Abstract
Background:
Wildland firefighters conducting prescribed burns are exposed to a complex mixture of pollutants, requiring an integrated measure of exposure.
Objective:
We used urinary mutagenicity to assess if systemic exposure to mutagens are higher in firefighters after working at prescribed burns versus after non-burn work days. Other biomarkers of exposure and oxidative stress markers were also measured.
Methods:
Using a repeated measures study design, we collected urine before, immediately after, and the morning after a work shift on prescribed burn and non-burn work days from 12 healthy subjects. Urines were analyzed for malondialdehyde (MDA), 8-isoprostane, 1-hydroxypyrene (OH-Pyrene), and mutagenicity in Salmonella YG1041 +S9. Particulate matter (PM2.5) and carbon monoxide (CO) measurements were collected by personal monitoring. Light-absorbing carbon (LAC) of PM2.5 was measured as a surrogate for black carbon exposure. Linear mixed-effect models were used to assess cross-work shift (pre- to post-work shift) changes in urinary biomarkers.
Results:
No significant differences occurred in creatinine-adjusted urinary mutagenicity across the work shift between burn days (48 samples) and non-burn day (21 samples). Firefighters lighting fires had a non-significant, 1.6-fold increase in urinary mutagenicity for burn- versus non-burn day exposures. Positive associations were found between cross-work shift (pre- to post-) changes in creatinine-adjusted urinary mutagenicity and MDA (p = 0.0010), OH-Pyrene (p = 0.0001), and mass absorption efficiency, which is the LAC/PM2.5 ratio (p = 0.2245), respectively. No significant effect of day type or work task on cross-work shift (pre- to post-) changes in MDA or 8-isoprostane was observed.
Conclusion:
Urinary mutagenicity may serve as a suitable measure of occupational smoke exposures among wildland firefighters, especially among those lighting fires for prescribed burns.
INTRODUCTION
Extensive studies show that smoke from wildland fires is associated with increased respiratory and cardiovascular disease among the general public (Adetona O et al. 2016; Reid et al. 2016; Black et al. 2017; Cascio 2018). Wood smoke alone is composed of hundreds of constituents, many of which are mutagenic and carcinogenic (Naeher et al. 2007). There has been only one study of the mutagenicity of smoke from a wildland fire, and organic extracts of particulate matter <10 μm in diameter (PM10) in which such fires in the Brazilian Amazon were found to be highly mutagenic in aSalmonella mutagenicity assay (de Oliveira Galvão et al. 2018). In addition, the International Agency for Research on Cancer (IARC) has classified the household combustion of biomass (primarily wood) as a probable (Group 2A) human carcinogen (IARC 2010).
Prescribed burning is a special category of wildland firefighting that involves the preplanned lighting of fires under the direction of certified management, usually for the prevention of wildland fires or to inhibit the advance of such fires, and these fires also can produce environmental and health impacts (Haikerwal et al. 2015; Adetona O et al. 2016). Individuals initiating prescribed burns may experience exposures in addition to wood smoke, such as emissions from drip-torches fueled by a mixture of diesel and gasoline (Adetona O et al. 2016). Diesel and gasoline emissions are also mutagenic and are classified, respectively, as known (Group 1) or possible (Group 2B) human carcinogens (IARC 2014). Although protective clothing is worn, wildland firefighters are not protected from inhalation exposures and typically do not wear protective masks, unlike municipal firefighters who use self-contained breathing apparatuses (SCBAs) with supplied fresh air.
Many studies document the elevated exposure of wildland firefighters to potential mutagens based on concentrations of hydroxylated urinary metabolites of polycyclic aromatic hydrocarbons (OH-PAHs) (Adetona O et al. 2016; Keir et al. 2017). Studies involving charcoal workers or people using wood-fired steam baths (temazcales) have also found that exposure to such wood smoke results in mutagenic urine, indicative of a systemic exposure to mutagenic and potentially carcinogenic compounds (Kato et al. 2004a; Long et al. 2014). Although there is a recent report showing elevated urinary mutagenicity in municipal firefighters (Keir et al. 2017), no such study is reported for wildland firefighters. The purpose of this pilot study was to evaluate systemic genotoxicity in wildland firefighters exposed to smoke from wood and/or diesel/gasoline fuel exhaust before and after a prescribed burn through the assessment of urinary mutagenicity in theSalmonella (Ames) mutagenicity assay.
Urinary mutagenicity provides an integrated measure of exposure to complex mixtures of genotoxic pollutants (Černá et al. 1997). Previous studies have found that urinary mutagenicity correlates with other biomarkers in subjects exposed to wood smoke as well as a variety of other pollutants, including benzidine dyes, cigarette smoke, heterocyclic amines, and nitrotoluenes (Černá et al. 1997; DeMarini et al. 1997; Kato et al. 2004b; Sabbioni et al. 2006; Shaughnessy et al. 2011; Long et al. 2014). Therefore, we explored the relationship between urinary mutagenicity, a variety of personal occupational exposure measurements, and urinary OH-PAHs among wildland firefighters at prescribed burns. Because air pollution exposure may lead to increased body burden of oxidative stress, (IARC 2016) we also measured urinary concentrations of two common biomarkers of oxidative stress, malondialdehyde (MDA) and 8-isoprostane, and we explored the relationships between each of these biomarkers and urinary mutagenicity.
We hypothesized that urinary mutagenicity would be significantly higher among firefighters after working at prescribed burns (post-work shift levels) compared to pre-work shift levels and compared to non-burn work days. We also hypothesized that work task would impact urinary mutagenicity, with firefighters lighting fires with diesel-gasoline-fueled drip-torches having higher urinary mutagenic potency compared to those performing other work tasks.
METHODS
Study Population and Design
Twelve healthy subjects (ten wildland firefighters and two work-certified volunteers) with the United States Department of Agriculture Forest Service at Savannah River Site (USFS-Savannah River), South Carolina, were monitored for occupational exposures during their work shifts at prescribed burns and on working days when prescribed burns were not conducted during January-July of 2015. Before and after-work-shift, spot urine samples were collected each sampling day. This study was approved by the University of Georgia Institutional Review Board, and written informed consent was obtained from each subject before their voluntary participation.
Baseline questionnaires and daily work activity questionnaires were administered to subjects to gain information on personal work history, length of firefighter career, health habits (i.e. exercise frequency, tobacco use), disease history, medication, diet (i.e. grilled-foods), daily work tasks, and other factors that could be considered influences on exposure and/or on mutagenic responses.
Subjects’ self-reported work tasks were categorized into four major categories, two of which were on burn days (“Holding” and “Lighting”), and two on non-burn work days (“Non-burn day—Exposures” and “Non-burn day—Office”). On burn days, work tasks included holding prescribed fire-lines (subjects were referred to as “Holders”) where firefighters used fire engine vehicles to patrol and contain fires from escaping established boundaries, and lighting (herein subjects were referred to as “Lighters”) frequently by hand using a drip-torch or less often by aerial methods (i.e. helicopter). Subjects also conducted various tasks on non-burn workdays, which included patrolling of areas where recent burns were conducted, field prep work, engine maintenance, etc. These tasks were all classified as “Non-burn day—Exposures” because subjects reported experiencing occupational exposures to vehicle exhaust, diesel, dust, or possible exposures to smoke from smoldering when patrolling areas where recent burns were conducted. Non-burn workdays also included subjects performing office work. An assigned primary work task was determined by reported time spent during a specific work task, i.e., the subject had spent more than 50% of the duration of a work-shift conducting the task.
Urine Sample Collection
Subjects were instructed on how to properly collect their urine samples in sterile polypropylene 4.5-ounce (~133 ml) cups. Spot urine samples were collected immediately before a work shift, immediately after a work shift, and the morning-after a work shift. Samples were frozen at −5°C immediately after collection and transported on dry ice to a −80°C freezer at the University of Georgia, Department of Environmental Health Science. The samples were thawed once to room temperature to prepare randomized aliquots into sterile, polypropylene, conical 50-ml tubes and again stored at −80°C until further analyses. All urine samples were analyzed in a blinded fashion.
Occupational Exposure Assessment
Gravimetric particulate matter with an aerodynamic diameter of ≤ 2.5 μm (PM2.5) and carbon monoxide (CO) were measured in the breathing zone of the subjects on burn and non-burn work days. The subjects did not use respiratory protection during the study. A detailed description of the sample collection methods and gravimetric analyses were reported previously (Adetona AM 2016) In brief, all exposure monitoring instruments were calibrated prior to use, and gravimetric analyses followed the United States Environmental Protection Agency (U.S. EPA) specifications (U.S. Environmental Protection Agency. Field Standard Operating Procedures for the PM2.5 Performance Evaluation Program 2006). PM2.5 samples were collected using MicroPEM™ (RTI International, Research Triangle Park, NC, USA) loaded with 25-mm polytetrafluoroethylene membrane filters (porosity: 3.0 μm) (Pall Life Sciences, Ann Arbor, MI, USA). Filters were weighed using the Cahn C-35 microbalance (sensitivity of ±1.0 μg; Thermo Electron, Waltham, MA, USA). All filter weights were blank-adjusted as described previously (Adetona AM 2016). After gravimetric analyses, PM on the filters was analyzed for light absorbing carbon (LAC) by reflectance analysis using the Evans Electroselenium Limited smoke stain reflectometer (Model 43D, Diffusion Systems Ltd, London, United Kingdom) as described (Adetona AM 2016), and used as a surrogate for black carbon (BC). Absorption coefficients (×10−5m−1) were calculated according to ISO 9835, and PM2.5 mass absorption efficiencies (×10−5m2/μg) were determined by dividing the absorption coefficients by gravimetric PM2.5 concentrations. Real-time CO was measured using the Dräger Pac III (DrägerSafety Inc., Pittsburgh, PA, USA). Time-weighted averages were calculated for both PM2.5 (μg/m3) and CO (ppm).
Malondialdehyde Analysis
Concentrations of malondialdehyde (MDA) were measured using a High-Performance Liquid Chromatography (HPLC) system coupled with a fluorescent detector (Lärstad et al. 2002). A 150-μl sample was added into a mixture of 750 μl phosphoric acid (440 mM) and 150 μl thiobarbituric acid (TBA, 42 mM). After 1-h incubation at 80°C, 20 μl of this final solution was injected into the HPLC system with the fluorescence detector set at 532 nm for the excitation wavelength and 553 nm for the emission wavelength. A Nova-Pak C18 column (Waters, Milford, MA, USA) was used with a mobile phase that was composed of 40% methanol and 60% water containing 50-mM KH2PO4 (pH = 6.8) at a flow rate of 0.8 ml/min. The detection limit, extraction recovery, and analytical precision of this method were 1.8 nM, 75.9%, and 2.2% (measured as relative standard deviation from 8 replicate injections), respectively. MDA was presented in nmol/L. Creatinine-adjusted concentrations, expressed as μmol MDA per mol creatinine, were calculated to correct for urine dilution. Urinary creatinine (mg/dL) was measured by a Beckman Coulter AU Analyzer for Creatinine (Beckman Coulter, Inc., Brea, CA, USA).
8-Isoprostane Analysis
Concentrations of 8-isoprostane in urine aliquots were measured using an enzyme linked immunosorbent assay kit (15-F2t-isoprostane [8-iso-PGF2α], NW Laboratories, LLC, ELISA kits, #NWK-ISO02). Urine samples were run in duplicate, standards were run in duplicate or triplicate, and creatinine-adjusted concentrations (μmol 8-isoprostane per mol creatinine) were calculated.
1-Hydroxypyrene Analysis
Urine aliquots underwent hydrolysis and solid phase extractions before analysis for OH-pyrene using an HPLC-fluorescence technique. A full description of the method has been reported (Birch 2017). The concentrations were expressed in pg/mL urine metabolite concentration and creatinine-adjusted concentrations (μmol OH-Pyrene/mol creatinine).
Sample Extraction and Preparation for Mutagenicity Assay
Organic extracts from the urine samples were prepared as described previously (Kato et al. 2004b). In brief, urine aliquots were thawed and filtered to remove urothelial cells. The volume of each sample was recorded, and the urine was enzymatically de-conjugated in 0.2-M (10% v/v) sodium acetate buffer (pH 5.0) (Sigma, St. Louis, MO, USA) containing β-glucuronidase (6 units/ml urine; Sigma, St. Louis, MO, USA) and sulphatase (2 units/ml urine, Sigma, St. Louis, MO, USA) for 16 h at 37°C. The de-conjugated urinary metabolites were then extracted and concentrated by pouring the urine through two C-18 silica-gel columns stacked in tandem (Waters Sep-Pak WAT04305, Milford, MA, USA). The eluted urine was discarded, and a new tube was placed under the column to collect the organics, which were eluted by pouring 10 ml of methanol through the column. The methanol extract was filtered through a 0.22-μm filter and then solvent-exchanged with dimethyl sulfoxide (DMSO) to produce an organic concentrate at 150×. These extracts were stored at 4°C until mutagenicity assays were conducted.
Mutagenicity Assay
The Salmonella (Ames) mutagenicity assay was performed using the plate-incorporation method (Maron and Ames 1983) to evaluate the organic extracts of the urines in a blinded fashion. A detailed description of the assay method has been reported (Kato et al. 2004a; Mutlu et al. 2015). Briefly, the urine concentrates were evaluated in seven batch experiments at 0.0 and 10.0, 0.0 and 12.0, or 0.0 and 15.0 ml-equivalents of urine/plate (ml-eq/plate) with four to seven doses per sample depending on the volume of urine sample available (Note: Dosing sometimes varied based on total urine volume available. i.e. 0.0, 0.5, 1.5, 3.0, 7.5, 12.0 or 15 ml-equivalents of urine/plate; 0.0, 4.0, 6.0, or 10.0 ml-equivalents of urine/plate; 0.0, 2.25, 4.0, 6.75, 9.0, 12 or 15 ml-eq/plate) in the presence of metabolic activation (S9 mix) made from Aroclor-induced, Sprague-Dawley rat-liver S9 (Moltox, Boone, NC, USA). The concentrate (not exceeding 100 μl of DMSO/plate) was added to 2.5-ml of top agar, along with 100 μl of overnight cell suspension, with 500 μl of S9 mix. The contents of the tube were vortexed and poured onto bottom-agar plates containing Vogel-Bonner Minimal E (VBME) medium, and plates were incubated at 37°C for 3 days (72 h), after which the colonies were counted using an automatic colony counter (ProtoCOL 3, Synbiosis, Frederick, MD, USA). Data were stored automatically in an Excel spreadsheet on the ProtoCOL3. Ten percent of the aliquots had replicate samples (identical urines that were divided into two sample tubes) and were analyzed according to the methods described herein. Method blanks were included per batch of experiments with an average ± standard deviation of 0.9 ± 0.3 ml-equivalents of urine/plate.
As described in Mutlu et al. (2015), TA98 [hisD3052 chl-1008 (bio uvrB gal) rfa-1004 pKM101+, Fels-1+, Fels-2+ Gifsy-1+ Gifsy-2+] detects frameshift mutagens (Mutlu et al. 2015). We used YG1041, a derivative of TA98 that over-expresses nitroreductase and acetyltransferase, permitting it to detect frameshift mutagens that are nitroarenes or aromatic amines. A previous study (Kato et al. 2004b) of wood smoke-associated urinary mutagenicity in charcoal workers in Brazil showed that strain YG1041 + S9 was the most sensitive detector of such mutagenicity. Likewise, this strain was the most sensitive among the strains used to evaluate the mutagenicity of PM10 from wildland fires in Brazil (de Oliveira Galvao et al. 2018). Thus, we used the same strain with S9 for this study.
A negative or solvent control (DMSO at 100 μl/plate) and two positive controls (2-nitrofluorene at 3 μg/plate and 2-aminoanthracene at 0.5 μg/plate) were included with each experiment (Note: The mean of seven batches of experiments that were conducted for the DMSO control for YG1041 +S9 was 60 revertants (rev)/plate, while the mean of the seven batches of experiments for 2-nitrofluorene for YG1041 −S9 and 2-aminoanthracene for YG1041 +S9 were 1715 and 1634 rev/plate, respectively). Three plates were used for each type of control for each experiment, and extracts were tested between 0.0 and 10.0, 0.0 and 12.0, or 0.0 and 15.0 ml-eq/plate with four to seven doses per sample depending on the volume of urine sample available. Due to limited urine volumes, only one independent experiment for each extract per one plate/dose was conducted. A positive mutagenic response was defined as a dose-related response with a twofold or greater increase in revertants (rev)/plate relative to the DMSO control plates. Urinary values were also creatinine-adjusted and reported as rev/μmole creatinine.
Statistical Analysis
Linear regressions over the linear portions of the dose-response curves were performed for all samples to determine the mutagenic potencies (expressed as rev/ml-eq). This included samples that did not approach a twofold increase over the solvent control and would otherwise be considered as a negative mutagenic response so that we could determine cross-work shift changes in mutagenicity that included these samples. However, a few urine samples showed clear cytotoxicity (n = 9 of the 150 analyzed), as evidenced by negative slopes of the linear regressions. These sample values were disregarded in determining cross-work shift changes and in the final statistical analyses because they were indicative of clear cytotoxicity and therefore mutagenicity was unmeasurable.
Heteroscedasticity and normality of the data were assessed for all variables. Log-transformations were applied to normalize the variables, including time-weighted averages of PM2.5 concentration, CO concentration, absorption coefficient (surrogate for black carbon), MDA concentration, 8-isoprostane concentration, OH-Pyrene concentration, and urinary mutagenicity data. Note that to achieve normality, mass absorption efficiencies (surrogate for black carbon/PM2.5 ratio) were arcsine-square root transformed. The results of the statistical analysis were back-transformed.
We assessed cross-work shift (pre- to post-; and pre- to morning-after) changes in urinary mutagenicity by day type (burn and non-burn day) and by work task (holding, lighting, non-burn day exposure, and non-burn day office), while also evaluating possible associations with the exposure measures including PM2.5, CO, absorption coefficient, mass absorption efficiency, and inhaled dose of PM2.5.
The approach used to estimate inhaled dose of PM2.5 has been reported previously (Adetona AM 2016). Briefly, accelerometer activity measurements recorded by the MicroPEM were used to estimate minute ventilation rate (L/min) using the linear regression equation published by Rodes et al. (i.e., V = m x ACCEL + b, where V is ventilation rate, m is the experimentally determined slope, ACCEL is the composite variable computed from the triaxial accelerometer measurements, and b is the intercept) (Rodes et al. 2012). Next, inhaled mass of PM2.5 (μg) was calculated by multiplying PM2.5 exposure concentrations by the total volume of inhaled air, which was estimated by multiplying the ventilation rate by the length of the monitoring period (Adetona AM 2016). Inhaled PM2.5 dose (μg/kg body weight) was then calculated by dividing the inhaled mass of PM2.5 by the subjects’ body weight (Adetona AM 2016).
Cross-work-shift changes in other outcomes, MDA, 8-isoprostane, and OH-Pyrene, according to day type and work task were also individually evaluated in a similar manner. Associations between urinary mutagenicity and the other outcomes, MDA, 8-isoprostane, and OH-Pyrene, were explored using the same statistical approach. To account for longitudinal within-subject correlations of the data, we included subject and date as random effect variables in the model. Using the forward-stepwise procedure, we also tested for the effects of possible confounding factors such as smokeless tobacco used (chew), grilled-foods, age, length of firefighter career, work-shift duration, and days since last prescribed burn. Only significant covariates were included in the final model.
Cross-work-shift changes are reported as post-work-shift/pre-work-shift ratios or morning-after-work-shift/pre-work-shift ratios, respectively. Therefore, cross-work-shift ratios were statistically different if the mean ratios’ corresponding 95% confidence limits (95% CL) did not include the value of 1. Descriptive statistics and linear mixed-effect models were conducted using SAS v.9.4 (SAS Institute, Cary, NC, USA). Statistical significance was set at p< 0.05, and the Bonferroni method was used for multiple comparisons.
RESULTS
A total of 201 spot urine samples were collected throughout the study period. One sample was spilled during analytical procedures and therefore could not be analyzed. Thus, 48 person-day paired (pre-post) samples were collected from 12 subjects on 7 prescribed burn days. We also collected 19 person-day paired (pre-post) samples from 8 subjects on 3 non-burn working days during the season. In addition, we collected morning after (MA) work day samples, resulting in 40 person-day paired (pre-post-MA) samples on burn days and 16 person-day paired (pre-post-MA) samples on non-burn days. Because some study participants were unable to give urine samples from time to time, there were a few occasions (6 in total) in which the MA in the sample set (pre, post, MA) was not completed, and these were not included in the person-day samples noted above.
Study population characteristics were described previously (Adetona AM 2016). In brief, the average age of the twelve subjects (nine men and three women) who participated in the study was 33 ± 5.4 years with subjects having less than 1 year to 22 years of experience as wildland firefighters. All subjects were non-smokers, although three subjects reported occasionally chewing smokeless tobacco. On average, 280 acres (Range: 38-1000 acres) were burned per day, and the duration of work-shifts averaged 4.5 h (Range: 1.9-9.4 h) on burn days and 6.2 h (Range: 3.9-7.8 h) on non-burn work days. The average PM2.5 and CO concentrations were 35.1 [95% CL: 15.9, 77.3] μg/m3 and 0.005 [95% CL: 0.002, 0.016] ppm, respectively, on non-burn work days, and 259.4 [95% CL: 156.1, 431.1] μg/m3 and 0.8 [95% CL: 0.4, 1.8] ppm, respectively, on burn days (Adetona AM 2016). Unadjusted arithmetic and geometric means of crude and creatinine-corrected urinary mutagenicity, MDA, and 8-isoprostane, and OH-Pyrene concentrations according to day type and time of sample collection are presented in Table 1. The number of days between the last prescribed burn and sample collection was from 3 to 30 days and from 1 to over 30 days for non-burn and burn day assessments respectively. Urinary creatinine values were between 10 and 382 mg/dL with 92% of the samples above the lower end of the normal range (30-300 mg/dL).
Table 1.
Unadjusted Arithmetic and Geometric Means of Urinary Mutagenicity, Malondialdehyde, 8-Isoprostane, and OH-Pyrene Concentrations by Day Type and Time of Sample Collection.
| Day Type | Sample Collection Time | Crude Urine Mutagenicity (rev/ml-eq) | Creatinine Adjusted Urine Mutagenicity (rev/μmole creatinine) | Crude MDA (nmol/l) | Creatinine Adjusted MDA μmol MDA/mole creatinine) | Crude 8-Isoprostane (ng/ml) | Creatinine Adjusted 8-Isoprostane (μg/g creatinine) | Crude OH-Pyrene (pg/ml) | Creatinine Adjusted OH-Pyrene (ng/g creatinine) |
|---|---|---|---|---|---|---|---|---|---|
|
Burn Day | |||||||||
| Pre-Work Shift | Sample Size n=50a | Sample Size n=50 | Sample Size n=41 | Sample Size n=50 | |||||
| Arithmetic Mean (95%CL) | 3.5 (2.4, 4.5) | 0.41 (0.29, 0.52) | 980.4 (789.3, 1171.5) | 105.6 (82.3, 128.8) | 1.5 (1.1, 1.8) | 0.5 (0.3, 0.7) | 333.5 (151.3, 515.6) | 235.7 (151.5, 320.0) | |
| Geometric Mean (95%CL) | 2.4 (1.9, 3.2) | 0.29 (0.23, 0.37) | 758.9 (607.5, 947.9) | 89.2 (76.4, 104.0) | 1.0 (0.7, 1.4) | 0.4 (0.3, 0.5) | 152.0 (108.6, 212.5) | 157.8 (124.4, 200.2) | |
| Ranges | 0, 23.6 | 0, 1.98 | 134.4, 3172.1 | 35.8, 551.4 | 0.1, 4.5 | 0.1, 3.5 | 17.5, 4237.9 | 32.9, 1793.8 | |
| Post-Work Shift | Sample Size n=50 | Sample Size n=50 | Sample Size n=41 | Sample Size n=41 | |||||
| Arithmetic Mean (95%CL) | 4.0 (2.9, 5.2) | 0.47 (0.27, 0.68) | 1074.1 (879.4, 1268.9) | 92.0 (80.5, 103.5) | 1.5 (1.1, 1.8) | 0.4 (0.3, 0.4) | 550.0 (299.0, 801.2) | 402.9 (254.1, 551.6) | |
| Geometric Mean (95%CL) | 2.9 (2.3, 3.8) | 0.28 (0.20, 0.37) | 894.6 (748.5, 1069.0) | 84.3 (74.9, 95.0) | 1.1 (0.9, 1.4) | 0.3 (0.2, 0.4) | 272.6 (197.3, 376.8) | 227.2 (168.7, 306.0) | |
| Ranges | 0.1, 28 | 0.01, 4.46 | 175.3, 3415.5 | 37.1, 212.5 | 0.2, 4.7 | 0.0, 0.9 | 18.1, 5245.3 | 40.7, 2343.0 | |
| Morning-after Work Shift | Sample Size n=43b | Sample Size n=44 | Sample Size n=32 | Sample Size n=50 | |||||
| Arithmetic Mean (95%CL) | 3.8 (2.9, 4.7) | 0.44 (0.26, 061) | 969.2 (798.0, 1140.3) | 82.6 (70.0, 95.2) | 1.6 (1.2, 2.0) | 0.3 (0.3, 0.4) | 397.2 (279.4, 515.0) | 257.4 (198.4, 316.5) | |
| Geometric Mean (95%CL) | 2.9 (2.3, 3.7) | 0.26 (0.19, 0.37) | 829.9 (694.1, 992.2) | 74.4 (65.0, 85.3) | 1.1 (0.7, 1.7) | 0.3 (0.2, 0.4) | 247.6 (181.1, 338.6) | 196.4 (155.8, 247.4) | |
| Ranges | 0, 13.6 | 0, 3.46 | 159.5, 3255.0 | 34.0, 204.3 | 0.0, 4.9 | 0.0, 0.8 | 40.2, 1527.2 | 32.0, 753.3 | |
|
Non-Burn Day | |||||||||
| Pre-Work Shift | Sample Size n=20c | Sample Size n=20 | Sample Size n=19 | Sample Size n=20 | |||||
| Arithmetic Mean (95%CL) | 3.3 (2.5, 4.1) | 0.77 (0.38, 1.15) | 611.1 (430.8, 791.4) | 90.8 (68.7, 112.8) | 1.0 (0.5, 1.4) | 0.3 (0.2, 0.4) | 165.0 (104.5, 225.4) | 204.5 (152.1, 256.9) | |
| Geometric Mean (95%CL) | 2.8 (2.1, 3.8) | 0.45 (0.27, 0.76) | 512.0 (383.4, 683.7) | 81.6 (65.8, 101.1) | 0.5 (0.3, 1.0) | 0.2 (0.2, 0.3) | 126.3 (88.5, 180.3) | 177.9 (137.7, 229.9) | |
| Ranges | 0, 7.3 | 0, 3.24 | 157.3, 1776.2 | 35.8, 551.4 | 0.1, 2.7 | 0.0, 0.6 | 23.5, 548.6 | 70.0, 466.6 | |
| Post-Work Shift | Sample Size n=20d | Sample Size n=20 | Sample Size n=17 | Sample Size n=20 | |||||
| Arithmetic Mean (95%CL) | 2.7 (1.9, 3.5) | 0.35 (0.21. 0.49) | 1089.3 (615.1, 1563.4) | 114.6 (77.1, 152.1) | 1.4 (0.8, 2.0) | 0.4 (0.3, 0.5) | 198.2 (126.6, 270.0) | 174.8 (130.0, 219.7) | |
| Geometric Mean (95%CL) | 2.3 (1.6, 3.2) | 0.27 (0.19, 0.38) | 828.6 (592.5, 1158.9) | 98.6 (77.5, 125.5) | 1.0 (0.7, 1.6) | 0.4 (0.3, 0.5) | 146.2 (99.2, 215.6) | 153.8 (121.0, 195.6) | |
| Ranges | 0, 7.3 | 0, 1.33 | 225.4, 4533.4 | 37.1, 212.5 | 0.3, 4.3 | 0.1, 0.8 | 33.1, 529.3 | 60.1, 460.5 | |
| Morning-after Work Shift | Sample Size n=18e | Sample Size n=18 | Sample Size n=14f | Sample Size n=18 | |||||
| Arithmetic Mean (95%CL) | 3.6 (2.3, 4.9) | 0.33 (0.20, 0.46) | 944.2 (783.0, 1105.3) | 93.6 (56.2, 131.1) | 1.4 (0.8, 1.9) | 0.4 (0.2, 0.5) | 247.2 (147.7, 346.6) | 178.6 (116.0, 241.2) | |
| Geometric Mean (95%CL) | 2.9 (1.9, 4.4) | 0.25 (0.16, 0.40) | 892.6 (750.2, 1062.1) | 78.8 (60.1, 103.2) | 1.1 (0.8, 1.6) | 0.3 (0.2, 0.4) | 172.2 (108.6, 272.8) | 134.3 (88.5, 204.0) | |
| Ranges | 0, 8 | 0, 0.89 | 489.0, 1778.8 | 34.0, 204.3 | 0.6, 3.6 | 0.1, 0.7 | 35.9, 647.6 | 29.4, 465.4 | |
Note: Revertants/ml-equivalent of urine (rev/ml-eq), Malondialdehyde (MDA), n=person-day samples, 95% Confidence Limits (95% CL).
For geometric mean, zero values were replaced with the LOD/√2
n=13 for creatinine adjusted 8-isoprostane morning-after work shift.
Cross-work Shift Changes in Urinary MutagenicityPercent positive results for mutagenicity and corresponding unadjusted arithmetic and geometric means by day type and time of sample collection are presented in Table 2. Twenty-eight percent of the post-work shift samples (14 of 50) on burn days were positive for mutagenicity, compared to 20% positive post-work shift samples (4 of 20) on non-burn work days (Table 2). Unadjusted means (95%CL) for crude and creatinine-corrected post-work shift urinary mutagenicity levels on burn days were 7.7 (95% CL: 4.2, 11.2) (arithmetic) rev/mL-eq and 0.83 (95% CL: 0.19, 1.48) (arithmetic) rev/μmol creatinine, and ranged from 4.4 to 28.0 rev/mL-eq and 0.24 to 4.46 rev/μmol creatinine, respectively (Table 2). On non-burn work days, unadjusted mean crude and creatinine-corrected post-work shift urinary mutagenicity levels were 4.8 (95% CL: 3.6, 5.9) (arithmetic) rev/mL-eq and 0.33 (95% CL: 0.19, 0.46) (arithmetic) rev/μmol creatinine, and ranged from 4.2 to 5.8 rev/mL-eq and 0.22 to 0.41 rev/μmol creatinine, respectively (Table 2).
Table 2.
Percent Positive Results for Mutagenicity and Corresponding Unadjusted Arithmetic and Geometric Means by Day Type and Time of Sample Collection
| Day Type | Sample Collection Time |
||
|---|---|---|---|
| Pre-Work Shift | Post-Work Shift | Morning-after Work Shift | |
|
Burn Day | |||
| Number and Percent Positive for Mutagenicity, n of total, % | 9 of 50 18% | 14 of 50 28% | 10 of 43 23% |
| Unadjusted Arithmetic Means (95%CL) | |||
| Crude Urine Mutagenicity (rev/ml-eq) | 8.4 (3.3, 13.5) | 7.7 (4.2, 11.2) | 7.8 (5.5, 10.0) |
| Creatinine-corrected Urine Mutagenicity (rev/μmole creatinine) | 0.44 (0.20, 0.68) | 0.83 (0.19, 1.48) | 0.63 (0.39, 0.88) |
| Unadjusted Geometric Means (95%CL) | |||
| Crude Urine Mutagenicity (rev/ml-eq) | 6.8 (4.1, 11.2) | 6.7 (5.1, 8.8) | 7.3 (5.5, 9.6) |
| Creatinine-corrected Urine Mutagenicity (rev/μmole creatinine) | 0.37 (0.24, 0.58) | 0.55 (0.34, 0.88) | 0.56 (0.39, 0.81) |
| Ranges (Min, Max) | |||
| Crude Urine Mutagenicity (rev/ml-eq) | 3.7, 23.6 | 4.4, 28.0 | 4.6, 13.6 |
| Creatinine-corrected Urine Mutagenicity (rev/μmole creatinine) | 0.20, 1.16 | 0.24, 4.46 | 0.32, 1.41 |
|
Non-Burn Day | |||
| Number and Percent Positive for Mutagenicity, n of total, % | 3 of 20 15% | 4 of 20 20% | 4 of 18 22% |
| Unadjusted Arithmetic Means (95%CL) | |||
| Crude Urine Mutagenicity (rev/ml-eq) | 5.9 (2.5, 9.4) | 4.8 (3.6, 5.9) | 7.4 (6.1, 8.6) |
| Creatinine-corrected Urine Mutagenicity (rev/μmole creatinine) | 0.61 (−0.29, 1.50) | 0.33 (0.19, 0.46) | 0.55 (0.30, 0.79) |
| Unadjusted Geometric Means (95%CL) | |||
| Crude Urine Mutagenicity (rev/ml-eq) | 5.8 (3.2, 10.7) | 4.7 (3.7, 6.0) | 7.3 (6.1, 8.7) |
| Creatinine-corrected Urine Mutagenicity (rev/μmole creatinine) | 0.54 (0.11, 2.57) | 0.32 (0.20, 0.49) | 0.53 (0.34, 0.84) |
| Ranges (Min, Max) | |||
| Crude Urine Mutagenicity (rev/ml-eq) | 4.5, 7.3 | 4.2, 5.8 | 6.3, 8.0 |
| Creatinine-corrected Urine Mutagenicity (rev/μmole creatinine) | 0.28, 1.00 | 0.22, 0.41 | 0.37, 0.74 |
Note: n= person-day samples; Revertants/ml-equivalent of urine (rev/ml-eq); 95% Confidence Limits (95%CL).
Adjusted cross-work shift (pre- to post-, and pre- to MA) changes in crude and creatinine-adjusted urinary mutagenicity according to day type are presented in Figures 1 (a–d). No statistically significant effect of day type on cross-work shift (pre- to post- or pre- to MA) changes in crude or creatinine-adjusted mutagenicity was observed (p> 0.05). However, data trended in the expected direction, with slightly higher (though not significant) cross-work shift creatinine-adjusted change observed on burn days compared to non-burn work days (1.0 [95% CL: 0.6, 1.6 and 0.6 [95% CL: 0.5, 1.3], respectively) (Figure 1b). Likewise, pre- to MA cross-work shift changes in creatinine-adjusted mutagenicity also trended in similar directions, with burn days having 1.4 times higher cross-work shift (pre- to MA) increases compared to non-burn work days (1.1 [95% CL: 0.5, 2.2] and 0.8 [95% CL: 0.3, 1.9], respectively) (Figure 1d).
Figures 1(a-d). Adjusted Cross-Work Shift Changes in Crude and Creatinine-adjusted Urinary Mutagenicity Concentrations according to Day Type.
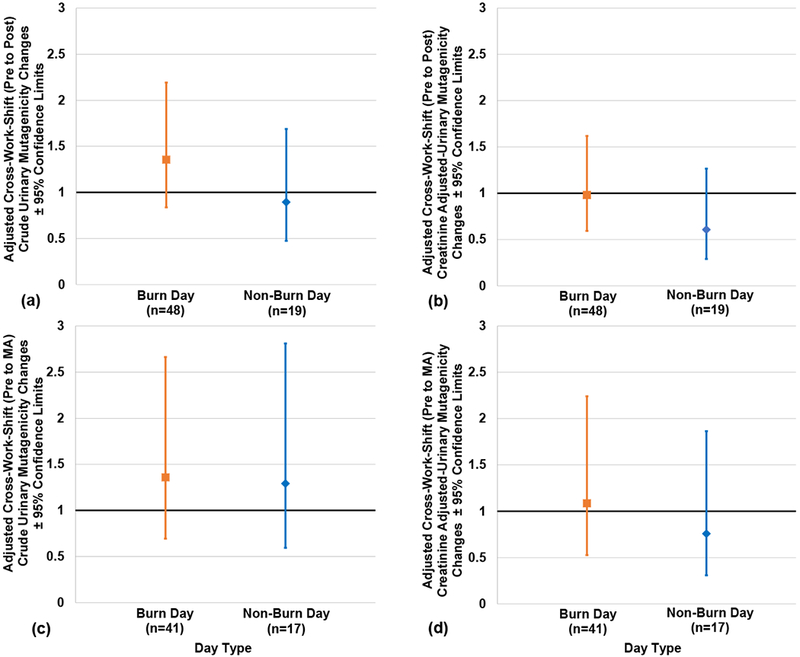
Pre- to post-work shift changes are depicted in Figs. 1a and 1b. Pre to morning-after (MA) work shift changes are depicted in Figs. 1c and 2d. No significant difference was observed between burn day and non-burn day samples for all figures. Note: n= person-day pre-post paired or pre-MA paired samples, respectively; Cross-work-shift changes are reported as post-work-shift/pre-work-shift ratios or morning-after-work-shift/pre-work-shift ratios, respectively. Where 95% confidence limits do not cross the x-axis, cross-work-shift changes are statistically different from 1 (p-values < 0.05).
Adjusted cross-work shift (pre- to post-, and pre- to MA) changes in crude and creatinine-adjusted urinary mutagenicity according to work tasks are presented in Figures 2 (a–d). Although lighters appeared to have the highest cross-work shift (pre- to post-) increase in urinary mutagenicity and were 1.6-fold higher compared to holding, no significant difference was observed between the two (p = 0.1487) (1.6, [95% CL: 1.0, 2.7] and 1.0 [95% CL: 0.5, 1.9], respectively) (Figure 2a). Likewise, no significant difference was seen between lighting and non-burn day exposures (p = 0.0901) (1.6, [95% CL: 1.0, 2.7] and 0.8 [95% CL: 0.4, 1.8], respectively) (Figure 2a). No significant overall effect of work task was seen among crude or creatinine-adjusted cross-work shift (pre- to post- [Figures 2a and 2b] and pre- to MA [Figures 2c and 2d]) changes.
Figures 2(a-d). Adjusted Cross-Work Shift Changes in Crude and Creatinine-adjusted Urinary Mutagenicity Concentrations according to Work Tasks.
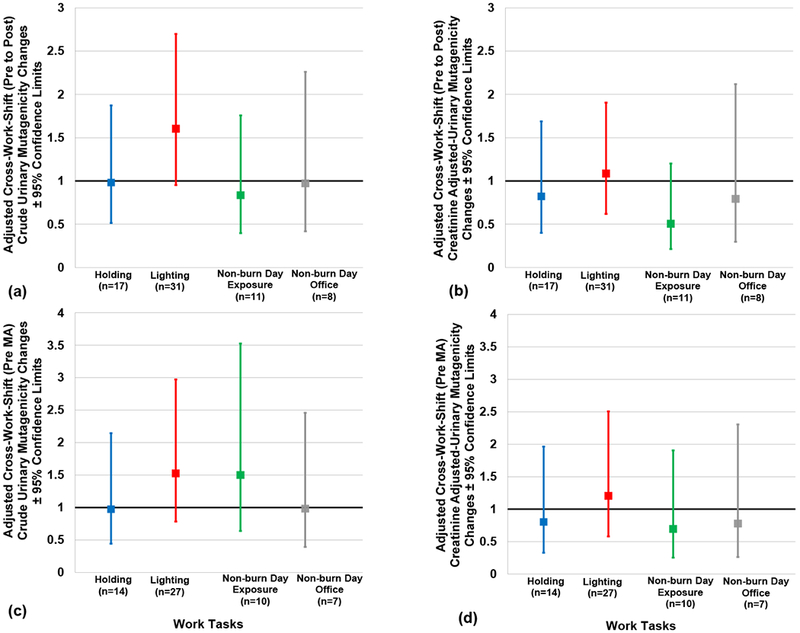
Pre-to post-work shift changes are depicted in Figs. 2a and 2b. Pre to morning-after (MA) work shift changes are depicted in Figs. 2c and 2d. No significant difference was observed across work tasks for all figures. Note: n= person-day pre-post paired or pre-MA paired samples, respectively; Cross-work-shift changes are reported as post-work-shift/pre-work-shift ratios or morning-after-work-shift/pre-work-shift ratios, respectively. Where 95% confidence limits do not cross the x-axis, cross-work-shift changes are statistically different from 1 (p-values < 0.05).
Cross-work Shift Changes Urinary Concentrations of MDAUnadjusted geometric mean crude and creatinine-adjusted post-work shift changes in MDA on burn days were 894.6 (95% CL: 748.5, 1069.0) nmole/l and 84.3 (95% CL: 74.9, 95.0) μmol MDA/mole creatinine, and ranged from 175.3 and 3415.5 nmole/l and 37.1 and 212.5 μmol MDA/mole creatinine, respectively (Table 1). On non-burn work days, unadjusted geometric mean crude and creatinine-adjusted post-work shift changes in MDA were 828.6 (95% CL: 592.5, 1158.9) nmole/l and 98.6 (95% CL: 77.5, 125.5) μmol MDA/mole creatinine, and ranged from 4225.4 and 4533.4 nmole/l and 37.1 and 212.5 μmol MDA/mole creatinine, respectively (Table 1).
Adjusted cross-work shift (pre- to post-, and pre- to MA) changes in crude and creatinine-adjusted MDA according to day type are presented in Figures 3 (a–d). No significant difference was observed between cross-work shift changes on burn day and non-burn days (Figures 3a, 3b, and 3d). However, a marginally significant difference in pre- to MA work shift crude MDA changes was observed between day types (p = 0.0502) (Figure 3c), with non-burn days having a higher crude MDA cross-work shift (pre- to MA) increase compared to burn days (1.7 [95% CL: 1.1, 2.7] and 1.1 [95% CL: 0.8, 1.5], respectively).
Figures 3(a-d). Adjusted Cross-Work Shift Changes in Crude and Creatinine-adjusted Urinary Malondialdehyde Concentrations according to Day Type.
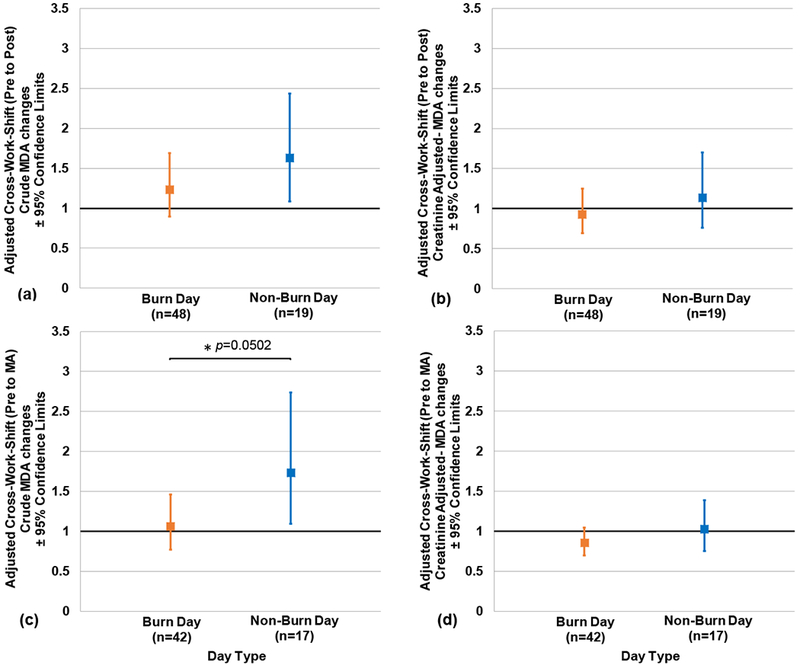
Pre- to post-work shift changes are depicted in Figs. 3a and 3b. Pre to morning-after (MA) work shift changes are depicted in Figs. 3c and 3d. No significant difference was observed between burn day and non-burn day samples for Figs. 3a, 3b, or 3d, however marginal significant differences were observed in Fig. 3c (p-value <0.05 was considered significant). Note: n= person-day pre-post paired or pre-MA paired samples, respectively; Cross-work-shift changes are reported as post-work-shift/pre-work-shift ratios or morning-after-work-shift/pre-work-shift ratios, respectively. Where 95% confidence limits do not cross the x-axis, cross-work-shift changes are statistically different from 1 (p-values < 0.05).
Adjusted cross-work shift (pre- to post-, and pre- to MA) changes in crude and creatinine-adjusted MDA according to work tasks are presented in Figures 4 (a–d). No significant effect of work tasks was observed across all cross-work shift changes (Figures 4a–d); however, non-burn work day exposures showed marginally significant higher crude MDA cross-work shift (pre- to MA) changes compared to lighting (p = 0.0565) (1.8 [95% CL: 1.1, 3.0] and 1.1 [95% CL: 0.8, 1.6], respectively) (Figure 4c).
Figures 4(a-d). Adjusted Cross-Work Shift Changes in Crude and Creatinine-adjusted Urinary Malondialdehyde Concentrations according to Work Tasks.
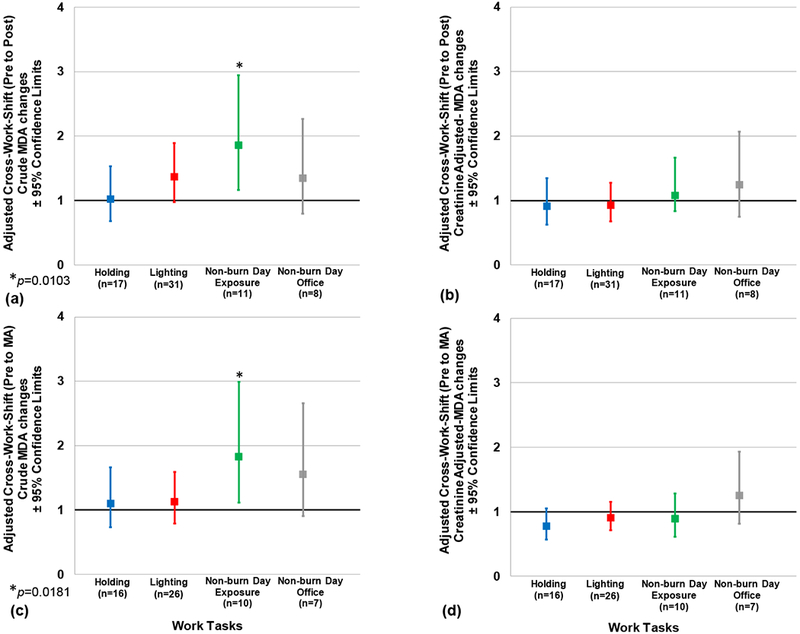
Pre-to post-work shift changes are depicted in Figs. 4a and 4b. Pre to morning-after (MA) work shift changes are depicted in Figs. 4c and 4d. No significant difference was observed across work tasks for all figures. Note: n= person-day pre-post paired or pre-MA paired samples, respectively; Cross-work-shift changes are reported as post-work-shift/pre-work-shift ratios or morning-after-work-shift/pre-work-shift ratios, respectively. Where 95% confidence limits do not cross the x-axis, cross-work-shift changes are statistically different from 1 (p-values < 0.05).
Association between Urinary Mutagenicity, Oxidative Stress, and ExposureWe found positive correlations between cross-work shift (pre- to post) changes in urinary mutagenicity and MDA (p = 0.0010) (Figure 5), and between cross-work shift (pre- to post-) changes in urinary mutagenicity and OH-Pyrene (p = 0.0001) (Figure 6). Additionally, a positive relationship, though not significant, was observed between cross-work shift (pre- to post-) changes in urinary mutagenicity and mass absorption efficiency (surrogate for BC/PM2.5 ratio) (p = 0.2245) (Figure 7).
Figure 5. Correlation between Log-transformed Cross-Work Shift (Pre- to Post-) Changes in Creatinine-adjusted Urinary Mutagenicity and Log-transformed Creatinine-adjusted MDA.
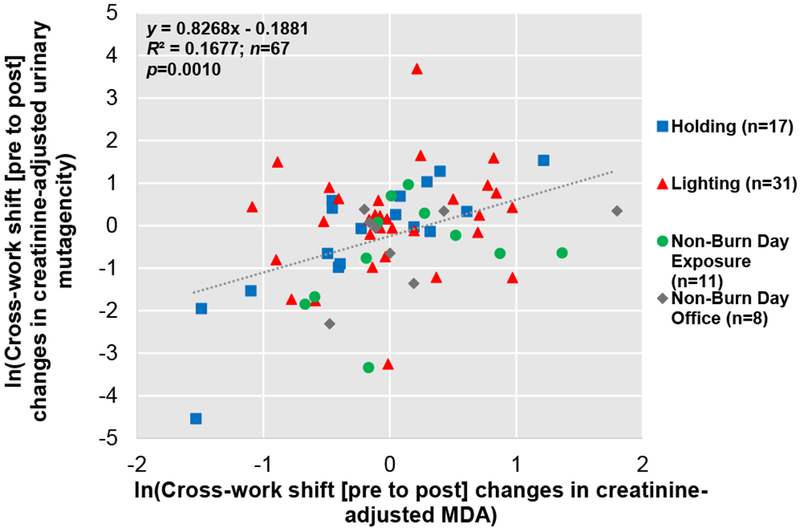
Person days are indicated as n. Linear mixed effect model results showed a positive significant correlation (p = 0.0010).
Figure 6. Correlation between Log-transformed Cross-Work Shift (Pre- to Post-) Changes in Creatinine-adjusted Urinary Mutagenicity and Log-transformed Creatinine-adjusted OH-Pyrene.
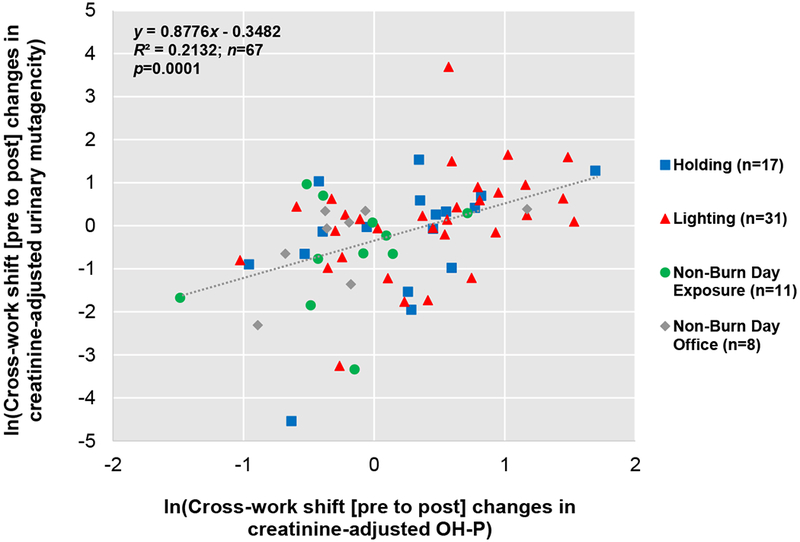
Person days are indicated as n. Linear mixed effect model results showed a positive significant correlation (p = 0.0001).
Figure 7. Correlation between Arcsine-square root Transformed Mass Absorption Efficiency (Surrogate for Black Carbon/PM2.5 Ratio) and Log-transformed Cross-Work Shift (Pre- to Post-) Changes in Creatinine-adjusted Urinary Mutagenicity.
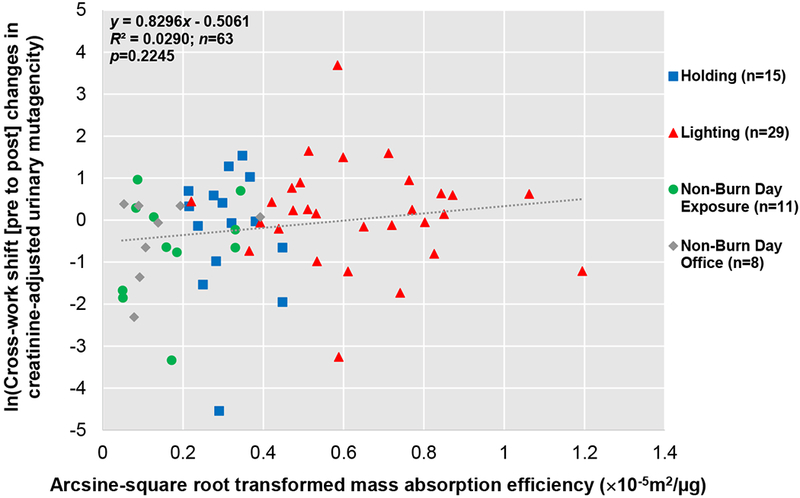
Person days are indicated as n. Linear mixed effect model results showed a cluster of data by work task and a positive (though not significant) correlation (p=0.2245).
No significant correlation was found between the remaining outcomes variables (PM2.5, inhaled dose of PM2.5, absorption coefficient) and cross-work shift changes in urinary mutagenicity, nor between the remaining exposure measures (PM2.5, inhaled dose of PM2.5, CO, absorption coefficient, and mass absorption efficiency (results not shown). Lastly, we found no significant effects for age; wildland firefighter career length; work-shift duration, days since last prescribed burn; use of smokeless tobacco (chew); or grilled foods on cross-work shift changes in MDA, 8-isoprostane, or urinary mutagenicity (results not shown).
DISCUSSION
Constituents of wood smoke and non-wood smoke occupational exposures such as diesel exhaust and wood smoke during wildland firefighting are mutagenic and carcinogenic (IARC 2010, 2014; Adetona O et al. 2015; Adetona O et al. 2016; IARC 2016). A prior study of wildland firefighters found measurably higher urinary metabolites of OH-PAHs after occupational exposure, indicating that subjects received an internal dose of exposure to possible mutagenic and carcinogenic PAHs (Adetona O et al. 2015). Because firefighters are exposed to a mixture of pollutants throughout a given work day and receive cumulative exposures throughout the length of their career, it was necessary to find an integrated approach in assessing internal (systemic) dose to smoke constituents in this population. Therefore, we explored the use of urinary mutagenicity in Salmonella YG1041 +S9 among firefighters working at prescribed burns and during non-burn work days.
Our results are comparable to the values reported in the literature (Kato et al. 2004b; Long et al. 2014). However, we observed generally lower geometric means for pre-, post- and MA- work shift creatinine-adjusted urinary mutagenicity (positive results) among our study subjects compared to the geometric means reported among non-smoking charcoal workers exposed to wood smoke in Brazil. Our burn day pre-work shift creatinine-adjusted urinary mutagenicity levels were 2.8× less than levels observed among the no-wood-smoke exposure group reported in Kato et al. (2004); and our burn day post-work shift and morning after-work shift levels were 4.6× and 4.1× less, respectively, compared to the low-wood-smoke exposure group reported by Kato et al. (Kato et al. 2004b). Personal or ambient exposure monitoring, i.e., PM2.5 or CO levels, was not measured in the charcoal study; rather, subjects were grouped based on task-related exposure levels (Kato et al. 2004b). However, it is possible that exposures in Kato et al. may have been significantly higher compared to our study. Exposures in our current study were among the lower levels measured among firefighters. This could have been due to the changes in prescribed burn operations that reduced both the size and period of burns conducted compared to historical data (Adetona O 2011; Adetona O, Simpson, et al. 2013; Hejl et al. 2013).
We found a non-significant 1.6-fold higher creatinine-adjusted urinary mutagenicity cross-work shift change on burn days compared to non-burn day samples. These results are in the expected direction and are consistent with previous findings reported in the literature. For instance, Kato et al. (2004) found that charcoal workers exposed to wood smoke had prevalence odds ratios of 2.33 (95% CL: 0.83, 6.57) for urinary mutagenicity at low-wood-smoke exposure levels and 5.31 (95% CL: 1.85, 15.27) at high-wood-smoke exposure levels compared to the non-exposed group of workers (Kato et al. 2004b). Likewise, another study on individuals from Mayan families from Guatemala (n = 32) who regularly used woodfired temazcales (steam baths) found that post-exposure samples were on average 1.7 times higher in urinary mutagenic potency compared to pre-exposure samples and also compared to control samples (n = 9, unexposed individuals from the same population)(Long et al. 2014).
In a study of occupational exposures to combustion emissions among structural firefighters, mutagenicity was assessed in urine collected before and after a fire event (Keir et al. 2017). Event-related increases in urinary mutagenicity were 4.3-fold higher in post-urine samples (Keir et al. 2017). One reason why our results may have differed compared to this study may be due to different levels of exposures to mutagenic compounds. Moreover, the PM2.5 exposure levels reported in our present study were at least 2 times lower compared to studies conducted at the same site and elsewhere (Reinhardt and Ottmar 2004; Adetona O, Simpson, et al. 2013; Hejl et al. 2013).
Our data suggest that lighters may have higher cross-work shift increases in crude urinary mutagenicity compared to other work tasks (Figure 2a). These results are understandable in that firefighters who light fires may be exposed to diesel and wood smoke particulate matter and, therefore, may have an additive or perhaps synergistic systemic dose to mutagenic compounds (Mutlu et al. 2015). A recent experimental study found that extractable organic material (EOM) from diesel exhaust particles had significantly 50-85% higher mutagenic potency compared to extracts from soy-biodiesel emission particles (Mutlu et al. 2015).
We found no significant effect of day type or work task on cross-work shift (pre- to post-) changes in MDA. However, we found a marginally significant higher cross-work shift (pre- to MA) increase in cross-work shift changes in crude MDA on non-burn days compared to burn days (Figure 3c), and a marginally significant higher cross-work shift (pre- to MA) increase in crude MDA for non-burn work day tasks that may have had other associated work exposures (Figure 4c). These results may be due to non-reported exposures on non-burn days. For instance, MDA levels in the body may be impacted by diet, such as lipid-rich foods (Richelle et al. 1999; Bloomer et al. 2010). For example, a human cross-over study among young healthy men (n = 9) found increased MDA blood levels after eating heavy whipping cream (Bloomer et al. 2010).
Our unadjusted creatinine-MDA concentrations are comparable to a similar study on wildland firefighters exposed to wood smoke (Our study: Unadjusted pre- and post- work shift arithmetic mean creatinine-adjusted MDA [95% CL]: 105.6 [95% CL: 82.3, 128.8] and 92.0 [95% CL: 80.5, 103.5], respectively [Table 1]; Adetona et al. (2013): Unadjusted pre- and post- work shift arithmetic mean creatinine-adjusted MDA ± SD: n=104, 88.14 ± 48.59 μmol MDA/mole creatinine and n=96, 107.35 ± 33.90 μmol MDA/mole creatinine, respectively) (Adetona O, Zhang, et al. 2013). Similarly, our unadjusted exposure concentrations are comparable to this study’s reported corresponding unadjusted geometric mean PM2.5 and CO exposure concentrations of 248 (n = 82, 95% CL: 184, 333) μg/m3 and 1.0 (n = 78, 95% CL: 0.07, 13) ppm, respectively (Adetona O, Zhang, et al. 2013). Also, similar to our study’s findings, no significant cross-work shift changes were observed for MDA in the urine samples of firefighters in the study of Adetona et al. (2013) (Adetona O, Zhang, et al. 2013). In contrast, results from a human chamber study found measurable increases in exhaled breath MDA levels after subjects were exposed to wood smoke (Barregard et al. 2008). (However, it is known that urinary MDA and exhaled breath MDA are not necessarily correlated (Gong et al. 2013)). We did not observe any significant cross-work shift changes in urinary 8-isoprostane. Although Keir et al. observed increases in urinary mutagenicity after structural fire events, they did not find significant cross-work shift changes in urinary 8-isoprostane levels (Keir et al. 2017).
We found a marginally significant correlation between adjusted cross-work shift (pre- to post-) changes in creatinine-adjusted urinary mutagenicity and cross-work shift (pre- to post-) changes in creatinine-adjusted MDA (Figure 5). This association is plausible because MDA is potentially mutagenic (Hartman 1983; Niedernhofer et al. 2003). MDA may act as an endogenous genotoxic product of lipid peroxidation of polyunsaturated fatty acids in the body (Niedernhofer et al. 2003). An in vitro study evaluated the mutagenic potential of MDA in human cells and found that MDA induced a 15-fold increase in mutation frequency in the supF reporter gene compared with untreated DNA (Niedernhofer et al. 2003). MDA induced multiple types of mutations, including large insertions and deletions (most frequently), base pair substitutions, and inter-strand cross-links (Niedernhofer et al. 2003).
Although urinary mutagenicity was not associated with PM2.5 exposure or inhaled dose of PM2.5, it was correlated with exposure to components of particulate matter. A significant correlation was observed between adjusted cross-work shift (pre- to post-) changes in creatinine-adjusted urinary mutagenicity and OH-Pyrene exposure (Figure 6). Various PAHs, found in wood smoke and diesel exhaust, are potentially mutagenic and carcinogenic (Kato et al. 2004b). Results from Kato et al. showed that urinary mutagenicity increased significantly after wood smoke exposure and was modified by smoking among 154 Brazilian charcoal workers (prevalence odds ratio of highly exposed workers versus non-exposed: 5.31 [95% CL: 1.85, 15.27]) (Kato et al. 2004b). Furthermore, the study reported significantly higher levels of 2-Naphthol and OH-Pyrene among the highly exposed group compared to the non-exposed (Kato et al. 2004b). Higher PAH exposures were also observed in structural firefighters with higher urinary mutagenicity levels (Keir et al. 2017).
Increased cross-work shift mutagenicity on non-burn days observed for some subjects could have been due to their exposures to mutagenic compounds. Some of the subjects reported performing tasks at which they could have experienced mutagenic exposures such as exhaust from engines, smoke from smoldering when patrolling areas where recent burns were conducted, or dust during field-prep work (Adetona AM 2016). In addition, exposures of a few subjects during the work-shift on non-burn days to OH-PAHs was apparent as indicated by their large increase in OH-Pyrene excretion across the work-shift (Figure 6).
We also observed a positive (though not significant) correlation between cross-work shift (pre- to post-) changes in creatinine-adjusted urinary mutagenicity and mass absorption efficiency (surrogate for BC/PM2.5 ratio) (Figure 7). This relationship suggests that urinary mutagenicity is dependent on the type of particles and composition of PM, i.e., BC content. Firefighters lighting with diesel-gasoline fueled drip-torches appear to have higher urinary mutagenicity as depicted in Figure 7 and suggested in Figure 2a. A previous study using extracted particles from diesel engine exhaust, which has a higher BC proportion compared to wood smoke, (Naeher et al. 2007; Ris 2007) had a higher mutagenic potency compared to soy-biodiesel particles (Mutlu et al. 2015).
Limitations
This study was a pilot study exploring the use of urinary mutagenicity among wildland firefighters, and the study sample size was powered initially using an effect size for a different health endpoint estimated from a previous piloted study (Hejl et al. 2013). Increasing the sample size in follow up studies will be important to determine small effects and ability to dissociate the effect of confounders. MDA could be influenced by dietary intake of high-lipid content foods. Biomarkers of lipid peroxidation that were not affected by lipid intake, such as urinary isoprostanes (Richelle et al. 1999), may be used as an alternative in future studies. Additionally, occupational exposures observed in this study were relatively low. Future studies should investigate mutagenicity at higher exposure levels because there was an association with particulate matter components in this study, and wood smoke is mutagenic (Kato et al. 2004b; Kim et al. 2018). Although our study was a repeated measures study design where subjects’ served as their own controls, inclusion of a non-exposed control group in the future would be valuable because wildland firefighters often perform various tasks on non-burn days that may result in inadvertent exposures to pollutants.
CONCLUSION
Results from this study suggest that healthy, non-smoking wildland firefighters are exposed to genotoxic compounds during prescribed burning. Urinary mutagenicity may serve as a suitable measure of occupational smoke exposures among this worker population when exposure levels are likely higher than what was observed in this study. No statistically significant cross-work shift increases were observed in creatinine-adjusted urinary mutagenicity between burn and non-burn days. However, our results suggest that firefighters using drip-torches to light fires have potentially higher urinary mutagenicity during prescribed burns than during other work tasks. Findings from this study suggest that occupational smoke exposure, especially related to tasks involving lighting fires on prescribed burns, may contribute to systemic exposure to mutagens.
Acknowledgements
Funding and support was provided by the National Institute of Occupational Safety and Health Education Research Center (NIOSH/ERC) Small Project/Pilot Study Grants via the University of Alabama at Birmingham (UAB) (Grant no.: 5T42OH008436-10), the National Institute of Environmental Health Sciences via the University of Washington Center for Exposures, Diseases, Genomics & Environment (P30ES007033), and the Interdisciplinary Toxicology Program at the University of Georgia to L.P.N. W.K.M. was supported by a pre-doctoral traineeship (National Research Service Award T32 ES007126) from the National Institute of Environmental Health Sciences. The mutagenicity analyses were funded by the intramural research program of the Office of Research and Development, U.S. Environmental Protection Agency, Research Triangle Park, NC and performed there. Sincere gratitude goes to William Crolly, Chris Hobson, Paul Varnedoe, John Blake, and the USFS-Savannah River crew and subjects who participated in the study.
Footnotes
Publisher's Disclaimer: Disclaimer
This article was reviewed by the National Health and Environmental Effects Research Laboratory, U.S. EPA, and approved for publication. Approval does not signify that the contents reflect the views of the agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Adetona AM. 2016. Wildland firefighter work task-related smoke exposures at prescribed burns and their effect on proinflammatory biomarkers and urinary mutagenicity. uga.
- Adetona O 2011. Occupational exposure to woodsmoke and associated changes in lung function and biomarkers of oxidative stress among wildland firefighters. Athens: College of Public Health, The University of Georgia Ph D diss Available at: http://dbsgalibugaedu/cgi-bin/ultimatecgi. [Google Scholar]
- Adetona O, Reinhardt T, Domitrovich J, Broyles G, Adetona A, Kleinman M, Ottmar R, Naeher L. 2016. Review of the health effects of wildland fire smoke on wildland firefighters and the public. Inhalation Toxicology. 28(3):95–139. [DOI] [PubMed] [Google Scholar]
- Adetona O, Simpson C, Li Z, Sjodin A, Calafat A, Naeher L. 2015. Hydroxylated polycyclic aromatic hydrocarbons as biomarkers of exposure to wood smoke in wildland firefighters. Journal of Exposure Science and Environmental Epidemiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adetona O, Simpson C, Onstad G, Naeher L. 2013. Exposure of wildland firefighters to carbon monoxide, fine particles, and levoglucosan. Annals of occupational hygiene.met024. [DOI] [PubMed] [Google Scholar]
- Adetona O, Zhang J, Hall D, Wang J, Vena J, Naeher L. 2013. Occupational exposure to woodsmoke and oxidative stress in wildland firefighters. Science of the Total Environment. 449:269–275. [DOI] [PubMed] [Google Scholar]
- Barregard L, Sällsten G, Andersson L, Almstrand A, Gustafson P, Andersson M, Olin A. 2008. Experimental exposure to wood smoke: effects on airway inflammation and oxidative stress. Occupational and environmental medicine. 65(5):319–324. [DOI] [PubMed] [Google Scholar]
- Birch S 2017. Evaluation of PAH Metabolites as Biomarkers for Occupational Wood Smoke Exposure in Wildland Firefighters (Dissertation 2017).
- Black C, Tesfaigzi Y, Bassein J, Miller L. 2017. Wildfire smoke exposure and human health: Significant gaps in researvh for a growing public health issue. Environ Toxicol Pharmacol. 55:186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer R, Kabir M, Marshall K, Canale R, Farney T. 2010. Postprandial oxidative stress in response to dextrose and lipid meals of differing size. Lipids in health and disease. 9(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio WE. 2018. Wildland fire smoke and human health. Sci Total Environ. 624:586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Černá M, Pastorková A, Myers S, Rössner P, Binková B. 1997. The use of a urine mutagenicity assay in the monitoring of environmental exposure to genotoxins. Mutat Ress. 391:99–110. [DOI] [PubMed] [Google Scholar]
- de Oliveira Galvão M, de Oliveira Alves N, Ferreira P, Caumo S, de Castro Vasconcellos P, Artaxo P, de Souza Hacon S, Roubicek D, de Medeiros S. 2018. Biomass burning particles in the Brazilian Amazon region: mutagenic effects of nitro and oxy-PAHs and assessment of health risks. Environ Pollut. 233:960–970. [DOI] [PubMed] [Google Scholar]
- DeMarini D, Brooks L, Bhatnagar V, Hayes R, Eischen B, Shelton M, Zenser T, Talaska G, Kashyap S, Dosemeci M. 1997. Urinary mutagenicity as a biomarker in workers exposed to benzidine: correlation with urinary metabolites and urothelial DNA adducts. Carcinogenesis. 18(5):981–988. [DOI] [PubMed] [Google Scholar]
- Gong J, Zhu T, Kipen H, Wang G, Hu M, Ohman-Strickland P, Lu S, Zhang L, Wang Y, Zhu P. 2013. Malondialdehyde in exhaled breath condensate and urine as a biomarker of air pollution induced oxidative stress. Journal of Exposure Science and Environmental Epidemiology. 23(3):322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haikerwal A, Reisen F, Sim M, Abramson M, Meyer C, Johnston F, Dennekamp M. 2015. Impact of smoke from presscribed burning: Is it a public health concern? J Air Waste manag Assoc. 65:592–598. [DOI] [PubMed] [Google Scholar]
- Hartman P 1983. Review: Putative mutagens and carcinogens in foods. IV. Malonaldehyde (malondialdehyde). Environmental mutagenesis. 5(4):603–607. [DOI] [PubMed] [Google Scholar]
- Hejl A, Adetona O, Diaz-Sanchez D, Carter J, Commodore A, Rathbun S, Naeher L. 2013. Inflammatory effects of woodsmoke exposure among wildland firefighters working at prescribed burns at the Savannah River Site, SC. Journal of occupational and environmental hygiene. 10(4):173–180. [DOI] [PubMed] [Google Scholar]
- IARC. 2010. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Household Use of Solid Fuels and High-Temperature frying. Lyon, France: 95. [PMC free article] [PubMed] [Google Scholar]
- IARC. 2014. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Diesel and Gasoline Engine Exhausts and Some Nitroarenes. Lyon, France: 105. [PMC free article] [PubMed] [Google Scholar]
- IARC. 2016. IARC Monographs on the Evaluation of Carcinogenic risks to Humans: Outdoor Air Pollution. Lyon, France: 109. [PMC free article] [PubMed] [Google Scholar]
- Kato M, Loomis D, Brooks L, Gattas G, Gomes L, Carvalho A, Rego M, DeMarini D. 2004a. Urinary biomarkers in charcoal workers exposed to wood smoke in Bahia State, Brazil. Cancer Epidemiology Biomarkers & Prevention. 13(6):1005–1012. [PubMed] [Google Scholar]
- Kato M, Loomis D, Brooks L, Gattas G, Gomes L, Carvalho A, Rego M, DeMarini D. 2004b. Urinary biomarkers in charcoal workers exposed to wood smoke in Bahia State, Brazil. Cancer Epidemiology Biomarkers & Prevention. 13(6):1005–1012. [PubMed] [Google Scholar]
- Keir J, Akhtar U, Matschke D, Kirkham T, Chan H, Ayotte P, White P, Blais J. 2017. Elevated Exposures to Polycyclic Aromatic Hydrocarbons and Other Organic Mutagens in Ottawa Firefighters Participating in Emergency, On-Shift Fire Suppression. Environmental science & technology. 51(21):12745–12755. [DOI] [PubMed] [Google Scholar]
- Kim Y, Warren S, Krantz Q, King C, Jaskot R, Preston W, George B, Hays M, Landis M, Higuchi M et al. 2018. Mutagenicity and lung toxicity of smoldering vs. flaming emissions from various biomass fuels: implications for health effects from wildland fires. Environmental Health Perspectives (Online). 126(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lärstad M, Ljungkvist G, Olin A, Torén K. 2002. Determination of malondialdehyde in breath condensate by high-performance liquid chromatography with fluorescence detection. Journal of Chromatography B. 766(1):107–114. [DOI] [PubMed] [Google Scholar]
- Long A, Lemieux C, Yousefi P, Ruiz-Mercado I, Lam N, Orellana C, White P, Smith K, Holland N. 2014. Human urinary mutagenicity after wood smoke exposure during traditional temazcal use. Mutagenesis. 29(5):367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron D, Ames B. 1983. Revised methods for the Salmonella mutagenicity test. Mutation Research/Environmental Mutagenesis and Related Subjects. 113(3–4):173–215. [DOI] [PubMed] [Google Scholar]
- Mutlu E, Warren S, Matthews P, King C, Walsh L, Kligerman A, Schmid J, Janek D, Kooter I, Linak W. 2015. Health effects of soy-biodiesel emissions: mutagenicity-emission factors. Inhalation toxicology. 27(11):585–596. [DOI] [PubMed] [Google Scholar]
- Naeher L, Brauer M, Lipsett M, Zelikoff J, Simpson C, Koenig J, Smith K. 2007. Woodsmoke health effects: a review. Inhalation toxicology. 19(1):67–106. [DOI] [PubMed] [Google Scholar]
- Niedernhofer L, Daniels J, Rouzer C, Greene R, Marnett L. 2003. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. Journal of Biological Chemistry. 278(33):31426–31433. [DOI] [PubMed] [Google Scholar]
- Reid C, Brauer M, Johnston F, Jerrett M, Balmes J, Elliott C. 2016. Critical review of health impacts of wildfire smoke exposure. Environ Health Perspect. 124:1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt T, Ottmar R. 2004. Baseline measurements of smoke exposure among wildland firefighters. Journal of occupational and environmental hygiene. 1(9):593–606. [DOI] [PubMed] [Google Scholar]
- Richelle M, Turini M, Guidoux R, Tavazzi I, Metairon S, Fay L. 1999. Urinary isoprostane excretion is not confounded by the lipid content of the diet. FEBS letters. 459(2):259–262. [DOI] [PubMed] [Google Scholar]
- Ris C 2007. US EPA health assessment for diesel engine exhaust: a review. Inhalation Toxicology. 19(sup1):229–239. [DOI] [PubMed] [Google Scholar]
- Rodes C, Chillrud S, Haskell W, Intille S, Albinali F, Rosenberger M. 2012. Predicting adult pulmonary ventilation volume and wearing complianceby on-board accelerometry during personal level exposure assessments. Atmospheric environment. 57:126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbioni G, Jones C, Sepai O, Hirvonen A, Norppa H, Järventaus H, Glatt H, Pomplun D, Yan H, Brooks L. 2006. Biomarkers of exposure, effect, and susceptibility in workers exposed to nitrotoluenes. Cancer Epidemiology Biomarkers & Prevention. 15(3):559–566. [DOI] [PubMed] [Google Scholar]
- Shaughnessy D, Gangarosa L, Schliebe B, Umbach D, Xu Z, MacIntosh B, Knize M, Matthews P, Swank A, Sandler R. 2011. Inhibition of fried meat-induced colorectal DNA damage and altered systemic genotoxicity in humans by crucifera, chlorophyllin, and yogurt. PloS one. 6(4):e18707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Field Standard Operating Procedures for the PM2.5 Performance Evaluation Program. 2006. [accessed 2016 July 7]. https://www3.epa.gov/ttnamti1/files/ambient/pm25/qa/fieldsoppm.pdf


