Abstract
Vitamin D3 reported in Italy in 2017 the net expenditure of almost €180 million, reaching the first place for consumption and the third place for conventional pharmaceutical spending. The aim of the study was to evaluate whether a shift of vitamin D3 prescriptions toward 100 000 IU formulation, less costly, could allow savings from the health-care perspective. An approach promoting the prescription of this formulation has been applied in a local health authority (ASL CN2) in Piedmont Region (Italy) starting from 2015. The retail pharmaceutical market and the consumption of vitamin D3 has been analyzed from year 2014 to 2017 in order to evaluate differences in expenditures. Despite an increase in consumption, the introduction of the new formulation enabled ASL CN2 to save about €280 000 in 2017 considering the regional average expenditure per 1000 inhabitants as a reference. If Piedmont Region had presented an average expenditure in line with that of ASL CN2, the annual regional savings would have exceeded €7 million in 2017 alone. A shift of vitamin D3 prescriptions toward 100 000 IU formulations would allow reducing costs from the payer perspective. Savings may be used to contain public pharmaceutical expenditure or can be allocated to fund other health-care technologies and services.
Keywords: vitamin D3, cholecalciferol, monthly formulation, economic evaluation, health policy
Introduction
Vitamin D3, also called cholecalciferol, is a hormone with an important role in terms of calcium and bone homeostasis. It is a fat-soluble vitamin, naturally present in few foods: fatty fish (salmon, tuna, eel, sardines, and swordfish), cod liver oil, egg yolks, beef liver, and some types of mushrooms. Dietary sources are represented by foods reinforced with vitamin D3 (supplements), such as milk, breakfast cereals, and so on. Vitamin D3 is also produced endogenously by the effect of ultraviolet rays of sunlight on the skin, which activate vitamin D3 synthesis.1
Whether obtained from sun exposure, foods or supplements, vitamin D3 is biologically inert and, to be activated, it must undergo 2 subsequent processes of hydroxylation in the body. The first occurs in the liver and converts vitamin D3 into 25-hydroxyvitamin D (25[OH]D), also known as calcidiol or calcifediol; the subsequent, which occurs mainly in the kidney, determines the hydroxylation of 25(OH)D into the physiologically active form, 1,25-dihydroxyvitamin D, calcitriol, or cholecalciferol, natural ligand of the vitamin D3 receptor.
In the last 20 years, vitamin D3 has been the subject of numerous observational studies that have correlated the blood levels of vitamin D3 with different pathological conditions such as diabetes, cancer, coronary heart disease, infections, and autoimmune and respiratory diseases.1–3
The role of vitamin D3 in intestinal calcium absorption and in the physiology of bone tissue is now known, but the data supporting an extended use of vitamin D in clinical practice are still few and the results of the randomized studies are conflicting and tendentially unsatisfactory.4
A recent meta-analysis claims that the positive effects on falls, fractures, cardiovascular events, neoplastic diseases, and mortality are in fact conflicting.5 Even the Italian Osteoporosis, Mineral Metabolism, and Skeletal Diseases Society (SIOMMMS)6 claims that the effects of vitamin D3 supplementation on bone mineral density are on average modest and documented mainly only in the femoral area. Based on the data available in the literature, vitamin D3 should be administered in prophylaxis in the newborn (especially in the premature), in the infant and in the pregnant woman (last trimester), in the woman who breastfeeds at the end of winter and in spring, in the institutionalized elderly patient, in cases of low sun exposure or malabsorption syndromes, in patients receiving anticonvulsants or long-term corticosteroids. The guidelines adopted by the American Institute of Medicine1 set the limit to 20 ng/mL, above which optimal calcium absorption and adequate levels of parathormone are guaranteed. The guidelines of the American Endocrine Society3 and SIOMMMS6 confirm this deficiency threshold but introduce the concept of “insufficiency” for levels between 20 and 30 ng/mL, shifting the normal limit between 30 and 100 ng/mL as reported from an “enthusiastic” study on vitamin D published in 2007.2
The difference between the 2 approaches is apparently small but has enormous repercussions on the population to be treated. Considering the data collected in the majority of Italian laboratories, extending the supplementary treatment to the so-called “insufficiency” area would mean applying a label of abnormality to almost the entire adult population.
Over the past 20 years, several studies on vitamin D status have been performed in Italy. Isaia et al reported in elderly Italian women aged 60 to 80 years, 25(OH)D circulating levels less than 12 ng/mL (30 nmol/L) in 76%.7 Fewer reports on vitamin D incidence, determinants, and metabolic consequences are available in young patients. An Italian study published by Adami et al reported in 608 young healthy premenopausal women, age range from 20 to 49, levels of 25(OH)D below 20 ng/mL were found in almost a third of the women and its levels were inversely (P < .001) related with age.8
Although the determination of the levels of 25(OH)D expresses a vitamin status of insufficiency in the vast majority of individuals, this does not justify the implementation of a screening of the general population regarding the blood levels of vitamin D3. No scientific organization, either national or international, currently recommends a generalized screening. There is a danger of incorrectly classifying vitamin D3 values because of the inaccurate threshold that identifies the deficiency status and because of the variability of laboratory tests. This can result in a very wide diagnosis of deficits, which can lead to unnecessary and unjustified treatment, or a narrow diagnosis of deficits, which determines the nontreatment of people who would need it.
The measurement of 25(OH)D levels is instead appropriate in patients with a vitamin D deficiency status associated with possible pathological conditions (eg, rickets, osteomalacia, osteoporosis, chronic renal failure, liver failure, malabsorption syndrome, primitive or secondary hyperparathyroidism) and therefore belonging to a risk category.
To estimate the vitamin D3 requirement, it is necessary to know the amount normally used by the body. This requirement of the organism has never been defined and must therefore be estimated empirically. The amount of vitamin D3 to be taken is normally expressed in international units (IU) of cholecalciferol. One IU corresponds to 0.025 µg (1 µg of cholecalciferol = 40 IU). International Defined Daily Dose (DDD) corresponds to 20 µg = 800 IU.
Based on the assays used in various trials for the treatment of osteoporosis, which involved the administration of vitamin D3 to patients with deficiency or inadequacy, it was estimated that to reach levels of 25(OH)D equal or higher than 75 nmol/L it is necessary to take between 1800 and 4000 IU of vitamin D3 daily. We can therefore assume an average daily requirement of vitamin D3 around 1500 to 2300 IU per day, taking into account that it may increase with age, body mass, fat mass, and calcium intake.6
The recommended dose of vitamin D3 is expressed as a daily dosage. However, for the same cumulative dose, vitamin D3 can also be administered through weekly boluses. To improve adherence to treatment, it is also possible to resort to monthly or quarterly boluses; in this case, the equivalent doses, according to some authors, should be greater than the cumulative daily or weekly doses. The intake of such high amounts of vitamin D3 single or tight, the so-called “annual boli”, considered by many compatible with the achievement and maintenance of “physiological” levels of 25(OH)D, was strongly challenged by the results of only 2 randomized controlled trials available today.9,10 In the groups treated with high oral or parenteral doses of vitamin D3, a significant paradoxical increase in falls and/or fractures was observed.
To understand the safety parameters of therapeutic vitamin D supplementation, it is important to know that 10 000 to 25 000 IU/d are made in the skin in response to adequate sunlight exposure.11 According to the Institute of Medicine, a serum 25(OH)D level of 25 ng/mL is adequate for most of the population while levels >50 are high.12 Serum 25(OH)D of 100 ng/mL is considered by several groups to be the laboratory upper normal limit.3 The safety parameters are guaranteed as a bolus of 100 000 IU of colecalciferol raises the levels of 25 (OH)D by 3 ng/mL for 3 months,13 one of 300 000 IU maintains levels above 32 ng/mL for at least 2 months14 while 600 000 IU of about 40 ng/mL for 3 months.15
The recommended dosing schedules should take into account potential interferences of other drugs or morbid conditions. The dosage of vitamin D3 to be recommended may therefore also vary depending on the clinical condition treated and the therapeutic objectives that are set. In patients who have not received any supplements in the last year, the cumulative therapeutic dose, to be divided into 8 to 12 weeks, is 600 000, 400 000, and 100 000 for base value 25(OH)D of <10 ng/mL or 25 nmol/L, 10 to 20 ng/mL or 25 to 50 nmol/L, and 20 to 30 ng/mL or 50 to 75 nmol/L, respectively. The daily maintenance dose (IU) for these categories is 2000, 1000, and 800, respectively.
Vitamin D3 is normally administered orally, limiting the use of the intramuscular route to patients with severe malabsorption syndromes.
In the last decade, there has been a progressive increase in the consumption of drugs for osteoporosis. The adequacy of the prescription of vitamin D is not simple since the authorized indications foresee the administration at a daily, weekly, monthly, or annual rate up to a maximum of 600 000 annual IU. Furthermore, vitamin D therapy is considered a safe drug with studies revealing that long-term doses up to 10 000 IU per day are not associated with toxicity.12
In Italy, the users of vitamin D in year 2017 have been estimated in 6.4 million people (10.3% of the total population).16 The ATC class “Vitamin D and analogues” recorded values, with exponential growth, going from a gross expenditure per capita of €2.0 in 2014 to €3.7 in 2016.17,18 Among them stands out the cholecalciferol, or vitamin D3, which reached in 2017 the net expenditure of nearly €180 million, up 30% compared to 2016, reaching the first place for consumption in DDD and the third place overall for pharmaceutical spending agreement.19
The present study investigated the prescriptions charged to the Italian National Health Service of vitamin D3 in from 2014 to 2017 years in order to evaluate possible saving strategies.
Methods
An accurate analysis of all the prescriptions charged to the Italian National Health Service of vitamin D3 from 2014 to 2017 has been carried out. Data were available from IMS Health related to SFERA Project (Spesa Farmaceutica Elaborazioni Regioni Asl). The data in the summary accounting statements, allocated in a specific database for the purpose of reimbursement of pharmacies, are used for the generation of expense reports. These reports show the main economic data of the territorial pharmaceutical assistance (number of recipes, gross expenditure, ticket, discount, etc) and the indicators related to them (total and per capita recipes, gross and net total and per-capita expenditure for period and by month, etc). No ethical issues are involved in the use of these data as they report aggregated indicators.
The analysis focused in particular on the trend of the retail medicine market and consumption of vitamin D3 considering the DDD, that is, the average daily dose taken for the main indication of a drug in an adult patient.
The commercial formulations of cholecalciferol were compared (Table 1) and the data were shared with a multidisciplinary approach with prescribers, general practitioners (GPs) and specialists, in a local health authority (ASL CN2) in Piedmont Region (Italy) in May 2015.
Table 1.
Reference Packages for Active Principle Cholecalciferol.a
| Reference Packs | Reference Price | Expired Patent |
|---|---|---|
| 10 000 IU/mL 10 mL GTT-ORAL USE | €4.50 | Yes |
| 25 000 IU/2.5 mL-ORAL USE | €4.50 | Yes |
| 100 000 IU/mL-6F IM OS | €4.00 | No |
| 300 000 IU/mL-6F IM OS | €3.50 | No |
| 50 000 IU/5 mL-ORAL USE | €7.90 | No |
| 50 000 IU/2.5 mL-ORAL USE | €8.50 | No |
aNational values, updated to March 17, 2015.
The formulations containing 25 000 IU of cholecalciferol were found to be the most expensive when compared to the cost of the packs of 100 000 IU and 300 000 IU (6 vials per pack), which can be administered both parenterally and orally (Tables 1 and 2). It was therefore agreed in ASL CN2, from May 2015, to follow a different therapeutic approach, taking into account the possible containment of pharmaceutical spending, using the same international units of cholecalciferol, promoting the orally assumption of the formulation of 100 000 IU a month, in single administration, instead of taking the formulation of 25 000 IU per week.
Table 2.
Annual Prophylaxis (P) and Treatment (T) Costs for the Various Formulations of Cholecalciferol.
| Dosage Form | Dose | Agreed Cost |
|---|---|---|
| Gtt (10 000 IU/mL 10 mL) | T: 40 gtt/die for 2 months | €27.00 |
| P: 4 gtt/die; 28 gtt/wk | €13.50 | |
| Vials OS 25 000 IU/2.5 mL | T: 50 000 IU/wk for 12 weeks | €108.00 |
| P: 25 000 IU/month | €54.00 | |
| Vials OS 50 000 IU/5 mL | T: 50 000 IU/wk for 12 weeks | €94.80 |
| P: 50 000 IU/2 months | €47.40 | |
| Vials 100 000 IU/mL (6 vials) | T: 200 000 IU/month for 3 months | €4.00 |
| P: 100 000 IU/month for 4 months | €2.00 | |
| Vials 300 000 IU/mL (2 vials) | T: 1 vial for 6 weeks | €3.50 |
| P: 1 vial for 12 months | €1.75 |
The different approach was evaluated by the specialist in endocrinology of this ASL, based on the SIOMMMS guidelines6 and supported by a randomized double blind controlled trial in which 2037 men and 649 women, older than 65 years, were treated with a 100 000 IU oral vitamin D3 (cholecalciferol) supplementation or matching placebo every 4 months over 5 years. This study claims that 4 monthly supplementations with 100 000 IU oral vitamin D may prevent fractures without adverse effects in men and women living in the general community.20
In order to evaluate the performances of the various local health authorities in the regional area, the lost savings for each local health authority have been compared to the best performer. Savings have been calculated according to the following formula:
A similar analysis estimated the incremental costs or savings for the local health authorities while considering the Piedmont Region average expenditure per 1000 inhabitants as a reference. The expenditure variations have been calculated as:
In order to test the difference in expenditure trends before and after the policy change, that is, the promotion of the assumption of 100 000 IU per month instead of 25 000 IU per week, a difference in differences (DID) model has been estimated.21
The DID estimate is a methodology used mainly in the microeconometric field to estimate the effect of a “treatment” (for example the introduction of a policy provision) on a group of subjects (“treated”), relative to a second group of subjects not exposed to the treatment (“control” group). The 2 groups are observed in 2 periods, one preceding and one following the treatment. Once the data set is defined, the regression model for the estimation takes the form y = β0 + β1dB + δ0d2 + δ1d2dB + u, where y is the variable of interest, observed in the periods 1 and 2; d2 is a dummy variable that assumes a value equal to 1 in the second period and 0 otherwise; dB is a dummy variable that assumes a value of 1 in the case of “treated” patients and 0 otherwise. In other words, this last variable captures the possible a priori differences between the treated group and the control group. The variable d2dB represents the interaction between d2 and dB and assumes a value of 1 in correspondence with the patients of the group of treated in the second period. The parameter of interest, that is, the one that expresses the effect of the treatment on the treated, is precisely the coefficient relative to the latter variable (δ1). The variable u indicates the error term.
Results
The majority of the population who assumed cholecalciferol was found to be in treatment with the formulations containing 25 000 IU (about 70% of consumption), although recent studies show that the use of such formulations is not related to a greater benefit for the patient.22,23
In the local health authority ASL CN2, in the first 4 months of 2015, a net expenditure of €447.86 per 1000 inhabitants was recorded in relation to the cholecalciferol molecule alone for the retail market, within a variance of more than 23% of the regional average, essentially doubling the spending in the same period of 2014.
In agreement with GPs and specialists of ASL CN2, it was decided to use a loading dose of 100 000 IU of cholecalciferol (1 vial every 2 weeks for 3 months, oral administration) followed by 100 000 IU every 2/4 months. This formulation would also be more advantageous in terms of therapeutic adherence, according to what is reported by the most recent SIOMMMS guidelines.6 The formulations in vials of 25 000 IU and 50 000 IU should be limited to cases of intolerance at the higher dosage or for problems related to the management of the vials. In the elderly, certainly deficient, particularly if institutionalized, it was agreed that there was no need to perform the dosage of vitamin D3 in the laboratory before treatment, thus eliminating a further cost to perform an examination that would surely confirm the deficiency.24
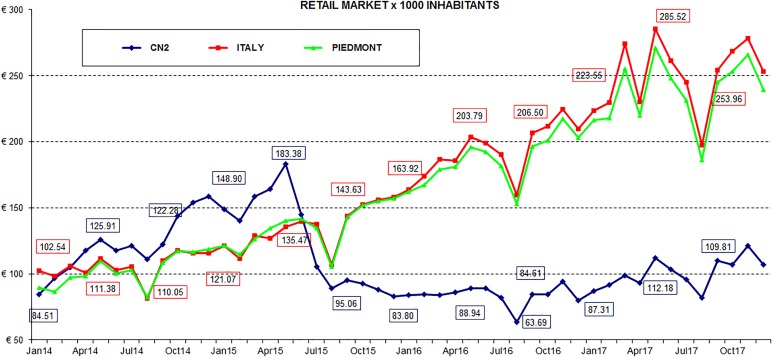
Following the new therapeutic approach, from June 2015, it was possible to observe an important slowdown in cholecalciferol expenditure: The monthly net expenditure per 1000 residents, charged to the National Healthcare System, increased significantly between January 2014 and May 2015, moving from €84.51 to €183.38, respectively. In the following 4 months, spending decreased rapidly, almost 50% compared to May. The lowest value was recorded in August 2016, equal to €63.69. Compared to the spending data of the Piedmont Region and Italy, the greatest deviation was recorded in March and May 2017, with an average regional and national value of about 2.5 times the average value of ASL CN2 (Figure 1).
Figure 1.
Net spending NHS per 1000 resident inhabitants from 2014 to 2017.
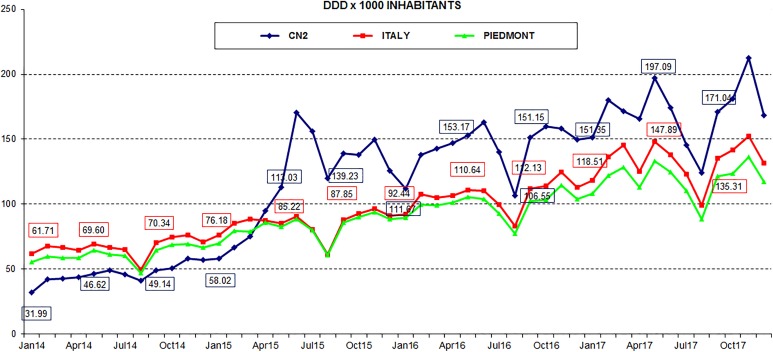
Figure 2 shows instead the consumption of the packages sent by ASL CN2 pharmacies expressed in terms of DDD per 1000 inhabitants. The graph shows a trend reversal of consumption since May 2015. The passage from colecalciferol packs containing 25 000 IU to 100 000 IU has recorded an increase in DDD, following the same trend of Piedmont Region and Italy.
Figure 2.
DDD per 1000 resident inhabitants from 2014 to 2017.
Table 3 shows the percentage of packages sent by the affiliated pharmacies of ASL CN2 divided by International Units, contained in the prescribed cholecalciferol packs. These data highlight how the percentage of packs of 25 000 IU decreased from 2014 to 2017 in contrast to the 100 000 IU packs which showed a significant increase. Also, the number of patients treated in the same period increased, affecting mainly adult female patients over 65 years of age (Supplementary Table 1 shows the data of the treated patients, divided by age and gender).
Table 3.
Percentage of Prescribed Cholecalciferol Packages Divided by International Units From 2014 to 2017.
| IU Dosage Form | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|
| 25 000 IU FL | 83.40% | 69.80% | 47.50% | 41.20% |
| 50 000 IU FL | - | 5.70% | 11.80% | 14.60% |
| 10 000 IU/mL 10 mL GTT | 15.00% | 12.80% | 14.20% | 13.80% |
| 100 000 IU 6F IM/OS | 0.90% | 11.10% | 25.80% | 29.60% |
| 300 000 IU 6F IM/OS | 0.70% | 0.60% | 0.70% | 0.80% |
Data related to year 2017 give even more relevance to the results obtained, as the cholecalciferol in Italy and Piedmont was found to be the third molecule in terms of net expenditure and the first in terms of consumption for the National Health System (Supplementary Tables 2 and 3) with percentages of increase compared to 2016 among the highest.
The choice of an appropriate packaging, promoted by ASL CN2 Pharmaceutical Service, has improved patient compliance and produced considerable savings. For ASL CN2, data show for year 2017 a very high consumption, with a value of DDD per 1000 inhabitants amounted to 2,041.63; on the other side, the net spending is much smaller, and cholecalciferol resulted only the 23rd molecule in terms of expenditure (Supplementary Table 4).
Finally, Table 4 shows the performances of the various local health authorities in the regional area in decreasing order of 2017 expenditure: the lost savings for each local health authority have been compared to the best performer (ASL CN2). The same table presents also the incremental costs or savings for the local health authorities while considering the Piedmont Region average expenditure per 1000 inhabitants as a reference.
Table 4.
Vitamin D3 Retail Market per 1000 Inhabitants in Year 2017 for the Different Local Health Authorities in Piedmont Region (Decreasing Order of Savings, in bold the areas under study).
| Local Health Authorities (ASL) and Region | Net Spending 2017 (€) | Number of Inhabitants 2017 | Net Spending per 1000 Inhabitants 2017 (€) | Lost Savings vs Best Performer (€) | Incremental Costs or Savings vs Piedmont Region Average Expenditure per 1000 Inhabitants (€) |
|---|---|---|---|---|---|
| TO1 | 1 827 108.19 | 463 123 | 3945.19 | 1 266 811.16 | 507 538.54 |
| NO | 1 239 129.80 | 343 400 | 3608.42 | 823 677.01 | 260 686.46 |
| AL | 1 560 679.14 | 439 343 | 3552.30 | 1 029 151.09 | 308 864.04 |
| VC | 586 305.79 | 173 949 | 3370.56 | 375 858.02 | 90 674.95 |
| PIEDMONT | 12 416 272.32 | 4 357 672 | 2849.29 | 7 144 250.13 | 0.00 |
| TO4 | 1 458 498.27 | 514 124 | 2836.86 | 836 498.73 | −6388.52 |
| VCO | 471 530.60 | 170676 | 2762.72 | 265 042.49 | −14 774.75 |
| TO3 | 1 524 193.22 | 577 405 | 2639.73 | 825 633.51 | −121 002.36 |
| AT | 518 932.08 | 205 030 | 2531.00 | 270 880.92 | −65 259.46 |
| TO2 | 980 694.31 | 406 189 | 2414.38 | 489 277.05 | −176 654.87 |
| BI | 411 969.45 | 173 182 | 2378.82 | 202 449.64 | −81 475.92 |
| TO5 | 698 105.16 | 305 000 | 2288.87 | 329 108.62 | −170 927.89 |
| CN1 | 934 459.20 | 417 069 | 2240.54 | 429 879.87 | −253 888.29 |
| CN2 | 204 667.14 | 169 172 | 1209.82 | 0.00 | −277 349.60 |
ASL CN2 invests about €1210 per 1000 inhabitants/year, compared with €2849 of regional average, but without, as we have seen, depriving patients of cholecalciferol supplementation; indeed, the choice to promote 100 000 IU formulations has led to greater use in terms of consumption. Considering the Regional average expenditure per 1000 inhabitants as a reference, this choice has allowed ASL CN2 to save about €280 000, only in year 2017, which should be added to the other proportional savings for year 2016 and 2015. By applying the same approach, savings of about €100 million could be estimated at national level.
The DID analysis has been performed in order to evaluate differences in trends for the average expenditure per 1000 inhabitants before and after the promotion of 100 000 IU monthly formulation. In this case, expenditures of ASL CN2 have been compared with the rest of Italy. The results are reported in Table 5. The differences-in-differences estimator (δ1) is equal to −124.350 and indicates the reduction of the average expenditure per 1000 inhabitants obtained through the promotion of 100 000 IU formulations; the cost reduction has a significant effect (P < .0001).
Table 5.
Regression Analysis Results for difference in differences model.
| Average Expenditure per 1000 Inhabitants | Coefficient | Robust Std. Err. | t | P > |t| | 95% Confidence Interval |
|---|---|---|---|---|---|
| Time | 91.695 | 9.036 | 10.150 | <.0001 | 73.749 to 109.642 |
| Treated | 19.671 | 6.619 | 2.970 | .004 | 6.525 to 32.818 |
| δ1 | −124.350 | 11.480 | −10.830 | <.0001 | −147.149 to−101.550 |
| _Cons | 109.735 | 2.912 | 37.690 | <.0001 | 103.952 to 115.518 |
Discussion
In Italy, vitamin D3 expenditure is a great concern: in 2017, the net expenditure was of almost €180 million, positioning it at the first place for consumption and at the third place for conventional pharmaceutical spending. The present study, to the best of our knowledge, is the first economic evaluation performed comparing the standard use of vitamin D3 prescriptions toward a different approach based on promoting the assumption of the formulation of 100 000 IU monthly, in single administration, instead of taking the formulation of 25 000 IU per week, in a local health authority in Piedmont region (ASL CN2) in Italy. The analyses started from the collection of the net expenditure and DDD of vitamin D3 per 1000 inhabitants in each regional (Piedmont) local health authority and for Italy.
The analyses showed that in ASL CN2 a shift of vitamin D3 prescriptions toward 100 000 IU formulations would allow to reduce the cost of vitamin D3 from the payer perspective. Despite an increase in consumption in comparison with past years, the introduction of the new formulation enabled ASL CN2 to save about €280 000 in 2017 considering the regional average expenditure per 1000 inhabitants as a reference. If the Piedmont region had presented an average expenditure in line with that of ASL CN2, the annual regional savings would have exceeded €7 million, in 2017 alone and, by applying the same approach, at national level, savings would have been about €100 million. The difference-in-differences analysis highlighted the statistically significant effect on the reduction of the average expenditure per 1000 inhabitants after the introduction of the new therapeutic approach.
The analyses showed also an increase in prescriptions, which could be indicative of a greater attention of GPs and specialists toward a widespread vitamin D3 deficiency, that also affects nonskeletal diseases but, above all, an increased risk of falls and fractures. These events mainly involve the population over 65 years, which in Italy is constantly increasing. The enhancement in prescriptions is therefore to be investigated from the point of view of evaluation and analysis of expenditure, in addition to the evaluation of “best practice”.
The study has few limitations. First, the different therapeutic approach promoting the use of monthly 100 000 IU formulation has been agreed with the endocrinologists and shared among medical specialists and GPs of ASL CN2, but it was not possible to measure to what extent the switch has been put in place in the clinical practice. Nevertheless, the analyses showed that this approach led to savings from the payer perspective and these savings should be considered conservative estimates. Secondly, although the difference-in-differences analysis showed a statistically significant trend of expenditures reduction after the promotion of the assumption of 100 000 IU per month, it was not possible to test whether other covariates may have influenced the savings. Thirdly, the analysis did not take into account the consequences related to the increase in prescriptions of vitamin D3 on the health-care system. If from one side an increased vitamin D3 utilization could rise the frequency of possible adverse events and consequently increase the expenditures related to their management, from the other side, a larger and adequate use of vitamin D3 could decrease mortality in elderly people living independently or in institutional care as reported by Bjelakovic and colleagues.25
Despite these limitations, the study provides for Regional and Italian estimates of potential savings from the health-care perspective due to the promotion of monthly vitamin D3 100 000 IU formulation instead of 25 000 IU formulation per week. These conclusions may help increasing the awareness that it is possible to achieve savings through a policy that endorses the use of less costly formulations. Savings may be used to contain public pharmaceutical expenditure or to fund innovative and cost-effective drugs or can be allocated to fund other health-care technologies and services.
Supplemental Material
Supplemental_material for Can a Different Formulation of Vitamin D3 Allow Savings? An Analysis From an Italian Regional Perspective by Mario Sanò, Patrizia Dutto, Stefano D’Anna and Carla Rognoni in Health Services Research and Managerial Epidemiology
Author Biographies
Mario Sanò holds a Master’s Degree in Pharmacy cum Laude, Specialization in Hospital Pharmacy and Specialization in Pharmacology. He attended various masters and courses. After experience in multinational pharmaceuticals companies with roles in pharmacoeconomics, market access and business, since 2000 he is an employee of NHS, with experience in various Local Health Authorities and Italian Regions. He is Hospital Pharmacy Director since 2008; at Local Health Authority CN2 since 2014.
Patrizia Dutto holds a Master’s Degree in Pharmacy and Specialization in Hospital Pharmacy. She is employee since 2010 at Local Health Authority CN2.
Stefano D’Anna holds a Master’s Degree in Pharmacy. He is attending the post graduate program in Hospital Pharmacy and he is a scholarship holder at Local Health Authority CN2 for the Regional Project on Pharmacovigilance.
Carla Rognoni holds a Master’s Degree in Computer Science and Engineering and PhD in Bioengineering and Bioinformatics (cum Laude). Since 2013, as researcher at CERGAS SDA Bocconi, supports national and international companies and institutes focusing on a variety of topics related to decision analysis, cost-effectiveness, budget impact analysis and decision modeling, in both public health and clinical medicine.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Carla Rognoni  https://orcid.org/0000-0002-6330-0591
https://orcid.org/0000-0002-6330-0591
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. In: Ross AC, Taylor CL, Yaktine AL, et al., eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press (US; ); 2011. [PubMed] [Google Scholar]
- 2. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 3. Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. [DOI] [PubMed] [Google Scholar]
- 4. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847–858. [DOI] [PubMed] [Google Scholar]
- 6. Italian Osteoporosis, Mineral Metabolism, and Skeletal Diseases Society. Linee guida su prevenzione e trattamento dell’ipovitaminosi D con colecalciferolo. 2011. http://www.siommms.it/linee-guida-su-prevenzione-e-trattamento-dellipovitaminosi-d-con-colecalciferolo/ (accessed 14 June 2019). [DOI] [PubMed]
- 7. Isaia G, Giorgino R, Rini GB, Bevilacqua M, Maugeri D, Adami S. Prevalence of hypovitaminosis D in elderly women in Italy: clinical consequences and risk factors. Osteoporos Int. 2003;14(7):577–582. [DOI] [PubMed] [Google Scholar]
- 8. Adami S, Bertoldo F, Braga V, et al. 25-hydroxy vitamin D levels in healthy premenopausal women: association with bone turnover markers and bone mineral density. Bone. 2009;45(3):423–426. [DOI] [PubMed] [Google Scholar]
- 9. Smith H, Anderson F, Raphael H, et al. Effect of annual intramuscular vitamin D on fracture risk in elderly men and women--a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford). 2007;46(12):1852–1857. [DOI] [PubMed] [Google Scholar]
- 10. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303(18):1815–1822. [DOI] [PubMed] [Google Scholar]
- 11. Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr.1999;69(5):842–856. [DOI] [PubMed] [Google Scholar]
- 12. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ilahi M, Armas LAG, Heaney RP. Pharmacokinetics of a single, large dose of vitamin D3. Am J Clin Nutr. 2008;87(3):688–691. [DOI] [PubMed] [Google Scholar]
- 14. Romagnoli E, Mascia ML, Cipriani C, et al. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab. 2008;93(8):3015–3020. [DOI] [PubMed] [Google Scholar]
- 15. Cipriani C, Romagnoli E, Scillitani A, et al. Effect of a single oral dose of 600,000 IU of cholecalciferol on serum calciotropic hormones in young subjects with vitamin D deficiency: a prospective intervention study. J Clin Endocrinol Metab. 2010;95(10):4771–4777. [DOI] [PubMed] [Google Scholar]
- 16. https://www.epicentro.iss.it/farmaci/pdf/fep2018/Da%20Cas%20Roberto.pdf (accessed 14 June 2019).
- 17. L’uso dei farmaci in Italia. Rapporto OsMed. 2014. http://www.agenziafarma co.gov.it/content/luso-dei-farmaci-italia-rapporto-osmed-2014 (accessed 14 June 2019).
- 18. L’uso dei farmaci in Italia. Rapporto OsMed. 2016. http://www.agenziafarmaco.gov.it/content/luso-dei-farmaci-italia-rapporto-osmed-2016 (accessed 14 June 2019).
- 19. L’uso dei farmaci in Italia. Rapporto OsMed. 2017. http://www.aifa.gov.it/content/luso-dei-farmaci-italia-rapporto-osmed-2017 (accessed 14 June 2019).
- 20. Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387): 469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imbens GW, Wooldridge JM. Recent developments in the econometrics of program evaluation. J Econ Lit. 2009;47(1):5–86. [Google Scholar]
- 22. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med. 2016;176(2):175–183. [DOI] [PubMed] [Google Scholar]
- 23. Hansen KE, Johnson RE, Chambers KR, et al. Treatment of vitamin D insufficiency in post-menopausal women a randomized clinical trial. JAMA Intern Med. 2015;175(10):1612–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Adami S, Romagnoli E, Carnevale V, et al. Linee Guida su prevenzione e trattamento dell’ipovitaminosi D con colecalciferolo [in Italian]. Reumatismo. 2011;63(3):129–147. [DOI] [PubMed] [Google Scholar]
- 25. Bjelakovic G, Gluud LL, Nikolova D, et al. Vitamin D supplementation for prevention of mortality in adults. Cochrane Database Syst Rev. 2014;(1):CD007470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_material for Can a Different Formulation of Vitamin D3 Allow Savings? An Analysis From an Italian Regional Perspective by Mario Sanò, Patrizia Dutto, Stefano D’Anna and Carla Rognoni in Health Services Research and Managerial Epidemiology