Abstract
Background:
The quadriceps tendon (QT) is increasingly considered for primary and revision anterior cruciate ligament reconstruction in skeletally immature patients, as it may be harvested as a purely soft tissue graft with considerable tissue volume. Because of distinct rectus tendon (RT) separation from the QT complex, the potential for RT retraction exists and could lead to QT weakness after QT graft harvest.
Purpose:
To describe the anatomy of the pediatric QT and clarify decussation of the RT and QT to avoid the risk of delayed RT retraction and QT weakness after QT graft harvest.
Study Design:
Descriptive epidemiology study.
Methods:
Nine cadaveric knee specimens (aged 4-11 years) underwent gross dissection. Coronal-plane width and depth of the QT were measured at intervals proximal to the superior pole of the patella at distances of 0.0, 0.5, 1.0, and 1.5 times the length of the patella. The distance was measured from the superior patellar pole to the point of RT separation from the remainder of the deeper/posterior QT.
Results:
The median patellar length was 28 mm (interquartile range, 26-37 mm). The coronal-plane width of the QT was larger superficially/anteriorly when closest to the patella but wider when measured deeper/posteriorly as the tendon extended proximally. The median distance between the superior pole of the patella and RT separation from the QT was 0.95 times the patellar length. The distance to widening of the deeper/posterior aspect of the QT was 1.14 times the patellar length proximal to the patella.
Conclusion:
The RT begins a distinct separation from the QT above the superior pole of the patella at a median of 0.95 times the patellar length in skeletally immature specimens. The deeper/posterior aspect of the QT begins to increase in coronal-plane width proximally after a distance of 1.14 times the patellar length above the knee, while the superficial/anterior aspect of the tendon continues to narrow. Awareness of the separation of the RT from the QT, and the coronal-plane width variation aspects of the QT proximally, is important for surgeons utilizing the QT as a graft to avoid inadvertent release of the RT from the rest of the QT complex.
Keywords: quadriceps tendon complex, anterior cruciate ligament, retraction, reconstructive surgery, patella
The incidence of anterior cruciate ligament (ACL) tears is increasing in skeletally immature athletes.14 Many factors, including increased participation in competitive youth sports and early sports specialization, may contribute to this increase in injuries, and the use of advanced imaging technology has increased diagnostic capabilities.14 Iatrogenic physeal damage during ACL reconstruction remains a concern, as the risk for growth arrest and resultant angular and length deformities after an intraoperative physeal injury has been well documented.6,13,26,34 Because of this risk, several new techniques for ACL reconstruction in the pediatric and adolescent populations have emerged.1,5,12,15 Although controversy remains about the optimal treatment of pediatric and adolescent ACL injuries, there is consensus emerging that surgical reconstruction is superior to nonoperative treatment in active patients.14,23–25 Furthermore, autograft tissue produces better clinical outcomes than allograft tissue, with a lower risk of graft ruptures.8,11,24 Because skeletally immature athletes are at the greatest risk for re-injury,5,11 the development of safe primary and revision autograft options for this patient population will be advantageous for pediatric ACL surgeons.
In addition to hamstring and iliotibial band grafts in skeletally immature patients, the quadriceps tendon (QT) is considered a viable option for ACL reconstruction because of its strong tensile strength and large cross-sectional area.10,20 Harvest of the QT graft also avoids direct proximal tibial physeal/apophyseal injuries and the risk of physeal arrest, in contrast to the traditional bone–patellar tendon–bone (BTB) graft. Recent studies have suggested that QT grafts may result in less kneeling and graft site pain than BTB grafts9,28 and produce similar stability to BTB and quadrupled hamstring grafts.19 Additionally, Albright et al1 advocated for the use of QT grafts in skeletally immature patients based on magnetic resonance imaging, showing that more native tendon is left in place after harvest compared with BTB grafts. In comparison to hamstring grafts, QT grafts may be superior in terms of knee flexion strength recovery after surgery.1,9,16
Despite these advantages, using the QT is not without risk, especially when considering this graft for anatomically small, skeletally immature patients.28 Intraoperative patellar fractures have been reported with adult harvest of QT–patellar bone grafts,9,17 and there may be a greater risk because of the smaller patellar anatomy in the pediatric and adolescent populations. A recent report of delayed-onset QT ruptures has been described in ACL reconstruction with adult QTs.21
Clinical experience with pediatric rectus transfer suggests that rectus tendon (RT) retraction after QT graft harvest is a realistic concern. During rectus transfer procedures, the distinct plane separating the RT from the QT complex allows for easy transfer of the RT to the hamstring to facilitate knee flexion during the swing phase.4,30 This distinct plane of separation between the RT and QT complex could allow for immediate or delayed RT or rectus femoris muscle retraction,27 leading to overall QT weakness after the use of a QT graft. The surgical experience with pediatric rectus transfer suggests that careful closure of the most proximal aspect of the QT harvest site may be essential to prevent early or delayed RT retraction after QT graft harvest, as this tendon retraction could lead to significant QT weakness.
The purpose of this study was to examine the QT and RT anatomy in pediatric cadaveric specimens, to develop a better understanding of the separation of the RT from the QT, and to direct safer techniques for QT graft harvest in the skeletally immature in order to avoid the risk of RT retraction and secondary QT weakness.
Methods
Before beginning this study, pediatric tissue was donated to AlloSource, a graft harvesting facility. Consent to use the tissue for research was obtained from all families. Because this study was performed on deidentified cadaveric tissue, it was exempt from institutional review board approval, as it met the following criteria: (1) no information that could potentially identify the tissue (patient identifiers, genetic information) was gathered, and (2) there was no contact between researchers and donor families.
Nine pediatric knees underwent gross dissection by a group of fellowship-trained orthopaedic surgeons (K.G.S., H.B.E., P.L.W., P.D.F., S.M., T.J.G.). During dissection, the surgeons measured dimensions of the patella and QT using digital calipers (measurement error ±0.005 mm). Demographics of the knee specimens are shown in Table 1.
TABLE 1.
Demographics of Dissected Kneesa
| Specimen | Age, y | Sex | Contralateral Knee Present? |
|---|---|---|---|
| 1 | 4 | Male | No |
| 2 | 4 | Female | Yes |
| 3 | 4 | Female | |
| 4 | 9 | Male | No |
| 5 | 9 | Female | Yes |
| 6 | 9 | Female | |
| 7 | 10 | Male | Yes |
| 8 | 10 | Male | |
| 9 | 11 | Male | No |
aKnees were donated from 6 children: 3 children donated both knees.
Measurements included patellar length (proximal-distal) and QT coronal-plane width (medial-lateral distance from both the superficial/anterior and deeper/posterior aspects of the tendon) and depth (anterior-deeper/posterior thickness of the tendon). The coronal-plane width and sagittal-plane depth of the QT were measured proximal to the superior pole of the patella at intervals of 0.0, 0.5, 1.0, and if possible 1.5 times the length of the patella to control for specimen size. The 0.0 interval corresponded to the width and depth of the QT at the superior patellar pole. Because of specimen preparation with division through the QT, not all QTs could be measured at the 1.5 interval. Four specimens were excluded from coronal-plane width and depth measurements superficially, while 7 were excluded from the deeper 1.5 interval.
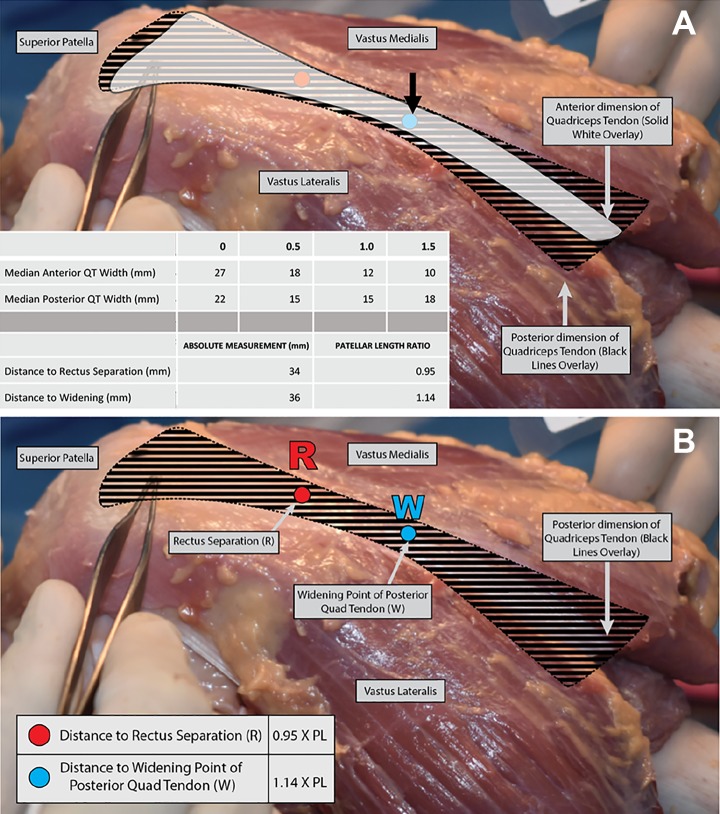
Additionally, measurements were recorded from the superior patellar pole to the point of separation between the RT and remainder of the QT (Figure 1A). Surgeons identified this point by placing a probe anterograde in the “cul-de-sac,” a pocket of potential space found between the RT superficially and remaining QT posteriorly (Figure 1B). Further, the distance was measured from the superior patellar pole to the point where the deeper QT distinctly began to widen, as identified posteriorly.
Figure 1.
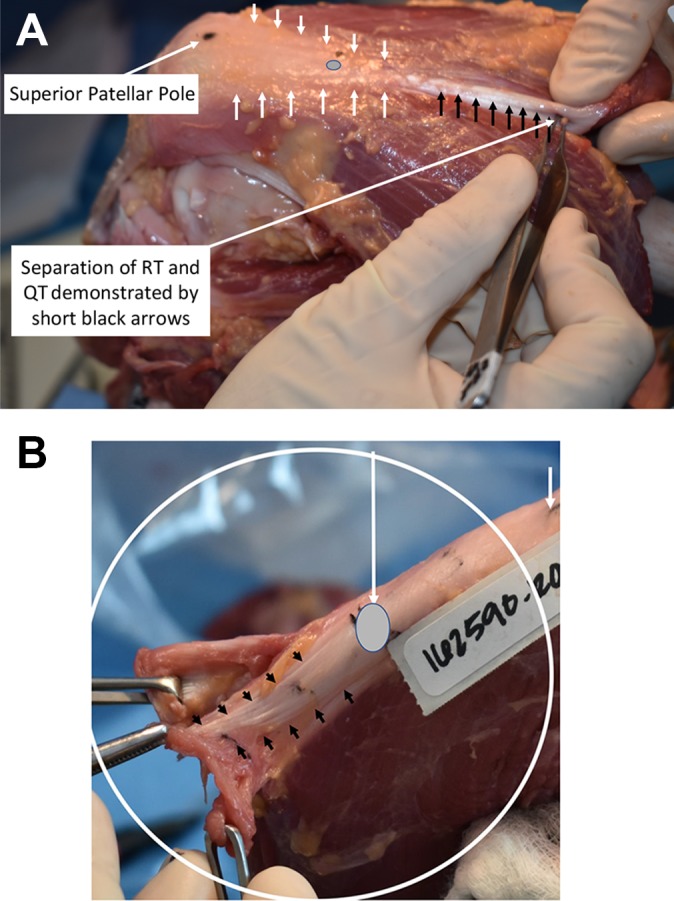
(A) Separation of the rectus tendon (RT) from the quadriceps tendon (QT) is shown with short black arrows, and this separation extends even more distally toward the gray oval on the QT. The short white arrows outline the QT. The gray oval demonstrates the distal extent of the “cul-de-sac” that separates the RT from the rest of the underlying QT. The proximal aspect of the femur is on the right, and the distal aspect is to the left. (B) Close-up of RT and QT separation. The short black arrows demonstrate the separation of the more superficial RT from the underlying QT. The short white arrow is at the superior pole of the patella. A thin layer of fat tissue may separate the RT from the deeper components of the QT below.
Statistical Analysis
Stata 13 (StataCorp) was used to perform statistical analyses. Descriptive statistics were reported as the median and interquartile range (IQR). Spearman correlation analysis for nonparametric data (r) was used to evaluate the relationship between QT parameters and specimen age. P values ≤.05 using 2-tailed analyses were considered significant.
Results
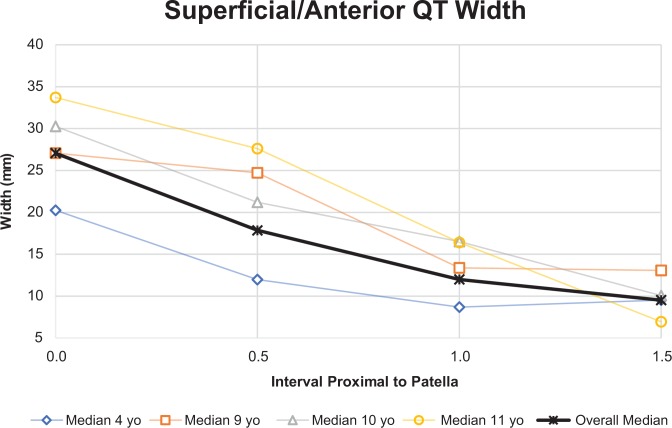
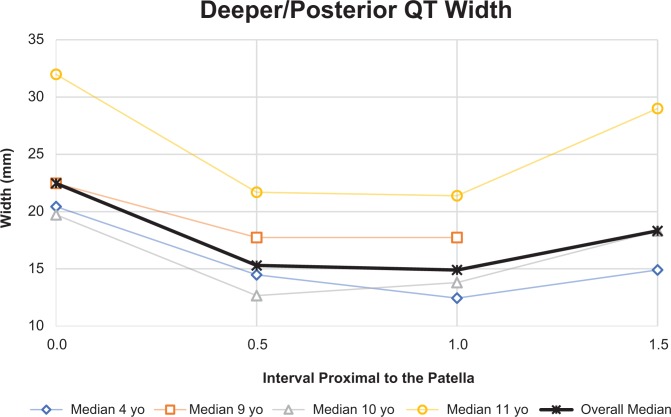
The median patellar length in all specimens was 28 mm (IQR, 26-37 mm). There was a statistically significant positive correlation between patellar length and age (r = 0.806, P = .009). Superficially, the mean coronal-plane width of the QT was 27 mm, 18 mm, 12 mm, and 10 mm at intervals proximal to the patellar pole equal to 0.0, 0.5, 1.0, and 1.5 times the patellar length, respectively. Deeper measurements at the same intervals yielded 22 mm, 15 mm, 15 mm, and 18 mm, respectively. The median depth of the QT in all specimens was 5 mm at the superior patellar pole (interval 0.0), 5 mm at interval 0.5, 4 mm at interval 1.0, and 4 mm at interval 1.5.
The deeper aspect of the QT narrowed and then widened, as shown in Figure 2A. The median distance from the superior pole of the patella to the point of divergence of the RT from the QT complex was 36 mm (IQR, 29-41 mm), as shown in Figure 2B. These absolute measurements were normalized to the length of the patella (distance to QT divergence/length of patella) and when normalized corresponded to a median ratio of 1.14 (IQR, 0.99-1.30). In contrast, the anterior QT coronal-plane width was greatest at its distal insertion and narrowed progressively more proximally, as shown in Figure 2A. These data are summarized in Figures 2 to 4.
Figure 2.
(A) Left knee specimen. The anterior portion of the quadriceps tendon (QT) or rectus tendon (RT; in white) narrows as the distance from the patella is increased, while the deeper QT complex (black striped pattern represents the remainder of the posterior QT complex) narrows and then widens. The point of widening is highlighted by the short black arrow in this specimen, occurring at a median of 0.95 times the patellar length for the group of specimens. The three 4-year-old QTs differed from the older specimens in that the tendon was never wider superficially, even at the superior patellar pole. The superior patellar pole is at the left, indicated by the tip of the forceps. (B) “R” denotes the distance from the superior pole of the patella to the separation of the anterior RT from the rest of the deeper QT complex, which occurred at 0.95 times the patellar length. “W” denotes the point of widening of the deeper aspect of the posterior QT complex, which occurred at 1.14 times the patellar length. The deeper/posterior QT complex is represented by the black striped pattern.
Figure 3.
The coronal-plane width of the anterior quadriceps tendon (QT) decreased when measuring the QT moving proximally from the patellar pole at distances of 0.0, 0.5, 1.0, and 1.5 times the patellar length. This trend held true for all ages, except that the measurement at the 1.5 interval was larger than the 1.0 interval for one 4-year-old specimen.
Figure 4.
There was a decreasing and then increasing trend observed for the coronal-plane width of the quadriceps tendon (QT) when measured posteriorly, moving proximally from the superior pole of the patella at distances of 0.0, 0.5, 1.0, and 1.5 times the patellar length. This trend held true for all specimens, except for when the QT was transected because of specimen preparation, which rendered a proximal measurement at the point 1.5 times the length of the patella impossible.
At the superior patellar pole (interval 0.0), the QT in 6 of the 9 specimens was wider superficially and anteriorly, and 3 of 9 (all 4 years old) had nearly identical superficial and deeper coronal-plane widths (within 1 mm). At the 0.5 interval, the same 6 of 9 specimens were wider superficially and anteriorly, while the same 3 were wider deeper. At interval 1.0, 7 of 9 QTs were wider posteriorly. The 2 specimens that were wider superficially were wider by less than 1 mm. At interval 1.5, none of the anterior measurements (5 specimens) were larger than the 2 posterior measurements able to be recorded. The 3-dimensional shape of the QT can be visualized, as seen in Figure 2A and 2B, and is graphically represented in Figures 3 and 4.
The distance between the superior patellar pole and the point of separation between the RT and remainder of the QT was measured. The median distance was 34 mm (IQR, 21-35 mm). When normalized to the length of the patella, these measurements corresponded to a median ratio of 0.95 (IQR, 0.80-1.01). There was a statistically significant positive correlation between patellar length and the distance to RT and QT separation (r = 0.750, P = .020). There were no significant relationships between specimen age and the distance to QT separation or between age and QT separation in terms of patellar length (P = .089 and .361, respectively). This separation can be clearly seen in Figure 1A and 1B.
When comparing the point of RT separation and the point of deeper and posterior QT widening, it was evident that the median point of widening (36 mm) was proximal to the separation of the RT (34 mm). Despite variability of this relationship, there was a statistically significant positive correlation between the distance to widening and the distance to RT separation (r = 0.786, P = .036).
Discussion
This anatomic study demonstrated that the RT becomes distinctly separate from the overall QT complex above the patella at about 0.95 times the patellar length. If QT graft harvest extends above this distance, the risk of separation of the RT from the QT complex may place the RT at risk of retraction and subsequent QT weakness.
The results of this study also suggest that the anatomy of the QT varies with age and changes with distance from the superior patellar pole. The superficial and anterior QT (which is composed of the RT) narrows as the distance from the patella is increased, while the deeper/posterior QT (composed of the vastus intermedius as well as contributions from the vastus lateralis and medialis obliquus)32 narrows and then widens. The three 4-year-old QTs differed from the older specimens in that the tendon was never wider superficially/anteriorly, even at the superior patellar pole.
The adult anatomy of the QT has been previously described using advanced imaging in living patients as well as in cadaveric studies.3,10,18,22,32,33 The QT is thought to be an acceptable graft choice for adult ACL reconstruction, namely because of its adequate depth, thickness, length, and volume.3,10,18,22,32,33 Other investigators have noted a “thin fatty layer” between the rectus femoris and intermedius tendons,3 although we note the importance of obtaining both portions of the tendon to harvest a graft of adequate depth, without regard for RT separation from the underlying structures. Studies have shown a similar decrease in anterior QT width working proximally18,33 and narrowing, followed by widening, when examining the width of the posterior aspect of the QT.22 One study even described a phenomenon similar to the “splay” described here: an “additional thickness corresponding to tendinous expansion” on the posterior aspect of the QT.22 Although QT thickness was not a focus of this study, our data do show a decreasing depth, or thickness, working proximally from the patella (unpublished data), similar to adult studies.22 Less is written about the QT anatomy of the skeletally immature, but Todd et al31 recently investigated the dimensions of the pediatric QT and reported measurements larger than those recorded in this study. This discrepancy could be because of the difference in measurement techniques, as this study employed cadaveric specimens while Todd et al31 used ultrasound with patients in the clinic, as well as the greater number of adolescent patients in their series.
Recent anatomic studies of the QT have focused on the details and separate layers of this complex structure, including the distinct separation of the RT from the other components of the QT.7,18 The point of separation between the RT and QT and the point of widening of the deeper and posterior QT are of interest. During QT graft harvest for ACL reconstruction, it is possible to separate the RT from the QT. For this group of specimens, RT separation was proximal to the superior pole of the patella at a distance of 0.95 times the patellar length (median patellar length in all specimens was 28 mm), which is less than half the length of the graft that would be selected for ACL reconstruction (generally more than 60 mm). Thus, routine QT graft harvest would extend ≥3 cm proximal to the zone of RT separation. Ideally, the graft is 10 mm in width.1
Several of the coauthors of this study have clinical, anatomic experience with rectus transfer for pediatric spasticity disorders, and this experience raised the concern of the potential for RT separation and secondary muscle retraction and/or recession with this graft harvest procedure. RT retraction is associated with QT weakness in adult patients. Traumatic RT ruptures are rare events in the pediatric population,29 but separation and retraction of the RT could contribute to QT weakness. Careful closure and visualization of the graft harvest interval that includes repair of the RT to the surrounding deep (vastus intermedius), medial (vastus medialis), and lateral (vastus lateralis) components of the QT may be advantageous to avoid secondary RT retraction and QT weakness. An incision allowing adequate exposure for visualization of this proximal graft harvest zone and consideration of this repair may be warranted in the immature population.
Limitations
This study has several limitations. The dimensions of this small group of knees may not be representative of the general skeletally immature population. It is possible that some patients were small for their age, but the normalization of measurements by patellar length partially addresses this constraint. Other limitations include specimen preparation and incomplete measurements on some samples, specifically at the 1.5 interval and at the distance to widening on the deeper/posterior aspect of the tendon. Finally, 6 of the 9 specimens were part of a single patient pair, which may violate the independent observations assumption required by the Spearman correlation coefficient. A few measurements between pairs were nearly identical, but there was considerable variability among others, especially in the older specimen pairs (9 and 10 years old). In fact, when measuring the QT width, the pairs were often more similar to an unrelated knee than to each other. However, access to pediatric knee specimens is limited, and despite these limitations in the number, size, and age of specimens, these data may offer value to surgeons, as physeal-respecting reconstruction techniques may benefit from the QT as a soft tissue graft source.2,15
Conclusion
During QT graft harvest for ACL reconstruction, a significant component of the RT may be removed, and extending the graft harvest site proximal to the separation between the RT and QT complex may be associated with complete separation of the RT from the QT complex. This could lead to RT and rectus muscle retraction, which may be associated with significant QT weakness. A better understanding of the QT complex anatomy will improve graft harvest techniques, allow for repair/reattachment of the RT to the QT complex, and may avoid RT retraction and secondary QT weakness.
Acknowledgment
The authors acknowledge the support and donation of the study specimens from Allosource. They honor the families that have made the gifts of tissue donation to allow them to perform this research to improve patient care.
Footnotes
One or more of the authors has declared the following potential conflict of interest or source of funding: Cadaveric specimens in this study were donated by AlloSource. K.G.S. has received nonconsulting fees from DePuy and research support from Sanofi-Aventis (contested by the author). H.B.E. has received educational support from Pylant Medical, nonconsulting fees from Smith & Nephew and Synthes, and hospitality payments from Arthrex. P.L.W. has received educational support from Pylant Medical. P.D.F. has received educational support from Smith & Nephew and hospitality payments from Medical Device Business Services. S.M. has received educational support from Smith & Nephew and Gemini Mountain Medical, consulting fees from Arthrex, and hospitality payments from Stryker. T.J.G. has received educational support from Arthrex and Liberty. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval was not sought for the present study.
References
- 1. Albright J, Lepon AK, Mayer S. Anterior cruciate ligament reconstruction in pediatric and adolescent patients using quadriceps tendon autograft. Sports Med Arthrosc. 2016;24(4):159–169. [DOI] [PubMed] [Google Scholar]
- 2. Anderson AF. Transepiphyseal replacement of the anterior cruciate ligament in skeletally immature patients: a preliminary report. J Bone Joint Surg Am. 2003;85(7):1255–1263. [DOI] [PubMed] [Google Scholar]
- 3. DeAngelis JP, Fulkerson JP. Quadriceps tendon: a reliable alternative for reconstruction of the anterior cruciate ligament. Clin Sports Med. 2007;26(4):587–596. [DOI] [PubMed] [Google Scholar]
- 4. Dreher T, Wolf SI, Maier M, et al. Long-term results after distal rectus femoris transfer as a part of multilevel surgery for the correction of stiff-knee gait in spastic diplegic cerebral palsy. J Bone Joint Surg Am. 2012;94(19):e142. [DOI] [PubMed] [Google Scholar]
- 5. Fabricant PD, Jones KJ, Delos D, et al. Reconstruction of the anterior cruciate ligament in the skeletally immature athlete: a review of current concepts. AAOS exhibit selection. J Bone Joint Surg Am. 2013;95(5):e28. [DOI] [PubMed] [Google Scholar]
- 6. Faunø P, Rømer L, Nielsen T, Lind M. The risk of transphyseal drilling in skeletally immature patients with anterior cruciate ligament injury. Orthop J Sports Med. 2016;4(9):2325967116664685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grob K, Manestar M, Filgueira L, Ackland T, Gilbey H, Kuster MS. New insight in the architecture of the quadriceps tendon. J Exp Orthop. 2016;3(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Group M. Effect of graft choice on the outcome of revision anterior cruciate ligament reconstruction in the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2014;42(10):2301–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Han HS, Seong SC, Lee S, Lee MC. Anterior cruciate ligament reconstruction: quadriceps versus patellar autograft. Clin Orthop Relat Res. 2008;466(1):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Harris NL, Smith DA, Lamoreaux L, Purnell M. Central quadriceps tendon for anterior cruciate ligament reconstruction, part I: morphometric and biomechanical evaluation. Am J Sports Med. 1997;25(1):23–28. [DOI] [PubMed] [Google Scholar]
- 11. Kaeding CC, Aros B, Pedroza A, et al. Allograft versus autograft anterior cruciate ligament reconstruction: predictors of failure from a MOON prospective longitudinal cohort. Sports Health. 2011;3(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kocher MS, Garg S, Micheli LJ. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents: surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1 pt 2):283–293. [DOI] [PubMed] [Google Scholar]
- 13. Kocher MS, Saxon HS, Hovis WD, Hawkins RJ. Management and complications of anterior cruciate ligament injuries in skeletally immature patients: survey of the Herodicus Society and the ACL Study Group. J Pediatr Orthop. 2002;22(4):452–457. [PubMed] [Google Scholar]
- 14. LaBella CR, Hennrikus W, Hewett TE; Council on Sports Medicine and Fitness, Section on Orthopaedics. Anterior cruciate ligament injuries: diagnosis, treatment, and prevention. Pediatrics. 2014;133(5):e1437–e1450. [DOI] [PubMed] [Google Scholar]
- 15. Lawrence JT, Bowers AL, Belding J, Cody SR, Ganley TJ. All-epiphyseal anterior cruciate ligament reconstruction in skeletally immature patients. Clin Orthop Relat Res. 2010;468(7):1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JK, Lee S, Lee MC. Outcomes of anatomic anterior cruciate ligament reconstruction: bone-quadriceps tendon graft versus double-bundle hamstring tendon graft. Am J Sports Med. 2016;44(9):2323–2329. [DOI] [PubMed] [Google Scholar]
- 17. Lee S, Seong SC, Jo CH, Han HS, An JH, Lee MC. Anterior cruciate ligament reconstruction with use of autologous quadriceps tendon graft. J Bone Joint Surg Am. 2007;89(suppl 3):116–126. [DOI] [PubMed] [Google Scholar]
- 18. Lippe J, Armstrong A, Fulkerson JP. Anatomic guidelines for harvesting a quadriceps free tendon autograft for anterior cruciate ligament reconstruction. Arthroscopy. 2012;28(7):980–984. [DOI] [PubMed] [Google Scholar]
- 19. Lund B, Nielsen T, Faunø P, Christiansen SE, Lind M. Is quadriceps tendon a better graft choice than patellar tendon? A prospective randomized study. Arthroscopy. 2014;30(5):593–598. [DOI] [PubMed] [Google Scholar]
- 20. Noyes FR, Butler DL, Grood ES, Zernicke RF, Hefzy MS. Biomechanical analysis of human ligament grafts used in knee-ligament repairs and reconstructions. J Bone Joint Surg Am. 1984;66(3):344–352. [PubMed] [Google Scholar]
- 21. Pandey V, Madi S, Joseph A, Acharya K. Late quadriceps tendon rupture at the donor site following cruciate ligament reconstruction using central quadriceps tendon graft. BMJ Case Rep. 2015;2015:BCR2015212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Potage D, Duparc F, D’Utruy A, Courage O, Roussignol X. Mapping the quadriceps tendon: an anatomic and morphometric study to guide tendon harvesting. Surg Radiol Anat. 2015;37(9):1063–1067. [DOI] [PubMed] [Google Scholar]
- 23. Shea KG, Belzer J, Apel PJ, Nilsson K, Grimm NL, Pfeiffer RP. Volumetric injury of the physis during single-bundle anterior cruciate ligament reconstruction in children: a 3-dimensional study using magnetic resonance imaging. Arthroscopy. 2009;25(12):1415–1422. [DOI] [PubMed] [Google Scholar]
- 24. Shea KG, Carey JL, Richmond J, et al. The American Academy of Orthopaedic Surgeons evidence-based guideline on management of anterior cruciate ligament injuries. J Bone Joint Surg Am. 2015;97(8):672–674. [DOI] [PubMed] [Google Scholar]
- 25. Shea KG, Grimm NL, Belzer JS. Volumetric injury of the distal femoral physis during double-bundle ACL reconstruction in children: a three-dimensional study with use of magnetic resonance imaging. J Bone Joint Surg Am. 2011;93(11):1033–1038. [DOI] [PubMed] [Google Scholar]
- 26. Shifflett GD, Green DW, Widmann RF, Marx RG. Growth arrest following ACL reconstruction with hamstring autograft in skeletally immature patients: a review of 4 cases. J Pediatr Orthop. 2016;36(4):355–361. [DOI] [PubMed] [Google Scholar]
- 27. Slone HS, Ashford WB, Xerogeanes JW. Minimally invasive quadriceps tendon harvest and graft preparation for all-inside anterior cruciate ligament reconstruction. Arthrosc Tech. 2016;5(5):e1049–e1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Slone HS, Romine SE, Premkumar A, Xerogeanes JW. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31(3):541–554. [DOI] [PubMed] [Google Scholar]
- 29. Temple HT, Kuklo TR, Sweet DE, Gibbons CL, Murphey MD. Rectus femoris muscle tear appearing as a pseudotumor. Am J Sports Med. 1998;26(4):544–548. [DOI] [PubMed] [Google Scholar]
- 30. Thawrani D, Haumont T, Church C, Holmes L, Dabney KW, Miller F. Rectus femoris transfer improves stiff knee gait in children with spastic cerebral palsy. Clin Orthop Relat Res. 2012;470(5):1303–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Todd DC, Ghasem AD, Xerogeanes JW. Height, weight, and age predict quadriceps tendon length and thickness in skeletally immature patients. Am J Sports Med. 2015;43(4):945–952. [DOI] [PubMed] [Google Scholar]
- 32. Waligora AC, Johanson NA, Hirsch BE. Clinical anatomy of the quadriceps femoris and extensor apparatus of the knee. Clin Orthop Relat Res. 2009;467(12):3297–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xerogeanes JW, Mitchell PM, Karasev PA, Kolesov IA, Romine SE. Anatomic and morphological evaluation of the quadriceps tendon using 3-dimensional magnetic resonance imaging reconstruction: applications for anterior cruciate ligament autograft choice and procurement. Am J Sports Med. 2013;41(10):2392–2399. [DOI] [PubMed] [Google Scholar]
- 34. Yoo WJ, Kocher MS, Micheli LJ. Growth plate disturbance after transphyseal reconstruction of the anterior cruciate ligament in skeletally immature adolescent patients: an MR imaging study. J Pediatr Orthop. 2011;31(6):691–696. [DOI] [PubMed] [Google Scholar]