Abstract
Background
Artemether–lumefantrine (AL) and dihydroartemisinin–piperaquine (DHA/PPQ) are the recommended first- and second-line treatments, respectively, for uncomplicated falciparum malaria in Somalia. The studies reported here were conducted to assess the efficacy of these artemisinin-based combinations and the mutations in Plasmodium falciparum K13-propeller (Pfk13) domain and amplification in Pfplasmepsin 2 (Pfpm2) gene in Somalia.
Methods
One-arm prospective studies were conducted to assess the clinical and parasitological responses to DHA/PPQ and AL at two sites in 2016 and 2017, respectively, using the standard WHO protocol. The patterns of molecular markers associated with artemisinin and PPQ resistance were investigated for the first time in Somalia.
Results
A total of 339 patients were enrolled with 139 for AL and 200 for DHA/PPQ. With AL, no parasite recurrence was observed among patients treated at either site, corresponding to 100% clinical and parasitological responses. For DHA–PPQ, an adequate clinical and parasitological response rate > 97% was observed. All study patients on both treatments at both sites were parasite-free on day 3. Of the 138 samples with interpretable results for the polymorphism in Pfk13, only one (0.7%), from Bosaso, contained a non-synonymous mutation (R622I), which is not one of the known markers of artemisinin resistance. No Pfpm2 amplification was observed among the 135 samples with interpretable results.
Conclusions
AL and DHA/PPQ were highly effective in the treatment of uncomplicated falciparum malaria, and there was no evidence of resistance to artemisinin or PPQ. These two combinations are thus relevant in the chemotherapeutic strategy for malaria control in Somalia.
Trial registration ACTRN12616001005448 (Jowhar DP study), ACTRN12616000553471 (Bosaso DP study), ACTRN12617001055392 (AL study in Bosaso and Jowhar)
Keywords: Artemether–lumefantrine, Dihydroartemisinin–piperaquine, Plasmodium falciparum, Artemisinin resistance, Piperaquine resistance, Somalia
Background
Malaria is still a major health problem: it caused an estimated 219 million cases and 435,000 deaths worldwide in 2017, most of which occurred in Africa [1]. Artemisinin-based combination therapy (ACT) is the currently recommended anti-malarial drug for the treatment of uncomplicated Plasmodium falciparum [2]. Although ACT has maintained its therapeutic efficacy in most malaria-endemic countries [3], the emergence and spread of resistance to artemisinins [4–6] and, more recently, to piperaquine (PPQ) [7, 8] in South-East Asia are of great concern. Artemisinin resistance, defined as delayed parasite clearance [3], has been associated with mutations in the P. falciparum k13-propeller (Pfk13) domain [9], and PPQ resistance has been linked to Pfplasmepsin 2 (Pfpm2) gene amplification [10]. A rapid increase in the treatment failure rate with dihydroartemisinin–piperaquine (DHA/PPQ) has been reported in Cambodia, Thailand and Viet Nam [7, 8, 11–13]. Non-synonymous Pfk13 mutations are so far rare in Africa, and the mutation most frequently observed is A578S, which is not associated with clinical or in vitro resistance to artemisinins [14, 15].
The World Health Organization (WHO) recommends routine monitoring of the efficacy of the recommended ACT at least every 2 years [16]. This is essential, particularly in view of the reported multidrug resistance to anti-malarial medicines and the declining efficacy of DHA/PPQ in South East Asia. A study of therapeutic efficacy in vivo, conducted with the standard WHO protocol [17], is the gold standard for monitoring the efficacy of anti-malarial medicines in order to guide effective malaria treatment. Analysis of molecular markers of drug resistance is an additional monitoring tool to support in vivo confirmation of parasite resistance.
The National Malaria Control Programme (NMCP) of Somalia recommended artesunate + sulfadoxine/pyrimethamine (AS + SP) as first-line treatment in 2006 and artemether–lumefantrine (AL) as second-line treatment in 2011 for uncomplicated falciparum malaria. Therapeutic efficacy studies conducted in 2011 and 2013 showed high AS + SP treatment failure rates (12–22%), with high levels of Pfdhfr/Pfdhps quadruple and quintuple mutations, while AL was highly efficacious [18, 19]. On the basis of this evidence, the NMCP recommended AL to replace AS + SP and DHA/PPQ as second-line treatment in 2016 [19]. The studies reported here were conducted to assess the therapeutic efficacy of DHA/PPQ and AL and to determine the mutations in Pfk13 and amplification in the Pfpm2 gene.
Methods
Study design, area and population
One-arm prospective studies were conducted with the standard WHO protocol [17] to evaluate clinical and parasitological responses to standard therapeutic doses of AL and DHA/PPQ in patients with uncomplicated falciparum malaria.
The studies were conducted at two sentinel sites (Fig. 1): Jowhar in the Middle Shabelle region and Bosaso in the North-east region (Puntland). Previous studies reported moderate and low malaria transmission in Middle Shabelle and North-east regions, respectively [20, 21]. In Bosaso district, very few malaria cases (0/970–2/949; 0–0.2%) were recorded through the HMIS before the 2013 outbreak, when the reported number of confirmed malaria cases reached 19% (NMCP, unpublished data). A mobile clinic was set up in Jowhar town to recruit patients from rural communities within 10 km. In Bosaso, patients were recruited at the out-patient department of the regional hospital. The studies were conducted during the malaria transmission season at both sites.
Fig. 1.
Map of Somalia showing the study sites (Jowhar and Bosaso)
Patients aged ≥ 6 months, excluding girls aged 12–17 years and unmarried women aged ≥ 18 years, as the local customs and culture would not allow pregnancy testing), with suspected uncomplicated malaria infection (axillary temperature ≥ 37.5 °C or history of fever during the previous 24 h) were tested for malaria parasites and were recruited if they had P. falciparum mono-infection with a parasite density of 500–200,000 asexual parasites/μL of blood and consented to participate in the study. There was no upper age limit. Patients were not enrolled if they presented with the following: severe malaria, mixed or mono-infection with non-falciparum species, known hypersensitivity to the study drugs, severe malnutrition, non-malaria febrile illness (measles, acute lower respiratory tract infection, severe diarrhoea with dehydration) or known underlying chronic diseases (e.g. cardiac, renal or hepatic disease, HIV/AIDS). In addition, patients on regular medication that might have interfered with the pharmacokinetics of the study ACT and those with a history of hypersensitivity reactions to the medicines were excluded.
Treatment and follow-up
Eligible patients were given a standard therapeutic oral dose of either AL (Artemether–lumefantrine, IPCA Laboratories India) twice daily for 3 days or DHA/PPQ (Duo-Cortecxin®, Holley-Cotec Pharmaceuticals China) once daily for 3 days. AL (20/120 mg) was administered according to the following weight ranges: one tablet was given to patient weighing 5–14 kg; two tablets for 15–24 kg; three tablets for 25–34 kg and four tablets for ≥ 35 kg. DHA/PPQ was given as follows: half, three-quarters, one tablet, two tablets, three tablets and four tablets to patients weighing 5 to < 8 kg, 8 to < 14 kg, 14 to < 25 kg, 25 to < 36 kg, 36 to < 60 kg and 60 to < 80 kg, respectively. The study medicines were provided by the WHO, and all treatment doses were given under the direct supervision of a designated study team member. Each patient was observed for 30 min after administration; if he or she vomited during this period, the full dose was given again. Patients who vomited a second time were withdrawn from the study and referred or admitted to hospital, where parenteral artesunate was administered according to the national treatment policy.
Patients were followed up daily for the first 3 days after the first dose (day 0) and then weekly from day 7 onwards, up to day 28 for the AL-treated groups. Follow-up was extended to day 42 for DHA/PPQ-treated groups because piperaquine has a longer half-life compared to lumefantrine. Patients were also assessed on an unscheduled day if symptoms occurred. Clinical and laboratory evaluations were undertaken during the follow-up visits. Adverse events and severe adverse events, defined according to the WHO protocol for monitoring therapeutic efficacy of anti-malarial medicines [18], were monitored clinically at each follow‐up visit.
Laboratory analysis
Microscopy
Thick and thin blood smears were taken from finger-prick blood on day 0 and during follow-up visits and stained with Giemsa. Parasites were counted on Giemsa-stained thick films and recorded as the number of asexual parasites per 200 white blood cells (or per 500, if the count was < 100 parasites/200 white blood cells). A smear was declared negative if no parasites were seen after 1000 white blood cells were counted. The presence of gametocytes at enrolment or follow-up days was recorded. In addition, 100 fields of the thick smear on day 0 were examined to exclude mixed infections; in case of any doubt, the thin film was examined for confirmation. Each blood smear was examined independently by two qualified microscopists, and parasite density (per µL) was calculated on the assumption of a leucocyte count of 8000 per µL blood. Final parasitaemia was calculated by averaging the readings of the two microscopists if they were in agreement (difference in parasite densities < 50%). If the two counts were discordant in terms of parasite positivity, species or density by > 50%, a third, independent microscopist re-examined the blood slides. For parasite species and positivity, two concordant results were considered the final result, while for parasite density, the average of the two closest estimates of parasitaemia was considered final.
Parasite genotyping
In order to differentiate recrudescence from re-infection, blood samples were collected on filter paper from a finger-prick on day 0 and on the day of parasite recurrence (day 7 onwards) for genotyping. Specimens were dried, stored in individual plastic bags with desiccants and protected from light, humidity and extreme temperatures. The samples were analysed at the Institut Pasteur, Cambodia. Each dried blood spot was punched with a sterile puncher, and the spots were placed in a 96-well plate in numerical order. Samples were lysed overnight in a saponin solution, and then DNA was extracted with Instagen Matrix resin, as previously described [22]. DNA samples (day 0 and day of recurrence) were analysed to genotype the highly polymorphic regions msp1, msp2 (merozoite surface proteins 1 and 2) and glurp (glutamate-rich protein) loci, as recommended by the WHO [23]. The results were classified as recrudescence if the recurrent parasites were of the same parasite strain as those on day 0, or as a new infection if they were a different strain.
Molecular markers
Parasite DNA was analysed on day 0 for the presence of mutations in the Pfk13 propeller domain, which is associated with artemisinin resistance. The propeller domain was amplified in a nested-PCR assay, amplicons were sequenced according to Sanger’s method (Macrogen, Republic of Korea), and DNA sequences were analysed to identify specific single nucleotide polymorphisms (SNPs) related to artemisinin resistance [9]. The amino acid sequences were compared with the 3D7 wild-type amino acid sequences PF3D7_1343700. The presence of SNPs was confirmed by reading both the forward and the reverse strands. Parasites with mixed alleles were considered mutants. The number of copies of Pfpm2 genes was assessed by reverse transcriptase-PCR (sybr green dye). The full method has been described by Witkowski et al. [10].
Classification of treatment outcome
According to the WHO criteria [17], treatment responses were classified as early treatment failure (ETF), late clinical failure (LCF), late parasitological failure (LPF) or adequate clinical and parasitological response (ACPR) before and after protein Polymerase Chain Reaction (PCR) correction [17]. The primary end point was PCR corrected ACPR on day 28 for AL and day 42 for DP. Other outcomes were loss to follow-up and withdrawals, which included protocol violation, withdrawal of consent, failure to complete study treatment (persistent vomiting, self- or third-party administration of anti-malarial drug or antibiotics with anti-malarial activity, occurrence during follow-up of concomitant disease that would interfere with a clear classification of the treatment outcome; detection of mono-infection with another malaria species during follow-up, or misclassification of a patient due to a laboratory error (parasitaemia) leading to administration of rescue treatment.
Sample size
Assuming a treatment failure of 5% for both AL and DHA/PPQ with 95% confidence level and 5% precision, a minimum sample of 73 patients per site per drug was estimated. With a 20% increase to allow loss to follow-up and withdrawals during the 28-day (AL) or 42-day (DHA/PPQ) follow-up period, 88 patients per site per drug were targeted.
Statistical analysis
The clinical and laboratory data of each patient were recorded on standard case record forms, double-entered independently and analysed with an WHO Excel® database specifically designed for studies of anti-malarial drug efficacy (https://www.who.int/malaria/areas/drug_resistance/efficacy-monitoring-tools/en/).
This software automatically computes treatment outcomes by both per-protocol and Kaplan–Meier analysis. For the per-protocol analysis, patients were excluded if they were lost to follow-up or withdrawn because of withdrawal and protocol violation. In addition, patients with reinfections or undetermined or missing PCR were also excluded from PCR corrected per-protocol analysis. For the Kaplan–Meier analysis, patients who were lost to follow-up or withdrawn were censored on the last day of follow-up according to the timetable. For the PCR corrected Kaplan–Meier analysis, patients with reinfection were censored on the day of reinfection while those with undetermined or missing PCR were totally excluded.
Baseline characteristics (age, gender, temperature, parasitaemia) at the two study sites were compared by study drug. Chi squared tests were used to compare categorical data. Differences in the baseline mean age and parasite density were evaluated in a two-sample Wilcoxon rank-sum (Mann–Whitney) test for non-normally distributed data. Gametocyte positivity rates on enrolment and on follow-up days were computed. In addition, clearance of gametocytaemia after treatment among gametocyte carriers at recruitment was assessed by Kaplan–Meier survival analysis. Confidence intervals were calculated for binomial proportions.
Ethical considerations
The study protocol was approved by the Ministry of Health of the Federal Government of Somalia, the Ministry of Health of Puntland and the WHO Research Ethics Review Committee. Patients or parents/guardians of study children were informed about the study and provided written consent. If a patient, parent or guardian was illiterate, he or she chose a witness to co-sign the consent form. Informed assent was obtained from children aged ≥ 12 years. Travel costs for scheduled and unscheduled visits were reimbursed. In Jowhar, community leaders of the study villages were informed about the study objectives and procedures and gave their permission.
Results
Baseline characteristics
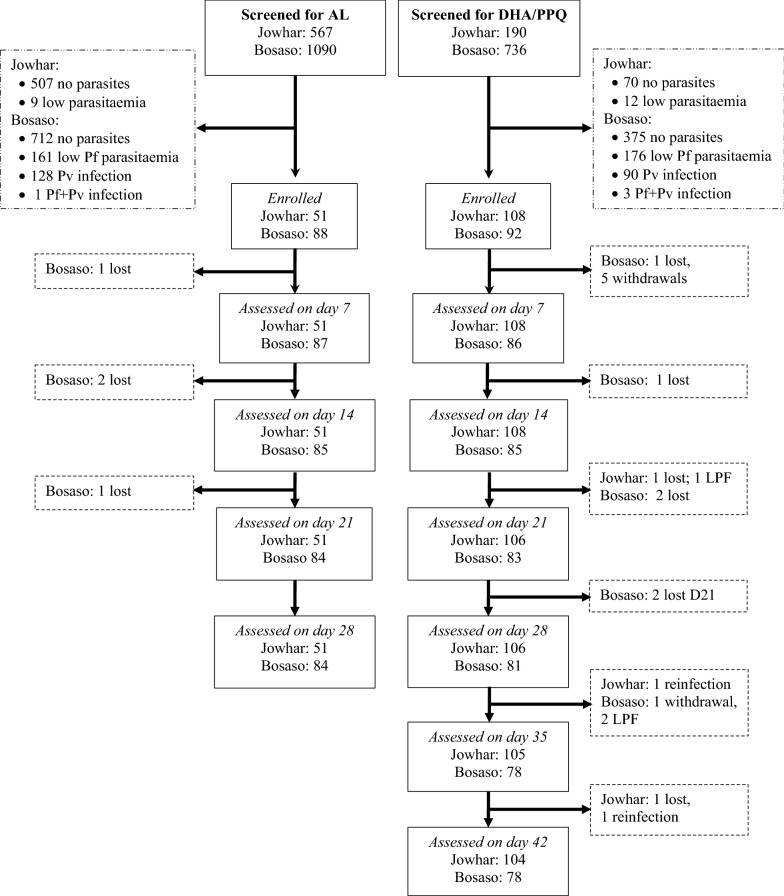
Potential study patients were screened in Jowhar from August to October 2016 (DHA/PPQ) and 2017 (AL), and in Bosaso from January to February (DHA/PPQ), and from November 2017 to February 2018 (referred as 2017 in the rest of the document) for AL. A total of 2583 febrile patients were screened for eligibility criteria and 339 patients were recruited (Fig. 2). Out of these, 200 patients (92 in Bosaso, 108 in Jowhar) were treated with DHA–PPQ and 139 (88 in Bosaso, 51 in Jowhar) with AL. The target sample of 88 patients could not be reached in Jowhar in 2017 because of the lower parasite positivity rate among screened febrile patients during the transmission season of 2017 (10.6%) than in 2016 (63.2%).
Fig. 2.
Patient flow chart for AL and DHA/PPQ cohorts in Jowhar and Bosaso sites. AL, artemether–lumefantrine; DHA/PPQ, dihydroartemisinin–piperaquine; Pf, Plasmodium falciparum; Pv, Plasmodium vivax
The baseline age, axillary temperature and parasitaemia of the study patients are summarized in Table 1. Significantly more males than females were recruited at both sites, which was expected, as girls aged 12–17 years and unmarried women aged ≥ 18 years were not enrolled. The mean age of patients in Bosaso was significantly (P < 0.0001) higher than that in Jowhar for both DHA/PPQ and AL groups. The geometric mean parasite density at enrolment was higher among patients in Jowhar than in Bosaso, although the difference was significant (P < 0.01) only between the groups treated with DHA/PPQ. The proportion of patients with detectable gametocytaemia at enrolment was higher among patients in Jowhar than in Bosaso for both treatment groups (Table 1), but the difference was significant only between the DHA/PPQ groups (X2= 13.9; P < 0.001) and not for the AL groups (X2 = 3.6; P = 0.06).
Table 1.
Baseline characteristics of study patients in Bosaso and Jowhar sites, Somalia
| Characteristic | DHA–PPQ (2016) | AL (2017) | ||
|---|---|---|---|---|
| Bosaso (n = 92) | Jowhar (n = 108) | Bosaso (n = 88) | Jowhar (n = 51) | |
| Males, n (%) | 83 (90.2) | 76 (70.4) | 65 (73.9) | 41 (80.4) |
| Mean age years (SD)a | 25 (14.6) | 9.7 (6.5) | 17 (10.4) | 10.2 (6) |
| Age group, n (%) | ||||
| < 5 years | 8 (8.7) | 15 (13.9) | 4 (4.5) | 5 (9.8) |
| 5 to 15 years | 19 (20.6) | 83 (76.8) | 38 (43.2) | 39 (76.5) |
| ≥ 15 (adults) | 65 (70.7) | 10 (9.3) | 46 (52.3) | 7 (13.7) |
| Axillary temperature (°C) | ||||
| Mean (SD) | 38.2 (0.3) | 38.0 (0.5) | 38 (0.4) | 38 (0.5) |
| Parasitaemia (per µL) | ||||
| Geometric meanb | 7466 | 11,074 | 8042 | 10,454 |
| Range (min–max) | 1300–74,768 | 597–144,000 | 811–83,163 | 1012–79,196 |
| Gametocytaemia, n (%) | 9 (9.8) | 34 (31.5) | 10 (11.4) | 12 (23.5) |
SD, standard deviation
aThe mean age of the Bosaso study population was significantly (P < 0.0001) greater than those of Jowhar for both AL and DP treatment groups
bDifference between the parasite density of Jowhar and Bosaso was significant (P < 0.02)
Treatment outcomes
Eight and six patients were lost to follow and withdrawn, respectively (Fig. 2). The reasons for withdrawal included death (n = 1), withdrawal of consent (n = 2), imprisonment (n = 1) and third-party treatment with antimalarial drug (n = 2). Table 2 summarizes the treatment outcomes at 28-days and 42-days of follow-up for AL and DHA/PPQ, respectively. In the DHA/PPQ-treated groups, five patients (two in Bosaso and three in Jowhar) showed parasite recurrence on day 14 (n = 1), day 28 (n = 3) and day 35 (n = 1). Only one of the three patients in Jowhar carried recrudescent parasites, while the other two cases were re-infections, giving a PCR-corrected ACPR rate of 99.0% (95% CI, 92.0%–99.4%). The samples from the two patients in Bosaso were not corrected by PCR because suitable samples were not available at the time of recurrence. Even in the worst-case scenario (both patients with recrudescence), however, the outcome would have led to an ACPR rate of 97.5% (95% CI 91.3%–99.7%). No parasite recurrence was observed in the AL-treated groups giving an ACPR rate of 100% (95% CI 93%–100%) in Jowhar and 100% (95% CI 95.7%–100%) in Bosaso (Table 2). All patients in both AL and DHA/PPQ treated groups were parasite-free on day 3, as verified by microscopy.
Table 2.
Treatment outcome in patients with uncomplicated falciparum infection treated with dihydroartemisinin–piperaquine (DHA/PPQ) or artemether–lumefantrine (AL) in Bosaso and Jowhar sites, Somalia
| Treatment responses | DHA-PP | AL | ||
|---|---|---|---|---|
| Bosaso (N = 92) | Jowhar (N = 108) | Bosaso (N = 88) | Jowhar (N = 51) | |
| Parasitaemia on day 3 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| PCR-uncorrected | ||||
| ETF, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LCF, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LPF, n (%) | 2 (2.5) | 3 (2.8) | 0 (0) | 0 (0) |
| ACPR, n (%) | 78 (97.5) | 103 (97.2) | 84 (100) | 51 (100) |
| Total per protocol | 80 | 106 | 84 | 51 |
| Lost/withdrawn, n (%) | 12 (13.0) | 2 (1.9) | 4 (4.5) | 0 (0) |
| Kaplan–Meier: cure rate | 78 (97.5) | 103 (97.2) | 84 (100) | 51 (100) |
| PCR-corrected | ||||
| ETF, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LCF, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| LPF, n (%) | NA* | 1 (1.0) | 0 (0) | 0 (0) |
| ACPR, n (%) | NA* | 103 (99.0) | 84 (100) | 51 (100) |
| Total per protocol | NA* | 104 | 84 | 51 |
| Lost/withdrawals/re-infection: n (%) | NA* | 4 (3.7) | 4 (4.5) | 0 (0) |
| Kaplan–Meier: cure rate | NA* | 103 (99.1) | 84 (100) | 51 (100) |
*PCR analysis to differentiate between recrudescence and new infection was not done for the two patients in the DHA/PPQ group in Bosaso because suitable samples were not available at the time of recurrence
ETF, early treatment failure; LCF, late clinical failure; LPF, late parasitological failure; ACPR, adequate clinical and parasitological response
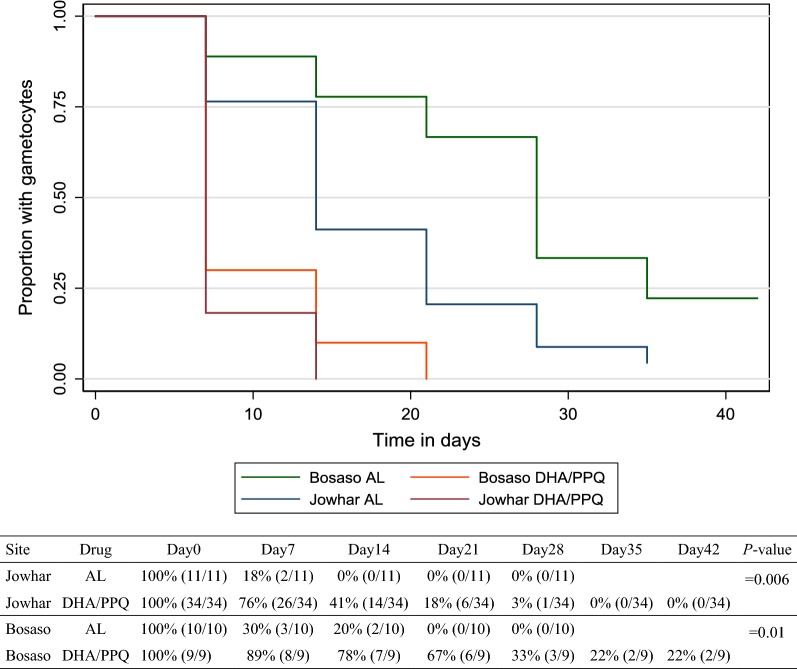
Of the patients with detectable gametocytaemia at enrolment, clearance of gametocytaemia was significantly faster with AL than with DP in Jowhar (HR: 3.0, 95% CI 1.4–6.5; P = 0.006) and in Bosaso (HR: 6.1, 95% CI 1.5–24.3; P = 0.01), as shown in Fig. 3. Only three and four patients who had no gametocytes at enrolment developed gametocytaemia after treatment AL and DHA/PPQ treatment, respectively.
Fig. 3.
Gametocyte clearance after treatment evaluated in patients with gametocytaemia at recruitment
Adverse events
One serious adverse event (death of a child) was recorded but it was not related to the study drug but to a car accident. The child, a girl aged 11 years, presented with an axillary temperature ≥ 38.2 °C and a parasite density of 44,466 asexual parasites/μL. She completed medication, and no parasites were detected on days 2 and 3 but did not attend the day 7 visit. Otherwise, no other adverse event was observed, and the drug was well tolerated.
PfK13 and PfPM2
Day-0 samples from the 139 patients in the AL study were tested for the presence of polymorphism in the Pfk13 gene and amplification of Pfpm2 genes. For the Pfk13 analysis, 138 samples gave interpretable results, and only one sample (0.7%) from Bosaso showed a non-synonymous mutation at codon 622 (R622I). Among the 139 samples analysed for Pfpm2 gene amplification, 135 gave interpretable results, and none showed PfPM2 amplification.
Discussion
The findings of the studies demonstrate that AL and DHA/PPQ are highly effective, supporting the recent recommendation of the Somalia NMCP for use of these artemisinin-based combinations as first-line and second-line treatments for uncomplicated malaria in Somalia. With respect to artemisinin resistance, none of the patients was parasitaemic on day 3 after either DHA/PPQ or AL, indicating an adequate response of the parasites to the artemisinin component. Pfk13 non-synonymous mutations (R622I) were present at a very low rate (1/138). The impact of this mutation on the parasite resistance phenotype is currently unknown, and it has also been detected in other locations in East Africa, such as Ethiopia [24]. Additional investigations, with in vitro testing of parasites carrying the R622I mutation are, therefore, essential to determine the extent to which circulation of this allele in East Africa is a threat.
The very high ACPR rate with both artemisinin-based combinations indicates excellent therapeutic activity of both partner drugs. This was confirmed genetically for PPQ by the absence of Pfpm2 amplification in the parasites collected from the study areas. The same analysis could not be conducted for lumefantrine resistance because of the absence of reliable molecular markers.
AL, the most commonly recommended ACT medicine in malaria-endemic countries, remains highly efficacious in Africa, despite its use as first-line treatment of uncomplicated falciparum malaria for more than a decade [25–41]. DHA/PPQ has recently been adopted as second-line treatment in several malaria-endemic countries in the African and Eastern Mediterranean regions, and the available data show high efficacy [25, 27, 30, 34–37, 39, 41, 42]. This high efficacy may be explained by the recent introduction of DHA/PPQ as second-line treatment, suggesting weak drug pressure through its limited use. Previous experience with use of DHA/PPQ in countries in the Greater Mekong sub-region nevertheless shows that, initial high efficacy (96–98%) [43, 44] can decrease rapidly [7, 8, 13, 45]. In Cambodia, DHA/PPQ was recommended as first-line treatment in 2008 in Pailin Province and in 2010 for the whole country [3] because of unacceptably high treatment failure with artesunate–mefloquine [46]. This was immediately followed by a substantial decrease in the efficacy of DHA/PPQ in Pailin (from 89% in 2008–2009 to 75% in 2010–2011) [47]. A study in 2012–2013 confirmed the rapid decrease in treatment efficacy and its spread to other provinces of Cambodia [13]. Recent studies have shown that DHA/PPQ treatment failure is associated with resistance to PPQ [13, 45]. Similarly, an increase in the DHA/PPQ treatment failure rate from 0 to 26% was found over 3 years in Viet Nam, with a background of increased molecular markers for artemisinin and PPQ resistance [7], as confirmed by Phuc et al. [8]. The explanation for this unprecedented, generalized failure of an ACT was later provided by demonstration of parasites that are multi-resistant to DHA and PPQ [8] and their spread in the Greater Mekong sub-region [48]. The reason for the rapid emergence of DHA/PPQ resistance in this sub-region is unknown; however, pre-existing circulation of parasites resistant to artemisinin or PPQ in this region was certainly a major facilitator for their evolution to multidrug resistance. This history of rapid resistance selection calls for combining any DHA/PPQ implementation initiative with regular, close clinical and biological monitoring of resistance.
The post-treatment rate of gametocytaemia clearance varied significantly between AL and DP treated groups, with DHA/PPQ being less potent, confirming previous reports on slower gametocyte clearance with DHA/PPQ treatment than with AL [49–54]. The cause of the differential effect of DHA/PPQ and AL on clearance of gametocytaemia is not clear but might be linked to the different partner drug activity.
Conclusions
The findings of these studies document high efficacy of AL and DHA/PPQ for the treatment of uncomplicated falciparum malaria as well as lack of evidence of resistance to artemisinins and PPQ, showing the relevance of these two combinations in the chemotherapeutic strategy for malaria control in Somalia. Regular monitoring of the efficacy of these artemisinin-based combinations and of markers of resistance for artemisinin and partner drugs should be continued in order to generate evidence to inform national malaria treatment policies.
Acknowledgements
The authors would like to thank the patients and the parents or guardians of the study children and the staff at the sentinel sites. We thank Dr. Abdikarim Muse for his support to the quality control of the slides, and Dr. Mx Petzold for his support to the data analysis.
Disclaimer
Pascal Ringwald is a staff member of the WHO and Marian Warsame is a recently retired from the WHO. They are responsible for the views expressed in this publication, which do not necessarily represent the decisions, policy or views of the WHO.
Abbreviations
- ACPR
adequate clinical and parasitological response
- ACT
artemisinin-based combination therapy
- AL
artemether–lumefantrine
- DHA/PPQ
dihydroartemisinin–piperaquine
- glurp
glutamate-rich protein gene
- NMCP
National Malaria Control Programme
- msp1
merozoite surface protein 1 gene
- msp2
merozoite surface protein 2 gene
- Pfk13
Plasmodium falciparum Kelch 13 gene
- Pfpm2
Plasmodium falciparum plasmepsin2 gene
- WHO
World Health Organization
Authors’ contributions
MW conceived the study concept, analysed the data and drafted the manuscript. AHH, AMJ and AHG, ZSN, MSM conducted the studies and collected data. AMH and AMA supervised conduct of the studies and controlled the quality of blood smears. AYM contributed to quality control of blood slides and data validation. FEY led data management and data validation. JGHA supported conduct of the studies conduct and contributed to the manuscript preparation. BW and NK analysed of parasite genotyping and molecular markers for artemisinin and PPQ resistance and contributed to writing the manuscript. All authors read and approved the final manuscript.
Funding
The studies were funded by the Bill & Melinda Gates Foundation (Grant No. OPP1140599). The funding body has no role in the design of the studies or in the collection, analysis or interpretation of data or in writing the manuscript.
Availability of data and materials
The datasets generated and analysed during the current studies have been shared with WHO to contribute to the WHO database.
Ethics approval and consent to participate
The study protocol was approved by the Ministry of Health Federal Government of Somalia, the Ministry of Health of Puntland and the WHO Research Ethics Review Committee (ERC0002740, ERC0002915). In Jowhar, community leaders of the study villages were informed about the study objectives and procedures and gave their permission. Adult patients or parents/guardians of study children provided written consent prior to enrolment. In addition to consent from parents/guardians, children aged ≥ 12 years provided assent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO. World Malaria Report 2017. Geneva: World Health Organization; 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/en/.
- 2.WHO. Guidelines for the treatment of malaria. 3rd edn. Geneva: World Health Organization; 2015. https://www.who.int/malaria/publications/atoz/9789241500470/en/. [PubMed]
- 3.WHO. Status report on artemisinin resistance and artemisinin-based combination therapy efficacy (August 2018). Geneva: World Health Organization; 2018. https://www.who.int/malaria/publications/atoz/artemisinin-resistance-august2018/en/.
- 4.Takala-Harrison S, Jacob CG, Arze C, Cummings MP, Silva JC, Dondorp AM, et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in southeast Asia. J Infect Dis. 2015;211:670–679. doi: 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashley EA, Dhorda M, Fairhurst RM, Amaratunga C, Lim P, Suon S, et al. Tracking resistance to artemisinin collaboration (T.R.A.C) spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–423. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tun KM, Imwong M, Lwin KM, Win AA, Hlaing TM, Hlaing T, et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis. 2015;15:415–421. doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thanh NV, Thuy-Nhien N, Tuyen NT, Tong NT, Nha-Ca NT, Dong LT, et al. Rapid decline in the susceptibility of Plasmodium falciparum to dihydroartemisinin–piperaquine in the south of Vietnam. Malar J. 2017;16:27. doi: 10.1186/s12936-017-1680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phuc BQ, Rasmussen C, Duong TT, Dong LT, Loi MA, Ménard D, et al. Treatment failure of dihydroartemisinin/piperaquine for Plasmodium falciparum Malaria, Vietnam. Emerg Infect Dis. 2017;23:715–717. doi: 10.3201/eid2304.161872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Witkowski B, Duru V, Khim N, Ross LS, Saintpierre B, Beghain J, et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype-genotype association study. Lancet Infect Dis. 2017;17:174. doi: 10.1016/S1473-3099(16)30415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spring MD, Lin JT, Manning JE, Vanachayangkul P, Somethy S, Bun R, et al. Dihydroartemisinin–piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect Dis. 2015;15:683–691. doi: 10.1016/S1473-3099(15)70049-6. [DOI] [PubMed] [Google Scholar]
- 12.Leang R, Taylor WR, Bouth DM, Song L, Tarning J, Char MC, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in Western Cambodia: dihydroartemisinin–piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59:4719–4726. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, et al. Dihydroartemisinin–piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis. 2016;16:357–365. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menard D, Khim N, Beghain J, Adegnika AA, Shafiul-Alam M, Amodu O, et al. A worldwide map of Plasmodium falciparum K13-propeller polymorphisms. N Engl J Med. 2016;374:2453–2464. doi: 10.1056/NEJMoa1513137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu F, Culleton R, Zhang M, Ramaprasad A, von Seidlein L, Zhou H, et al. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med. 2017;376:991–993. doi: 10.1056/NEJMc1612765. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Global report on antimalarial drug efficacy and drug resistance: 2000–2010. Geneva: World Health Organization; 2010. https://www.who.int/malaria/publications/atoz/9789241500470/en/.
- 17.WHO. Methods for surveillance of antimalarial drug efficacy. Geneva: World Health Organization; 2009. https://www.who.int/malaria/publications/atoz/9789241597531/en/.
- 18.Warsame M, Hassan AM, Barrette A, Jibril AM, Elmi HH, Arale AM, et al. Treatment of uncomplicated malaria with artesunate plus sulfadoxine–pyrimethamine is failing in Somalia: evidence from therapeutic efficacy studies and Pfdhfr and Pfdhps mutant alleles. Trop Med Int Health. 2015;20:510–517. doi: 10.1111/tmi.12458. [DOI] [PubMed] [Google Scholar]
- 19.Warsame M, Hassan AH, Hassan AM, Arale AM, Jibril AM, Mohamud SA, et al. Efficacy of artesunate + sulphadoxine/pyrimethamine and artemether + lumefantrine and dhfr and dhps mutations in Somalia: evidence for updating the malaria treatment policy. Trop Med Int Health. 2017;22:415–422. doi: 10.1111/tmi.12847. [DOI] [PubMed] [Google Scholar]
- 20.Warsame M, Perlmann H, Ali S, Hagi H, Farah S, Lebbad M, Björkman A. The seroreactivity against Pf155 (RESA) antigen in villagers from a mesoendemic area in Somalia. Trop Med Parasitol. 1989;40:412–414. [PubMed] [Google Scholar]
- 21.Noor AM, Moloney G, Borle M, Fegan GW, Shewchuk T, Snow RW. The use of mosquito nets and the prevalence of Plasmodium falciparum infection in rural South Central Somalia. PLoS One. 2008;3:e2081. doi: 10.1371/journal.pone.0002081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Canier L, Khim N, Kim S, Sluydts V, Heng S, Dourng D, et al. An innovative tool for moving malaria PCR detection of parasite reservoir into the field. Malar J. 2013;12:405. doi: 10.1186/1475-2875-12-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. Geneva: World Health Organization; 2008. http://apps.who.int/iris/bitstream/10665/43824/1/9789241596305_eng.pdf.
- 24.Bayih AG, Getnet G, Alemu A, Getie S, Mohon AN, Pillai DR. A unique Plasmodium falciparum K13 gene mutation in Northeast Ethiopia. Am J Trop Med Hyg. 2016;94:132–135. doi: 10.4269/ajtmh.15-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogutu BR, Onyango KO, Koskei N, Omondi EK, Ongecha JM, Otieno GA, et al. Efficacy and safety of artemether–lumefantrine and dihydroartemisinin–piperaquine in the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children aged less than five years: results of an open-label, randomized, single-centre study. Malar J. 2014;13:33. doi: 10.1186/1475-2875-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paczkowski M, Mwandama D, Marthey D, Luka M, Makuta G, Sande J, et al. In vivo efficacy of artemether–lumefantrine and artesunate–amodiaquine for uncomplicated Plasmodium falciparum malaria in Malawi, 2014. Malar J. 2016;15:236. doi: 10.1186/s12936-016-1281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sow D, Ndiaye JL, Sylla K, Ba MS, Tine RCK, Faye B, et al. Evaluation of the efficacy and safety of three 2-drug combinations for the treatment of uncomplicated Plasmodium falciparum malaria in Senegal: artesunate–amodiaquine, dihydroartemisinin–piperaquine, and artemether lumefantrine. Med Sante Trop. 2016;26:45–50. doi: 10.1684/mst.2015.0524. [DOI] [PubMed] [Google Scholar]
- 28.de Wit M, Funk AL, Moussally K, Nkuba DA, Siddiqui R, Bil K, et al. In vivo efficacy of artesunate–amodiaquine and artemether–lumefantrine for the treatment of uncomplicated falciparum malaria: an open-randomized, non-inferiority clinical trial in South Kivu, Democratic Republic of Congo. Malar J. 2016;15:455. doi: 10.1186/s12936-016-1444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogouyèmi-Hounto A, Azandossessi C, Lawani S, Damien G, de Tove YS, Remoue F, et al. Therapeutic efficacy of artemether–lumefantrine for the treatment of uncomplicated falciparum malaria in northwest Benin. Malar J. 2016;15:37. doi: 10.1186/s12936-016-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ursing J, Rombo L, Rodrigues A, Kofoed PE. Artemether–lumefantrine versus dihydroartemisinin–piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in children aged less than 15 years in Guinea-Bissau—an open-label non-inferiority randomised clinical trial. PLoS One. 2016;1:e0161495. doi: 10.1371/journal.pone.0161495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salvador C, Rafael B, Matsinhe F, Candrinho B, Muthemba R, De Carvalho E, et al. Efficacy and safety of artemether–lumefantrine for the treatment of uncomplicated falciparum malaria at sentinel sites in Mozambique, 2015. Acta Trop. 2017;171:146–150. doi: 10.1016/j.actatropica.2017.03.032. [DOI] [PubMed] [Google Scholar]
- 32.Konaté A, Barro-Kiki PCM, Angora KE, Bédia-Tanoh AV, Djohan V, Kassi KF, et al. Efficacy and tolerability of artesunate-amodiaquine versus artemether–lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria at two sentinel sites across Côte d’Ivoire. Ann Parasitol. 2018;64:49–57. doi: 10.17420/ap6401.132. [DOI] [PubMed] [Google Scholar]
- 33.Roth JM, Sawa P, Makio N, Omweri G, Osoti V, Okach S, et al. Pyronaridine–artesunate and artemether–lumefantrine for the treatment of uncomplicated Plasmodium falciparum malaria in Kenyan children: a randomized controlled non-inferiority trial. Malar J. 2018;15(17):199. doi: 10.1186/s12936-018-2340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith SJ, Kamara ARY, Sahr F, Samai M, Swaray AS, Menard D, et al. Efficacy of artemisinin-based combination therapies and prevalence of molecular markers associated with artemisinin, piperaquine and sulfadoxine–pyrimethamine resistance in Sierra Leone. Acta Trop. 2018;185:363–370. doi: 10.1016/j.actatropica.2018.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dama S, Niangaly H, Djimde M, Sagara I, Guindo CO, Zeguime A, Dara A, et al. A randomized trial of dihydroartemisinin–piperaquine versus artemether–lumefantrine for treatment of uncomplicated Plasmodium falciparum malaria in Mali. Malar J. 2018;17:347. doi: 10.1186/s12936-018-2496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandara CI, Kavishe RA, Gesase S, Mghamba J, Ngadaya E, Mmbuji P, et al. High efficacy of artemether–lumefantrine and dihydroartemisinin–piperaquine for the treatment of uncomplicated falciparum malaria in Muheza and Kigoma Districts, Tanzania. Malar J. 2018;17:261. doi: 10.1186/s12936-018-2409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davlantes E, Dimbu PR, Ferreira CM, Florinda Joao M, Pode D, Félix J, et al. Efficacy and safety of artemether–lumefantrine, artesunate–amodiaquine, and dihydroartemisinin–piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in three provinces in Angola, 2017. Malar J. 2018;17:144. doi: 10.1186/s12936-018-2290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayalew MB. Therapeutic efficacy of artemether–lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Ethiopia: a systematic review and meta-analysis. Infect Dis Poverty. 2017;6:157. doi: 10.1186/s40249-017-0372-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yeka A, Wallender E, Mulebeke R, Kibuuka A, Kigozi R, Bosco A, et al. Comparative efficacy of artemether–lumefantrine and dihydroartemisinin–piperaquine for the treatment of uncomplicated malaria in Ugandan children. J Infect Dis. 2019;219:1112–1120. doi: 10.1093/infdis/jiy637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebenebe JC, Ntadom G, Ambe J, Wammanda R, Jiya N, Finomo F, et al. Efficacy of artemisinin-based combination treatments of uncomplicated falciparum malaria in under-five-year-old Nigerian children ten years following adoption as first-line antimalarials. Am J Trop Med Hyg. 2018;99:649–664. doi: 10.4269/ajtmh.18-0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kakolwa MA, Mahende MK, Ishengoma DS, Mandara CI, Ngasala B, Kamugisha E, et al. Efficacy and safety of artemisinin-based combination therapy, and molecular markers for artemisinin and piperaquine resistance in Mainland Tanzania. Malar J. 2018;17:369. doi: 10.1186/s12936-018-2524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandara CI, Francis F, Chiduo MG, Ngasala B, Mandike R, Mkude S, et al. High cure rates and tolerability of artesunate-amodiaquine and dihydroartemisinin–piperaquine for the treatment of uncomplicated falciparum malaria in Kibaha and Kigoma, Tanzania. Malar J. 2019;18:99. doi: 10.1186/s12936-019-2740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janssens B, van Herp M, Goubert L, Chan S, Uong S, Nong S, et al. A randomized open study to assess the efficacy and tolerability of dihydroartemisinin–piperaquine for the treatment of uncomplicated falciparum malaria in Cambodia. Trop Med Int Health. 2007;12:251–259. doi: 10.1111/j.1365-3156.2006.01786.x. [DOI] [PubMed] [Google Scholar]
- 44.Song J, Socheat D, Tan B, Seila S, Xu Y, Ou F, et al. Randomized trials of artemisinin–piperaquine, dihydroartemisinin–piperaquine phosphate and artemether–lumefantrine for the treatment of multi-drug resistant falciparum malaria in Cambodia–Thailand border area. Malar J. 2011;10:231. doi: 10.1186/1475-2875-10-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amato R, Lim P, Miotto O, Amaratunga C, Dek D, Pearson RD, et al. Genetic markers associated with dihydroartemisinin–piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype–phenotype association study. Lancet Infect Dis. 2017;17:164–173. doi: 10.1016/S1473-3099(16)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denis MB, Tsuyuoka R, Poravuth Y, Narann TS, Seila S, Lim C, et al. Surveillance of the efficacy of artesunate and mefloquine combination for the treatment of uncomplicated falciparum malaria in Cambodia. Trop Med Int Health. 2006;11:1360–1366. doi: 10.1111/j.1365-3156.2006.01690.x. [DOI] [PubMed] [Google Scholar]
- 47.Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, et al. Efficacy of dihydroartemisinin–piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob Agents Chemother. 2013;57:818–826. doi: 10.1128/AAC.00686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Imwong M, Hien TT, Thuy-Nhien NT, Dondorp AM, White NJ. Spread of a single multidrug resistant malaria parasite lineage (PfPailin) to Vietnam. Lancet Infect Dis. 2017;17:1022–1023. doi: 10.1016/S1473-3099(17)30524-8. [DOI] [PubMed] [Google Scholar]
- 49.Mens PF, Sawa P, van Amsterdam SM, Versteeg I, Omar SA, Schallig HD, et al. A randomized trial to monitor the efficacy and effectiveness by QT-NASBA of artemether–lumefantrine versus dihydroartemisinin–piperaquine for treatment and transmission control of uncomplicated Plasmodium falciparum malaria in western Kenya. Malar J. 2008;7:237. doi: 10.1186/1475-2875-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zwang J, Ashley EA, Karema C, D’Alessandro U, Smithuis F, Dorsey G, et al. Safety and efficacy of dihydroartemisinin–piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One. 2009;4:e6358. doi: 10.1371/journal.pone.0006358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smithuis F, Kyaw MK, Phe O, Win T, Aung PP, Oo AP, et al. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect Dis. 2010;10:673–681. doi: 10.1016/S1473-3099(10)70187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kakuru A, Jagannathan P, Arinaitwe E, Wanzira H, Muhindo M, Bigira V, et al. The effects of ACT treatment and TS prophylaxis on Plasmodium falciparum gametocytemia in a cohort of young Ugandan children. Am J Trop Med Hyg. 2013;88:736–743. doi: 10.4269/ajtmh.12-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sawa P, Shekalaghe SA, Drakeley CJ, Sutherland CJ, Mweresa CK, Baidjoe AY, et al. Malaria transmission after artemether–lumefantrine and dihydroartemisinin–piperaquine: a randomized trial. J Infect Dis. 2013;207:1637–1645. doi: 10.1093/infdis/jit077. [DOI] [PubMed] [Google Scholar]
- 54.WWARN Gametocyte Study Group Gametocyte carriage in uncomplicated Plasmodium falciparum malaria following treatment with artemisinin combination therapy: a systematic review and meta-analysis of individual patient data. BMC Med. 2016;14:79. doi: 10.1186/s12916-016-0621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analysed during the current studies have been shared with WHO to contribute to the WHO database.