Abstract
Background
This study investigated the epidemiological and molecular aspects of dicrocoeliosis in extensive sheep farms.
Methods
From 2013 to 2014, copromicroscopical analyses in 190 dairy sheep farms and anatomo-pathological inspections in six slaughterhouses were carried in Sardinia, Italy. Rectal faecal samples were analyzed using the FLOTAC® method, and anatomo-pathological examinations were based on detecting thickened terminal bile ducts (TTBDs). In addition, genetic analyses were conducted on representative DNA samples of adult Dicrocoelium spp.
Results
Ninety-seven (51.1%) out of 190 sheep farms were coprologically positive for Dicrocoelium spp. In the liver, on the surface and cut surface, TTBDs were reported in 40.1% (309/770) and 15.3% (118/770) of the animals examined, respectively, with an overall prevalence of 25.5% (196/770). No intraspecific genetic variation was observed among the Dicrocoelium dendriticum isolates.
Conclusions
Our survey reveals the widespread presence of D. dendriticum in Sardinia, although seasonal, geographical and climatic conditions might be key factors in modulating the infection prevalence. Examining typical lesions due to D. dendriticum in the liver in abattoirs can be used as a marker for tracking chronic dicrocoeliosis infection.
Keywords: Sheep, Breeding, Trematoda, Dicrocoelium dendriticum, Epidemiology
Background
Dicrocoeliosis is a disease caused by several species of the genus Dicrocoelium Dujardin, 1845 (Trematoda: Digenea), which live in the hepatic bile ducts and gall-bladder of domestic and wild ruminants [1]. Liver lesions due to dicrocoeliosis, such as abscesses, granulomas and fibrosis, as well as bile duct proliferation have also been described in the New World camelids (llamas and alpacas) [2–4]. Occasionally, Dicrocoelium spp. can also infect rabbits, pigs, dogs, horses and humans [5]. The various species of Dicrocoelium have different geographical distributions, with D. dendriticum being the most widespread globally, being found in Europe, Asia (China and the Indo-Malayan region), Japan, North Africa and Australia [3, 5], while Dicrocoelium hospes, Dicrocoelium chinensis and Dicrocoelium suppereri [3] have a limited distribution in Africa, Asia and some areas of western Europe, respectively [6–9]. To complete its life-cycle, Dicrocoelium develops within the body of some land snails and ant species, which act as first and second intermediate hosts, respectively [10].
Dicrocoeliosis is commonly considered to be of negligible economic importance, resulting only in livers being discarded during meat inspection at slaughterhouses [11, 12]. In reality however, production performance losses in animals are often not associated with dicrocoeliosis, as the infection remains underestimated in field conditions because of its subclinical evolution [13]. The pathological effects related to dicrocoeliosis in ruminants can sometimes be overshadowed by concurrent liver infections (i.e. cystic echinococcosis, cysticercosis caused by Taenia hydatigena, fasciolosis); consequently, veterinarians and farmers may underestimate the importance of this disease [14–16]. Infected animals with a parasitic burden of under 1000 individuals of D. dendriticum usually do not show any clinical manifestations [17] and even infections with 4000 parasites can cause mild symptoms [8]. In fact, in previous work we reported that only 33.3% of practitioners diagnose dicrocoeliosis according to clinical symptoms [18].
In addition, sheep with D. dendriticum are often co-infected with other parasites (e.g. gastrointestinal and bronchopulmonary nematodes) making it quite difficult to identify the specific outcomes of each individual parasitosis [5].
Another aspect that may lead to an underestimation of dicrocoeliosis is that this parasitosis is generally not diagnosed with an appropriate coprodiagnostic analysis, thus infected animals are not identified [19]. As a consequence, the infection becomes increasingly persistent, with cumulative effects [19, 20]. Moreover, serological techniques do not provide reliable information for diagnosing dicroceliosis, although these tests may be useful when investigating prepatent infections [21].
Sardinia (Italy) has a long-established sheep farming tradition with over 3,200,000 sheep, which represent 45% of the entire stock of the Italian sheep population [22]. Due to the insularity and the high concentration of animals, which all belong to the Sardinian sheep breed, the island is regarded as a unique geographical area for epidemiological studies on parasites [15, 16, 23–28].
Most of the data on sheep dicrocoeliosis regarding Sardinia have demonstrated that this parasitosis is endemic, though much of the data is not recent [29, 30].
In this study, we investigated sheep dicrocoeliosis in Sardinia with particular emphasis on parasitological and molecular aspects in order to provide new insights into its epidemiology in extensive sheep farms.
Methods
Copromicroscopical survey in sheep farms
The sample size of the farms studied was estimated considering 15,387 Sardinian sheep farms (National Data Bank of the Italian Ministry of Health; https://www.vetinfo.it) with an expected Dicrocoelium spp. prevalence of 15%, and confidence level of 95% (http://www.raosoft.com/samplesize.html).
A total of 190 dairy sheep farms in Sardinia (Fig. 1) were investigated from 2013 to 2014. Within each flock, 15 individual rectal faecal samples from sheep older than 3 years of age were collected. These samples were then split into three different faecal pools from five animals, which were then analysed using the FLOTAC® method with a heavy saturated zinc sulphate solution, specific gravity (SG) 1350) [31, 32].
Fig. 1.
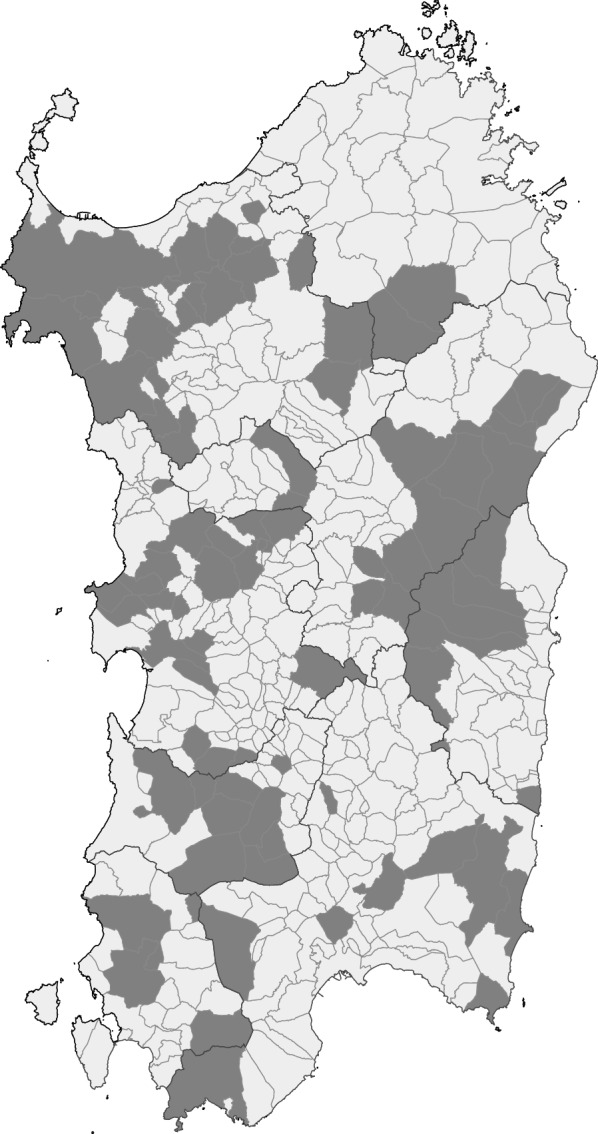
Map of the municipalities of Sardinia showing the sampling sites
Data were processed for each farm considering the eggs per gram (EPG) mean of the three faecal pools.
The data were then stratified by geolocalisation in the four provinces of Sardinia (Sassari, Cagliari, Nuoro and Oristano) (Fig. 1). Farms were grouped according to the EPG means values into four classes: (i) ≤ 50 EPG; (ii) > 50 and ≤ 100 EPG; (iii) > 100 and ≤ 300; (iv) > 300 EPG.
The mean intensity (MI) was obtained considering the arithmetic mean of the EPG values in the total number of the infected animals.
Epidemiological survey in slaughterhouses
The sample size of the studied animals was determined considering a total of 3,206,821 heads of Sardinian dairy sheep (National Data Bank of the Italian Ministry of Health; https://www.vetinfo.it) with an expected Dicrocoelium spp. prevalence of 15%, and confidence level of 95% (http://www.raosoft.com/samplesize.html).
From 2013 to 2014, 770 Sarda sheep slaughtered in six different abattoirs in Sardinia, were submitted to anatomo-pathological examination to detect the liver parasites and to evaluate the typical thickened terminal bile duct (TTBD) lesions [33, 34], following the EEC Inspection Regulation No. 854 of 2004 (Annex 1, Section IV, Chapter II, point 5) [35] and the methods illustrated by Marcato [36].
To perform the anatomo-pathological examination, seven hepatic areas were selected: right lobe (RL) and left lobe (LL) of both the diaphragmatic face (DF) and visceral face (VF), quadrate lobe (QL), caudate lobe (CL) and finally the cut surface. For each area the severity/extension of the lesions indicative of a TTBD pattern were scored as follows: (0) absence of TTBD; (1) presence of rare TTBD; (2) ≤ 5 cm2; (3) 5–7.5 cm2; (4) 7.5–10 cm2; and (5) > 10 cm2.
Subsequently, according to the guidelines of the World Association for the Advancement of Veterinary Parasitology (WAAVP) [37], an incision on the gall-bladder wall was performed, and the entire liver parenchyma was cut into 0.5–1.0 cm slices, in order to identify and count the adult parasites. The parasitic burdens were classified into five classes, based on the number of parasites found in the organ: (i) ≤ 50; (ii) 50–100; (iii) 100–300; (iv) 300–1000; and (5) > 1000. Five adult Dicrocoelium spp. were taken from each liver in order to confirm the species based on published morphological keys [6].
Genetic analysis
DNA from 15 adult Dicrocoelium spp. representing all four provinces of Sardinia was extracted using a commercial kit, PureLink® Genomic DNA Mini Kit (Invitrogen, Carlsbad, CA, USA) by following the manufacturer’s instructions. DNA samples were amplified by PCR for the regions internal transcribed spacer (ITS2) with the primers and the methods described elsewhere [6, 40]. PCR products were purified using a commercial kit (Nucleospin Gel and PCR Clean Up; Macherey-Nagel, Düren, Germany) and sent to an external sequencing service (Eurofins Genomics, Ebersberg, Germany). Sequences were assembled manually with the aid of the CLUSTAL W multiple alignment program [38], and analyzed using the basic local alignment search tool (BLAST) available on the NCBI website (https://blast.ncbi.nlm.nih.gov/Blast.cgi.).
Statistical analysis
Data were processed using MINITAB v.12.1 (Minitab Inc., State College, PA, USA) and EpiInfo v.6.04 (CDC, Atlanta, GA, USA). A Chi-square test was performed to compare the prevalence in the four provinces. In order to compare the prevalence rates found in the different seasons, a chi-square trend test was used and odds ratio (OR) values were calculated. Mann–Whitney and Kruskal–Wallis non-parametric tests were used to compare the EPG means. Pearsonʼs correlation test was performed in order to evaluate the correlations between the parasite burden and TTBD score.
Results
Copromicroscopic survey
Ninety-seven out of the 190 examined farms were coprologically positive for Dicrocoelium spp. (51.1%; 95% CI: 43.91–58.07%). Quantitative coprological analysis of Dicrocoelium spp. showed a EPG mean (± standard deviation, SD) of 31.2 ± 68.7 and a MI of 61.1 EPG. Interestingly, dicrocoeliosis prevalence was significantly lower in the summer (37.5%) compared to the winter (90.9%), when the odds ratio (OR) values were four times higher than in other seasons (Table 1).
Table 1.
Seasonal trend of prevalence, EPG mean excretion and odds ratio values for D. dendriticum in farm faecal pools samples
| Season | Total no. of farms | No. of positive farms | % positive farmsa | EPG Mean ± SDb | Odds ratio |
|---|---|---|---|---|---|
| Spring | 13 | 9 | 69.2 | 26.8 (29.4) | 1.00 |
| Summer | 120 | 45 | 37.5 | 22.1 (65.4) | 0.27 |
| Autumn | 46 | 33 | 71.7 | 52.8 (80.3) | 1.13 |
| Winter | 11 | 10 | 90.9 | 44.9 (68.8) | 4.44 |
aχ2 trend = 11.558, df = 3, P < 0.0007
bKruskal–Wallis test: H = 24.74, P < 0.0001
Table 2 shows the prevalence, farm EPG mean, MI values for Dicrocoelium spp. in the faecal pools and OR values in the four provinces. Regarding the Dicrocoelium spp. prevalence, the four provinces showed significant differences (χ2 = 23.89, df = 3, P < 0.0001); there were also statistically significant differences in EPG means (Kruskal–Wallis H-test: χ2 = 30.88, P < 0.0001). The province of Nuoro showed the highest prevalence and EPG means for dicrocoeliosis, as well as the highest OR values (OR = 9) (Table 2).
Table 2.
Prevalence, EPG mean values, mean intensity and odds ratio values for D. dendriticum in farm faecal samples in each province of Sardinia
| Province | Total no. of farms | No. of positive farms | Prevalence (%)a | EPG Meanb | Mean intensity (EPG) | Odds ratio |
|---|---|---|---|---|---|---|
| Cagliari | 32 | 16 | 50.0 | 55.5 | 111.0 | 1.00 |
| Oristano | 44 | 22 | 50.0 | 21.2 | 42.4 | 1.00 |
| Sassari | 84 | 32 | 38.1 | 16.4 | 43.0 | 0.62 |
| Nuoro | 30 | 27 | 93.3 | 61.4 | 66.8 | 9.00 |
aχ2 = 23.89, df = 3, P < 0.0001
bKruskal–Wallis test: H = 30.88, P < 0.0001
On-farm overall prevalence of Dicrocoelium by year was 36.3% (45/124) in 2013 and 78.8% (52/66) in 2014. There were statistically significant differences in prevalence between the two years (χ2 = 31.13, df = 1, P < 0.0001), as well as in the EPG means, which were 20.7 ± 63 EPG in 2013 and 50.8 ± 89.3 EPG in 2014 (Mann–Whitney U-test: U = 10554.0, P = 0.0004).
A total of 156 (82.1%) of the farms investigated were negative or with EPG mean values of ≤ 50 EPG, while 17 (9%) had EPG mean values of 50–100 EPG, 13 (6.8%) had EPG mean values of 100–300 EPG and only four (2.1%) had EPG mean values of > 300 EPG. These values were statistically different (χ2 = 443.09, df = 3, P < 0.0001).
Epidemiological survey in slaughterhouses
The anatomo-histopathological examination of the livers showed a Dicrocoelium spp. prevalence of 25.5% (95% CI: 0.22–0.28%) (196/770), 54.1% of which harboured less than 50 adult parasites per organ, while only 3% harboured over 1000 parasites (Table 3).
Table 3.
Prevalence and odds ratios of D. dendriticum in livers examined at abattoirs
| Infection classa | No. of positive livers | Prevalence (%)b | Odds ratio exposure score |
|---|---|---|---|
| ≤ 50 | 106 | 54.1 | 1.00 |
| > 50 to ≤ 100 | 37 | 18.9 | 0.20 |
| > 100 to ≤ 300 | 25 | 12.8 | 0.12 |
| > 300 to ≤ 1000 | 22 | 11.2 | 0.11 |
| > 1000 | 6 | 3.0 | 0.03 |
aNo. of adult parasites
bχ2 trend =147.25, df = 3, P < 0.0001
TTBD on the surface and the cut surface were reported in 40.1% (309/770) and 15.3% (118/770) of examined livers, respectively (Fig. 2). The hepatic areas most involved were the RL of VF and CL with a prevalence of 24.8% (191/770) and 16.8% (129/770), respectively. TTBD was not observed in the quadrate lobe. Our results did not show any match between the presence of parasites in the examined livers (25.5%) and the TTBD both on the surface (40.1%), and the cut surface (15.3%) (χ2 = 121.62, df = 2, P < 0.0001). The score values were higher in RL VF than the other hepatic localizations. Detailed data are reported in Table 4.
Fig. 2.
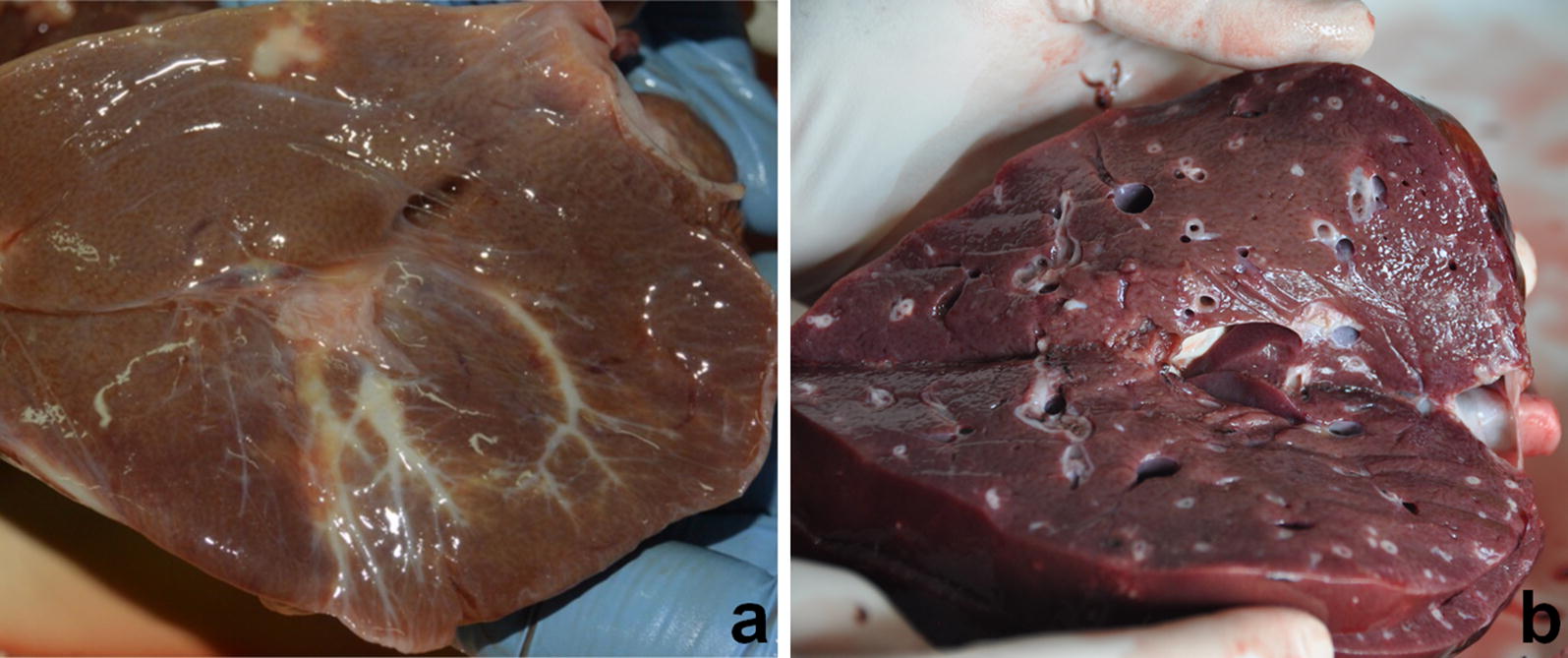
Thickened terminal bile duct (TTBD) on the surface (a) and cut surface (b) of the liver
Table 4.
Score of livers reporting thickened terminal bile duct (TTBD) in the different examined areas: right lobe (RL) and left lobe (LL) of diaphragmatic face (DF) and visceral face (VF), caudate lobe (CL) and cut surface
| Localisation TTBD | No. positive (%) | Score, no. positive (%) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| RL DF | 45 (5.8) | 34 (4.4) | 11 (1.4) | 0 (0) | 0 (0) | 0 (0) |
| LL DF | 5 (0.7) | 5 (0.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| RL VF | 191 (24.8) | 95 (12.3) | 79 (10.3) | 6 (0.8) | 11 (1.4) | 0 (0) |
| LL VF | 124 (16.1) | 67 (8.7) | 51 (6.6) | 6 (0.8) | 0 (0) | 0 (0) |
| CL | 129 (16.8) | 6 (0.8) | 107 (13.9) | 10 (1.3) | 6 (0.8) | 0 (0) |
| Cut surface | 118 (15.3) | 45 (5.8) | 45 (5.8) | 11 (1.4) | 17 (2.2) | 0 (0) |
There was a significant positive correlation between the parasite burden and the scores for the severity/extension of TTBD as follows: RL of DF (r = 0.538, P < 0.0001), RL VF (r = 0.484, P < 0.0001), LL VF (r = 0.374, P < 0.0001), CL (r = 0.351, P < 0.0001) and the cut surface (r = 0.338, P < 0.0001). According to Colton [39], the correlation, based on reported r-values, between the parasitic burden and RL VF was moderate to good, while the correlations between the parasitic burden and the other hepatic localizations were quite good. Using the morphological examination, all Dicrocoelium spp. were identified as D. dendriticum [6].
Genetic analysis
No intraspecific variations were observed for the ITS2 gene sequence (GenBank: MG004688) among the D. dendriticum isolates. In addition, the same isolates showed a homology of 99%, with the Iranian ITS-B haplotype isolate (GenBank: JQ966973) [40], and a homology of 99% and 98% with the Italian isolates DQ379986.2 [41] and EF547132.1 [6], respectively. The sequence alignment of ITS showed a T/A substitution at the 153 codon with an index diversity of 0.002, compared with the sequence of the above mentioned Iranian isolate.
Discussion
The present survey provides an update of various epidemiological aspects of sheep dicrocoeliosis in Sardinia. Our copromicroscopic survey on the farms revealed the widespread presence of D. denditricum. However, the distribution of this parasite does not appear to be homogeneous across the island, with significantly more farms affected in the province of Nuoro, which is located in the central part of the island. It should also be highlighted that this area is characterised by the highest altitude with an average altitude of 496 meters) (http://www.comuni-italiani.it/20/clima.html) and the lowest average temperature compared with the other provinces (http://www.sar.sardegna.it/pubblicazioni/riepiloghimensili/mensili.asp).
The EPG means and OR values appear to be statistically higher in the winter in other regions of Italy [33] and also in other countries [42–44]. In Spain it has been reported that the mountainous pastures located over 600 meters and with temperatures of < 11.8 °C present the highest risk of infection with D. dendriticum [44]. These findings suggest that in some geographical areas and, especially during the winter, it is important to monitor and carry out anthelmintic treatments against Dicrocoelium spp. in sheep. We also found a different prevalence and EPG means values between the two years studied, thus suggesting that the epidemiology of the dicrocoeliosis could also be influenced by annual climatic conditions.
Our results show that the prevalence of dicrocoeliosis in Sardinia appears to be lower compared with other sheep-farming areas of Italy, such as Umbria (80%) [45], southern Apennines (67.5%) [1], Campania (67%) [46] and Basilicata (62%) [47].
Our survey demonstrated that inspections at slaughterhouses can detect the presence of the typical lesions due to D. dendriticum in the liver, and can thus be used to monitor the presence of chronic infections in a given flock. Underestimating the numbers of infected sheep is thus leading to the spread of parasitosis in Sardinia, which probably explains the high prevalence among sheep flocks on the island.
According to Ambrosi [33], infections with threshold values of under 100 adult parasites are not easily detected by copromicroscopical analysis. The same author [33] reported that approximately 7% of farms with EPG means values over 100 EPG could incur production losses. However, we found that only 3% of the examined livers in slaughterhouses showed a burden of over 1000 D. dendriticum. At the same time, the mild clinical signs might contribute to chronic infection and potentially to a loss of productive performance, which could be an interesting research line for further studies on this parasite.
Although previous papers have reported a high variability within D. dendriticum [48], both in terms of genetic and morphological parameters, no intraspecific variation was observed within our isolates and our results were consistent with findings in other surveys carried out in Italy [6, 41] and in Iran [40].
Conclusions
This present study show the widespread presence of D. dendriticum in Sardinia and highlights the key role of abattoirs and of the coprological analysis in the monitoring of parasitic diseases, through which farmers and practitioners can be given the data needed for diagnosing D. dendriticum and thus for setting up specific anthelmintic treatments.
Acknowledgments
The authors would like to thank Francesco Salis, head technician at the Department of Veterinary Medicine, for his valuable assistance in the laboratory, and Raffaele Marrosu and Giuseppe Bianco, ASL N.1 of Sassari for their help during the sampling.
Abbreviations
- TTBD
thickened terminal bile duct
- EPG
eggs per gram
- MI
mean intensity
- OR
odds ratio
- RL
right lobe
- VF
visceral face
- CL
caudate lobe
- QL
quadrate lobe
- DF
diaphragmatic face
- LL
left lobe
- ITS
internal transcribed spacer
- NDB
National Data Bank
- SD
standard deviation
Authors’ contributions
Conceived and designed the experiments: AS. Performed the experiments: GD, GS, CT, AV and GPS. Analyzed the data: AS, AV, CT and CL. Contributed reagents/materials/analysis tools: SC, PJ, AC and CL. Wrote the paper: AV, AS and CL. Collected biological samples: CT, GD, GS and GPS. Revised the manuscript: AV, AS, PJ and CL. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
All relevant data are included in the article. The newly generated sequence was submitted to the GenBank database under the Accession Number MG004688.
Ethics approval and consent to participate
This study was performed following the recommendations of European Council Directive (86/609/EEC) on the protection of animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Antonio Scala and Claudia Tamponi are equal contributors
Contributor Information
Antonio Scala, Email: scala@uniss.it.
Claudia Tamponi, Email: claudiatamponi@yahoo.it.
Giorgia Dessì, Email: giorgia.dessi87@tiscali.it.
Giampietro Sedda, Email: giampisedda@gmail.com.
Giuliana Sanna, Email: giulianasanna@yahoo.it.
Silvia Carta, Email: scarta1@uniss.it.
Andrea Corda, Email: andreacorda@uniss.it.
Philippe Jacquiet, Email: p.jacquiet@envt.fr.
Antonio Varcasia, Email: varcasia@uniss.it.
Ciriaco Ligios, Email: ciriaco_ligios@izs-sardegna.it.
References
- 1.Cringoli G, Rinaldi L, Veneziano V. A cross-sectional coprological survey of liver flukes in cattle and sheep from an area of the southern Italian Apennines. Vet Parasitol. 2002;108:137–143. doi: 10.1016/S0304-4017(02)00183-8. [DOI] [PubMed] [Google Scholar]
- 2.Wenker Ch, Halt J-M, Hertzberg H, Ossen P, Hänichen T, Brack A, Isenbügel E. Dicrocoeliosis in South American camelids. Tierarztl Prax. 1998;26:355–361. [PubMed] [Google Scholar]
- 3.Gunsser I, Hänichen T, Maierl J. Liver fluke infestation in New World camelids Parasitology, pathology, clinical findings and therapy. Tierärztl Prax. 1999;27:187–192. [PubMed] [Google Scholar]
- 4.Hilbe M, Robert N, Pospischil A, Gerspach C. Pulmonary arterial lesions in New World camelids in association with Dicrocoelium dendriticum and Fasciola hepatica infection. Vet Pathol. 2015;52:1202–1209. doi: 10.1177/0300985814564978. [DOI] [PubMed] [Google Scholar]
- 5.Otranto D, Traversa D. Dicrocoeliosis of ruminants: a little-known fluke disease. Trends Parasitol. 2003;19:12–15. doi: 10.1016/S1471-4922(02)00009-0. [DOI] [PubMed] [Google Scholar]
- 6.Otranto D, Rehbein S, Weigl S, Cantacessi C, Parisi A, Lia RP, et al. Morphological and molecular differentiation between Dicrocoelium dendriticum (Rudolphi, 1819) and Dicrocoelium chinensis (Sudarikov and Ryjikov, 1951) Tang and Tang, 1978 (Platyhelminthes: Digenea) Acta Trop. 2007;104:91–98. doi: 10.1016/j.actatropica.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 7.Lucius R. Untersuchungen zur Biologie, Pathologie ünd Ökologie von Dicrocoelium hospes Looss, 1907 (Trematodes, Dicrocoeliidae). Dissertation zur Erlangung des Grades eines Doktors der Naturwissenschaften vorgelegt der Fakultät II (Biologie) der Universität Hohenheim. University of Hohenheim, Germany; 1981.
- 8.Hinaidy HK. Dicrocoelium suppereri nomen novum (syn. D. orientalis Sudarikov et Ryjikov,1951), ein neuer Trematode fur die Parasitenfauna Osterreichs. Zentralbl Vetmed B. 1951;1983(30):576–589. [PubMed] [Google Scholar]
- 9.Tang C, Tang Z, Tang L, Cui Q, Lu H, Qian Y. Studies on the biology and epizootics of Dicrocoelium chinensis in the eastern Inner Mongol Autonomous region. Acta Zool Sin. 1983;29:340–349. [Google Scholar]
- 10.Campo R, Manga-Gonzalez MY, Gonzalez-Lanza C. Relationship between egg output and parasitic burden in lambs experimentally infected with different doses of Dicrocoelium dendriticum (Digenea) Vet Parasitol. 2000;87:139–149. doi: 10.1016/S0304-4017(99)00165-X. [DOI] [PubMed] [Google Scholar]
- 11.Theodoridis Y, Sotiraki S, Papadopoulos E. Observations on the blood figure of sheep infected with Dicrocoelium dendriticum. Bull Hellenic Vet Med Soc. 1999;50:300–304. doi: 10.12681/jhvms.15725. [DOI] [Google Scholar]
- 12.Arias M, Lomba C, Dacal V, Vazquez L, Pedreira J, Francisco I, et al. Prevalence of mixed trematode infections in an abattoir receiving cattle from northern Portugal and north-west Spain. Vet Rec. 2011;168:408. doi: 10.1136/vr.d85. [DOI] [PubMed] [Google Scholar]
- 13.Urqhhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW. Parassitologia veterinaria. Edizione italiana a cura di Claudio Genchi. Torino: UTET; 1998. [Google Scholar]
- 14.Otranto D, Traversa D. A review of dicrocoeliosis of ruminants including recent advances in the diagnosis and treatment. Vet Parasitol. 2002;107:317–335. doi: 10.1016/S0304-4017(02)00121-8. [DOI] [PubMed] [Google Scholar]
- 15.Tamponi C, Varcasia A, Pipia AP, Zidda A, Panzalis R, Dore F, et al. ISCOM ELISA in milk as screening for Neospora caninum in dairy sheep. Large Anim Rev. 2015;21:213–216. [Google Scholar]
- 16.Varcasia A, Tanda B, Giobbe M, Solinas C, Pipia AP, Malgor R, et al. Cystic echinococcosis in Sardinia: farmers’ knowledge and dog infection in sheep farms. Vet Parasitol. 2011;181:335–340. doi: 10.1016/j.vetpar.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Rojo-Vazquez FA, Cordero-Del-Campillo M, Diez-Baños P, Chaton-Schaffner M. Relation existant entre le nombre d’oeufs dans les feces et la charge parasitaire lors des infestations naturelles a Dicrocoelium dendriticum chez les ovins. Rev Med Vet. 1981;132:601–607. [Google Scholar]
- 18.Scala A, Maron P, Salamina V, Sanna G. Un “Questionnair survey” sui trattamenti antielmintici contro la dicroceliosi in una realtà ad alta vocazione per l’allevamento ovino quale la Sardegna. Large Animal Review, Acta XX Congreso Nazionale SIPAOC, Siracusa, Italy. 2012;131–3.
- 19.Cringoli G, Rinaldi L, Veneziano V, Capelli G, Scala A. The influence of flotation solution, sample diluition and the choice of McMaster slide area (volume) on the reliability of the McMaster technique in estimating the faecal egg counts of gastrointestinal strongyles and Dicrocoelium dendriticum in sheep. Vet Parasitol. 2004;123:121–131. doi: 10.1016/j.vetpar.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 20.Ducommun D, Pfister K. Prevalence and distribution of Dicrocoelium dendriticum and Fasciola hepatica infections in cattle in Switzerland. Parasitol Res. 1991;77:364–366. doi: 10.1007/BF00930918. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez-Andrade R, Paz-Silva A, Suarez JL, Arias M, Lopez C, Morrondo P, et al. Serum antibodies to Dicrocoelium dendriticum in sheep from Sardinia (Italy) Prev Vet Med. 2003;57:1–5. doi: 10.1016/S0167-5877(02)00215-5. [DOI] [PubMed] [Google Scholar]
- 22.ISTAT. Consistenza del bestiame ovino, caprino ed equino, per categoria (numero di capi) al 1° dicembre. Dettaglio per regione. 2015. http://agri.istat.it/jsp/dawinci.jsp?q=plB040000010000012000&an=2015&ig=1&ct=204&id=8A%7C16A%7C72A%7C13A%7C9A. Accessed 18 Oct 2018.
- 23.Scala A, Paz-Silva A, Suárez JL, López C, Díaz P, Díez-Baños P, et al. Chronobiology of Oestrus ovis (Diptera: Oestridae) in Sardinia, Italy: Guidelines to chemoprophylaxis. J Med Entomol. 2002;39:652–657. doi: 10.1603/0022-2585-39.4.652. [DOI] [PubMed] [Google Scholar]
- 24.Scala A, Garippa G, Varcasia A, Tranquillo VM, Genchi C. Cystic echinococcosis in slaughtered sheep in Sardinia (Italy) Vet Parasitol. 2006;135:33–38. doi: 10.1016/j.vetpar.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Scala A, Demontis F, Varcasia A, Pipia AP, Poglayen G, et al. Toltrazuril and sulphonamide treatment against naturally Isospora suis infected suckling piglets: Is there an actual profit? Vet Parasitol. 2009;163:362–365. doi: 10.1016/j.vetpar.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 26.Scala A, Pipia AP, Dore F, Sanna G, Tamponi C, Marrosu R, et al. Epidemiological updates and economic losses due to Taenia hydatigena in sheep from Sardinia, Italy. Parasitol Res. 2015;114:3137–3143. doi: 10.1007/s00436-015-4532-x. [DOI] [PubMed] [Google Scholar]
- 27.Sanna G, Varcasia A, Serra S, Salis F, Sanabria R, Pipia AP, et al. Calicophoron daubneyi in sheep and cattle of Sardinia, Italy. Helminthologia. 2016;53:87–93. doi: 10.1515/helmin-2015-0069. [DOI] [Google Scholar]
- 28.Varcasia A, Pipia AP, Dessì G, Zidda A, Tamponi C, Pau M, et al. Morphology and genetic variability within Taenia multiceps in ruminants from Italy. Vet Parasitol. 2016;223:181–185. doi: 10.1016/j.vetpar.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 29.Sanna E, Garippa G, Asoni G. Attuale diffusione delle parassitosi ovine in Sardegna. Atti SISVET. 1989;43:1299–1302. [Google Scholar]
- 30.Scala A. Dicrocoeliosi ovina in Sardegna: tassi di prevalenza, lesioni anatomoistopatologiche e tests ematochimici. Atti FEMESPRUM. 1991;1:209–214. [Google Scholar]
- 31.Rinaldi L, Coles GC, Maurelli MP, Musella V, Cringoli G. Calibration and diagnostic accuracy of simple flotation, McMaster and FLOTAC for parasite egg counts in sheep. Vet Parasitol. 2011;177:345–352. doi: 10.1016/j.vetpar.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Rinaldi L, Biggeri A, Musella V, De Waal T, Hertzberg H, Mavrot F, et al. Sheep and Fasciola hepatica in Europe: the GLOWORM experience. Geospat Health. 2015;9:309–317. doi: 10.4081/gh.2015.353. [DOI] [PubMed] [Google Scholar]
- 33.Ambrosi M. Parassitologia zootecnica. Bologna: Edagricole; 1995. [Google Scholar]
- 34.Camara L, Pfister K, Aeschlimann A. Analyse histopathologique de foie de bovin infesté par Dicrocooelium dendriticum. Vet Res. 1996;27:87–92. [PubMed] [Google Scholar]
- 35.European Parliament and Council. EEC Inspection Regulation No. 854 of 2004 (Annex 1, Section IV, Chapter II, point 5). Strasbourg: Official Journal of the European Union; 2004.
- 36.Marcato SP. Patologia animale e ispezione sanitaria delle carni fresche - Testo e Atlante. Bologna: Edagricole; 1995. [Google Scholar]
- 37.Wood IB, Amaral NK, Bairden K, Duncan JL, Kassai T, Malone JB, et al. World Association for the Advancement of Veterinary Parasitology (WAAVP) second edition of guidelines for evaluating the efficacy of anthelmintics in ruminants (bovine, ovine, caprine) Vet Parasitol. 1995;58:181–213. doi: 10.1016/0304-4017(95)00806-2. [DOI] [PubMed] [Google Scholar]
- 38.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colton T. Statistica in medicina. Padova: Piccin Nuova Libraria SPA; 1991. [Google Scholar]
- 40.Gorjipoor S, Moazeni M, Sharifiyazdi H. Characterization of Dicrocoelium dendriticum haplotypes from sheep and cattle in Iran based on the internal transcribed spacer 2 (ITS-2) and NADH dehydrogenase gene (nad1) J Helminthol. 2015;89:158–164. doi: 10.1017/S0022149X13000679. [DOI] [PubMed] [Google Scholar]
- 41.Maurelli MP, Rinaldi L, Capuano F, Perugini AG, Veneziano V, Cringoli G. Characterization of the 28S and the second internal transcribed spacer of ribosomal DNA of Dicrocoelium dendriticum and Dicrocoelium hospes. Parasitol Res. 2007;101:1251–1255. doi: 10.1007/s00436-007-0629-1. [DOI] [PubMed] [Google Scholar]
- 42.Godara R, Katoch R, Yadav A, Borah MK. Dicrocoeliosis in goats in Jammu, India. J Parasitic Dis. 2014;38:201–204. doi: 10.1007/s12639-012-0212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pezeshki A, Aminfar H, Aminzare M. An analysis of common foodborne parasitic zoonoses in slaughtered sheep and cattle in Tehran, Iran, during 2015–2018. Vet World. 2018;11:1486–1490. doi: 10.14202/vetworld.2018.1486-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diaz P, Paz-Silva A, Sanchez-Andrade R, Suarez JL, Pedreira J, Arias M, et al. Assessment of climatic and orographic conditions on the infection by Calicophoron daubneyi and Dicrocoelium dendriticum in grazing beef cattle (NW Spain) Vet Parasitol. 2007;149:285–289. doi: 10.1016/j.vetpar.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Venditti G, Grelloni V, Filippini G. Applicazione dei Sistemi Informatici Geografici (GIS) per la definizione di mappe parassitologiche nelle regioni Umbria e Marche: indagine sulle endoparassitosi ovine. SPV Webzine. 2010;60:28–33. [Google Scholar]
- 46.Musella V, Catelan D, Rinaldi L, Lagazio C, Cringoli G, Biggeri A. Covariate selection in multivariate spatial analysis of ovine parasitic infection. Prev Vet Med. 2011;99:69–77. doi: 10.1016/j.prevetmed.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 47.Cringoli G. Parassiti in Basilicata-Quadro parassitologico di base negli allevamenti bovini ed ovini. Napoli: Mappe Parassitologiche; 2012. [Google Scholar]
- 48.Sandoval H, Manga-González Y, Campo R, García P, Castro JM, la Vega M. Preliminary study on genetic variability of Dicrocoelium dendriticum determined by random amplified polymorphic DNA. Parasitol Int. 1999;48:21–26. doi: 10.1016/S1383-5769(98)00035-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are included in the article. The newly generated sequence was submitted to the GenBank database under the Accession Number MG004688.


