Abstract
The aim of the study was to investigate health-related quality of life among Vietnamese breast cancer women who were treated at National Cancer Hospital, Hanoi, Vietnam, in 2018. Information about physical functioning, Role Physical, Bodily Pain, General Health, vitality, Social Functioning, Role Emotional, and Mental Health of 200 patients with breast cancer was collected through face-to-face interview, using short form-36 questionnaire. We found that the older patients (older than 50 years) had higher score of Mental Health than patients at age 50 and lower (P < .05). The patients who had better economic status had significantly higher score of Vitality (P < .05). Patients who were married and living with their partners/husband had better quality of life in General Health (P<0.05). The patients who had less than 6 months of treatment had better physical functioning score (P < .05) than the patients who had treatment longer than 6 months. Patients with caring supports from family members had higher scores of Bodily Pain, Social Functioning, Role Emotional, and Mental Health. Patients who have stressed feelings had significantly lower scores of all domains, except for Physical Functioning. The participants who usually stay up late reported lower scores of all components except for Physical Functioning and Role Physical. In conclusion, it is needed to develop psychosocial services, enhance early screening, and diagnose for the women in Vietnam.
Keywords: breast cancer, quality of life, Vietnam, women, SF-36 questionnaire
Introduction
Breast cancer is the most common cancer among women in developed and developing countries. Approximately 2 million women were diagnosed worldwide in 2018, which amounted to 25% of all cancers.1 In Vietnam, the incidence of breast cancer has increased rapidly in recent years, with over 15 000 new diagnoses each year.1 Improvements in early detection and advanced treatment of breast cancer have led to longer survival. However, even after being cured, more struggles still lie ahead for patients, including depression, anxiety, fear, and side effects of treatment including pain. The disease also affects not only the patients themselves but also their families, throughout the whole process of treatment. Quality of life (QoL) is defined as “an individual’s perception of his/her position in life in the context of the culture and value systems in which he/she lives and in relation to his/her goals, expectations, standards, and concerns.”2(p.1-2) Overall, breast cancer causes a great impact upon the QoL of the patients, and not just on their physical condition. Quality of life in patients with breast cancer has received increasing attention due to an increasing number of new cases, an improved survival rate, and the important vital role of women in the family.3 In cancer treatment, QoL is an important indicator to evaluate effectiveness of treatment, especially for curable cancers such as breast cancer.3 Results from many studies illustrate that QoL of breast cancer survivors is associated with the age at cancer diagnosis, level of education, marital status, income, and time period since cancer diagnosis.4-6 However, there were limited studies on QoL and related factors in Vietnamese breast cancer women. The aim of the study was to investigate the health-related QoL among patients with breast cancer who were hospitalized at the National Cancer Hospital in 2018 and some related factors.
Methods
Study Design and Settings
In a descriptive case series study which implemented during January 1, 2018, to August 30, 2018, women at the age 18 and older who were diagnosed and/or treated for breast cancer at the National Cancer Hospital recruited to the study.
The National Cancer Hospital in Vietnam is the biggest specialized oncology center in the country, with 2000 inpatient beds. According to an unpublished annual report, the hospital received 417 000 visits for diagnosis and treatment in 2018. Most of breast cancer women come there for diagnosis and treatment.
Sample Size and Sampling
The sample size for estimating the mean was calculated, using the following formula:
where n was minimum sample size; α was confidence level. In this research, we use Z(1 − α/2) = 1.96; σ = 6.32: population variance, which was based on the research in 2016 carried out by Xiao et al7; ∊ = 0.174: margin of error; µ = 50.46: the total QOL score measured by short form-36based on the research of Xiao and colleagues.7 From the formula, the minimum sample size is 199 patients. In this study, we recruited 200 patients.
Breast cancer women were selected by purposive sampling technique. The minimum sample size was estimated by the formula to determine sample size for estimating the mean, with 95% confidence intervals; the mean score of total QoL was 50.46 based on previous study. The minimum sample size requirement was 200 participants.
Data Collection
Data were collected by face-to-face interviews. Interviewers were the authors listed above. The questionnaire was piloted on 5 breast cancer women to identify barriers and difficulties during the interview. Then, interviewers discussed how to overcome barriers before starting data collection for the study. Written informed consent was obtained from participants prior to data collection. During the interview, we collected information on demographic characteristics (education, marital status, economic status, duration time of treatment) and health-related QoL according to SF-36 questionnaire. It was estimated that 20% of participants were randomly selected by the principal investigator for rechecking information concerning to demographic characteristics and information related to QoL.
Ethical Issue
Ethical approval for the study was obtained from the institutional review board of the National Cancer Hospital (No P20 CA210300, dated June 8, 2017). Patients decided to participate in the study and signed a consent form before answering the questionnaire.
Measurements
The instrument was a structured questionnaire about participant’s socioeconomic, clinical, and behavioral characteristics together with the SF-36 questionnaire. The SF-36 is a standardized questionnaire with 36 questions and is one of the most widely used regarding health-related quality-of-life measures. The validity and reliability of SF-36 questionnaire was confirmed by several studies implemented previously in different populations at developed and developing countries, including patients with breast cancer.8-10 These are multidimensional measures of self-reported health status. The SF-36 questionnaire measures physical and Mental Health status in relation to 8 health domains: physical functioning, Role Physical Functioning (role limitations due to physical health), Bodily Pain, General Health Perceptions, vitality (energy/fatigue), Social Functioning, Role Emotional Functioning (role limitations due to emotional health), and general Mental Health (psychological distress/well-being). In each health domain, the relation with variables on marital status, economic situation, duration time of treatment, stress feeling, and sleeping late habit were evaluated.
Statistical Analysis and Statistical Method
Specific software was used to calculate the score of SF-36. Responses to each of the SF-36 items are scored and summed according to a standardized scoring protocol (Ware et al)11 and expressed as a score on a 0 to 100 scale for each of the 8 health concepts. Higher scores represent better self-perceived health. Then, SPSS version 20.0 software was used for data analysis. The descriptive and comparison analysis, using t test, analysis of variance, or Mentel-Henzel, depends on relevant variables. All values were 2 sided, the P values less than .05 were considered statistically significant. The scoring system and interpretation of the SF-36 have been fully described elsewhere.12
Results
Sample Characteristics
There were 200 breast cancer women participated in the study with a response rate of 100%. The mean age of breast cancer women was 50.6 ± 10.1 years old. The percentage of patients who did not graduate high school was 57.5%; the proportion of patients who were married was 78.5%. Regarding occupation, the most common occupation among patients was farming (45.0%). The rate of poor/near-poor was 23.0%; nearly all (98.0%) had health insurance. The participants who underwent fewer than 6 months of treatment accounted for 58.0%. The percentage of patients at the second stage is highest, accounting for 61.5%. Of all, 74.5% of patients reported having caregivers, such as husbands, parents, children, or siblings. All patients in the study did not smoke nor drink alcohol currently; only 1% endorsed a prior drinking history but had since stopped. Over half had felt stress during the past week, accounting for 47.5% (Table 1)
Table 1.
Characteristics of Breast Cancer Women.
| Characteristic | N = 200 | % |
|---|---|---|
| Age | ||
| 21-50 | 96 | 48.0 |
| >50 | 104 | 52.0 |
| Average age | 50.6 ± 10.5 (min = 21; max = 80) | |
| Education | ||
| <High school | 115 | 57.5 |
| ≥High school | 85 | 42.5 |
| Marital status | ||
| Married | 157 | 78.5 |
| Single/divorced/widowed/missing | 43 | 21.5 |
| Main occupation | ||
| Farmer | 90 | 45.0 |
| Worker | 17 | 8.5 |
| Public servant | 44 | 22.0 |
| Business job | 22 | 11.0 |
| Retired/housewife | 27 | 13.5 |
| Health insurance | ||
| Do not have | 4 | 2.0 |
| 40% | 6 | 5.0 |
| 80% | 101 | 50.5 |
| 95% | 29 | 14.5 |
| 100% | 60 | 30.5 |
| Economic status | ||
| Poor/near poor | 46 | 23.0 |
| No | 154 | 77.0 |
Health-Related QoL
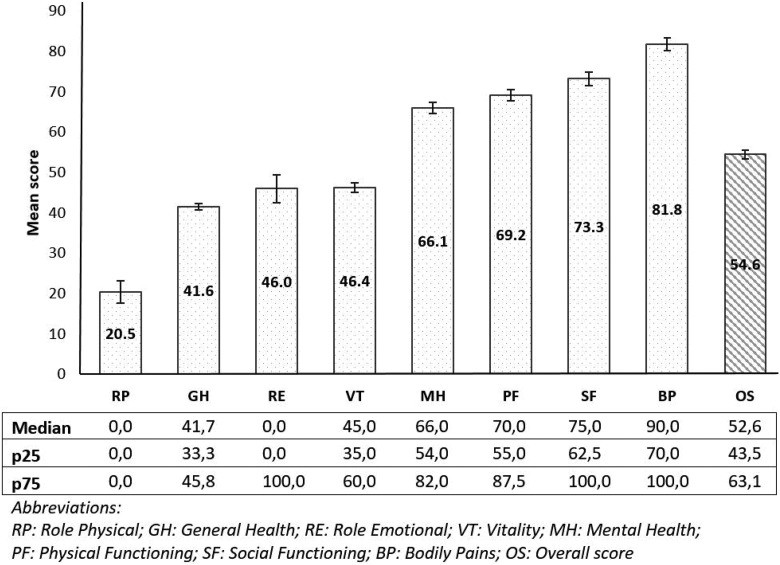
The mean score of total QoL in breast cancer women was 54.6 points (95% CI: 52.5-56.7). The QoL to be measured among 8 domains in which Role Physical to be reported by the patients with breast cancer at the lowest score. It means that the patients could not perform the daily activities that result from physical health (95% CI: 15.1-25.9). In contrast, the patients reported the highest score of Bodily Pain, with 81.3 points (95% CI: 78.7-85.0; Figure 1).
Figure 1.
Quality of life of breast cancer women by 8 components.
In this study, we analyzed the data to find out the relation between the QoL score of each 8 separate domains and the general QoL score of patients with breast cancer with some associated factors, including age, marital status, economic status, duration of treatment, caregivers, stress feeling, and stay up late habit (Table 2).
Table 2.
Difference in QoL in Components Between Characteristics.
| Related Factors | QoL Mean Score (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Physical Functioning | Role Physical | Bodily Pain | General Health | Vitality | Social Functioning | Role Emotional | Mental Health | Overall | |
| Age | |||||||||
| Under 50 | 72.0 (68.3-75.7) | 23.7 (15.3-32.1) | 82.6 (77.9-87.2) | 42.0 (39.9-44.0) | 44.5 (41.3-47.6) | 70.2 (65.1-75.3) | 45.1 (35.3-55.0) | 63.4a (59.8-67.0) | 55.0 (51.9-58.1) |
| Over 50 | 66.6 (62.4-70.9) | 17.5 (10.4-24.7) | 81.2 (76.8-85.6) | 41.2 (39.2-43.1) | 48.1 (44.8-51.4) | 76.1 (71.1-80.7 | 46.8 (37.0-56.5) | 68.5a (64.9-72.1) | 54.1 (51.1-57.2) |
| Marital status | |||||||||
| Married | 69.6 (66.7-73.2) | 21.2 (14.9-27.4) | 81.4 (77.7-85.1) | 42.2a (40.7-43.8) | 47.3 (44.7-49.8) | 73.8 (70.0-77.6) | 47.1 (39.4-54.9) | 66.6 (63.8-69.5) | 55.2 (52.-57.7) |
| Single/divorced/widowed | 66.6 (60.6-72.7) | 18.0 (6.5-29.6) | 83.4 (76.9-89.9) | 39.1a (35.7-42.6) | 43.0 (38.0-48.0) | 71.2 (63.2-79.3) | 41.9 (26.5-57.2) | 64.0 (58.0-70.0) | 52.2 (47.5-56.8) |
| Economic status | |||||||||
| Poor/near poor | 66.6 (60.7-73.3) | 19.0 (8.0-30.0) | 77.9 (69.7-86.0) | 39.8 (36.8-42.7) | 40.9a (36.0-45.8) | 67.1 (59.4-74.9) | 37.0 (22.8-51.1) | 62.7 (56.9-68.5) | 50.9 (46.6-55.1) |
| No | 70.0 (59.4-74.9) | 20.9 (14.6-27.3) | 83.0 (79.7-86.4) | 42.1 (40.5-43.7) | 48.0a (45.5-50.5) | 75.1 (71.3-78.9) | 48.7 (40.8-56.6) | 67.1 (64.2-69.9) | 55.7 (53.2-58.2) |
| Treatment duration | |||||||||
| ≤6 months | 71.1a (67.0-75.1) | 23.1 (15.5-30.6) | 83.5 (79.6-87.5) | 41.8 (40.1-43.6) | 46.9 (44.0-49.7) | 72.4 (67.9-76.9) | 43.7 (34.7-52.7) | 66.0 (62.8-69.3) | 55.3 (52.4-58.1) |
| >6 months | 66.7a (62.8-70.5) | 17.0 (9.2-24.7) | 79.5 (74.2-84.8) | 41.2 (38.8-43.5) | 45.7 (41.9-49.5) | 74.4 (69.0-79.8) | 49.2 (38.5-59.9) | 66.1 (61.9-70.3) | 53.6 (50.4-56.9) |
| Caregivers | |||||||||
| Husband | 68.4 (63.9-73.0) | 20.9 (12.6-29.2) | 85.4a (74.2-84.9) | 42.5 (40.4-44.5) | 48.1 (44.7-51.5) | 79.6a (80.6-90.2) | 58.0a (47.5-68.6) | 70.5a (66.8-74.2) | 56.6a (53.4-59.8) |
| Children | 70.8 (64.0-77.5) | 23.3 (10.7-36.0) | 84.5a (71.9-83.1) | 42.4 (39.6-45.2) | 49.6 (44.9-54.2) | 77.5a (78.5-90.5) | 41.5a (26.8-56.1) | 67.9a (62.8-73.0) | 56.1a (51.3- 61.0) |
| Parents/siblings | 71.1 (62.2-79.9) | 26.3 (4.5-48.1) | 83.6a (55.1-77.8) | 39.0 (34.4-43.6) | 42.6 (34.9-50.4) | 66.4a (73.4-93.7) | 59.6a (36.0-83.3) | 65.9a (55.3-76.4) | 55.6a (49.6-61.7) |
| Self-take care | 68.5 (63.3-73.7) | 15.2 (5.3-25.0) | 72.9a (54.5-68.6) | 40.3 (37.0-43.6) | 42.0 (37.1-46.8) | 61.5a (66.0-79.7) | 24.8a (12.7-37.0) | 57.1a (52.1-62.0) | 49.5a (45.1-53.8) |
| Stress | |||||||||
| Stress feeling | 68.3 (61.2-72.4) | 14.2a (7.3-21.1) | 77.2a (72.3-82.2) | 39.6a (37.7-41.5) | 41.9a (39.1-44.8) | 66.7a (61.3-72.1) | 24.2a (15.4-33.0) | 57.9a (54.5-61.2) | 49.5a (46.6-52.5) |
| No stress | 70.1 (66.1-74.1) | 26.2a (17.9-34.4) | 86.0a (82.0-90.0) | 43.3a (41.3-45.4) | 50.3a (47.0-53.6) | 79.2a (75.1-83.3) | 65.7a (56.8-74.6) | 73.4a (70.2-76.7) | 59.1a (56.3-62.0) |
| Stay up late habit | |||||||||
| Always | 71.3 (64.1-78.3) | 20.5 (5.4-35.7) | 79.9a (70.6-89.2) | 39.9 (36.0-43.7) | 47.5a (41.8-53.2) | 82.1a (72.8-91.5) | 47.6a (28.2-67.0) | 65.9 (59.3-72.3) | 55.7a (50.0-61.4) |
| Usually | 66.6 (59.7-73.5) | 20.9 (8.7-33.1) | 75.1a (68.0-82.2) | 39.1 (36.7-41.6) | 40.5a (35.7-45.2) | 64.8a (57.3-72.3) | 32.6a (18.3-46.8) | 59.6 (53.7-65.5) | 50.1a (45.5-54.8) |
| Sometimes | 67.6 (62.8-72.5) | 19.3 (10.2-28.4) | 81.1a (75.2-87.0) | 42.4 (39.6-45.1) | 46.3a (42.0-50.6) | 68.8a (63.3-74.2) | 39.5a (28.1-50.9) | 68.0 (63.8-72.2) | 53.4a (49.6-57.2) |
| Never | 72.0 (66.9-77.2) | 21.6 (11.0-32.2) | 88.6a (84.0-93.0) | 43.1 (40.7-45.6) | 50.2a (46.3-54.0) | 80.5a (74.3-86.7) | 62.7a (50.0-75.4) | 68.5 (63.7-73.4) | 58.7a (55.0-62.4) |
Abbreviations: CI, confidence interval; QoL, quality of life.
a P < .05.
Using the Mann-Whitney and t test, the study showed that the mean score of health quality of life (HQoL) in Mental Health was higher in age-group older than 50 years (P < .05); health quality of life in General Health was higher in married women (P < .05); the mean score of QoL in Vitality was lower in poor/near-poor patients (P < .05); QoL in Physical Functioning was lower at group of patients who had time treatment longer 6 months and patients with comorbidity (P < .05; Table 2).
Concerning caretakers of the participants, the results showed that in almost all measured domains, the patients who take care by themselves had lower QoL mean score than the others. The difference had the statistical significance (P < .05) with the domains Role Emotional, Mental Health, Social Functioning, and Bodily Pain (Table 2).
In all domains of QoL, the mean score of breast cancer women who has stress feeling was lower than patients who had no stress feeling. The difference in all 7 domains was statistically significant (P < .05), except for physical functioning.
Patients who never stayed up late had the highest score of QoL, while those who usually stayed up late have the lowest score of QoL. The significant difference between the group “never stay up late” and the group “usually stay up late” was found at Role Emotional, Vitality, Social Functioning, and Bodily Pain (P < .05; Table 2)
Discussion
Quality-of-Life Measurement by 8 Health-Related Domains
The results from our study showed that the mean score of Role physical was lowest, at 20.5 points. The questionnaire about the physical role is intended to assess the difficulty of the patient’s performance of work or daily activities due to the effects of physical activity performance capacity. This score was lower than those of the general patients with cancer in the study from Vu (57.6 points)13 and patients with colorectal cancer by Tran (35.2 points).14 However, the score from our study was lower than results from studies in other countries, including the United States, Brazil, Iran, and Denmark.4, 15–17 It also can be seen that, when suffering from breast cancer, the patient has a lot of difficulties in doing things according to her own preferences or desires, and daily tasks become big challenges. Participating in a breast cancer club may help suffered women to share experiences and support one to another.
Regarding Bodily Pain, the result showed that pain freedom reported by the participants from our study was slightly higher than other studies where scores ranged from 66.4 to 75.8.4,14,15,18 Studies applied other instruments as European Organization for Research and Treatment of Cancer core quality of life questionnaire (Version 3.0) reported that QoL score in Bodily Pain varied.13,16,19,20 In the present study, we noted a huge difference in the QoL scores between Bodily Pain and Role Physical (81.8 vs 20.5). This can be explained by the characteristics of breast cancer. Although pain is very common and terribly scared in other cancers such as liver cancer or pancreatic cancer, it seems not common for breast cancer. In contrast, the cancer usually has significant influence on the performance of daily activities which is important for breast cancer women in Vietnam. This can explain the relatively low QoL score of Role Physical compared to Bodily Pain in our study.
Quality of Life–Related Factors
Results from our study demonstrated that marital status, economic situation, duration of treatment, stress feeling, and sleeping late habit had significant impact on QoL of Vietnamese breast cancer women.
Age-group
Participants older than 50 years had QoL scores higher than those younger than 50 years concerning Role Emotional, Vitality, Social Functioning, and Mental Health. However, only the QoL in Mental Health was statistically significant at P < .05. The study by Dieu et al about QoL assessment of patients with breast cancer showed patients aged ≤45 have a higher QoL than those older than 45 years of age.19 Additionally, the study of “QoL over 5 years after a breast cancer screening in the United States in 2012” showed that old women have lower QoL than young women, but the study did not clarify specific age-group associations.21 Younger women had lower QoL score because they usually had to take care of their family while maintaining their responsibilities at works. This implies the necessity of psychological and social supports for young breast cancer women in Vietnam.
Marital status
When analyzing the difference between patients with breast cancer who are married/currently living with their spouse and the single/divorced/widow groups, the QoL of the married group was higher than the other in almost areas except Bodily Pain and the difference was statistically significant with P < .05 in the General Health. When a woman had cancer, the husband is the crucial supporter in body and spirit that helps the patients overcome the disease and have a better QoL. The study in Denmark in 2006 also found the link in this regard, in particular that the married group had a higher QoL than the other groups.4 Health education for husbands or family members of patients with breast cancer is needed to improve QoL for these women.
Economic status
According to the analysis, in all items, patients with breast cancer whose households were poor/near-poor had lower QoL than others, with a statistically significant difference in Vitality. As regulated by Ministry of Labour—Invalids and Social Affairs of Vietnam, a household is classified as poor or near-poor when their income is lower than 1 200 000 VND/person/month (around US$55) in rural area and 1 400 000 VND/person/month (around US$65) in urban area. As such, even without illness, the life of poor/near-poor patients had already been full of hardship; their economic status is a crucial factor affecting their mental and social function. In addition, even though health insurance coverage in Vietnam now is more than 85%, insurance only can cover about 50% of the cost of most oncology drugs. These findings were similar to Tran’s study on 205 patients with colon cancer in National Cancer Hospital in 2017, which found a relationship between QoL and family’s economic status.14 Moreover, Binh et al’s study on 175 patients with cancer in Hanoi Medical University Hospital in 2015 and several other studies in the world had not found any similar relation.22 Currently, at the National Cancer Hospital, aside from improving the effectiveness of treatment, social activities including providing psychosocial supports for patients have been made a priority by the Director Board, especially for disadvantaged patients and children.
Duration for treatment
In most items, patients with the duration of disease younger than 6 months had higher QoL than the ones above 6 months. However, only in “Physical Functioning” was there a significant difference with P < .05. Otherwise, for Role Emotional, Mental Health, and Social Functioning, the patients with younger than 6 months disease actually had lower QoL, but these differences were not statistically significant. Several studies on QoL of patients with cancer in Vietnam and in the world had found the relation between disease duration and patients’ QoL. Binh et al’s study at Hanoi Medical University Hospital found that the patients with cancer with disease duration from 3 to 6 months had the highest QoL, and lowest among the ones whose disease duration were longer than 3 years22; Duong’s study in Thai Nguyen showed patients with breast cancer who, at earlier stages of disease, had higher QoL20; Ali Montazeri’s study in Iran reported that QoL of patients with breast cancer was highest after diagnosis, following by after-3-month and lowest among above-18-month.17 Even though we as well as other studies found there is a relationship between QoL and disease duration, the findings were still varied and were not similar. One thing worth noticing in our study was that for Role Emotional, Mental Health, and Social Functioning, it seems like the longer the duration, the more QoL had improved. It could be explained as the longer the duration, the more time the patients had to cope with and accept their disease; they no longer felt shocked, fear, or despair as before, leading to fewer impacts upon their social, emotional, and mental well-being compared to new patients. Through this, it is recommended that more attention should be paid to taking care and improving the QoL regarding emotional and social well-being for newly diagnosed patients so that they could have the motivation to overcome the disease, having more faith and better QoL.
Caregivers
The result from our study showed that in most components, the patients who take care of themselves have lower QoL level than the others who have got the caregivers. The lowest score belongs to the group who were taking care of themselves. The role of caregiver is very important which is illustrated in many studies, especially in Asian culture. It becomes even more important when the cancer treatment is long and costly.
Stress
It was observed in our study that patients with breast cancer who had more feelings of stress had lower QoL than the others. The difference in all 7 components had statistical significance at P < .05, except for “Physical Functioning.” In fact, anyone who feels stressed usually becomes fatigued and have bad emotions, which can easily happen to patients with cancer. A similar result was also found in studies around the world such as Xiao’s study in the United States in 2015, which found that stress reduces the QoL of patients.15 The study of Daldoul in 2017 that investigated related factors of QoL among patients with breast cancer in Tunisia using Hospital Anxiety and Depression Scale reported that considering the anxiety and depression in patients with cancer, 12.9% of patients have real anxiety and 21.4% of patients have real depression which significantly reduced the QoL of patients.23 Therefore, the treatment and support for patients with breast cancer requires coordination between physicians, psychologists, psychiatrists, and social support organizations.
Staying up late
The study showed that patients who never stay up late have some of the highest scores of QoL, while those who usually do have the lowest QoL. Sufficient sleep is extremely important for long-term health. When a person is able to fall asleep early, they are more likely to have a good night’s sleep, then they are able to relieve anxiety, feel relaxed and relieve anger, and have a social relationship and better QoL as well. This has been well analyzed in the descriptive study in United States on the link between sleep and QoL in patients with breast cancer. The Pittsburgh Quality of Sleep Index and the SF-36 questionnaire were used in that study. The result found that 61% of patients with breast cancer had significant sleep problems and these patients had a significant reduction in QoL in all areas. The reduction has significant statistics concerning Role Physical Functioning, Bodily Pain, Vitality, Social Functioning, Role Emotional Functioning, and Mental Health. In addition, we also found that up to 42% of patients with breast cancer were prescribed sleep medications in the past 4 weeks, of which 21% of them have at least 3 times per week.7 As a result, our research is similar to that in the United States. By understanding the effect of sleeping habits of patients with breast cancer at K Hospital today, we can implement some measures such as reminding patients to go sleep early to improve their health and improve treatment efficacy as well as improve QoL in a more positive way.
Limitations
The study was conducted using a cross-sectional descriptive and the purposive selection of sampling, so we could only describe at a selected point of time at the National Cancer Hospital and the result may not be able to represent the whole community with just a limited population. In regard with patients with breast cancer, the research has not yet looked at more specific groups, such as patients with chemotherapy, radiotherapy, surgery, and so on. The limitation of sample size influenced to do multiple regression. Therefore, more research on other cancers should be carried out with bigger sample size in order to better understand the QoL of patients with cancer at National Cancer Hospital and general medical facilities as well. In addition, each type of cancer should be researched on more specific groups, from that we can be able to assess the QoL of each disease more accurately and in detail. In addition, the SF-36 Measurement Questionnaire itself also contained its limitations.
Conclusion
Quality of life among Vietnamese breast cancer women was related to age, duration of treatment, stress feeling, and late sleeping habit. Among patients with breast cancer, those who were married and living with their husband had significantly higher scores of QoL than those who did not. In addition to medical treatment, mental and emotional supports are extremely important for breast cancer women in Vietnam. Therefore, it is necessary to develop comprehensive psychosocial services and enhance early screening and diagnose programs to meet the various demands of different patients with breast cancer in Vietnam.
Acknowledgments
The authors would like to express sincere thanks to the patients who participated in this survey.
Authors’ Note: This study was conducted under approval of National Cancer Hospital Director Board.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. GLOBOCAN(2018)704-viet-nam-fact-sheets.pdf. http://gco.iarc.fr/today/data/factsheets/populations/704-viet-nam-fact-sheets.pdf. Published 2019 Accessed February 27, 2019.
- 2. van der Steeg AFW, Vries JD, Roukema JA. Quality of life and health status in breast carcinoma. Eur J Surg Oncol. 2004;30(10):1051–1057. doi:10.1016/j.ejso.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 3. Montazeri A. Health-related quality of life in breast cancer patients: a bibliographic review of the literature from 1974 to 2007. J Exp Clin Cancer Res. 2008;27:32 doi:10.1186/1756-9966-27-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peuckmann V, Ekholm O, Rasmussen NK, et al. Health-related quality of life in long-term breast cancer survivors: nationwide survey in Denmark. Breast Cancer Res Treat. 2007;104(1):39–46. doi:10.1007/s10549-006-9386-6. [DOI] [PubMed] [Google Scholar]
- 5. Ganz PA, Greendale GA, Petersen L, Kahn B, Bower JE. Breast cancer in younger women: reproductive and late health effects of treatment. J Clin Oncol. 2003;21(22):4184–4193. doi:10.1200/JCO.2003.04.196. [DOI] [PubMed] [Google Scholar]
- 6. Casso D, Buist DS, Taplin S. Quality of life of 5–10 year breast cancer survivors diagnosed between age 40 and 49. Health Qual Life Outcomes. 2004;2(1):25 doi:10.1186/1477-7525-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fortner BV, Stepanski EJ, Wang SC, Kasprowicz S, Durrence HH. Sleep and quality of life in breast cancer patients. J Pain Symptom Manage. 2002;24(5):471–480. doi:10.1016/S0885-3924(02)00500-6. [DOI] [PubMed] [Google Scholar]
- 8. Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:2050312116671725 doi:10.1177/2050312116671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and psychosocial recovery in the year after primary treatment of breast cancer. J Clin Oncol. 2011;29(9):1101–1109. doi:10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295(23):2742 doi:10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 11. John EW. SF-36 Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 12. Monica 1776 Main Street Santa, California 90401-3208. 36-Item Short Form Survey from the RAND Medical Outcomes Study. https://www.rand.org/health/surveys_tools/mos/36-item-short-form.html. Published 1994 Accessed August 25, 2018.
- 13. Vu VV, Hanh VTX. Investigation on the pain and quality of life of cancer patients in long term in Ho Chi Minh Oncology hospital 7/2009-7/2010. J Med Ho Chi Minh City. 2010;14(4):811–822. [Google Scholar]
- 14. Huong T, Hung LV, Quang NT. Quality of life and related factors in colon cancer patients in National Cancer Hospital. Med Res J. 2018;119(3):65–73. [Google Scholar]
- 15. Xiao C, Miller AH, Felger J, Mister D, Liu T, Torres MA. A prospective study of quality of life in breast cancer patients undergoing radiation therapy. Adv Radiat Oncol. 2016;1(1):10–16. doi:10.1016/j.adro.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lôbo SA, Fernandes AFC, de Almeida PC, de L Carvalho CM, Sawada NO. Quality of life in women with breast cancer undergoing chemotherapy. Acta Paul Enferm. 2014;27(6):554–559. doi:10.1590/1982-0194201400090. [Google Scholar]
- 17. Montazeri A, Vahdaninia M, Harirchi I, Ebrahimi M, Khaleghi F, Jarvandi S. Quality of life in patients with breast cancer before and after diagnosis: an eighteen months follow-up study. BMC Cancer. 2008;8(1):330. doi:10.1186/1471-2407-8-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee SH, Min YS, Park HY, Jung TD. Health-related quality of life in breast cancer patients with lymphedema who survived more than one year after surgery. J Breast Cancer. 2012;15(4):449 doi:10.4048/jbc.2012.15.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dieu B, Thuan TV, Nga NTH, Quynh Anh N, Khoa TD, Minh LH. Quality of life of breast cancer patients in Vietnam. Vietnam Cancer J. 2016;2016:13–21. [Google Scholar]
- 20. Duong NV. Quality of life assessment of cancer patients in oncology center—Thai Nguyen General Hospital. J Cancer Vietnam. 2015;Special number. [Google Scholar]
- 21. Maly RC, Liu Y, Liang LJ, Ganz PA. Quality of life over 5 years after a breast cancer diagnosis among low-income women: effects of race/ethnicity and patient-physician communication: quality of life and breast cancer. Cancer. 2015;121(6):916–926. doi:10.1002/cncr.29150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Binh BV, Anh DT, Dinh DT, Trung TQ. Assessment of quality of life of cancer patients and related factors at Ha Noi Medical Hospital University in 2015. In: Vol 1380 Military Hospital; 103; 2015. [Google Scholar]
- 23. Daldoul A, Khechine W, Bhiri H, et al. Factors predictive of quality of life among breast cancer patients. Asian Pac J Cancer Prev. 2018;19(6):1671–1675. doi:10.22034/APJCP.2018.19.6.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]