Abstract
Rationale: Data are limited regarding the safety of 12-dose once-weekly isoniazid (H, 900 mg) plus rifapentine (P, 900 mg) (3HP) for latent infection treatment during pregnancy.
Objectives: To assess safety and pregnancy outcomes among pregnant women who were inadvertently exposed to study medications in two latent tuberculosis infection trials (PREVENT TB or iAdhere) evaluating 3HP and 9 months of daily isoniazid (H, 300 mg) (9H).
Methods: Data from reproductive-age (15–51 yr) women who received one or more study dose of 3HP or 9H in either trial were analyzed. Drug exposure during pregnancy occurred if the estimated date of conception was on or before the last dose date.
Results: Of 126 pregnancies (125 participants) that occurred during treatment or follow-up, 87 were exposed to study drugs. Among these, fetal loss was reported for 4/31 (13%) and 8/56 (14%), 3HP and 9H, respectively (difference, 13% − 14% = −1%; 95% confidence interval = −17% to +18%) and congenital anomalies in 0/20 and 2/41 (5%) live births, 3HP and 9H, respectively (difference, 0% − 5% = −5%; 95% confidence interval = −18% to +16%). All fetal losses occurred in pregnancies of less than 20 weeks. Of the total 126 pregnancies, fetal loss was reported in 8/54 (15%) and 9/72 (13%), 3HP and 9H, respectively; and congenital anomalies in 1/37 (3%) and 2/56 (4%) live births, 3HP and 9H, respectively. The overall proportion of fetal loss (17/126 [13%]) and anomalies (3/93 [3%]) were similar to those estimated for the United States, 17% and 3%, respectively.
Conclusions: Among reported pregnancies in these two latent tuberculosis infection trials, there was no unexpected fetal loss or congenital anomalies. These data offer some preliminary reassurance to clinicians and patients in circumstances when these drugs and regimens are the best option in pregnancy or in women of child-bearing potential.
This work used the identifying trial registration numbers NCT00023452 and NCT01582711, corresponding to the primary clinical trials PREVENT TB and iAdhere (Tuberculosis Trials Consortium Study 26 and 33).
Keywords: pregnancy outcomes, latent tuberculosis infection treatment, safety assessment
Treatment of latent tuberculosis (TB) infection (LTBI) in high-risk populations is an important strategy for TB prevention and elimination. In 2014, almost half a million women died from TB worldwide (1, 2). Women of reproductive age (15–51 yr) may show higher rates of progression of LTBI to TB compared with men of similar age (3–7). In addition, mortality during pregnancy and in the postpartum period is particularly high in women with concurrent human immunodeficiency virus (HIV) and Mycobacterium tuberculosis disease (8).
The prevalence of LTBI among pregnant women in the United States is not known with certainty, and has been assumed to be similar to the estimated LTBI prevalence in women in the U.S. population (4.4%) (9) (see Table E1 in the online supplement). Estimated LTBI prevalence is substantially higher among foreign-born compared with U.S.-born persons (20.5% vs. 1.5%) (9). Although treatment of LTBI may be a low priority for most pregnant women, some pregnant women are high priority for treatment, such as those with HIV, and contacts of persons with TB. Therefore, knowledge of the safety profiles of available LTBI therapies is of public health importance.
For pregnant women with LTBI who are at high risk of developing active TB, the current recommendation is to initiate, without delay, daily or twice weekly isoniazid for 9 months; for women who are not at high TB risk, the recommendation is to delay LTBI treatment until after delivery to avoid the possibly increased risk of hepatotoxicity during pregnancy and the early postpartum period (10–12). The Tuberculosis Trials Consortium (TBTC) PREVENT TB trial demonstrated that a 12-dose once-weekly regimen of isoniazid (H, 900 mg) plus rifapentine (P, 900 mg) given under direct observation (3HP) was as safe and effective as the standard 9-month self-administered daily isoniazid (H, 300 mg) (9H) regimen (13). The TBTC iAdhere trial demonstrated (in a preplanned subgroup analysis) that treatment completion of self-administered 3HP was noninferior in the United States to treatment completion of 3HP given under directly observed therapy (14). Although pregnancy was an exclusion criterion in these trials due to lack of safety data on rifapentine during pregnancy (15), some participants became pregnant and were inadvertently exposed to study medications. The objective of this analysis was to assess the safety of 3HP and 9H among these pregnant women and their newborns.
Some of the results of this study have been previously reported in the form of an abstract (16).
Methods
The TBTC PREVENT TB trial (TBTC Study 26) was a phase 3, open-label, randomized trial that compared 3HP to 9H for treatment of LTBI; participants were enrolled from the United States/Canada (n = 7,460), Brazil (n = 793), Spain (n = 270), and Peru (n = 65) (13). The TBTC iAdhere trial (TBTC Study 33) was a phase 4, open-label, randomized trial that compared adherence between participants assigned to receive either self-administered or directly observed 3HP; participants were enrolled from the United States (n = 774), Spain (n = 100), South Africa (n = 83), and Hong Kong (n = 45) (14). All participants provided written informed consent for participation in the studies. Institutional review boards at the U.S. Centers for Disease Control and Prevention and at participating clinical sites approved the study protocols.
In both trials, women who were pregnant, planning to become pregnant before completing treatment, or breastfeeding were excluded. A pregnancy test was performed on all women of reproductive potential (as determined locally by site staff) within 14 days before enrollment, and during treatment if pregnancy was suspected. Women of reproductive potential allocated to 3HP were advised to practice a barrier method of birth control (due to the concern of possible interaction of rifapentine and hormonal methods), and were discouraged from getting pregnant due to the unknown safety of 3HP during pregnancy. After pregnancy was diagnosed, treatment was immediately discontinued for women receiving 3HP. They were offered to change treatment to 9H. Participants receiving 9H at the time of diagnosis of pregnancy were given the option to continue treatment. Nonpregnant women of reproductive age were included in this analysis to compare demographic characteristics and adverse events to those seen in pregnant women.
Treatment completion was defined as taking at least 11 of 12 doses within 10–16 weeks for 3HP or at least 240 of 270 doses within 35–52 weeks for 9H. Men, women under 15 or over 51 years of age, persons determined after enrollment to be ineligible, and those who never initiated treatment were excluded from this analysis.
Participants receiving 3HP made clinical evaluation visits at Weeks 4, 8, and 12. Those receiving 9H had nine monthly visits during treatment. Participants in PREVENT TB were followed for 33 months after enrollment. Adverse events, weight, symptoms of TB, and concomitant medications were assessed at each visit. We asked about the following outcomes of pregnancies that occurred during treatment or follow-up after the last dose of study drug: spontaneous abortion, elective abortion, live birth, fetal death, and congenital anomalies, or birth defect in live birth or fetal death products. These outcomes were not defined on the reporting forms. For the purpose of this analysis, we defined spontaneous abortion as fetal loss at less than 20 weeks gestation and stillbirth as fetal loss at or over 20 weeks gestation (17). We accepted any report of congenital anomaly defined by the local investigator. The case report forms requested a “date of onset” and an “estimated date of delivery” (EDD) for reported pregnancy, and “date of outcome” for follow-up reports on pregnancies. Reporting of these dates was inconsistent: site staff variably reported the date when the pregnancy was first diagnosed, the date when the participant had notified the site about the pregnancy, or the first day of the last menstrual period (LMP) before the pregnancy. We reviewed in detail the case report form for each pregnant participant to obtain the date of the LMP (not a required field) as well as any additional information reported in open text fields of the forms. In some cases, we contacted site staff for clarification of notes on the report forms.
To evaluate drug exposure during pregnancy, Estimated Date of Conception (EDC) was derived for each participant following an algorithm, based on information available (EDC, EDD, date of birth [DOB], and/or LMP). We assumed an average pregnancy length of 266 days (18). Women were considered exposed to the study drugs during pregnancy if the EDC was on or before the last study dose date.
The 95% confidence intervals (CIs) of individual proportions (Fisher exact [Clopper-Pearson]) of fetal loss and congenital anomalies for each regimen were calculated. The 95% CIs (with continuity correction) were calculated for the difference of proportions between regimens for fetal loss and congenital anomalies (19, 20). The proportion of fetal loss in this study was compared with the proportion estimated for the United States (21). Only the point estimate for the U.S. proportion of congenital anomalies is provided in Reference 22 (3%).
Results
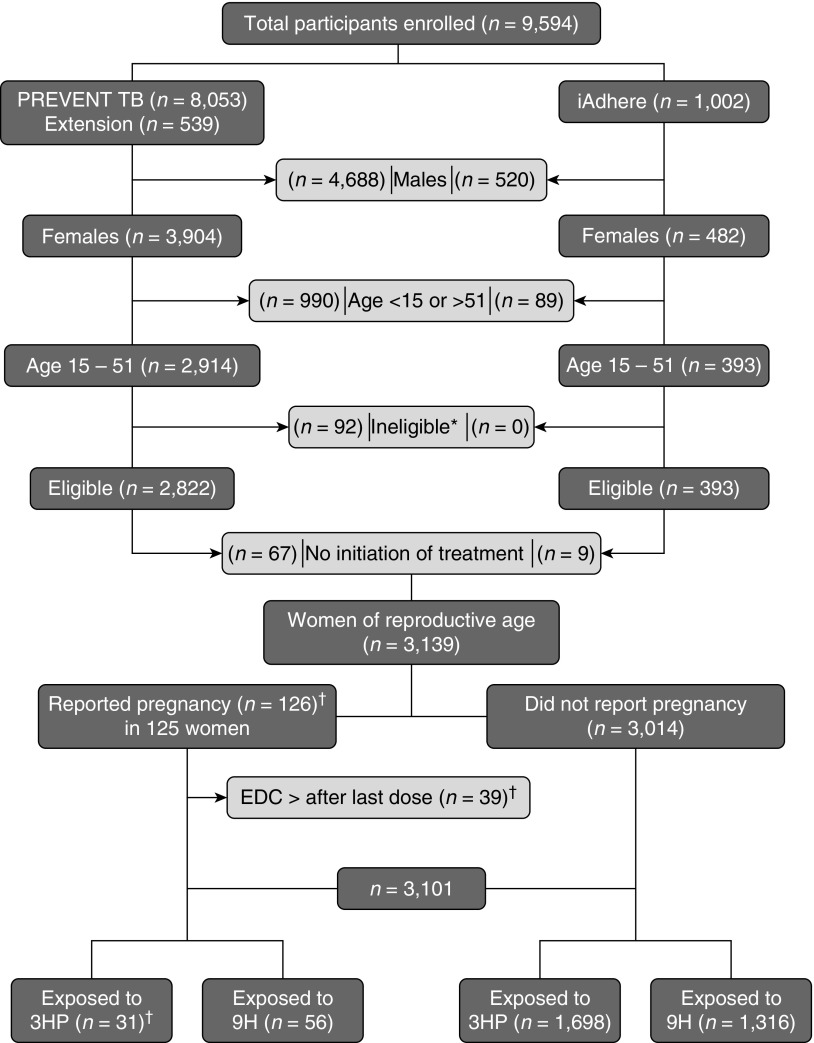
Of 9,594 participants enrolled in both trials, 6,455 were excluded (5,208 males, 1,079 women <15 or >51 yr of age, 92 considered ineligible after enrollment, and 76 participants who did not initiate treatment). Of the remaining 3,139 women of reproductive age, 3,014 women did not report pregnancy (3HP = 1,698 [56%] and 9H = 1,316 [44%]). EDC occurred within 184 days after the last study dose in 39 pregnancies. One woman reported two pregnancies (Figure 1). Thus, 87 pregnancies were judged more likely to have been exposed to study drugs and 39 were deemed less likely to have been exposed (although the fetuses may have had some exposure given the long half-lives of the drugs). Results are reported among those likely to have been exposed and separately among all pregnancies.
Figure 1.
Pregnancy events safety assessment. This figure shows the total number of participants who were enrolled in the PREVENT TB and the iAdhere trials. Certain groups were excluded to select women of reproductive age who were evaluated in this analysis. In addition, pregnancies in which the estimated date of conception occurred after the last study dose were excluded to identify pregnancies that had been exposed to the study drugs. *Reasons for ineligibility: false positive TST (n = 3), lack of sensitivity test for the index case (n = 4), negative tuberculosis culture of the source (n = 31), isoniazid and rifapentine resistant of the source (n = 53), tuberculosis at enrollment (n = 1). †One participant reported 2 pregnancies (EDC occurred before and after the LD for each pregnancy). 3HP = 12-dose once-weekly regimen of isoniazid (900 mg) plus rifapentine (900 mg); 9H = 9-month daily isoniazid (300 mg); EDC = estimated date of conception; LD = last study dose; TST = Tuberculin Skin Test.
A total of 31 (36%) pregnant women were likely exposed to 3HP and 56 (64%) to 9H. Median ages at enrollment were 23 and 25 years for participants receiving 3HP and 9H, respectively. Most demographic characteristics at enrollment between pregnant women exposed to 3HP or 9H, and between pregnant and nonpregnant women, were similar. However, completion of high school was lower among women who became pregnant on either treatment regimen, and unemployment was higher for pregnant women on the 3HP regimen. We found no differences in alcohol intake or cigarette smoking between pregnant and nonpregnant women (Table 1 and Tables E2 and E3).
Table 1.
Demographic characteristics among participants who reported pregnancy and were considered exposed to study drugs
| Characteristic | Regimen 3HP (n = 31) n (%) | Regimen 9H (n = 56) n (%) |
|---|---|---|
| Age, median (IQR) | 23 (21–30) | 25 (21–29) |
| Enrollment site | ||
| Brazil | 2 (6.9) | 1 (1.8) |
| Hong Kong | 0 (0) | 0 (0) |
| Peru | 0 (0) | 0 (0) |
| South Africa | 0 (0) | 0 (0) |
| Spain | 0 (0) | 0 (0) |
| United States/Canada | 29 (93.5) | 55 (98.2) |
| Race | ||
| American Indian/Alaskan Native | 0 (0) | 0 (0) |
| Asian/Pacific Islander | 6 (19.4) | 9 (16.1) |
| Black | 3 (9.7) | 9 (16.1) |
| Other | 1 (3.2) | 1 (1.8) |
| White | 21 (67.7) | 37 (66.1) |
| Alcohol | ||
| No | 19 (61.3) | 33 (58.9) |
| Use | 11 (35.5) | 22 (39.3) |
| Abuse | 1 (3.2) | 1 (1.8) |
| Unknown | 0 (0) | 0 (0) |
| LTBI treatment* | ||
| Contact | 19 (61.3) | 40 (71.4) |
| Fibrosis | 2 (6.5) | 1 (1.8) |
| HIV positive | 0 (0) | 1 (1.8) |
| LTBI (+) | 2 (6.5) | 0 (0) |
| TST converter | 8 (25.8) | 14 (25) |
| Education | ||
| High School | 19 (61.3) | 33 (58.9) |
| <High School | 12 (38.7) | 23 (41.1) |
| Unknown | 0 (0) | 0 (0) |
| Ethnic origin | ||
| Non–United States/Canada | 2 (6.5) | 1 (1.8) |
| United States/Canada: Hispanic | 15 (48.3) | 31 (55.4) |
| United States/Canada: non-Hispanic | 14 (45.2) | 24 (42.9) |
| HIV infection | ||
| No | 20 (64.5) | 29 (51.8) |
| Unknown | 11 (35.5) | 26 (46.4) |
| Yes | 0 (0) | 1 (1.8) |
| IDU history† | ||
| No | 31 (100) | 56 (100) |
| Yes | 0 (0) | 0 (0) |
| Unknown | 0 (0) | 0 (0) |
| Jail | ||
| No | 31 (100) | 56 (100) |
| Yes | 0 (0) | 0 (0) |
| Current smoker | ||
| No | 24 (77.4) | 47 (83.9) |
| Yes | 7 (22.6) | 9 (16.1) |
| Unemployed | ||
| No | 25 (80.6) | 51 (91.1) |
| Yes | 6 (19.4) | 5 (8.9) |
| Homeless | ||
| No | 31 (100) | 56 (100) |
| Yes | 0 (0) | 0 (0) |
Definition of abbreviations: 3HP = 12-dose once-weekly regimen of isoniazid (900 mg) plus rifapentine (900 mg); 9H = 9-month daily isoniazid (300 mg); HIV = human immunodeficiency virus; IDU = intravenous drug use; IQR = interquartile range; LTBI = latent tuberculosis infection; TST = Tuberculin Skin Test.
Subjects were counted only once in the order presented. The total number of HIV-infected persons who were enrolled in the study is listed separately in this table.
Intravenous drug use ever.
We accepted the EDC reported by investigators in 14/87 (16%) pregnancies, because the reported EDD and EDC were consistent with the expected length of pregnancy. For the remaining pregnancies, we estimated EDC based on the algorithm in Table 2. To calculate EDC, 14 days were added to the LMP date in 31/87 (37%), 266 days were subtracted from EDD in 37/87 (43%), 266 days were subtracted from the date the baby was born in 4/87 (5%), and EDC was calculated from the gestational age in 1/87 (1%) (Table 2). All fetal losses occurred in pregnancies under 20 weeks.
Table 2.
Algorithm for the adjustment of the estimated date of conception
| Calculation around EDC as Reported by the Site | LMP Reported | Interpretation | Method for Estimating/Adjusting EDC | 3HP (n = 31) n | 9H (n = 56) n | Total (n = 87) n (%) |
|---|---|---|---|---|---|---|
| EDD(r) − EDC(r) = 266 ± 1 d | Yes or No | EDC reported was consistent | Accepted as reported | 3 | 11 | 14 (16.1) |
| EDC(r) − (LMP + 14) = n ± 55 d | Yes | EDC reported was not consistent, used LMP | EDC estimated as, EDC = LMP + 14 | 13 | 18 | 31 (36.5) |
| 1) EDD(r) − DOB(r) in live birth = n ± 44 d, or | No | EDC reported was not consistent, used EDD | EDC estimated as, EDC = EDD − 266 | 13 | 24 | 37 (42.5) |
| 2) 233 < = EDD(r) − EDC(r) in non-live birth ≤283 | ||||||
| EDD(r) − DOB(r) = n + 48 d | No | EDC reported was not consistent, used reported gestational age at birth | EDC estimated as, EDC = DOB − (gestational age in weeks × 7) | 1 | 0 | 1 (1.1) |
| EDD(r) − DOB(r) = n ≤93 or EDD(r) − EDC(r), n ≥300 | No | EDC reported was not consistent, used DOB | EDC estimated as, DOB − 266 | 1 | 3 | 4 (4.6) |
Definition of abbreviations: 3HP = 12-dose once-weekly regimen of isoniazid (900 mg) plus rifapentine (900 mg); 9H = 9-month daily isoniazid (300 mg); DOB = date of birth; DOB(r) = DOB reported by the site; EDC = estimated date of conception; EDC(r) = EDC reported by the site; EDD = estimated delivery date; EDD(r) = EDD reported by the site (e.g., if a child was born prematurely, the calculation of EDD − 266 is still considered accurate, as EDD was calculated by the clinician at enrollment, most likely using LMP; however, LMP was not reported); LMP = last menstrual period.
Among the 87 pregnancies judged likely to have been exposed to study drugs, fetal loss was reported for 4/31 (13%) and 8/56 (14%) for 3HP and 9H, respectively (difference, 13% − 14% = −1%; 95% CI = −17% to +18%), and congenital anomalies in 0/20 and 2/41 (5%) live births for 3HP and 9H, respectively (difference, 0% − 5% = −5%; 95% CI = −18% to +16%) (Table 3). The median number of days between EDC and fetal loss was 48 and 53 in women who received 3HP and 9H, respectively. The reported infant with Turner syndrome was not part of this group.
Table 3.
Pregnancy outcomes of those considered exposed to study drugs versus all reported pregnancies
| Outcome | Pregnancies Exposed to Study Drugs |
All Reported Pregnancies |
||||
|---|---|---|---|---|---|---|
| 3HP (n = 31) | 9H (n = 56) | Total (n = 87) | 3HP (n = 54) | 9H (n = 72) | Total (n = 126) | |
| Pregnancy outcomes | ||||||
| Live birth, n (%) | 20 (65) | 41 (73) | 61 (70) | 37 (69) | 56 (78) | 93 (74) |
| Elective abortion, n (%) | 7 (23) | 7 (13) | 14 (16) | 9 (17) | 7 (10) | 16 (13) |
| Fetal loss (all are spontaneous abortion <20 wk), n (%) | 4 (13)* | 8 (14)* | 12 (14)† | 8 (15)‡ | 9 (13)‡ | 17 (13)§ |
| Fetal loss, n (%) 95% CI of individual proportion | 4/31 (13) (4–30) | 8/56 (14) (6–26) | 12/87 (14) (7–23) | 8/54 (15) (7–27) | 9/72 (13) (6–22) | 17/126 (13) (8–21) |
| Infant outcomes | ||||||
| Congenital anomalies, n (%)ǁ | 0¶ | 2 (5)¶ | 2 (3)** | 1 (3)†† | 2 (4)†† | 3 (3)‡‡ |
| Congenital anomalies, n (%) 95% CI of individual proportion | 0/20 (0) (0–17) | 2/41 (5) (1–17) | 2/61 (3) (0–11) | 1/37 (3) (0–14) | 2/56 (4) (0–12) | 3/93 (3) (1–9) |
Definition of abbreviations: 3HP = 12-dose once-weekly regimen of isoniazid (900 mg) plus rifapentine (900 mg); 9H = 9-month daily isoniazid (300 mg); CI = confidence interval.
The 95% CI of individual proportions (Fisher Exact [Clopper-Pearson]) of fetal loss and congenital anomalies for each regimen (e.g., number of fetal losses among pregnancies exposed to 3HP [numerator] divided by the number of pregnancies exposed to 3HP [denominator]); 95% CI (with continuity correction) for the difference of proportions between regimens for fetal loss and congenital anomalies (e.g., number of fetal losses among pregnancies exposed to 3HP [numerator] divided by number of pregnancies exposed to 3HP [denominator] minus number of fetal losses among pregnancies exposed to 9H [numerator] divided by number of pregnancies exposed to 9H [denominator]). Proportion of fetal loss in this study compared to the proportion estimated for the United States (e.g., total number of fetal losses [numerator] divided by number of pregnancies exposed to study drugs during the trial [denominator] minus the estimated U.S. proportion of fetal loss [1,118/6,578 (17%)]).
Fetal loss (all are spontaneous abortion <20 wk) among pregnancies exposed to study drugs (n = 87):
Fetal loss in 3HP (4/31 [13%]) and 9H (8/56 [14%]): difference, 13% − 14% = −1%; 95% CI = −17% to +18%.
Fetal loss (12/87 [14%]) and the estimated U.S. rates (1,118/6,578 [17%]) (21): difference, 14% − 17% = −3%; 95% CI = −9% to +6%.
Fetal loss (all are spontaneous abortion <20 wk) among all reported pregnancies (n = 126):
Fetal loss in 3HP (8/54 [15%]) and 9H (9/72 [13%]): difference, 15% − 13% = 2%; 95% CI = −11% to +17%
Fetal loss (17/126 [13%]) and the estimated for United States (1,118/6,578 [17%]) (21): difference, 13% − 17% = −4%; 95% CI = −9% to +4%.
ǁDenominator is number of total births.
Congenital anomalies among pregnancies exposed to study drugs (n = 87):
Congenital anomalies in 3HP (0/20 [0%]) and 9H (2/41 [5%]): difference, 0% − 5% = −5%; 95% CI = −18% to +16%
Congenital anomalies (2/61 [3%]) and the U.S. estimate (3%) (only the point estimate is provided in the reference) (22): difference, 3% − 3% = 0.
One infant with congenital heart anomaly, bilateral cleft lip, and palate (mother’s age = 29 yr, 80 9H doses received); one infant with pyloric stenosis (mother’s age = 26 yr, 26 9H doses received).
Congenital anomalies among all reported pregnancies (n = 126):
Congenital anomalies in 3HP (1/37 [3%]) and 9H (2/56 [4%]): difference, 3% − 4% = −0.9%; 95% CI = −11% to +13%.
Congenital anomalies (3/93 [3%]) and the U.S. estimate (only the point estimate is provided in the reference) (3%) (22): difference, 3% − 3% = 0.
One infant with congenital heart anomaly, bilateral cleft lip and palate (mother’s age = 29, 80 9H doses received); one infant with pyloric stenosis (mother’s age = 26 yr, 26 9H doses received); one Turner syndrome (mother’s age = 35 yr, 12 3HP doses received), conception date was estimated as 53 days after the last study dose.
Among all reported pregnancies (126 [3HP = 54, 9H = 72]), fetal loss was reported for 8/54 (15%) pregnant women taking 3HP, and for 9/72 (13%) taking 9H. Among live births, the frequency of reported congenital anomalies was 1/37 (3%) and 2/56 (4%) for 3HP and 9H, respectively; all three of these were reported from North American sites. The overall proportions of fetal loss (17/126 [13%]) and of anomalies (3/93 [3%]) were similar to those estimated for the United States, 17% and 3%, respectively (Table 3). Among live births, reported congenital anomalies included: one infant with a congenital heart anomaly and bilateral cleft lip and palate whose mother (age 29 yr) had received 80 doses of 9H; one infant with pyloric stenosis whose mother (age 26 yr) had received 26 doses of 9H; and one infant with karyotype-confirmed Turner syndrome whose mother (age 35 yr) had received 12 doses of 3HP (in this participant, the EDC [based on EDD − 266] was 53 days after the last dose of study drug) (Table 4).
Table 4.
Congenital anomalies
| Congenital anomaly | Reg. | EDC | First/Last Dose Date | Preg. Out. Date (Gestational Age) | Doses Received | Mother’s Age | Diagnosis Made by | Days of Drug Exposure Comment |
|---|---|---|---|---|---|---|---|---|
| Congenital heart anomaly, bilateral cleft lip, bilateral cleft palate, jaundice; exposed to study drugs EDC < LD | 9H | March 18, 2005 (by EDD − 266) | April 3, 2005/July 5, 2005 | December 6, 2005 (38 wk) | 80 | 29 | Physician report: | 94 d of drug exposure. No information about the type of heart anomaly. On the July 12, 2006 evaluation, it was reported that lip repair had been done, and that palate repair surgery was pending. |
| 1) Congenital heart anomaly NOS | ||||||||
| 2) Bilateral cleft lip | ||||||||
| 3) Bilateral cleft palate | ||||||||
| 4) Fetal jaundice NOS | ||||||||
| 5) Infant infectious | ||||||||
| Pyloric stenosis; exposed to study drugs EDC < LD | 9H | December 23, 2006 (by EDD − 266) | November 28, 2006/January 3, 2007 | August 6, 2007 (32 wk) | 26 | 26 | Symptoms of pyloric stenosis and abdominal ultrasound | 12 d of drug exposure. Baby boy developed frequent, nonbilious vomiting after discharge from the hospital; abdominal ultrasound: elongation of the pyloric channel up to 2.3 cm in length and circumferential thickening of the pyloric muscle measuring 2.7–4.1 mm; surgery: Fredet-Ramstedt pyloromyotomy. |
| Turner syndrome; not exposed to study drugs EDC > LD | 3HP | April 26, 2002 (by EDD − 266) | December 17, 2001/March 4, 2002 | July 25, 2002 (13 wk) | 12 | 35 | Ultrasound at Weeks 11 and 17: cystic hygroma and possible heart anomaly; amniocentesis karyotype confirmed Turner syndrome. | EDC 53 d after the last study dose. Confirmed diagnosis of Turner syndrome prompted elective medical abortion. Two pregnancies during the study. Diagnosis of hypochromic microcytic anemia on December 10, 2001. Received folic acid before conception. Past medical history of migraines treated with Amerge (naratriptan). |
| Down syndrome not exposed to study drugs EDC > LD | 3HP | October 15, 2007 (by LMP) | July 13, 2007/October 8, 2007 | February 22, 2008 (19 wk) | 12 | 36 | Positive quad test; no chromosomal analysis | EDC 7 d after the last study dose. On February 20, 2008, pregnant woman with morbid obesity was admitted to the hospital with vaginal bleeding and no fetal movement. Spontaneous abortion occurred 2 d later. Placenta was notable for extensive acute chorioamnionitis. Fetus had cystic hygroma at the neck with hydrops, and ears abnormally set. |
Definition of abbreviations: 3HP = 12-dose once-weekly regimen of isoniazid (900 mg) plus rifapentine (900 mg); 9H = 9-month daily isoniazid (300 mg); EDC = estimated date of conception; EDD = estimated date of delivery; LD = last study dose; LMP = last menstrual period; NOS = not otherwise specified; Preg. Out. = pregnancy outcome date; Reg. = regimen.
Rates: 1) cleft lip/palate and congenital anomaly, 1/940 live births (Division of Birth Defects and Developmental Disabilities, National Center on Birth Defects and Developmental Disabilities [NCBDDD], U.S. Center for Disease Control and Prevention [CDC; https://www.cdc.gov/ncbddd/birthdefects/data.html]); 2) pyloric stenosis, 0.18–30.70 per 10,000 live births (U.S. Department of Health and Human Services Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research: evaluating the risk of drug exposure in human pregnancies, April 2005). 3) Turner syndrome, 1/2,500 live births (http://www.vivo.colostate.edu/hbooks/genetics/medgen/chromo_eg/turners.html); 4) Down syndrome, 1/691 live births (Division of Birth Defects and Developmental Disabilities, NCBDDD, CDC (https://www.cdc.gov/ncbddd/birthdefects/data.html). EDC < LD: EDC preceded the last study dose; EDC > LD: EDC followed the last study dose.
We received no reports of maternal death, fetal death, or neonatal/postneonatal death. One suspected case of Down syndrome was reported in a pregnancy ending in spontaneous abortion. The mother (age 36 yr) had received 12 doses of 3HP; her EDC (based on the LMP) was 7 days after the last dose of study drug. Down syndrome was suspected based on quadruple screen and cystic hygroma, but chromosomal analysis was not done, preventing confirmation of Down syndrome. We do not know if any of the remaining 16 fetal losses were assessed for anomalies.
Among the pregnancies judged likely exposed to study drugs, in 9/31 (29%) and 14/56 (25%) on 3HP and 9H, respectively, the pregnancy was discovered after they had completed treatment. None of the 22 women who became pregnant before completing 3HP changed therapy to 9H. The median lengths of study drug exposure during pregnancy were 20 and 33 days for women taking 3HP and 9H, respectively. Discontinuation of treatment because of an adverse event, after becoming pregnant, was reported for one woman receiving 9H (fatigue and weakness after 60 doses of 9H). Another participant on 9H developed hepatotoxicity. No additional adverse events were reported for pregnant women receiving 3HP (Table 5 and Table E4).
Table 5.
Pregnancy safety assessment during exposure with 3HP or 9H treatment
| 3HP Reported Pregnancy (n = 31)* | 9H Reported Pregnancy (n = 56)* | |
|---|---|---|
| Duration of study drug exposure, d | 718 | 2,297 |
| Median (IQR) | 20 (1–80) | 32.5 (1–242) |
| Study drug doses received, median (range) | 8 (4–12) | 160 (76–241) |
| Completed study treatment, n (%) | 9 (29) | 14 (25) |
| Pregnancy outcome after last study dose | 8† | 7‡ |
| Participants who reported an adverse event, n (%) | 0 (0) | 7 (12.5) |
| Discontinued due to an adverse event, n (%) | 0 (0) | 1 (1.8) |
| Toxicity grade, n (%)§ | ||
| Grade 1 or 2 | 0 (0) | 6 (10.7) |
| Grade 3 or 4 | 0 (0) | 1 (1.8) |
| Grade 5 (death) | 0 (0) | 0 (0) |
| Adverse event classification, n (%)ǁ | ||
| Drug-related adverse event | ||
| Hepatotoxicity only | 0 (0) | 1 (1.8) |
| Systemic drug reaction | 0 (0) | 0 (0) |
| Rash only | 0 (0) | 0 (0) |
| Other drug-related adverse event | 0 (0) | 3 (5.4) |
| Non–drug-related adverse event | 0 (0) | 3 (5.4) |
Definition of abbreviations: 3HP = 12-dose once-weekly regimen of isoniazid (900 mg) plus rifapentine (900 mg); 9H = 9-month daily isoniazid (300 mg); IQR = interquartile range.
Adverse event other than pregnancy;
Women were considered exposed to the study drug(s) during pregnancy if they took at least one study dose and if the estimated date of conception was on or before the last study dose date.
Live birth (6), elective abortion (2).
Live birth (6), fetal loss (1).
For participants reporting multiple adverse events with same toxicity, the earliest adverse event is reported.
ǁParticipants with multiple adverse events can be present in more than one grade of toxicities and in more than one adverse event classification.
Discussion
In this study of pregnancies inadvertently exposed to 3HP or 9H, there were no unexpected patterns of fetal loss or congenital anomalies. Women who became pregnant while taking 3HP or 9H for LTBI treatment in the PREVENT TB or the iAdhere trial experienced fetal loss in 13%–14% of pregnancies exposed to each regimen. This is comparable to the U.S. background in the general pregnant population. In 2008, fetal losses (all gestational ages) reported by 45 states among 6,578,000 pregnancies in women through age 44 years was 17% (21). The rate of spontaneous abortion ranged from 14% to 23% among 3,269 pregnancies reported by 1,572 women (age 15–44 yr) in New York and Vermont between 1980 and 1990 (23). In Finland, among 3,000 women aged 18–44 years, spontaneous abortion ranged from 12% to 21% (24), whereas, in the United Kingdom, it was reported as 12% for all ages (25, 26).
The proportion of reported defects among the 93 live births was comparable to U.S. estimates (3%) (22), and not statistically significantly different by regimen. The prevalence of defects among infants and fetuses from 1968 to 2003 in the five counties of metropolitan Atlanta was 2.7% (22, 27). Diagnosis was confirmed in three of the four congenital anomalies reported in this study. The suspected case of Down syndrome (suspected based on a positive quadruple screen and presence of cystic hygroma) was not confirmed by karyotype. The quadruple screen, which measures α-fetoprotein, unconjugated estriol, human chorionic gonadotropin, and inhibin A in maternal serum, has a 5% false-positive rate and a detection rate of 80% (28). Cystic hygroma is more frequently associated with Turner syndrome than with Down syndrome, and can also occur in the absence of any chromosomal anomaly (29). It has been suggested that environmental factors, including drug exposure, could affect maternal oocytes before pregnancy, perhaps causing chromosome nondisjunction that could lead to Down or Turner syndrome (30). Thus, prepregnancy drug exposures might affect pregnancy outcomes.
The U.S. Food and Drug Administration has released the Pregnancy and Lactation Labeling Final Rule (PLLR) to replace the former pregnancy risk categories based on letters (A, B, C, D, and X), with the intention of providing more information to patients and health care providers. Under PLLR labeling, pregnancy risk information is summarized under the subtitles of: pregnancy, lactation, and female and male reproductive potential (31).
Presently, isoniazid and rifapentine remain classified as risk category C, as their labels have not yet been updated under the PLLR. Category C means that animal studies have shown adverse effects on the fetus, but data are not available in humans. In addition, the human response to drugs cannot always be predicted reliably from animal studies. It has been recommended that category C drugs be used in pregnancy only if the benefit justifies the potential risk (32). Treatment of active TB in a pregnant woman has been shown to be beneficial for the mother and the baby by decreasing the risk for low birth weight, preterm labor, pre-eclampsia, and early fetal death (33). However, it is not clear whether LTBI should be treated during pregnancy. The available data do not consistently suggest that pregnancy increases the risk of progression to active TB (34–39). Even if pregnancy alone does not increase the risk of TB, some women are at increased risk of progression, and may benefit from LTBI treatment (e.g., those with HIV coinfection or recently infected with M. tuberculosis). Isoniazid has not been found to be associated with congenital anomalies, even if it is given early in pregnancy, making 6–9 months of daily isoniazid the recommended treatment for pregnant women at risk of developing TB (10). We found no well-controlled studies of pregnant women exposed to rifapentine. We are not aware of any reports of human birth defects associated with the use of rifapentine; embryo-fetal toxicities have been reported in rats and rabbits (40). Clinical hypoprothrombinemia manifested by postpartum and infant bleeding due to exposure to rifampin during the last weeks of pregnancy has been reported in humans (33). A case report described a limb anomaly in the child of a mother with no other risk factors who received isoniazid and rifampin during the first 2 months of pregnancy for treatment of TB (41). Other congenital anomalies, such as hydrocephalus, anencephaly, and limb defects, have been inconsistently reported with the use of rifampin (42, 43).
Our study provides important information on exposure to rifapentine early in pregnancy. The results of this study will provide clinicians comfort while counseling pregnant women who may have been exposed to 3HP. Additional safety data will come from the IMPAACT 2001 trial. This ongoing study aims to enroll 82 HIV-infected and HIV-uninfected pregnant (second and third trimesters) and postpartum women with latent TB to evaluate the pharmacokinetics, tolerability, and safety of 3HP in these participants (44). Inclusion of pregnant women in clinical trials would provide good evidence to develop standards for prevention and treatment of TB during pregnancy (45).
In 29% of 31 pregnant women exposed to 3HP in these two studies, pregnancy was diagnosed after completion of treatment, and no adverse events were reported among this group. For seven of the nine pregnant women who completed 3HP treatment, the pregnancy outcome occurred after the last study dose (live birth [n = 5]; fetal loss [n = 2]). The only event of hepatotoxicity occurred in a woman exposed to 9H. Franks and colleagues (12) reported five cases of hepatitis attributed to isoniazid among 3,681 pregnant women who received LTBI treatment during and soon after delivery, of whom 2 died; they reported a 2.5-times increase in the risk of isoniazid hepatitis among pregnant and postpartum Hispanic women compared with nonpregnant women.
Contradictory beliefs about the effect of pregnancy on the progression of TB have been present for centuries. In the 19th century, it was thought that tuberculous cavities could be compressed by an enlarged uterus for therapeutic benefit; a century later, induced abortion was commonly practiced to treat TB among pregnant women (38). More recently, studies have evaluated the risk of TB reactivation in pregnancy with inconsistent findings. A case–control study in the Dominican Republic found no association between pregnancy and the risk of developing active TB among HIV-infected and noninfected women (34). A study of 177 pregnant women with TB in the United Kingdom found that the rate of TB during pregnancy and postpartum was significantly higher than in nonpregnant women; after adjusting for confounding factors, the risk of TB was statistically higher during the 180-day postpartum period, but not during pregnancy (39). It has been suggested that immunologic changes during pregnancy, such as suppression of the T helper 1 response and cytokines (IL-12, IFN-ϒ), increase the risk of reactivation of endogenous M. tuberculosis and development of TB (46). Older immunological models hypothesized that the maternal immune system controlled responses to the developing fetus and external micro-organisms. Recent studies support an integrational model, where the immune responses of the placenta, the fetus, and the mother are integrated and coordinated. This coordinated response model suggests that there is not an increased susceptibility to infectious diseases during pregnancy (47).
Although our sample size is small, ours is the only report to date of women who became pregnant while exposed to 3HP treatment for LTBI. Our study is limited by evaluation of safety outcomes in this subgroup not being a primary study aim of either trial, and pregnant women were excluded from enrollment in both trials. Because both trials were open label, participants receiving the weekly 3HP regimen under observation therapy may have received more careful monitoring or more frequent counseling about avoiding pregnancy during treatment. This may partly explain the lower frequency of pregnancy in the 3HP (31/1,729) compared with the 9H group (56/1,372). In addition, the longer duration of 9H treatment contributes to the higher frequency of pregnancy in this group. We have no information on many potential confounders, such as reproductive history, medical comorbidities, or drug use. The two trials did not capture some important pregnancy-related outcomes, such as intrauterine growth restriction or pre-eclampsia. The length of drug exposure may have been over- or underestimated based on the inconsistent reporting of date of conception. We tried to estimate the date of conception in cases where LMP had not been reported. A minor limitation is that our definition of drug exposure does not account for the different half-lives of the study drugs (2–5 h for isoniazid and 12–15 h for rifapentine) (40). In terms of drug exposure, it is important to note that participants on directly observed therapy probably ingested optimal dosing of 3HP, which may be different from participants who self-administered 3HP. Another important limitation is that comparisons between proportions of pregnancy outcomes in this study and those reported for the United States have not been adjusted for differences in the demographics of female participants in this study from those of the U.S. female population (for example, our study population was 59% white, 24% black, and 13% Asian; the U.S. female population aged 15–44 yr in 2010 was 77% white, 15% black, and 6% Asian) (48).
In conclusion, these data offer some preliminary reassurance to clinicians and patients in circumstances in which these drugs and regimens are the best option in pregnancy or in women of child-bearing potential. More definitive safety data will come from systematic and prospective efforts to collect information regarding pregnancy outcomes among women treated with TB preventive therapy in clinical practice, and by including pregnant women in clinical trials whenever possible, safe, and appropriate.
Supplementary Material
Acknowledgments
Acknowledgment
The study investigators and coordinators gratefully acknowledge all study participants. Carla Winston, Ph.D., M.A., Stuart K. Shapira, M.D., Ph.D., and Lawrence Barker, Ph.D., provided helpful comments during the clearance process, and Michael Chen, Ph.D., provided statistical advice.
Footnotes
Supported by the U.S. Centers for Disease Control and Prevention (CDC), Division of Tuberculosis Elimination, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Sanofi (Sanofi-Aventis [Paris, France, and Bridgewater, NJ]) donated the rifapentine used in this study; Sanofi has cumulatively donated more than $2.9 million since 2007 to the CDC Foundation to supplement available U.S. federal funding for rifapentine research; R.N.M. and N.A.S. were employed by the CDC Foundation at the time this analysis was conducted.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Sanofi did not participate in the collection, analysis, or interpretation of the data, in drafting of the manuscript, or in the decision to submit the manuscript for publication.
Author Contributions: Conception or design of the work—R.N.M. and T.R.S.; statistical analysis— R.N.M., N.A.S., and A.V.; interpretation of data and critical review—R.N.M., N.A.S., A.V., N.K.T., S.V.G., K.S., C.-C.L., N.W.S., R.W.B., R.E.C., M.N., E.S.M., M.L., J.S., M.E.V., and T.R.S.; drafting of the manuscript—R.N.M., A.V., N.K.T., S.V.G., and T.R.S.; accountability for all aspects of the work—R.N.M., A.V., S.V.G., and T.R.S.; R.N.M. and N.A.S. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention . Controlling tuberculosis in the United States: recommendations from the American Thoracic Society, CDC, and the Infectious Diseases Society of America. Atlanta: U.S. Department of Health and Human Services; 2005MMWR 2005;54(No. RR-12):1–69 [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2015. Geneva: World Health Organization; 2015. WHO/HTM/TB/2015 22. [Google Scholar]
- 3.Crampin AC, Glynn JR, Floyd S, Malema SS, Mwinuka VK, Ngwira BM, et al. Tuberculosis and gender: exploring the patterns in a case control study in Malawi. Int J Tuberc Lung Dis. 2004;8:194–203. [PubMed] [Google Scholar]
- 4.Rhines AS. The role of sex differences in the prevalence and transmission of tuberculosis. Tuberculosis (Edinb) 2013;93:104–107. doi: 10.1016/j.tube.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Holmes CB, Hausler H, Nunn P. A review of sex differences in the epidemiology of tuberculosis. Int J Tuberc Lung Dis. 1998;2:96–104. [PubMed] [Google Scholar]
- 6.Hudelson P. Gender differentials in tuberculosis: the role of socio-economic and cultural factors. Tuber Lung Dis. 1996;77:391–400. doi: 10.1016/s0962-8479(96)90110-0. [DOI] [PubMed] [Google Scholar]
- 7.Kochi A. Tuberculosis: distribution, risk factors, mortality. Immunobiology. 1994;191:325–336. doi: 10.1016/S0171-2985(11)80437-7. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A, Nayak U, Ram M, Bhosale R, Patil S, Basavraj A, et al. Byramjee Jeejeebhoy Medical College-Johns Hopkins University Study Group. Postpartum tuberculosis incidence and mortality among HIV-infected women and their infants in Pune, India, 2002–2005. Clin Infect Dis. 2007;45:241–249. doi: 10.1086/518974. [DOI] [PubMed] [Google Scholar]
- 9.Miramontes R, Hill AN, Yelk Woodruff RS, Lambert LA, Navin TR, Castro KG, et al. Tuberculosis infection in the United States: prevalence estimates from the National Health and Nutrition Examination Survey, 2011–2012. PLoS One. 2015;10 doi: 10.1371/journal.pone.0140881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control & Prevention Targeted tuberculin testing and treatment of latent tuberculosis infection. Atlanta: U.S. Department of Health and Human Services; 2000MMWR 2000;49(No. RR-6):1–54 [Google Scholar]
- 11.Centers for Disease Control & Prevention Latent tuberculosis infection: a guide for primary health care providers. Atlanta, GA: National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention; 2013. 1–40 [Google Scholar]
- 12.Franks AL, Binkin NJ, Snider DE, Jr, Rokaw WM, Becker S. Isoniazid hepatitis among pregnant and postpartum Hispanic patients. Public Health Rep. 1989;104:151–155. [PMC free article] [PubMed] [Google Scholar]
- 13.Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, et al. TB Trials Consortium PREVENT TB Study Team. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–2166. doi: 10.1056/NEJMoa1104875. [DOI] [PubMed] [Google Scholar]
- 14.Belknap R, Holland D, Feng PJ, Millet JP, Caylà JA, Martinson NA, et al. TB Trials Consortium iAdhere Study Team. Self-administered versus directly observed once-weekly isoniazid and rifapentine treatment of latent tuberculosis infection: a randomized trial. Ann Intern Med. 2017;167:689–697. doi: 10.7326/M17-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanofi. PRIFTIN (rifapentine) package insert. Bridgewater, New Jersey: Sanofi-Aventis U.S. LLC; 2017 Aug [revised 2017 Aug; accessed 2018 Mar 3]. Available from: http://products.sanofi.us/priftin/priftin.pdf.
- 16.Moro RN, Scott N, Vernon A, Goldberg S, Schwartzman K, Narita M, et al. Pregnancy safety assessment of 3 months of once-weekly rifapentine and isoniazid and 9 months of daily isoniazid: a post-hoc analysis of the PREVENT TB and the iAdhere trials [abstract] Am J Respir Crit Care Med. 2016;193:A7859. [Google Scholar]
- 17.Centers for Disease Control and Prevention. National Vital Statistics System. Atlanta, GA: U.S. Department of Health & Human Services; 2016 Dec 22 [updated 2016 Dec 22; accessed 2018 Mar 2]. Available from: https://www.cdc.gov/nchs/nvss/fetal_death.htm.
- 18.Cunningham L, Bloom S, Dashe H, Casey S. Chapter 7: Embryogenesis and fetal morphological development. In: Hill M, editor. Williams obstetrics, 24th edition. New York: McGraw-Hill Education; 2014. [Google Scholar]
- 19.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 20.Lowry R. The confidence interval for the difference between two independent proportions, 2001–2018. [Accessed 2018 Mar 2]. Available from: http://vassarstats.net/prop2_ind.html.
- 21.Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep. 2012;60:1–21. [PubMed] [Google Scholar]
- 22.U.S. Centers for Disease Control and Prevention. Update on overall prevalence of major birth defects, Atlanta, Georgia, 1978–2005. Atlanta: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 23.Gray RH, Wu LY. Subfertility and risk of spontaneous abortion. Am J Public Health. 2000;90:1452–1454. doi: 10.2105/ajph.90.9.1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemminki E, Forssas E. Epidemiology of miscarriage and its relation to other reproductive events in Finland. Am J Obstet Gynecol. 1999;181:396–401. doi: 10.1016/s0002-9378(99)70568-5. [DOI] [PubMed] [Google Scholar]
- 25.Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. BMJ. 1989;299:541–545. doi: 10.1136/bmj.299.6698.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black S, Eskola J, Siegrist CA, Halsey N, Macdonald N, Law B, et al. Importance of background rates of disease in assessment of vaccine safety during mass immunisation with pandemic H1N1 influenza vaccines. Lancet. 2009;374:2115–2122. doi: 10.1016/S0140-6736(09)61877-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Correa A, Cragan JD, Kucik J, Alverson CJ, Gilboa SM, Balakrishnan R, et al. Metropolitan Atlanta Congenital Defects Program 40th Anniversary Edition Surveillance Report: reporting birth defects surveillance data 1968–2003. Birth Defects Res A Clin Mol Teratol. 2007;79:65–186. doi: 10.1002/bdra.20350. [DOI] [PubMed] [Google Scholar]
- 28.Wald NJ, Kennard A, Hackshaw A, McGuire A. Antenatal screening for Down’s syndrome. J Med Screen. 1997;4:181–246. doi: 10.1177/096914139700400402. [DOI] [PubMed] [Google Scholar]
- 29.Sanhal CY, Mendilcioglu I, Ozekinci M, Yakut S, Merdun Z, Simsek M, et al. Prenatal management, pregnancy and pediatric outcomes in fetuses with septated cystic hygroma. Braz J Med Biol Res. 2014;47:799–803. doi: 10.1590/1414-431X20143895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman SL, Freeman SB, Allen EG, Lamb NE. Risk factors for nondisjunction of trisomy 21. Cytogenet Genome Res. 2005;111:273–280. doi: 10.1159/000086900. [DOI] [PubMed] [Google Scholar]
- 31.Food and Drug Administration, HHS. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling: final rule. Fed Regist. 2014;79:72063–72103. [PubMed] [Google Scholar]
- 32.National Institute of Health. U.S. Department of Health and Human Services. 2017 Mar [updated 2018 Mar 3; accessed 2018 Mar 3]. Available from: https://aidsinfo.nih.gov/drugs/123/isoniazid/0/professional.
- 33.Bothamley G. Drug treatment for tuberculosis during pregnancy: safety considerations. Drug Saf. 2001;24:553–565. doi: 10.2165/00002018-200124070-00006. [DOI] [PubMed] [Google Scholar]
- 34.Espinal MA, Reingold AL, Lavandera M. Effect of pregnancy on the risk of developing active tuberculosis. J Infect Dis. 1996;173:488–491. doi: 10.1093/infdis/173.2.488. [DOI] [PubMed] [Google Scholar]
- 35.Malhamé I, Cormier M, Sugarman J, Schwartzman K. Latent tuberculosis in pregnancy: a systematic review. PLoS One. 2016;11:e0154825. doi: 10.1371/journal.pone.0154825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis. 2012;55:1532–1549. doi: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz N, Wagner SA, Keeler SM, Mierlak J, Seubert DE, Caughey AB. Universal tuberculosis screening in pregnancy. Am J Perinatol. 2009;26:447–451. doi: 10.1055/s-0029-1214244. [DOI] [PubMed] [Google Scholar]
- 38.Snider D. Pregnancy and tuberculosis. Chest. 1984;86(3 suppl):10S–13S. doi: 10.1378/chest.86.3.10s. [DOI] [PubMed] [Google Scholar]
- 39.Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: a national, primary care–based cohort and self-controlled case series study. Am J Respir Crit Care Med. 2012;185:779–784. doi: 10.1164/rccm.201106-1083OC. [DOI] [PubMed] [Google Scholar]
- 40. Sanofi. PRIFTIN (rifapentine) package insert. Bridgewater, New Jersey: Sanofi-Aventis U.S. LLC; 2010 May 11 [revised 2010 May; accessed 2018 Mar 3]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021024s009lbl.pdf.
- 41.Kalayci T, Erener-Ercan T, Buyukkale G, Cetinkaya M. Limb deformity in a newborn. Is rifampicin just an innocent bystander? Eur Rev Med Pharmacol Sci. 2015;19:517–519. [PubMed] [Google Scholar]
- 42.Snider DE, Jr, Layde PM, Johnson MW, Lyle MA. Treatment of tuberculosis during pregnancy. Am Rev Respir Dis. 1980;122:65–79. doi: 10.1164/arrd.1980.122.1.65. [DOI] [PubMed] [Google Scholar]
- 43.Steen JS, Stainton-Ellis DM. Rifampicin in pregnancy. Lancet. 1977;2:604–605. doi: 10.1016/s0140-6736(77)91447-7. [DOI] [PubMed] [Google Scholar]
- 44.National Institute of Health. Clinical Trials web page. 2016 Jan 8 [updated 2017 Oct 3; accessed 2018 Mar 3]. Available from: https://clinicaltrials.gov/show/nct02651259.
- 45.Gupta A, Mathad JS, Abdel-Rahman SM, Albano JD, Botgros R, Brown V, et al. Toward earlier inclusion of pregnant and postpartum women in tuberculosis drug trials: consensus statements from an international expert panel. Clin Infect Dis. 2016;62:761–769. doi: 10.1093/cid/civ991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45:1192–1199. doi: 10.1086/522182. [DOI] [PubMed] [Google Scholar]
- 47.Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. National Center for Health Statistics. 2015 Nov 6 [updated 2014 Mar 28; accessed 2018 Mar 3]. Available from: http://www.cdc.gov/nchs/hus/contents2012.htm#001.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.