Key Points
Question
What is the prevalence of undiagnosed or silent myocardial infarction scars among individuals who experienced sudden cardiac death?
Findings
In this case-control study, 3122 individuals experienced coronary artery disease–associated sudden cardiac death without prior knowledge of coronary artery disease, and 1322 (42.4%) of these had scarring associated with silent myocardial infarction at autopsy. Of those with electrocardiograms recorded prior to death, 67% had abnormal findings.
Meaning
In this analysis, a substantial number of individuals who experienced coronary artery disease–associated sudden cardiac death had had prior myocardial infarction without a diagnosis of coronary artery disease; some of these individuals might have been identifiable by premortem electrocardiography.
This case-control study uses existing medical records and autopsy data to assess the prevalence of silent myocardial infarction and electrocardiographic abnormalities in individuals who experienced sudden cardiac death without a prior diagnosis of coronary artery disease.
Abstract
Importance
Myocardial infarction in the absence of major or unrecognized symptoms are characterized as silent (SMI). The prevalence of SMI among individuals who experience sudden cardiac death (SCD), with or without concomitant electrocardiographic (ECG) changes, has not previously been described in detail from large studies to our knowledge.
Objective
To determine the prevalence of SMI in individuals who experience SCD without a prior diagnosis of coronary artery disease (CAD) and to detect ECG abnormalities associated with SMI-associated SCD.
Design, Setting, and Participants
This case-control study compared autopsy findings, clinical characteristics, and ECG markers associated with SMI in a consecutive cohort of individuals in the Finnish Genetic Study of Arrhythmic Events (Fingesture) study population who were verified to have had SCD. The Fingesture study consists of individuals who had autopsy-verified SCD in Northern Finland between 1998 and 2017. Individuals who had SCD with CAD and evidence of SMI were regarded as having had cases; those who had SCD with CAD without SMI were considered control participants. Analyses of ECG tests were carried out by investigators blinded to the SMI data. Data analysis was completed from October 2018 through November 2018.
Main Outcomes and Measures
Silent MI was defined as a scar detected by macroscopic and microscopic evaluation of myocardium without previously diagnosed CAD. Clinical history was obtained from medical records, previously recorded ECGs, and a standardized questionnaire provided to the next of kin. The hypothesis tested was that SMI would be prevalent in the population who had had SCD with CAD, and it might be detected or suspected from findings on ECGs prior to death in many individuals.
Results
A total of 5869 individuals were included (2459 males [78.8%]; mean [SD] age, 64.9 [12.4] years). The cause of SCD was CAD in 4392 individuals (74.8%), among whom 3122 had no history of previously diagnosed CAD. Two individuals were excluded owing to incomplete autopsy information. An ECG recorded prior to SCD was available in 438 individuals. Silent MI was detected in 1322 individuals (42.4%) who experienced SCD without a clinical history of CAD. The participants with SMI were older than participants without MI scarring (mean [SD] age, 66.9 [11.1] years; 65.5 [11.6] years; P < .001) and were more often men (1102 of 1322 [83.4%] vs 1357 of 1798 [75.5%]; P < .001). Heart weight was higher in participants with SMI (mean [SD] weight, 483 [109] g vs 438 [106] g; P < .001). In participants with SMI, SCD occurred more often during physical activity (241 of 1322 [18.2%] vs 223 of 1798 [12.4%]; P < .001). A prior ECG was abnormal in 125 of the 187 individuals (66.8%) who had SCD after SMI compared with 139 of 251 (55.4%) of those who had SCD without SMI (P = .02).
Conclusions and Relevance
Many individuals who experienced SCD associated with CAD had a previously undetected MI at autopsy. Previous SMI was associated with myocardial hypertrophy and SCD during physical activity. Premortem ECGs in a subset with available data were abnormal in 67% of the individuals who had had a SCD after an SMI.
Introduction
Sudden cardiac death (SCD) is a worldwide major public health burden, accounting for more than 50% of all cardiac deaths and 20% of all deaths in the United States.1,2 Coronary artery disease (CAD) is the most common underlying cause of SCD.3 Often, SCD is the first recognized clinical manifestation of underlying heart disease, and the ability to recognize this specific risk before the event is severely limited.4
Currently, there are highly effective treatments for acute myocardial infarction (MI), including percutaneous coronary interventions, antithrombotic therapy, and secondary prevention.5 Still, 2 groups of patients with acute MI have not benefitted from this progress: patients whose first manifestation is SCD and patients who have atypical symptoms or no symptoms associated with the acute phase of MI.6
Approximately one-half of all MIs are estimated to be clinically unrecognized.7,8,9 Along with pathological Q waves, several other ECG abnormalities have been studied in attempts to determine the presence of a myocardial scarring or MI and associated increased risk of SCD. These include fragmented QRS complexes (fQRS), prolonged QRS duration, and inverted T waves.10,11,12,13,14 Myocardial scarring owing to infarction creates a potentially arrhythmogenic substrate for fatal arrhythmias.15 The risk of SCD, heart failure, and other adverse outcomes have been observed, with similar incidences in association with both silent MI and clinically recognized MI, but the outcome of events has been shown to be worse with silent myocardial infarction (SMI).7,15,16
There are limited data regarding the prevalence of SMI in individuals who experience SCD. The aim of this study was to determine the prevalence and evaluate the characteristics of SMI among a large, autopsy-verified population of individuals who experienced SCD without a history of CAD. In addition to autopsy findings and medical records, we investigated prior ECGs that were available for evidence of coronary heart disease in general and a preexisting SMI in particular.
Methods
Study Population
The Finnish Genetic Study of Arrhythmic Events (Fingesture) is a unique study consisting of 5869 consecutive individuals who experienced SCD in Northern Finland between 1998 and 2017. All cases were autopsy verified. The medicolegal autopsies were performed by experienced forensic pathologists who each performed more than 100 autopsies per year at the Department of Forensic Medicine of the University of Oulu and National Institute for Health and Welfare in Oulu, Finland. Contemporary guidelines were used for diagnosis of the cause of death. In Finland, all unexpected deaths undergo medicolegal autopsy based on Finnish law (Act on the Inquest into the Cause of Death, 459/1973, seventh paragraph: Finnish Law), which minimizes the possibility of selection bias in individuals who experienced SCD. The autopsy rate in Finland is the highest in Western countries,17 and the quality of postmortem investigations is set at a high standard and ensured by national laws and guidelines.18
Sudden death was defined as witnessed death within 6 hours of the onset of symptoms or an unwitnessed death within 24 hours of when the deceased individual was last seen in a stable state of health. The Fingesture study included only sudden deaths considered to be caused by a cardiac disease. Data obtained regarding the individuals who experienced SCD included death certificates, medical records, autopsy reports, standardized questionnaire to the next of kin, and police reports at the scene where the individual who experienced SCD was found dead. All 12-lead ECGs recorded incidentally before SCD were collected from medical records.
This study complies with the Declaration of Helsinki and has been approved by the ethics committee of the University of Oulu and Finland's Ministry of Social Affairs and Health. The National Supervisory Authority for Welfare and Health (which is also known as Valvira) and the National Institute for Health and Welfare approved the review of autopsy data by the investigators.
Autopsy Data
Causes of death were determined by forensic pathologists in medicolegal cause-of-death investigations, based on police reports, medical records, autopsy findings, and complementary analyses. For study purposes, additional data were gathered via questionnaires. The inclusion of cases was based on all the available data assessed by forensic pathologist and clinician in consensus. Each cause of death was reported according to the International Classification of Diseases, Tenth Revision code classifications. Precise cardiac investigations were performed to all deceased individuals, including macroscopic investigation and dissection of myocardium and coronary arteries. Histological samples of the myocardium were obtained (3 to 5 sections) from areas of the anterior and posterior walls of the left ventricle and septum. A toxicological examination was performed if intoxication had to be excluded as the cause of death. The details of the method for classification of causes of SCD were reported in a previous study.19 An old myocardial infarction was identified as a focal myocardial scar detected by macroscopic evaluation of the dissected ventricles and histologic analysis of the myocardial samples. Silent MI in this study was defined by the generally accepted definition of unrecognized MI: as a failure of patients to recognize or report symptoms during or after the event, combined with an absence of a history of previously diagnosed CAD and a myocardial scar in the absence of evidence of an acute MI in the area of the scar or elsewhere in the heart at autopsy. Included individuals were classified into 4 body mass index categories (calculated as weight in kilograms divided by height in meters squared): lean (<20), normal weight (20.0-24.9), overweight (25.0-29.9), and obese (>30).
Electrocardiographic Analysis
Standard 12-lead ECGs were recorded in the supine position with a paper speed of 50 mm/s and calibration of 1 mV/10 mm. If multiple ECGs were available, the most recent one was used. The criteria of fQRS have been reported in a previous study.14 A possible Q wave was considered pathological when it was 30 milliseconds or longer, at least 0.1 mV deep in 2 contiguous leads, and reflecting the vascular bed of the same coronary branch. The QRS duration was calculated as the mean duration from all leads, and duration of 110 milliseconds or more was considered clinically significantly prolonged based on prior evidence of clinical significance in a general population at this cutpoint.12 The T waves were classified as inverted when T-wave amplitude was −0.1 mV or less in at least 2 contiguous leads. The ECG abnormalities was categorized as anterior (V1-V3), lateral (I, aVL, or V4-V6), and inferior (II, III, or aVF).
Statistical Analysis
Two-sided t tests for contiguous variables and χ2 tests for categorical variables were used for comparisons between study groups. All P values less than .05 were considered statistically significant, and all P values were 2-sided. Continuous variables are reported as mean (SD) values. All analyses were performed with the Statistical Package for Social Studies version 24.0 (IBM).
Results
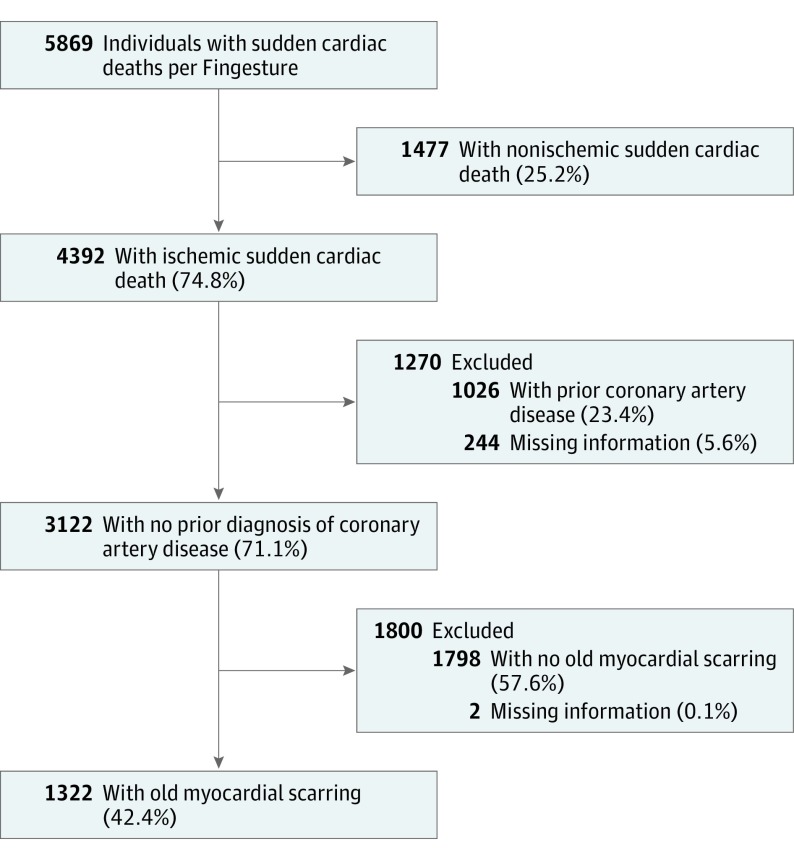
Among all individuals who experienced SCD, 4392 individuals (74.8%) had CAD as an underlying cause of death. A total of 3122 individuals (71.1%) had no history of CAD prior to death, with SCD being the first manifestation of underlying heart disease. In 244 individuals (5.6%), there was no information available whether CAD had been diagnosed prior to SCD, and those cases were excluded from this study. Two individuals were excluded owing to incomplete autopsy information. The study design is shown in the Figure.
Figure. Study Population Derivation From the Finnish Genetic Study of Arrhythmic Events (Fingesture) Study.
Clinical Characteristics and Circumstances of SCD
Among individuals who experienced SCD without previously diagnosed CAD (3122 individuals), 42.4% had myocardial scar consistent with an SMI at autopsy (1322 individuals). The characteristics of those with and without SMI are presented in Table 1. The individuals with SMI were slightly older (mean [SD] age: SMI group, 66.9 [11.1] years vs non-SMI group, 65.5 [11.6] years; P < .001) and were more likely to be male (SMI group, 1102 of 1322 [83.4%] vs non-SMI group, 1357 of 1798 [75.5%]; P < .001) compared with individuals with SCD without SMI. Statistically significant differences between SMI and non-SMI groups were also observed in total heart weight in both men (mean [SD] weight: SMI group, 497.2 [107.0] g vs non-SMI group, 454.7 [105.0] g; P < .001) and women (mean [SD] weight: SMI group, 411.3 [85.7] g vs non-SMI group, 386.1 [91.2] g; P = .001), left ventricular hypertrophy (SMI group, 938 of 1322 [71.0%] vs non-SMI group, 965 of 1798 [53.7%]; P < .001), and heart weight–to–body surface area ratio (mean [SD] ratio: SMI group, 251.6 [43.6] vs non-SMI group, 230.4 [41.1]; P < .001). There were no statistically significant differences in the history of diabetes mellitus, hypertension, dyslipidemia, angina pectoris, or exercise dyspnea.
Table 1. Clinical Characteristics and Autopsy Findings in Individuals Without a History of Coronary Artery Disease.
| Characteristic | Individuals, No. (%) | P Value | |
|---|---|---|---|
| With Silent Myocardial Infarction (n = 1322) | Without Silent Myocardial Infarction (n = 1798)a | ||
| Age, mean (SD), y | 66.9 (11.1) | 65.5 (11.6) | .001 |
| ≥50 y | 1244 (94.1) | 1646 (91.5) | .08 |
| Male | 1102 (83.4) | 1357 (75.5) | <.001 |
| BMI, mean (SD) | 26.9 (5.1) | 26.7 (5.6) | .22 |
| <20 | 87 (6.6) | 196 (10.9) | .001 |
| 20.00-24.9 | 395 (29.9) | 517 (28.8) | .65 |
| 25.0-29.9 | 525 (39.7) | 641 (35.7) | .03 |
| ≥30 | 315 (23.8) | 444 (24.7) | .21 |
| Total heart weight, mean (SD), g | 482.9 (108.6) | 437.8 (105.9) | <.001 |
| Men | 497.2 (107.0) | 454.7 (105.0) | <.001 |
| Women | 411.3 (85.7) | 386.1 (91.2) | .001 |
| Left ventricular hypertrophy | 938 (71.0) | 965 (53.7) | <.001 |
| Heart weight/body surface area, mean (SD) | 251.6 (43.6) | 230.4 (41.1) | <.001 |
| Total occlusion of coronary artery | 519 (39.3) | 291 (16.2) | <.001 |
| Diabetes mellitus | 249 (18.8) | 312 (17.4) | .07 |
| Hypertension | 523 (39.6) | 688 (38.3) | .68 |
| Angina | 81 (6.1) | 104 (5.8) | .72 |
| Dyslipidemia | 149 (11.3) | 200 (11.1) | .92 |
| Dyspnea | 45 (3.4) | 53 (2.9) | .48 |
| Heart failure | 95 (7.2) | 88 (4.9) | .07 |
| Circumstances at death | |||
| Unwitnessed; dead on initial contact | 1104 (83.5) | 1551 (86.3) | .18 |
| During physical activity | 241 (18.2) | 223 (12.4) | <.001 |
| In hospital, health center, or ambulance | 84 (6.4) | 125 (7.0) | .55 |
| Outdoors | 265 (20.0) | 268 (14.9) | .001 |
| In sauna | 21 (1.6) | 78 (4.3) | .03 |
| Time of death | |||
| No. | 824 | 908 | |
| 12 am–6 am | 149 (18.1) | 193 (21.3) | .10 |
| 6 am–12 pm | 248 (30.1) | 251 (27.6) | .27 |
| 12 pm–6 pm | 279 (33.9) | 279 (30.7) | .17 |
| 6 pm–12 am | 148 (18.0) | 185 (20.4) | .22 |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
A total of 1800 individuals were in the group without silent myocardial infarctions, but 2 individuals had incomplete autopsy information and were omitted from the Table.
The individuals with SMI died more often during physical activity (SMI group, 241 of 1322 [18.2%] vs non-SMI group, 223 of 1798 [12.4%]; P < .001) and outdoors (SMI group, 265 of 1322 [20.0%] vs non-SMI group, 268 of 1798 [14.9%]; P = .001). Surprisingly, SCDs in saunas were more common in individuals without SMI (78 of 1798 [4.3%]) compared with individuals with SMI (21 of 1322 [1.6%]; P = .03).
Electrocardiographic Measurements
We were able to collect a total of 438 ECGs prior to SCD (187 in individuals with SMI and 251 in the previously diagnosed group). The ECG abnormalities are presented in Table 2. Fragmented QRS complex in at least 2 contiguous leads was detected in about one-half of both groups. Prevalence was 54.5% (102 of 187 individuals) in the SMI group and 45.8% (115 of 251 individuals) in the non-SMI group; however, this finding was not statistically significant (P = .08). No significant difference was detected in mean QRS duration between groups or in the prevalence of prolonged QRS duration of 110 milliseconds or longer (34 of 187 [18.2%] in the SMI group and 36 of 251 [14.3%] in the non-SMI group; P = .29). The SMI group had more frequently inverted T waves (31 of 187 [16.6%] vs 21 of 251 [8.4%]; P = .01) and pathologic Q waves (24 of 187 [12.8%] vs 17 of 251 [6.8%]; P = .045) compared with the non-SMI group. Overall, having at least 1 of the ECG abnormalities mentioned above (fQRS, Q wave, T-wave inversion, or QRS ≥110 milliseconds) was more common in the SMI group (125 of 187 [66.8%]) compared with the non-SMI group (139 of 251 [55.4%]; P = .02).
Table 2. Potential Electrocardiographic Markers of Silent Myocardial Infarction Among Individuals Who Experienced Sudden Cardiac Death Without a History of Coronary Artery Disease.
| Variable | Individuals, No. (%) | P Value | |
|---|---|---|---|
| With Silent Myocardial Infarction (n = 187) | Without Silent Myocardial Infarction (n = 251) | ||
| Fragmented QRS complexes | 102 (54.5) | 115 (45.8) | .08 |
| Anterior | 34 (18.2) | 38 (15.1) | .44 |
| Inferior | 85 (45.5) | 94 (37.5) | .10 |
| Lateral | 36 (19.3) | 51 (20.3) | .81 |
| Q wave | 24 (12.8) | 17 (6.8) | .05 |
| Anterior | 10 (5.3) | 5 (2.0) | .07 |
| Inferior | 14 (7.5) | 10 (4.0) | .14 |
| Lateral | 2 (1.1) | 5 (2.0) | .70 |
| Inverted T wave | 31 (16.6) | 21 (8.4) | .01 |
| Anterior | 3 (1.6) | 7 (2.8) | .53 |
| Inferior | 13 (7.0) | 5 (2.0) | .01 |
| Lateral | 22 (11.8) | 14 (5.6) | .02 |
| QRS, mean (SD), ms | 98.6 (19.0) | 96.7 (18.3) | .28 |
| ≥110 ms | 34 (18.2) | 36 (14.3) | .29 |
| Any electrocardiographic abnormality | 125 (66.8) | 139 (55.4) | .02 |
Discussion
The aim of the present study was to determine the prevalence of SMI among individuals who experienced SCD with autopsy defined CAD. Only one-fifth of ischemic individuals who experienced SCD had a history of CAD before death, which is not surprising, considering that in most cases, SCD is the first manifestation of the underlying heart disease, particularly in CAD.2,3,4 However, only unexpected and sudden deaths undergo medicolegal autopsy in Finland, which is why this study might not include all deaths from known heart disease. The main finding in this study is that 42.4% of the study population without known CAD had a myocardial scar at autopsy, indicating a previous, unrecognized MI. In additional, individuals with SMI were older and more often male, with a higher proportion of myocardial hypertrophy, and their SCDs more often occurred during physical activity. Some previously studied ECG markers, presumably associated with myocardial scarring (Q waves and T-wave inversions), were associated with SMIs. Among the subgroup with antemortem ECGs, two-thirds of the individuals who experienced SCD with SMI had an abnormal ECG result.
Comparing the prevalence of SMI in individuals who experienced SCD in this study (1322 of 3122 individuals [42.4%]) with a previous study conducted in the United States (24 of 71 individuals [34%])20 shows that the burden of SMI in the present study was even higher. However, in the prior study, the number of included individuals was notably lower, and only individuals between age 25 and 60 years were included, which likely lowered the prevalence of detected SMIs. In addition, the difference in the prevalence may be derived from divergence between autopsy methods or geographical differences.
The large proportion of individuals who experienced SCD with SMI may partly be owing to diversity in the perceived symptoms of MI. It is likely that some patients had unrecognized symptoms or signs of an MI and did not recognize and report the severity of their symptoms or that there is variation between patients in cardiac receptors and autonomic nervous system characteristics. Moreover, in patients with diabetes, there may be some impairment of recognizing angina because of diabetic neuropathy.8 It has also been noted that patients who experience unrecognized MIs have higher pain thresholds.21
Myocardial scarring owing to a previous MI may be a substrate for arrhythmias,15 and myocardial ischemia can be one of the triggering factors. Indeed, there is evidence that myocardial scarring has been associated with SCD during physical exercise,22 which was also observed in these data. We observed a higher proportion of SCDs during physical activity among individuals with myocardial scars. Individuals with scars also died more frequently outdoors. A recent study from Sweden showed that cold weather has an independent role in the occurrence of MI.23 In the previous study of the Fingesture population, cold weather has been noted to associate with increased risk of SCD caused by ischemic heart disease.24 Additionally, individuals with SMI were unaware of their disease and presumably did not limit their physical exertion when unrecognized symptoms occurred. Also, individuals with SMI most likely did not have medication targeted at halting progression or preventing CAD, since by definition these individuals did not have a prior diagnosis of CAD in this study.
In general, the prevalence of SMI among people with diabetes seems to be higher compared with those who are nondiabetic,6,16,25 and they have been reported to have high rates of asymptomatic myocardial ischemia.8 Nonetheless, diabetes was not more prevalent in individuals with SMI than without SMI in the present study. About one-fifth of the scar group had a history of diabetes. Admittedly, diabetic neuropathy may impair recognition of infarction. However, this neurologic dysfunction usually occurs in more advanced cases of diabetes, which can explain the discordant outcomes. Second, individuals with overt diabetes are generally under more intense health surveillance and medical control than those without diabetes, and infarctions are consequently more likely to be detected.8 However, diagnosing type 2 diabetes post mortem is problematic, and many individuals who experienced SCD may in fact have had undiagnosed diabetes at the time of death.
In the study, individuals who experienced SCD with SMI more commonly had cardiac hypertrophy. The mean heart weight was higher in individuals with SMI, both in men and women. Myocardial infarction is often followed by hypertrophy of myocardium as a long-term compensatory response to maintain stroke volume.26 The magnitude of hypertrophy partly depends on the size of the infarct. The presence of left ventricular hypertrophy is an independent risk factor for SCD,27 which, combined with myocardial scarring, may further worsen the prognosis. The association of cardiac hypertrophy with healed infarct at autopsy has been observed in previous studies,28,29 as well as the association of cardiac hypertrophy and healed infarct with vulnerability toward cardiac arrhythmias and SCD.30,31,32
The present findings support the view that the prevalence of SMI increases within age6,16 and incidence is higher in men.33 As observed in the Atherosclerosis Risk in Communities study,33 there are race and sex differences in the incidence and prognostic significance of SMI. However, in the Atherosclerosis Risk in Communities SMI study, the diagnosis of SMI was determined only by ECG, and in this study, autopsy data were combined with ECG results. These observations support the notion that assessing risk for SMI should be personalized.
In the present study, at least 1 abnormality on ECG (fQRS, prolonged QRS, Q wave, or T-wave inversion), indicating possible myocardial scarring, was more common in the SMI group. The prevalence of inverted T waves and pathologic Q waves was higher in individuals with SMI. We found that fQRS was the most common marker of a scar in the SMI group. In our previous study on exercise-associated SCD, fQRS was also the most common finding among participants with MI scarring.34 The fQRS complex is probably a sensitive marker of myocardial scarring, but its specificity is not very high.14 In some cases, a myocardial scar caused by SMI could have been detected in an ECG recording, even though the methods are not specific. It would be more effective to use multiple ECG markers instead of 1. Among patients in whom SCD without a prior MI is the first sign of cardiac disease, a previous ECG result is likely to be normal.35 However, ECGs were available only in 187 individuals with SMI, so the data are not sufficient to draw definite conclusions. Rather, they support motivation for further studies on this question.
In the future, other, more efficient methods might be useful for diagnosing SMI, in addition to standard ECGs. These include the likelihood that greater sensitivity can be achieved by imaging techniques, such as cardiac magnetic resonance imaging (CMRI),15 but the cost-effectiveness for CMRI screening is likely to be unreasonable. Therefore, screening high-risk populations with ECG to identify individuals for further examinations would probably be reasonable. After ECG screening, identifying candidates for CMRI screening with echocardiographic strain analyses would be an additional possibility to lower the costs of SMI screening in selected populations at high risk for CAD. Recent studies have shown promising correlations between strain measurements and CMRI fibrosis.29 In addition, a recent study in Iceland36 showed that individuals with SMI findings by CMRI have a risk of death congruent with that of individuals with prior clinical MI. After diagnosing previous SMI, secondary prevention strategies should be initiated, as in clinical MI.
Limitations
There are a few limitations in this study. First, autopsy data only revealed if there was a scar detected in the myocardium but not the size of it. Nevertheless, autopsy verification of SMI is superior to only ECG analyses used in the previous studies on this topic. Second, the symptoms prior to death or at the onset of cardiac arrest cannot be reliably assessed, and a subset of individuals with SCD may have understated their premonitory symptoms. Finally, not all individuals had an ECG recorded prior to death. Besides, if SMI occurred between latest ECG recording and SCD, no ECG changes were detected.
Conclusions
Among individuals who experienced SCDs determined to be caused by ischemic heart disease, SMIs indicated by a scar in the left ventricle at autopsy in the absence of a clinical history of CAD or MI was found in 42.4%. The presence of SMI in individuals who experienced SCD is associated with older age, male sex, left ventricular hypertrophy, and sudden death during exercise. Inverted T waves and Q waves may be seen on prior ECG results, but many events occur in patients without prior ECG changes. Further studies are needed to determine how to recognize and prevent SMIs to prevent SCDs.
References
- 1.Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med. 2001;345(20):1473-1482. doi: 10.1056/NEJMra000650 [DOI] [PubMed] [Google Scholar]
- 2.Hayashi M, Shimizu W, Albert CM. The spectrum of epidemiology underlying sudden cardiac death. Circ Res. 2015;116(12):1887-1906. doi: 10.1161/CIRCRESAHA.116.304521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125(8):1043-1052. doi: 10.1161/CIRCULATIONAHA.111.023846 [DOI] [PubMed] [Google Scholar]
- 4.Wellens HJJ, Schwartz PJ, Lindemans FW, et al. . Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J. 2014;35(25):1642-1651. doi: 10.1093/eurheartj/ehu176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van de Werf F, Bax J, Betriu A, et al. ; ESC Committee for Practice Guidelines (CPG) . Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29(23):2909-2945. doi: 10.1093/eurheartj/ehn416 [DOI] [PubMed] [Google Scholar]
- 6.Arenja N, Mueller C, Ehl NF, et al. . Prevalence, extent, and independent predictors of silent myocardial infarction. Am J Med. 2013;126(6):515-522. doi: 10.1016/j.amjmed.2012.11.028 [DOI] [PubMed] [Google Scholar]
- 7.Pride YB, Piccirillo BJ, Gibson CM. Prevalence, consequences, and implications for clinical trials of unrecognized myocardial infarction. Am J Cardiol. 2013;111(6):914-918. doi: 10.1016/j.amjcard.2012.11.042 [DOI] [PubMed] [Google Scholar]
- 8.Sheifer SE, Manolio TA, Gersh BJ. Unrecognized myocardial infarction. Ann Intern Med. 2001;135(9):801-811. doi: 10.7326/0003-4819-135-9-200111060-00010 [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB, Abbott RD. Incidence and prognosis of unrecognized myocardial infarction: an update on the Framingham study. N Engl J Med. 1984;311(18):1144-1147. doi: 10.1056/NEJM198411013111802 [DOI] [PubMed] [Google Scholar]
- 10.Michael MA, El Masry H, Khan BR, Das MK. Electrocardiographic signs of remote myocardial infarction. Prog Cardiovasc Dis. 2007;50(3):198-208. doi: 10.1016/j.pcad.2007.05.003 [DOI] [PubMed] [Google Scholar]
- 11.Das MK, Maskoun W, Shen C, et al. . Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010;7(1):74-80. doi: 10.1016/j.hrthm.2009.09.065 [DOI] [PubMed] [Google Scholar]
- 12.Aro AL, Anttonen O, Tikkanen JT, et al. . Intraventricular conduction delay in a standard 12-lead electrocardiogram as a predictor of mortality in the general population. Circ Arrhythm Electrophysiol. 2011;4(5):704-710. doi: 10.1161/CIRCEP.111.963561 [DOI] [PubMed] [Google Scholar]
- 13.Teodorescu C, Reinier K, Uy-Evanado A, et al. . Prolonged QRS duration on the resting ECG is associated with sudden death risk in coronary disease, independent of prolonged ventricular repolarization. Heart Rhythm. 2011;8(10):1562-1567. doi: 10.1016/j.hrthm.2011.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das MK, Khan B, Jacob S, Kumar A, Mahenthiran J. Significance of a fragmented QRS complex versus a Q wave in patients with coronary artery disease. Circulation. 2006;113(21):2495-2501. doi: 10.1161/CIRCULATIONAHA.105.595892 [DOI] [PubMed] [Google Scholar]
- 15.Kwong RY, Chan AK, Brown KA, et al. . Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113(23):2733-2743. doi: 10.1161/CIRCULATIONAHA.105.570648 [DOI] [PubMed] [Google Scholar]
- 16.Valensi P, Lorgis L, Cottin Y. Prevalence, incidence, predictive factors and prognosis of silent myocardial infarction: a review of the literature. Arch Cardiovasc Dis. 2011;104(3):178-188. doi: 10.1016/j.acvd.2010.11.013 [DOI] [PubMed] [Google Scholar]
- 17.Saukko P. Medicolegal investigative system and sudden death in Scandinavia. Nihon Hoigaku Zasshi. 1995;49(6):458-465. [PubMed] [Google Scholar]
- 18.Lahti RA. From findings to statistics: an assessment of Finnish medical cause-of-death information in relation to underlying-cause coding. https://core.ac.uk/download/pdf/14915925.pdf. Published 2005. Accessed May 28, 2019.
- 19.Hookana E, Junttila MJ, Puurunen VP, et al. . Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. 2011;8(10):1570-1575. [DOI] [PubMed] [Google Scholar]
- 20.Adabag AS, Peterson G, Apple FS, Titus J, King R, Luepker RV. Etiology of sudden death in the community: results of anatomical, metabolic, and genetic evaluation. Am Heart J. 2010;159(1):33-39. doi: 10.1016/j.ahj.2009.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Øhrn AM, Nielsen CS, Schirmer H, Stubhaug A, Wilsgaard T, Lindekleiv H. Pain tolerance in persons with recognized and unrecognized myocardial infarction: a population-based, cross-sectional study. J Am Heart Assoc. 2016;5(12):e003846. doi: 10.1161/JAHA.116.003846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toukola T, Hookana E, Junttila J, et al. . Sudden cardiac death during physical exercise: characteristics of victims and autopsy findings. Ann Med. 2015;47(3):263-268. doi: 10.3109/07853890.2015.1025824 [DOI] [PubMed] [Google Scholar]
- 23.Mohammad MA, Koul S, Rylance R, et al. . Association of weather with day-to-day incidence of myocardial infarction: a Swedeheart nationwide observational study. JAMA Cardiol. 2018;3(11):1081-1089. doi: 10.1001/jamacardio.2018.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryti NRI, Mäkikyrö EMS, Antikainen H, et al. . Risk of sudden cardiac death in relation to season-specific cold spells: a case-crossover study in Finland. BMJ Open. 2017;7(11):e017398. doi: 10.1136/bmjopen-2017-017398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scirica BM. Prevalence, incidence, and implications of silent myocardial infarctions in patients with diabetes mellitus. Circulation. 2013;127(9):965-967. doi: 10.1161/CIRCULATIONAHA.113.001180 [DOI] [PubMed] [Google Scholar]
- 26.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81(4):1161-1172. doi: 10.1161/01.CIR.81.4.1161 [DOI] [PubMed] [Google Scholar]
- 27.Zehender M, Faber T, Koscheck U, Just H, Meinertz T. Ventricular tachyarrhythmias, myocardial ischemia, and sudden cardiac death in patients with hypertensive heart disease. Clin Cardiol. 1995;18(7):377-383. doi: 10.1002/clc.4960180705 [DOI] [PubMed] [Google Scholar]
- 28.Burke AP, Farb A, Liang YH, Smialek J, Virmani R. Effect of hypertension and cardiac hypertrophy on coronary artery morphology in sudden cardiac death. Circulation. 1996;94(12):3138-3145. doi: 10.1161/01.CIR.94.12.3138 [DOI] [PubMed] [Google Scholar]
- 29.Kaikkonen KS, Kortelainen ML, Huikuri HV. Comparison of risk profiles between survivors and victims of sudden cardiac death from an acute coronary event. Ann Med. 2009;41(2):120-127. doi: 10.1080/07853890802213295 [DOI] [PubMed] [Google Scholar]
- 30.Warnes CA, Roberts WC. Sudden coronary death: relation of amount and distribution of coronary narrowing at necropsy to previous symptoms of myocardial ischemia, left ventricular scarring and heart weight. Am J Cardiol. 1984;54(1):65-73. doi: 10.1016/0002-9149(84)90305-9 [DOI] [PubMed] [Google Scholar]
- 31.Scott RF, Briggs TS. Pathologic findings in pre-hospital deaths due to coronary atherosclerosis. Am J Cardiol. 1972;29(6):782-787. doi: 10.1016/0002-9149(72)90495-X [DOI] [PubMed] [Google Scholar]
- 32.Davies MJ, Bland JM, Hangartner JRW, Angelini A, Thomas AC. Factors influencing the presence or absence of acute coronary artery thrombi in sudden ischaemic death. Eur Heart J. 1989;10(3):203-208. doi: 10.1093/oxfordjournals.eurheartj.a059467 [DOI] [PubMed] [Google Scholar]
- 33.Zhang ZM, Rautaharju PM, Prineas RJ, et al. . Race and sex differences in the incidence and prognostic significance of silent myocardial infarction in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2016;133(22):2141-2148. doi: 10.1161/CIRCULATIONAHA.115.021177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Toukola T, Junttila MJ, Holmström LTA, et al. . Fragmented QRS complex as a predictor of exercise-related sudden cardiac death. J Cardiovasc Electrophysiol. 2018;29(1):55-60. doi: 10.1111/jce.13341 [DOI] [PubMed] [Google Scholar]
- 35.Junttila MJ, Castellanos A, Huikuri HV, Myerburg RJ. Risk markers of sudden cardiac death in standard 12-lead electrocardiograms. Ann Med. 2012;44(7):717-732. doi: 10.3109/07853890.2011.594807 [DOI] [PubMed] [Google Scholar]
- 36.Acharya T, Aspelund T, Jonasson TF, et al. . Association of unrecognized myocardial infarction with long-term outcomes in community-dwelling older adults: the Iceland MI study. JAMA Cardiol. 2018;3(11):1101-1106. doi: 10.1001/jamacardio.2018.3285 [DOI] [PMC free article] [PubMed] [Google Scholar]