, 0.26-17.33]), and mitral regurgitation (OR, 1.74 [95% CI, 0.32-9.24]) (eResults 2 in the Supplement). Sensitivity analysis using GRS that excluded SNPs known to be associated with any of the established cardiovascular risk factors did not show any significant change in the estimations for aortic stenosis (OR, 3.82 [95% CI, 1.70-8.56]), aortic regurgitation (OR, 2.42 [95% CI, 0.66-8.84]), and mitral regurgitation (OR, 1.90 [95% CI, 0.89-4.02]) (eResults 3 in the Supplement). Further adjustment for use of BP-lowering treatment did not have a substantial association with the effect sizes for aortic stenosis (OR, 3.21 [95% CI, 1.47-6.98]), aortic regurgitation (OR, 2.31 [95% CI, 0.67-7.97]), and mitral regurgitation (OR, 2.10 [95% CI, 1.03-4.28]) (eResults 4 in the Supplement). Furthermore, the positive and negative control analysis using our main GRS revealed strong associations between systolic BP and stroke (OR, 3.59 [95% CI, 2.12-6.06]), HF (OR, 2.57 [95% CI, 0.99-6.64]), and CHD (OR, 2.51 [95% CI, 1.86-3.39], and a null association with COPD (OR, 1.00 [95% CI, 0.66-1.51]) further suggests that our instrumental variable was valid (Figure 2). In addition, sensitivity analysis was associated with no material change in the risk estimates after exclusion of patients with a diagnosis of CHD for aortic stenosis (OR, 3.76 [95% CI, 1.55-9.09]), aortic regurgitation (OR, 3.40 [95% CI, 0.90-12.83]), and mitral regurgitation (OR, 2.15 [95% CI, 0.94-4.93]) or HF for aortic stenosis (OR, 2.92 [95% CI, 1.30-6.53]), aortic regurgitation (OR, 2.53 [95% CI, 0.70-9.09]), and mitral regurgitation (OR, 2.45 [95% CI, 1.15-5.23]) (Figure 3).
Figure 2. Mendelian Randomization Estimates for the Association Between Systolic Blood Pressure per 20-mm Hg and Stroke, Heart Failure, and Coronary Heart Disease (CHD) as Positive Controls and Chronic Obstructive Pulmonary Disease (COPD) as Negative Outcome.
The control analyses showed that the instrumental variable is valid.
Figure 3. Sensitivity Analysis With Exclusion of Patients With Coronary Heart Disease (CHD) or Heart Failure (HF) as Possible Mediators of Valvular Disease.
Main estimation included all cases; with CHD and HF exclusions, all types were excluded from the analysis.
Discussion
This mendelian randomization study suggests that elevated systolic BP is associated with an increased risk of aortic stenosis, mitral regurgitation, and aortic regurgitation. When considered together as a composite outcome, each 20-mm Hg higher genetically associated systolic BP was found to increase the odds of VHD by nearly 3-fold.
A few prospective studies have reported positive associations between higher BP and risk of VHD. In 2 large-scale studies that used data from electronic health records with BP as a continuous variable as in our study, over a median of 9 years’ follow-up, exposure to 20-mm Hg higher BP was associated with a 41% greater risk of aortic stenosis, 38% greater risk of aortic regurgitation, and 26% greater risk of mitral regurgitation.10,12 Our estimates are stronger than those reported in those large cohort studies. This difference might be related to residual confounding in earlier studies, which can bias associations toward the null.28 In addition, mendelian randomization studies often show stronger associations because they capture lifetime exposure instead of assessing risk exposure at a specific time.29 The stronger risk estimates that we observed are in keeping with epidemiologic studies that have shown that cumulative risk exposure has better predictive ability than typically measured associations based on single measures of risk exposure.30,31
Considering that the association between high BP and VHD was similar to that of other cardiovascular diseases, such as stroke, HF, and CHD, for which BP-lowering treatment has been shown to be effective in primary and secondary prevention trials,32,33 it is reasonable to assume that BP reduction may accrue similar relative effects in patients at risk for VHD. Considering the paucity of therapeutic options currently available for prevention of VHD, BP–lowering treatment may potentially play a role in the prevention of this condition. To date, clinical guidelines make no specific recommendations for medical prevention of VHD,3,34 and no drugs have demonstrated compelling efficacy in reversing or curbing disease progression.7 In addition, the current epidemic of hypertension may herald a significant increase in the incidence of VHD, which is already on the rise owing to population aging.
The heavy health care burden and cost associated with valve replacement therapy, which is the only treatment currently available for end-stage valvular disease, underscore the importance of VHD prevention. Our findings suggest that BP-lowering treatment might be a useful strategy to prevent VHD. However, further research is warranted to clarify the underlying mechanisms for the observed association between elevated systolic BP and VHD. As previously suggested, it is possible that elevated BP exerts its structural effects on valves through different mechanisms. For instance, for aortic stenosis, a cascading cycle of arteriosclerosis, left ventricular hypertrophy, and elevated BP might lead to mechanical stress and calcification, whereas BP-induced aortic root dilation might be the pathophysiologic consequence of elevated BP in aortic regurgitation.35 As for mitral regurgitation, a process of direct valve leaflet damage and indirect ring dilatation might be involved.36,37 Further mechanistic research could explore the different pathways.
Strengths and Limitations
The main strength of this study is the fact that, by using a robust GRS as a proxy for high systolic BP, we were able to address uncontrolled confounding and reverse causality that may have affected previous observational studies. Thus, by relying on nature’s randomization, we demonstrate that previously observed associations are unlikely to be due to residual confounding or reverse causality. However, we acknowledge that mendelian randomization is a quasi-experimental study and its findings do not necessarily provide proof of causality.
Our study findings should be interpreted in light of its limitations. First, the numbers of some types of VHD investigated were low, leading to relatively wide 95% CIs and, in particular, limiting the statistical power for aortic regurgitation as an individual outcome. Nonetheless, consistent with previous epidemiologic studies that had generated the hypothesis for the present study, the point estimates for all 3 valvular diseases were broadly consistent and their aggregation into a single outcome had sufficient statistical power. Second, although our various sensitivity analyses supported the main study findings, we lacked statistical power for detection of modest mediating associations. In particular, for mitral regurgitation, one would expect a certain degree of mediation through diseases of the left ventricle. In our study, however, we saw no evidence of statistical heterogeneity between our main results and mediator-adjusted results, although the 95% CI crossed 1 for CHD-excluded analysis but did not for HF-excluded analysis. In a previous epidemiologic study, the percentage of excess risk mediated by such proximate causes of secondary mitral regurgitation was only 13% (95% CI, 6.1%-20%), and accounting for them showed little modification of the long-term association between BP and mitral regurgitation (confounder- and mediator-adjusted hazard ratio [HR], 1.22; 95% CI, 1.20-1.25 compared with confounder-adjusted HR, 1.26; 95% CI, 1.23-1.29).12 Irrespective of the outcomes of such mediation, however, we believe that for disease prevention considerations, the overall associations are more relevant than those that exclude potential indirect effects. Third, we acknowledge a degree of misclassification owing to the fact that outcome definition relied mainly on data retrieved from linked electronic health records with no echocardiographic data for direct case ascertainment and assessment of the severity of disease. However, previous studies showed that diagnostic validity of electronic health records is approximately 90% compared with echocardiographic assessment and recorded cases typically represented moderate to severe disease.38,39 Fourth, our study was restricted to a population of European descent, which, despite the benefit of greater genetic homogeneity, limits the generalizability of our results to other ethnicities. It would be useful to investigate whether the observed associations are present in populations from different genetic backgrounds.
Conclusions
The present study suggests that lifetime exposure to elevated systolic BP may be associated with an increased risk of major VHD. Our mendelian randomization study is less prone to confounding and reverse causation and thus suggests that BP-lowering treatment may be a useful strategy for prevention of VHD.
eFigure 1. Study Design Schematic for Initial Exclusion Criteria and Genetic Data Quality Control
eTable 1. Systolic Blood Pressure Related Variants Used for Generation of Weighted Genetic Risk Score
eMethods. Statistical Power Analysis for the Mendelian Randomisation With Binary Outcomes
eTable 2. Statistical Power Analysis for Mendelian Randomisation With Binary Outcome
eTable 3. Correlation Coefficient (r) Between Weighted Genetic Risk Score, Measured Systolic Blood Pressure, and Potential Confounders
eTable 4. Associations Between Systolic Blood Pressure (per 20 mmHg) and Three Major Outcomes of Valve Disease Using Unweighted Genetic Risk Score as Instrumental Variable
eFigure 2. Regression Line Represent Intercorrelations Between Weighted Genetic Risk Score and Systolic Blood Pressure Originated From 130 SNPs in the Last Published GWAS Study
eFigure 3. MR-Egger Intercept Test to Assess Validity of the Instrumental Variable
eResults 1. Case Definition Sensitivity Analyses
eResults 2. Sensitivity Analysis for Assessing the Effect of Severe Cases Including Aortic Valve Replacement and Mitral Valve Replacement Surgery
eResults 3. Sensitivity analysis for assessing the pleiotropic effect of variants included in the genetic risk score
eResults 4. Sensitivity Analysis for Assessing the Effect of Adjustment For Blood Pressure Lowering Treatment on the Estimations
eReferences.
References
- 1.Watkins DA, Johnson CO, Colquhoun SM, et al. . Global, regional, and national burden of rheumatic heart disease, 1990-2015. N Engl J Med. 2017;377(8):713-722. doi: 10.1056/NEJMoa1603693 [DOI] [PubMed] [Google Scholar]
- 2.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006;368(9540):1005-1011. doi: 10.1016/S0140-6736(06)69208-8 [DOI] [PubMed] [Google Scholar]
- 3.Baumgartner H, Falk V, Bax JJ, et al. ; ESC Scientific Document Group . 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38(36):2739-2791. doi: 10.1093/eurheartj/ehx391 [DOI] [PubMed] [Google Scholar]
- 4.Smith CR, Leon MB, Mack MJ, et al. ; PARTNER Trial Investigators . Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187-2198. doi: 10.1056/NEJMoa1103510 [DOI] [PubMed] [Google Scholar]
- 5.Otto CM, Prendergast B. Aortic-valve stenosis—from patients at risk to severe valve obstruction. N Engl J Med. 2014;371(8):744-756. doi: 10.1056/NEJMra1313875 [DOI] [PubMed] [Google Scholar]
- 6.Pate GE. Association between aortic stenosis and hypertension. J Heart Valve Dis. 2002;11(5):612-614. [PubMed] [Google Scholar]
- 7.Stewart BF, Siscovick D, Lind BK, et al. . Clinical factors associated with calcific aortic valve disease: Cardiovascular Health Study. J Am Coll Cardiol. 1997;29(3):630-634. doi: 10.1016/S0735-1097(96)00563-3 [DOI] [PubMed] [Google Scholar]
- 8.Lindroos M, Kupari M, Valvanne J, Strandberg T, Heikkilä J, Tilvis R. Factors associated with calcific aortic valve degeneration in the elderly. Eur Heart J. 1994;15(7):865-870. doi: 10.1093/oxfordjournals.eurheartj.a060602 [DOI] [PubMed] [Google Scholar]
- 9.Singh JP, Evans JC, Levy D, et al. . Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol. 1999;83(6):897-902. doi: 10.1016/S0002-9149(98)01064-9 [DOI] [PubMed] [Google Scholar]
- 10.Rahimi K, Mohseni H, Kiran A, et al. . Elevated blood pressure and risk of aortic valve disease: a cohort analysis of 5.4 million UK adults. Eur Heart J. 2018;39(39):3596-3603. doi: 10.1093/eurheartj/ehy486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yan AT, Koh M, Chan KK, et al. . Association between cardiovascular risk factors and aortic stenosis: the CANHEART Aortic Stenosis Study. J Am Coll Cardiol. 2017;69(12):1523-1532. doi: 10.1016/j.jacc.2017.01.025 [DOI] [PubMed] [Google Scholar]
- 12.Rahimi K, Mohseni H, Otto CM, et al. . Elevated blood pressure and risk of mitral regurgitation: a longitudinal cohort study of 5.5 million United Kingdom adults. PLoS Med. 2017;14(10):e1002404. doi: 10.1371/journal.pmed.1002404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennett DA, Holmes MV. Mendelian randomisation in cardiovascular research: an introduction for clinicians. Heart. 2017;103(18):1400-1407. doi: 10.1136/heartjnl-2016-310605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sudlow C, Gallacher J, Allen N, et al. . UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins R. What makes UK Biobank special? Lancet. 2012;379(9822):1173-1174. doi: 10.1016/S0140-6736(12)60404-8 [DOI] [PubMed] [Google Scholar]
- 16.Biobank UK. Protocol for a large-scale prospective epidemiological resource: protocol UKBB-BROT-09-06 (main phase). https://www.ukbiobank.ac.uk/wp-content/uploads/2011/11/UK-Biobank-Protocol.pdf. Accessed November 6, 2018.
- 17.biobank. Genetic data. http://www.ukbiobank.ac.uk/scientists-3/genetic-data/. Accessed November 6, 2018.
- 18.Bycroft C, Freeman C, Petkova D, et al. . The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203-209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evangelou E, Warren HR, Mosen-Ansorena D, et al. . Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat Genet. 2018; 50(10):1412-1425. doi: 10.1038/s41588-018-0205-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purcell S, Neale B, Todd-Brown K, et al. . PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559-575. doi: 10.1086/519795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S, Thompson SG. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation. Taylor & Francis Group; 2015. doi: 10.1201/b18084 [DOI] [Google Scholar]
- 22.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133-1163. doi: 10.1002/sim.3034 [DOI] [PubMed] [Google Scholar]
- 23.Brion M-JJA, Shakhbazov K, Visscher PM. Calculating statistical power in mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497-1501. doi: 10.1093/ije/dyt179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pierce BL, VanderWeele TJ. The effect of non-differential measurement error on bias, precision and power in mendelian randomization studies. Int J Epidemiol. 2012;41(5):1383-1393. doi: 10.1093/ije/dys141 [DOI] [PubMed] [Google Scholar]
- 25.Burgess S, Thompson SG. Erratum to: interpreting findings from mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):391-392. doi: 10.1007/s10654-017-0276-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yavorska OO, Burgess S. Mendelian randomization: an R package for performing mendelian randomization analyses using summarized data. Int J Epidemiol. 2017;46(6):1734-1739. doi: 10.1093/ije/dyx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manichaikul A, Mychaleckyj JC, Rich SS, Daly K, Sale M, Chen W-M. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26(22):2867-2873. doi: 10.1093/bioinformatics/btq559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenland S, Robins JM, Pearl J. Confounding and collapsibility in acusal inference. Stat Sci. 1999;14(1):29-46. doi: 10.1214/ss/1009211805 [DOI] [Google Scholar]
- 29.Smith GD, Ebrahim S. Mendelian randomization: prospects, potentials, and limitations. Int J Epidemiol. 2004;33(1):30-42. doi: 10.1093/ije/dyh132 [DOI] [PubMed] [Google Scholar]
- 30.Ayala Solares JR, Canoy D, Raimondi FED, et al. . Long-term exposure to elevated systolic blood pressure in predicting incident cardiovascular disease: evidence from large-scale routine electronic health records. J Am Heart Assoc. 2019;8(12):e012129. doi: 10.1161/JAHA.119.012129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tielemans SMAJ, Geleijnse JM, Menotti A, et al. . Ten-year blood pressure trajectories, cardiovascular mortality, and life years lost in 2 extinction cohorts: the Minnesota Business and Professional Men Study and the Zutphen Study. J Am Heart Assoc. 2015;4(3):e001378. doi: 10.1161/JAHA.114.001378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ettehad D, Emdin CA, Kiran A, et al. . Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet. 2016;387(10022):957-967. doi: 10.1016/S0140-6736(15)01225-8 [DOI] [PubMed] [Google Scholar]
- 33.Emdin CA, Callender T, Cao J, McMurray JJV, Rahimi K. Meta-Analysis of Large-Scale Randomized Trials to Determine the Effectiveness of Inhibition of the Renin-Angiotensin Aldosterone System in Heart Failure. Am J Cardiol. 2015;116(1):155-161. doi: 10.1016/j.amjcard.2015.03.052 [DOI] [PubMed] [Google Scholar]
- 34.Nishimura RA, Otto CM, Bonow RO, et al. . 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017;70(2):252-289. doi: 10.1016/j.jacc.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 35.Lindman BR, Clavel M-A, Mathieu P, et al. . Calcific aortic stenosis. Nat Rev Dis Primers. 2016;2:16006. doi: 10.1038/nrdp.2016.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Antoine C, Mantovani F, Benfari G, et al. . Pathophysiology of degenerative mitral regurgitation: new 3-dimensional imaging insights. Circ Cardiovasc Imaging. 2018;11(1):e005971. doi: 10.1161/CIRCIMAGING.116.005971 [DOI] [PubMed] [Google Scholar]
- 37.Patel H, Desai M, Tuzcu EM, Griffin B, Kapadia S. Pulmonary hypertension in mitral regurgitation. J Am Heart Assoc. 2014;3(4):e000748. doi: 10.1161/JAHA.113.000748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thanassoulis G, Campbell CY, Owens DS, et al. ; CHARGE Extracoronary Calcium Working Group . Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368(6):503-512. doi: 10.1056/NEJMoa1109034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andell P, Li X, Martinsson A, et al. . Epidemiology of valvular heart disease in a Swedish nationwide hospital-based register study. Heart. 2017;103(21):1696-1703. doi: 10.1136/heartjnl-2016-310894 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study Design Schematic for Initial Exclusion Criteria and Genetic Data Quality Control
eTable 1. Systolic Blood Pressure Related Variants Used for Generation of Weighted Genetic Risk Score
eMethods. Statistical Power Analysis for the Mendelian Randomisation With Binary Outcomes
eTable 2. Statistical Power Analysis for Mendelian Randomisation With Binary Outcome
eTable 3. Correlation Coefficient (r) Between Weighted Genetic Risk Score, Measured Systolic Blood Pressure, and Potential Confounders
eTable 4. Associations Between Systolic Blood Pressure (per 20 mmHg) and Three Major Outcomes of Valve Disease Using Unweighted Genetic Risk Score as Instrumental Variable
eFigure 2. Regression Line Represent Intercorrelations Between Weighted Genetic Risk Score and Systolic Blood Pressure Originated From 130 SNPs in the Last Published GWAS Study
eFigure 3. MR-Egger Intercept Test to Assess Validity of the Instrumental Variable
eResults 1. Case Definition Sensitivity Analyses
eResults 2. Sensitivity Analysis for Assessing the Effect of Severe Cases Including Aortic Valve Replacement and Mitral Valve Replacement Surgery
eResults 3. Sensitivity analysis for assessing the pleiotropic effect of variants included in the genetic risk score
eResults 4. Sensitivity Analysis for Assessing the Effect of Adjustment For Blood Pressure Lowering Treatment on the Estimations
eReferences.