Abstract
Background
Supplemental oxygen therapy is widely used in hospitals and in the home for chronic care. However, there are several fundamental problems with the application of this therapy such that patients are often exposed to arterial oxygen concentrations outside of the intended target range. This paper reports volume-averaged tracheal oxygen concentration measurements (FtO2) from in vitro experiments conducted using a physiologically realistic upper airway model. The goal is to provide data to inform a detailed discussion of the delivered oxygen dose.
Methods
A baseline FtO2 dataset using a standard, straight adult nasal cannula was established by varying tidal volume (Vt), breathing frequency (f), and continuous oxygen flow rate (QO2) between the following levels to create a factorial design: Vt = 500, 640, or 800 ml; f = 12, 17, or 22 min− 1; QO2 = 2, 4, or 6 l/min. Further experiments were performed to investigate the influence on FtO2 of variation in inspiratory/expiratory ratio, inclusion of an inspiratory or expiratory pause, patient interface selection (e.g. nasal cannula versus a facemask), and rapid breathing patterns in comparison with the baseline measurements.
Results
Oxygen concentration measured at the trachea varied by as much as 60% (i.e. from 30.2 to 48.0% of absolute oxygen concentration) for the same oxygen supply flow rate due to variation in simulated breathing pattern. Among the baseline cases, the chief reasons for variation were 1) the influence of variation in tidal volume leading to variable FiO2 and 2) variation in breathing frequency affecting volume of supplemental oxygen delivered through the breath.
Conclusion
For oxygen administration using open patient interfaces there was variability in the concentration and quantity of oxygen delivered to the trachea over the large range of scenarios studied. Of primary importance in evaluating the oxygen dose is knowledge of the breathing parameters that determine the average inhalation flow rate relative to the oxygen flow rate. Otherwise, the oxygen dose cannot be determined.
Electronic supplementary material
The online version of this article (10.1186/s12931-019-1104-0) contains supplementary material, which is available to authorized users.
Keywords: Oxygen, Nasal cannula, Facemask, Fraction of inspired oxygen, Supplemental oxygen, Oxygen therapy
Introduction
Supplemental oxygen therapy is widely used in hospitals for acute care and in the home for chronic care. Oxygen is one of the most commonly administered medications in patients receiving emergency or hospital-based care [1]. In the United States alone, more than 1 million patients receive home oxygenation therapy [2].
Despite this widespread use, potential hazards due to inaccurate dosing causing hypoxia and hyperoxia have been identified [3]. For example, it has been shown that toxic reactive oxygen species in lung capillary endothelial cells increased with hyperoxic ventialation of 70% oxygen [4]. On the other hand, many babies died due to inadequate oxygenation to avoid retrolental fibroplasia [5]. Over that last 10 years or so there have been several surveys of clinicians in various countries and disciplines to document their comprehension of oxygen therapies [6, 7]. Furthermore, other clinical studies have reported on the actual practices employed and the success of these practices in maintaining adequate oxygenation as measured by blood gas analysis or pulse oximetry (resulting in a peripheral capillary oxygen saturation, SpO2, measurement) [8–21]. The essence of these studies can be gleaned from the conclusion written by Helmerhorst and colleauges, that most ICU [Intensive Care Unit] clinicians understand the danger of prolonged exposure to hyperoxia; however, they report higher arterial oxygen levels than target ranges [6]. Furthermore, for those patients receiving supplemental oxygen in the home there are frequent and varied problems, particularly a lack of access to effective instruction and adequate portable systems. Thus, there is a need to promote access to equipment and services tailored to each patient’s needs [2].
Consider the conviction, which is also the regulatory norm, that oxygen is a drug [1, 22]; as correct dosing is a fundamental aspect in all drug administration, it is a topic of inherent interest for supplemental oxygen therapy. As a step in the direction of a better overall understanding of the dose associated with the administration of supplemental oxygen therapy, in this paper tracheal oxygen concentration measurements from in vitro experiments using a physiologically realistic upper airway model are reported. The in vitro methodology follows that recently presented by Chen et al. [23, 24] to compare pulsed oxygen delivery from portable oxygen concentrators to continuous flow oxygen delivery. More generally, in vitro methods incorporating realistic or idealized upper airway geometries have been widely used in characterizing inhalation drug delivery systems [25–28].
Here our results are presented in terms of average oxygen concentration percentage at the trachea (FtO2) compared to the average oxygen concentration inhaled (FiO2), as well as the volume of oxygen delivered per breath and per minute.
Methods
Conceptual framework
The conceptual framework for this study is to address the passage of supplemental oxygen mixed with air at the patient interface and through the upper airway to the trachea. In this paper, the key locations where the dose is assessed are at the interface (an estimate) and at the trachea (a measurement). In previous papers, under different conditions the passage through the tracheobronchial tree to the terminal bronchiole and the transfer to blood (oxygen flux, VO2) have been considered, but these are beyond the scope of the present paper, which is to highlight the variability in oxygen dosing occurring before supplementary oxygen reaches the gas exchange regions of the respiratory system [23, 29, 30]. However, these tracheal measurements can be considered as boundary conditions for further analysis of the complete transport to the blood that would accurately combine and characterize both gas transport in the upper airways and gas exchange.
Interface estimate
For all of the cases considered herein (with the exception of the Oxyarm interface, discussed below) the oxygen dose being delivered at the patient interface to the airway opening is estimated by taking the flow-weighted average concentration between the ambient inhaled air and the supplemental oxygen flows. Eq. 1 is based on the assumptions that the total average inspiratory flow rate is tidal volume Vt divided by inspiratory time ti, and that the ambient air infiltration (Qair) is the total average inspiratory flow less the supplemental oxygen flow (Qsup). Eq. 2 is the estimated FiO2 in the total (mixed) inspiratory flow entering the model airway:
| 1 |
| 2 |
The estimated volume of inhaled oxygen is then, ViO2 = FiO2 ∗ Vt./100
In vitro model
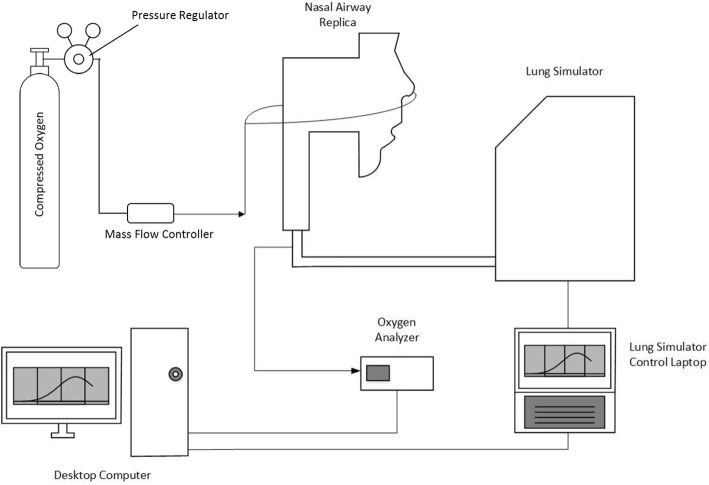
The general in vitro methodology is described by Chen et al. [23, 24]. Briefly, with reference to Fig. 1, a realistic adult nose-throat airway replica was connected to a lung simulator (ASL 5000 Breathing Simulator, IngMar Medical USA) used to simulate tidal breathing. A photo of the experimental setup is also shown in Fig. 1. The volume of the chamber of the test lung was recorded during simulated breathing at a sampling frequency of 512 Hz using the ASL 5000 software. The airway replica was developed previously using medical images from an adult volunteer and 3D-printing techniques [31]. The replica outlet, corresponding to the entrance to the trachea, was connected to the lung simulator breathing circuit with 22 mm internal diameter tubing. The total internal volume of this tubing was 135 cm3, representative of the conducting airway volume from the trachea to the gas exchange regions of the lungs for an average adult with 3 L functional residual capacity. Continuous flows of oxygen were delivered to the replica via nasal cannula (or other patient interfaces as indicated) from a regulated compressed oxygen cylinder through a calibrated mass flow controller (MCMC-Series Mass Flow Controller, Alicat, USA). Gas was sampled at the exit of the replica (representing the trachea) and oxygen concentrations were measured using a laser diode analyzer (GA-200, iWorx, USA) at a frequency of 34.88 Hz.
Fig. 1.
Schematic and photo of the experimental apparatus. Arrows indicate direction of gas flow on the schematic [23]
Real-time oxygen concentrations from the analyzer and volume vs. time data from the lung simulator were streamed via virtual instrument software architecture (VISA) serial and TCP/IP connection ports, respectively, to a program written in LabVIEW (National Instruments, USA) for collection and synchronization. Total gas flows at each point in time were then calculated using forward differences from the volume data. To synchronize the two signals (oxygen concentration and flow), we initially allowed a continuous stream of oxygen to bleed into the apparatus under a condition of zero flow. Once we were able to observe an increase in the oxygen concentration over time at the trachea, we induced an inspiratory effort using the test lung, resulting in a sudden jump in the oxygen concentration. The two signals could then be synchronized by matching the time of the start of the inspiratory flow with the jump in oxygen concentration. The synchronized flow and oxygen concentration waveforms were consistent with oxygen waveform features (e.g. presence of the anatomic reservoir at the start of inspiration) previously described in the literature [32]. In addition, this synchronization method was able to minimize the difference between the total inhaled and exhaled volumes of oxygen. During steady state with ideal synchronization, this difference would be zero, given that the in vitro experimental setup did not include an oxygen uptake mechanism.
The flow rate of oxygen passing through the trachea over time was calculated by multiplying inspiration flow with measured oxygen concentrations at the same time point. Inspiration start and end times were identified as times of zero flow rate. These oxygen flow rates were then numerically integrated using the trapezoidal rule from the start to the end of inspiration to determine a volume of oxygen inhaled per breath. Tidal volume, Vt, was calculated by integrating inspiration flow rates over the inspiratory time period. The volume of inhaled oxygen was then divided by Vt to obtain a volume-averaged value for that breath, which we call here FtO2 to represent the concentration of gas passing the trachea during inhalation. FtO2 for each test was calculated as the average of five consecutive breaths after a steady state in expiratory oxygen concentration was observed (after at least 50 breaths).
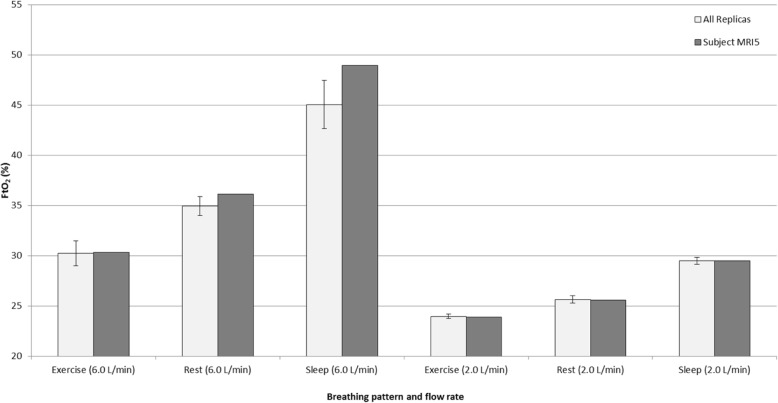
Previous testing of both continuous and pulsed oxygen delivery through realistic adult nose-throat airway replicas has been reported by Chen et al. [23] In that work, tests were repeated in 15 replicas, and FtO2 data were presented as average values along with standard deviations between replicas. These tests provide a subset of data through which the influence of nasal airway geometry on FtO2 may be explored. For further testing described herein, a single replica has been selected, to allow a wider range of breathing parameters to be explored while limiting the total number of experimental repetitions. Fig. 2 displays FtO2 values measured for the replica selected for the present study (labeled MRI5) compared with average values for the 15 replicas studied by Chen et al. [23]. It can be seen that FtO2 measured in the selected replica was in reasonable agreement with the average for the flow rates and breathing patterns previously evaluated. Also of note is the relatively small magnitude of error bars reflecting standard deviation between airway geometries previously studied. This suggests that the large intersubject variability in inhaled oxygen concentrations reported by previous authors [33, 34] may not be due to variability in upper airway geometry, but rather due to uncontrolled variability in breathing pattern, and supports the use of a single upper airway geometry in the present experiments.
Fig. 2.
Comparison of FtO2 measured in nose-throat replica MRI5 vs. average data obtained from the larger set of 15 adult nose-throat replicas. Error bars denote one standard deviation. Exercise, Rest, and Sleep breathing patterns are described in Chen et al. [23]. Data labels ‘2.0 L/min’ and ‘6.0 L/min’ refer to tests performed with continuous oxygen delivery at the flow rate indicated
The design of the experiments followed a philosophy to mimic physiology as much as possible within the limits of a mechanical system. The strength of in vitro bench model is that it is highly repeatable and allows for the parametric controlled experiments that are reported herein. Thus, the error analysis we employed follows a different approach than would be the case for in vivo human testing, where variability between subjects is comparatively large. That is, in lieu of a statistical analysis, repeatability assessments were conducted for all baseline parameter combinations for the baseline tests described below across five simulated breaths and for experimental repetitions conducted over three separate days, which included repositioning of the cannula between repetitions. The average standard deviation of FtO2 among all the tests was 0.3% oxygen reading. Accordingly, the controlled bench experiments described herein were highly repeatable, and experimental uncertainty was deemed negligible relative to the variation in FtO2 between experiments.
In vitro dosing experiments
The apparatus and techniques were applied to 105 separate in vitro dosing experiments consisting of a baseline set plus several other sets to investigate fundamental oxygen therapy variables. Thus, the purpose of the baseline dataset is to provide a basis of comparison for the other oxygen therapy variables considered in a parametric methodology; such that we report the oxygen dose first for the baseline cases and then as more or less from the baseline for the single variable that was modified. Additional file 1: Table S1 provides a list of each experiment’s parameters. A description of the experiment sets follows.
Baseline dataset
The baseline dataset was established using a standard, straight adult nasal cannula (Model 1103; Hudson RCI, USA) patient interface and by varying tidal volume (Vt), breathing frequency (f), and oxygen flow rate (QO2) between the levels specified below in a factorial design: Vt = 500, 640, or 800 ml; f = 12, 17, or 22 min− 1; QO2 = 2, 4, or 6 l/min. The inspiratory/expiratory (i/e) ratio was held constant at approximately 0.5 to match inhaled and exhaled volumes in user-defined breaths simulated by the ASL 5000 instrument (i/e deviated slightly from 0.5 as indicated in Additional file 1: Table S1 with no end-expiratory pause nor end-inspiratory pause in the simulated breaths). Inspiratory and expiratory phases of the breath are modeled as half-sine waves.
Influence of rapid breathing
For patients that develop respiratory failure, Sim et al. [35] report that there is no universal breathing pattern, and that there is little in the scientific literature regarding breathing patterns during respiratory failure. However, they relate clinical observations that a pattern of high respiratory rate with small tidal volumes is often seen in respiratory distress syndrome, left ventricular failure, diaphragmatic splinting, and pulmonary fibrosis. Separately, Bateman and Leach [36] provide a representative breathing pattern for a patient undergoing respiratory failure with Vt = 750 ml and f = 40 min− 1, indicating rapid but not shallow breathing. In many cases, patients experiencing respiratory failure may receive high flow oxygen through nasal cannula or face mask, where FiO2 may be less influenced by breathing pattern. However, as some patients may receive low-flow oxygen delivery through a nasal cannula, tests with simulated rapid breathing representative of the above clinical observations were performed. Testing was conducted with Vt of 250 ml and 750 ml, f = 30 min− 1, i/e = 1.0, and QO2 of 2, 4, and 6 l/min.
Influence of deviations from characteristic breathing pattern
Three deviations from the characteristic baseline breathing pattern were performed i) for an inspiratory/expiratory ratio i/e = 1.0, ii) an i/e ratio of 0.5, and with the addition of an end-expiratory pause (EEP) occupying 20% of the total breath time, and iii) with an i/e ratio of 0.5, and with the addition of an end-inspiratory pause (EIP) occupying 10% of the total breath time.
For each type of deviation from the characteristic breathing pattern, a subset of tests were repeated from the baseline dataset. The repeated combinations of tidal volume and breathing pattern were Vt = 500 ml and f = 12 min− 1, Vt = 640 ml and f = 17 min− 1, and Vt = 800 ml and f = 22 min− 1. Each of these combinations was evaluated with QO2 of 2, 4, and 6 l/min, for the three deviations from the characteristic breathing pattern outlined above.
Influence of patient Interface
Five additional patient interface types besides the standard cannula used in the baseline tests were evaluated i) a flared cannula (1104; Hudson RCI, USA), ii) a second model of straight cannula (1600Q-7-50; Salter Labs, USA), iii) a simple oxygen mask (GK 1041; Glenwood Labs, Canada), iv) the Oxymask (OM-1125-8; Southmedic, Canada) where testing was performed with the cuff sealed to the replica face using adhesive putty, and v) the Oxyarm (OA-PLUS-1125-8; Southmedic, Canada) where the oxygen outlet was positioned above the replica’s face without contact following the manufacturer’s illustrative instructions. For each patient interface, experiments were performed for three combinations of tidal volume and breathing pattern: Vt = 500 ml and f = 12 min− 1, Vt = 640 ml and f = 17 min− 1, and Vt = 800 ml and f = 22 min− 1. Each of these combinations was evaluated with QO2 of 2, 4, and 6 l/min.
Results
Baseline experiments
The results in terms of oxygen concentration for the 27 baseline experiments that cover a range of breathing patterns and oxygen supply flow rates are given in Additional file 1: Table S1. The data presented in terms of oxygen concentration, oxygen flux VO2 and volume of oxygen delivered per breath estimated at the interface and measured at the trachea. Data for all 105 cases are also provided in the same table.
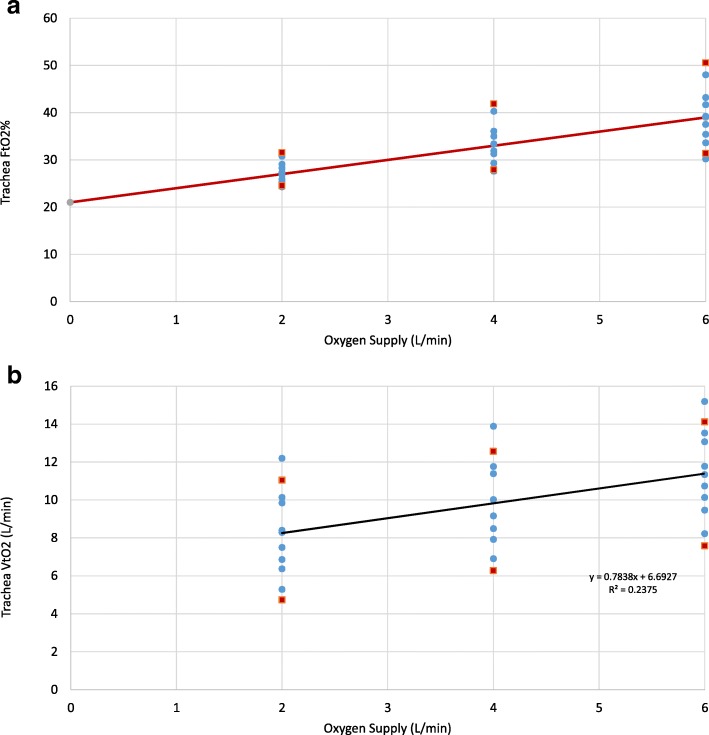
The dose variability can be interpreted through the plots shown in Figs. 3, 4, 5 and 6. As shown in Fig. 3a, there is substantial variation (a relative variation by as much as 60%, from 30.2 to 48.0% of oxygen, at the same 6 L/min oxygen supply flow rate) in oxygen concentration measured at the trachea for the same oxygen supply flow rate. This result underscores the serious drawback to prescribing supplemental oxygen therapy based solely on the oxygen supply flow rate. A typical estimate of FiO2 [37, 38] as indicated by the solid red line is 21 + (L/min O2 * 3). By sight, it follows the average trend accurately, but cannot account for the variations (for the regression line that overlaps it R2 = 0.49). Fig. 3b indicates that this drawback of variability in dose based on oxygen supply flow rate persists if the dose is considered as volume/time; i.e., in L/min. In fact, the relative variability in volume/time reaches 130% at the 2 L/min supply flow rate.
Fig. 3.
a Tracheal oxygen concentration (FtO2) as a function of the supplemental oxygen supply flow rate for the baseline experiments (circles) and for the rapid breathing cases (squares). The solid red line represents a typical clinical estimate of FiO2 as is 21 + (L/min O2 * 3)) [37, 38]. Note that the regression line is not visible behind the clinical estimate. b Trachea oxygen flow (VtO2) as a function of supplemental oxygen supply flow rate for the baseline experiments, circles, and for the rapid breathing cases, squares
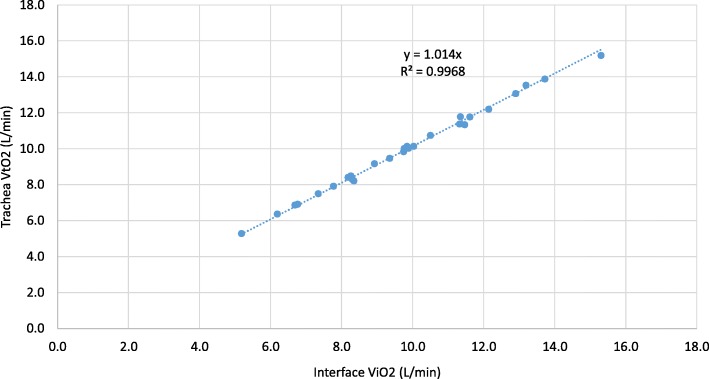
Fig. 4.
Trachea VtO2 as a function of the linear estimate of the interface ViO2 for the baseline experiments
Fig. 5.
Trachea VtO2 as a function of the trachea oxygen concentration for the baseline experiments
Fig. 6.
a The effect of characteristic breathing pattern deviation on tracheal oxygen concentration for the baseline subset experiments. b The effect of patient interface on tracheal oxygen concentration for the baseline subset experiments
Figure 4 shows experimentally measured VtO2 as a function of the estimated oxygen minute ventilation ViO2. The slope of the trend line, 1.0132, shows that the estimate is a very good indicator, but also that the trachea measurement is marginally greater than the estimate. One source of increased oxygen at the trachea is due to pooling (a breathing frequency effect) in the upper airways that is not accounted for in the estimate [32]. It is important to recognize, that while the estimate itself is accurate, it requires knowledge of the inhalation flow rate, derived from the tidal volume and inspiratory time (see Eqs. 1 and 2), parameters that are not generally known for patients using open interfaces. This result is in consonance with the recent paper by Dupriez et al. that showed the importance of inhalation flow rate on delivered oxygen fraction [39].
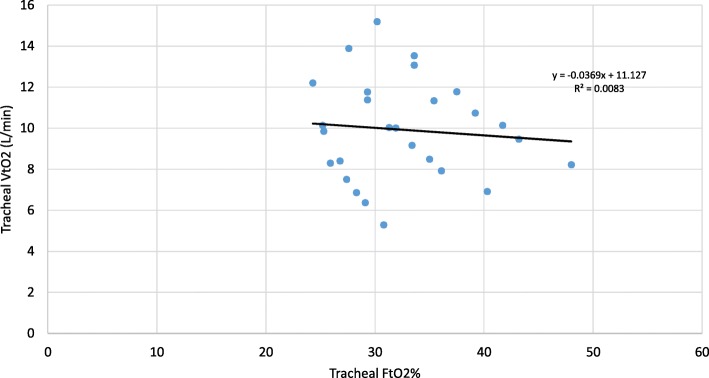
In Fig. 5 the tracheal VtO2 is compared to the tracheal concentration FtO2%. It is noteworthy that for the same oxygen concentration the amount of oxygen actually passing the trachea can be very different because of variation in minute ventilation. For example, at about 31% concentration the VtO2 varies between 2 L/min and over 5 L/min.
Rapid breathing
The rapid breathing cases are indicated by squares in Fig. 3. The low tidal volume cases resulted in high FtO2% but low VtO2 in comparison to the baseline cases. To the contrary, the high tidal volume cases resulted in low FtO2% but high VtO2.
Influence of characteristic breathing pattern deviation
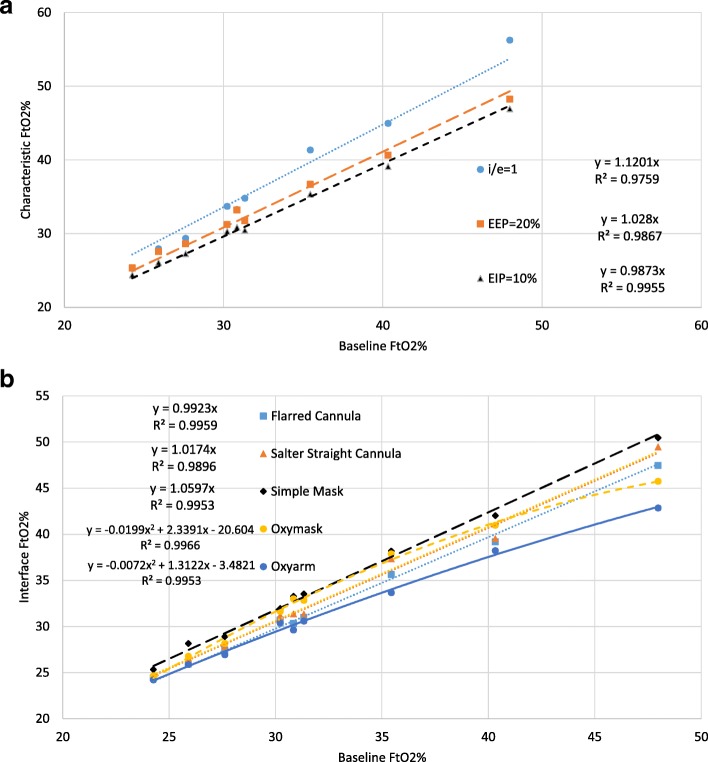
To investigate the effect of deviations from the characteristic breathing pattern used for the baseline set of cases, for a subset of cases the inspiratory/expiratory (i/e) ratio was changed from 0.5 to 1. Additional subsets of cases were studied with i/e maintained at 0.5 but with the addition of an end expiratory pause (EEP) of 20%, or an end inspiratory pause (EIP) of 10%. These data, evaluated in terms of the measured FtO2%, are plotted versus the baseline case with equivalent tidal volume and frequency in Fig. 6a. From the slopes of the trend lines it can be observed that increasing i/e ratio increases oxygen concentration (1.12 > 1), in contrast adding the EEP or EIP had much less effect (1.028~1; and 0.987~1). Changing the i:e ratio has a more significant influence on FtO2 than adding EEP because variation in the inspiratory time has a greater influence of FtO2 than does the pooling effect.
Influence of patient Interface
FtO2 measured for the subset of baseline cases with five additional patient interfaces is compared to the baseline cases using the Hudson straight cannula in Fig. 6b. For the two additional nasal cannula types and the simple mask, a linear trend line fits the data relatively well. For these cases it can be seen that there is very little difference in FtO2 between the Hudson model straight cannula used for the baseline experiments and the Salter model straight cannula (slope of 1.0174). For the flared cannula as well there was very little difference in results for FtO2% (slope of 0.9953). This suggests that commentary presented herein can be applied to low flow oxygen delivery via nasal cannula in general, and are not specific to any particular make or model of nasal cannula. Using a simple mask, the FtO2 was higher than for nasal cannula by about 6% relative (slope of 1.0597). For the Oxymask and Oxyarm a quadratic trend line fit the data better than a linear fit. Both of these unique interfaces tend to provide similar FtO2 as do nasal cannula at lower flow rates, but tend to underperform as the flow rate, and hence FtO2, increases.
Discussion
Results of volume-averaged tracheal oxygen concentration measurements (FtO2) from in vitro experiments conducted using a physiologically realistic upper airway model are reported. These data indicate that using a standard cannula and over a wide range of breathing parameters and oxygen flow rates, there is significant variability is the oxygen dose delivered to the trachea. This is true in terms of the concentration volume percentage and in the volume of oxygen delivered per breath. These data formed a baseline for the comparison of further changes in breathing (such as inspiratory to expiratory ratio) and patient interface (e.g., a simple mask). It is also shown that if the respiratory parameters were known, in particular the inspiratory flow rate, the oxygen dose could be accurately characterized. This is consistent with recent in vitro results presented by Duprez et al. [39]; however, the present experiments advance in vitro methodology through incorporation of a realistic upper airway geometry that allowed several patient interfaces to be investigated. As shown by Fig. 4, the linearized or average inspiratory flow rate, based on tidal volume and inspiratory time will suffice to make accurate predictions, though the instantaneous values integrated over the inspiratory time would be more rigorously accurate. Consistent with the conclusions of Duprez et al. [39], our results indicate that prediction formulas commonly used by clinicians, which do not include inspiratory flow rate, could lead to over- or under-prediction of inspired oxygen fractions (Fig. 3a).
The context of the results presented herein can be considered in regard to a recent review paper: Walsh and Smallwood [40] provided a captivating historical synopsis of oxygen treatment. Although their focus was on pediatric treatment, several historical milestones had similar influence on the treatment of adults. For many decades oxygen administration dosing was based on the observation of skin color, as well as the breathing frequency, regularity, and perceived work of breathing. It was not until the 1960s that sampling of blood gases became common practice in hospitals. More recently, pulse oximetry has become widely available to provide a simple to obtain measure of oxygenation, though with some limitations in accuracy that can be consequential [40, 41]. For example, due to artifacts arising from low perfusion, noise, or motion, and because of the sigmoid shape of the oxyhemoglobin dissociation curve, pulse oximetry may not detect hypoxemia in some patients [41, 42].
Walsh and Smallwood note that the overall goal of oxygen therapy is to achieve adequate oxygenation using the lowest fraction of delivered oxygen. However, achieving this goal is complicated by several factors. Foremost is that despite more than 75 years of routine oxygen administration, normoxia (administration that avoids the detrimental effects of hypoxia on the one hand and those caused by hyperoxia on the other) is not clearly or universally defined [43–45], leading to wide variations in practice [46]. Even the definition of adequate oxygenation is not clear [47]. Certainly adequate oxygenation implies a balance between oxygen delivery to the tissues and their rate of oxygen consumption. However, another definition may include oxygen delivery that allows the cells to consume oxygen for energy normally; otherwise anaerobic metabolism or cell death can occur, depending on cell type [40].
Another key to maintaining adequate oxygenation is the understanding that there are two components that contribute to oxygen uptake from the alveoli: oxygen carrying capacity (e.g., hematocrit) and perfusion (blood flow). Thus, a patient can have adequate oxygen carrying capacity but low cardiac output and suffer from inadequate oxygen uptake, as well as the problems associated with inadequate ventilation in the lungs. Beyond patient variability due to underlying pathology, other physical characteristics (e.g., sex, age, size) may also influence oxygen dose. Furthermore, variability in the application of oxygen therapy by clinicians suggests that they in some cases lack adequate knowledge in the use of oxygen delivery equipment [48]; as well as in the concepts of oxygen delivery and equipment used to monitor the effects of oxygen therapy [49]. Walsh and Smallwood maintain that “unfortunately, adverse reactions from the therapeutic use of oxygen are not well documented in pediatric patients. Therefore, it is imperative that oxygen therapy be provided at accurate and safe levels with the lowest possible fractional concentration of inspired oxygen (FiO2)” [40]. These concerns also apply to adult patients. Indeed, in a recent review article Branson [50] noted that “The role of oxygen in COPD exacerbation has the ability to be therapeutic and toxic”.
The recognition of these issues had promoted initiatives to improve supplemental oxygen therapy. One such initiative that reflects the state-of-the-art is a 2017 guideline promulgated by the British Thoracic Society [51]. The key aspects of the guideline related to this paper are: 1) “The essence of this guideline can be summarised simply as a requirement for oxygen to be prescribed according to a target saturation range and for those who administer oxygen therapy to monitor the patient and keep within the target saturation range”; and 2) “The oxygen saturation should be checked by pulse oximetry in all breathless and acutely ill patients, ‘the fifth vital sign’ (supplemented by blood gases when necessary), and the inspired oxygen concentration should be recorded on the observation chart with the oximetry result. (The other vital signs are pulse rate, blood pressure, temperature and respiratory rate)” [51].
In parallel to the new recognition that supplemental oxygen therapy should be guided by a SpO2 target has been the development of devices to control oxygen delivery for closed inhalation circuits based on algorithms relating SpO2 to the inhaled oxygen fraction, FiO2 [49, 50]. In effect such devices provide the feedback automatically that otherwise is performed by clinicians. For an open inhalation circuit (e.g., nasal cannula, facemask) the FiO2 can be estimated based upon an initially selected flow rate of 100% oxygen based on well understood methods available in respiratory therapy textbooks (e.g. [52]). However, as demonstrated above (Fig. 3), variability around such estimates is considerable due to variations in breathing pattern. Thus, the inspired oxygen concentration cannot be recorded such that clinicians in practice are left to beg the question: what oxygen flow rate will maintain a particular FiO2 that in turn will provide a target SpO2? There now exist automatic feedback control devices for open patient interfaces that control the supplemental oxygen flow rate in response to the SpO2 target [3, 53]. However, as shown above, controlling only the supplemental oxygen flow rate does not control the actual delivered dose of oxygen.
These difficulties with the understanding of dose are rooted in the complex physiology and pathology related to insufficient oxygenation. In West’s [54] canonical text on respiratory physiology perfusion limited and diffusion limited uptake to the blood are discussed. These limitations are related to the number of oxygen molecules that can pass from the gas phase into the blood. Furthermore, the effect of ventilation-perfusion inequality on overall gas exchange is reviewed in a separate chapter. This ventilation limitation is in effect a lack of oxygen molecules present in some alveoli. Supplemental oxygen therapy has very little effect on perfusion nor diffusion limitations, but is clearly aimed to alleviate a deficiency in the presence of oxygen molecules in the alveoli. Thus, it is possible that better knowledge of the dose could lead to better understanding of the manifestation of pathology for individual patients in their response to supplemental oxygen therapy.
The definition of a dose is the quantity of drug to be taken within a given time period. It is evident that specifying the oxygen dose using SpO2 target range falls outside this definition, because neither a specific quantity nor time period are indicated. Thus, a more relevant specification is VO2. Indeed, from a physiological perspective, VO2 is the most relevant variable. For example, Ye et al. [55] note that oxygen uptake kinetics (changes in VO2) “is an important physiological parameter for the determination of functional health status and muscle energetics during physical exercise” [56]. In addition, the VO2 provides a useful assessment of the body’s ability to support a change in metabolic demand and an insight into the circulatory and metabolic response to exercise. Several studies confirmed that oxygen consumption is mainly controlled by intramuscular factor related metabolic system [57, 58]. Different from heart rate, the oxygen uptake cannot be affected by mood, stress, etc., and is generally considered as the most accurate measurement of the fitness for the cardiorespiratory system [59, 60].
It can be supposed, that a better understanding and determination of the true dose all along the route of administration as discussed herein should provide better tracking toward the SpO2 target. In the sense of comparing the oxygen content in the blood to the oxygen delivered, the ratio of SpO2/FiO2 is providing guidance for more effective oxygen delivery [61]. However, the problem of accurately estimating FiO2 when using open interfaces has been identified [62, 63]. Another promising direction for research would recognize the importance of VO2, such that the deviation of SpO2 could be tracked to where the VO2 was deficient in order to pinpoint manifestations of pathology.
The present study may have a broader application, in that it could be extrapolated to better understand the administration of other gases such as nitrous oxide and nitric oxide. That is, the fundamental respiratory physiology will also have a profound effect on the open circuit administration dose of these other gases.
There are several limitations to this study. While a large number of scenarios have been considered, the study is not exhaustive. Notably not all types of patient interface were tested, such as venturi and rebreather masks. The in vitro model only considers inhalation with a closed mouth and does not model gas exchange and the effect of carbon dioxide on the oxygen dose, which results in slightly higher oxygen concentrations at the entrance to the respiratory system than would be observed in vivo. While the discussion provided above has included reference to previous literature describing oxygen delivery to adults as well as children, the present experiments were conducting using an adult airway replica only. It may be that Eqs. 1 and 2 provide reasonable estimation of oxygen delivery in pediatric and neonatal settings as well as for adults, by accounting for variation across age groups in tidal volume, inhalation time, and typical supplemental oxygen flows; however, this has not been demonstrated in the present study. Further studies incorporating pediatric and neonatal upper airway replicas [64, 65] are needed, given the importance of maintaining targeted ranges of arterial oxygen content in children [66], as in adults [67]. Additional interface types may also be relevant to the pediatric and neonatal settings.
Conclusions
This paper has presented data describing oxygen dose in the form of concentration or flow (quantity per unit time) that was measured using a physiological realistic in vitro upper airway model. In particular, these data reveal that for low flow oxygen delivery using open patient interfaces there is great variability in the quantity, or dose, of oxygen delivered over a large range of scenarios. Among the baseline cases studied, the chief reasons for variation were 1) influence of variation in tidal volume leading to variable FiO2 and 2) variation in breathing frequency affecting volume of supplemental oxygen delivered through the breath. These data and conclusions are consistent with the understanding of expert respiratory clinicians, and they are added to the medical literature to emphasize the need for the medical community to better understand these fundamental concepts of oxygen dosing.
Additional file
Table S1. List of experimental cases and results. (DOCX 53 kb)
Authors’ contributions
IK designed the study, analyzed the data, and wrote the manuscript; JC performed experiments, analyzed the data, and read the manuscript; KD performed experiments and read the manuscript; KZ read the manuscript; MP designed the study and read the manuscript; GC designed the study and read the manuscript; and AM designed the study, analyzed the data, and read the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by Air Liquide Santé International.
Availability of data and materials
All supporting data is available on request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors’ declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ira Katz, Phone: +33 7 72 11 27 93, Email: ira.katz@airliquide.com.
John Chen, Email: jzc@ualberta.ca.
Kelvin Duong, Email: Kelvin@ualberta.ca.
Kaixian Zhu, Email: Kaixian.ZHU@airliquide.com.
Marine Pichelin, Email: marine.pichelin@airliquide.com.
Georges Caillibotte, Email: Georges.Caillibotte@airliquide.com.
Andrew R. Martin, Email: armartin@ualberta.ca
References
- 1.Beasley R, Thayabaran D, Bardsley G. The acute use of oxygen therapy in adults. Malta Med J. 2017;29(2).
- 2.Jacobs SS, Lindell KO, Collins EG, Garvey CM, Hernandez C, McLaughlin S, et al. Patient perceptions of the adequacy of supplemental oxygen therapy. Results of the American Thoracic Society nursing assembly oxygen working group survey. Ann Am Thorac Soc. 2018;15(1):24–32. doi: 10.1513/AnnalsATS.201703-209OC. [DOI] [PubMed] [Google Scholar]
- 3.Lellouche F, L’Her E. Automated oxygen flow titration to maintain constant oxygenation. Respir Care 1 août. 2012;57(8):1254–1262. doi: 10.4187/respcare.01343. [DOI] [PubMed] [Google Scholar]
- 4.Brueckl C, Kaestle S, Kerem A, Habazettl H, Krombach F, Kuppe H, et al. Hyperoxia-induced reactive oxygen species formation in pulmonary capillary endothelial cells in situ. Am J Respir Cell Mol Biol avr. 2006;34(4):453–463. [DOI] [PubMed]
- 5.Bolton DP, Cross KW. Further observations on cost of preventing retrolental fibroplasia. Lancet Lond Engl 16 mars. 1974;1(7855):445–448. [DOI] [PubMed]
- 6.Helmerhorst HJ, Schultz MJ, van der Voort PH, Bosman RJ, Juffermans NP, de Jonge E, et al. Self-reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4(1):23. doi: 10.1186/s13613-014-0023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly CA, Maden M. How do health-care professionals perceive oxygen therapy? A critical interpretive synthesis of the literature how do health-care professionals perceive oxygen therapy? A critical interpretive synthesis of the literature. Chron Respir Dis 1 févr 2015;12(1):11–23. [DOI] [PubMed]
- 8.Bellomo R, Bailey M, Eastwood GM, Nichol A, Pilcher D, Hart GK, et al. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15(2):R90. doi: 10.1186/cc10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PH, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12(6):R156. doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eastwood G, Bellomo R, Bailey M, Taori G, Pilcher D, Young P, et al. Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med. 2012;38(1):91–98. doi: 10.1007/s00134-011-2419-6. [DOI] [PubMed] [Google Scholar]
- 11.Farquhar H, Weatherall M, Wijesinghe M, Perrin K, Ranchord A, Simmonds M, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. Am Heart J. 2009;158(3):371–377. doi: 10.1016/j.ahj.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 12.Hovaguimian F, Lysakowski C, Elia N, Tramèr MR. Effect of intraoperative high inspired oxygen fraction on surgical site infection, postoperative nausea and vomiting, and pulmonary FunctionSystematic review and meta-analysis of randomized controlled trials. Anesthesiol J Am Soc Anesthesiol. 2013;119(2):303–316. doi: 10.1097/ALN.0b013e31829aaff4. [DOI] [PubMed] [Google Scholar]
- 13.Ihle JF, Bernard S, Bailey MJ, Pilcher DV, Smith K, Scheinkestel CD. Hyperoxia in the intensive care unit and outcome after out-of-hospital ventricular fibrillation cardiac arrest. Crit Care Resusc. 2013;15(3):186. [PubMed] [Google Scholar]
- 14.Janz DR, Hollenbeck RD, Pollock JS, McPherson JA, Rice TW. Hyperoxia is associated with increased mortality in patients treated with mild therapeutic hypothermia after sudden cardiac arrest. Crit Care Med. 2012;40(12):3135. doi: 10.1097/CCM.0b013e3182656976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. Jama. 2010;303(21):2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 16.Rachmale S, Li G, Wilson G, Malinchoc M, Gajic O. Practice of excessive FIO2 and effect on pulmonary outcomes in mechanically ventilated patients with acute lung injury. Respir Care. 2012;57(11):1887–1893. doi: 10.4187/respcare.01696. [DOI] [PubMed] [Google Scholar]
- 17.Raj R, Bendel S, Reinikainen M, Kivisaari R, Siironen J, Lang M, et al. Hyperoxemia and long-term outcome after traumatic brain injury. Crit Care. 2013;17(4):R177. doi: 10.1186/cc12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rincon F, Kang J, Vibbert M, Urtecho J, Athar MK, Jallo J. Significance of arterial hyperoxia and relationship with case fatality in traumatic brain injury: a multicentre cohort study. J Neurol Neurosurg Psychiatry. 2014;85(7):799–805. doi: 10.1136/jnnp-2013-305505. [DOI] [PubMed] [Google Scholar]
- 19.Wijesinghe M, Perrin K, Ranchord A, Simmonds M, Weatherall M, Beasley R. Routine use of oxygen in the treatment of myocardial infarction: systematic review. Heart. 2009;95(3):198–202. doi: 10.1136/hrt.2008.148742. [DOI] [PubMed] [Google Scholar]
- 20.Young P, Beasley R, Bailey M, Bellomo R, Eastwood GM, Nichol A, et al. The association between early arterial oxygenation and mortality in ventilated patients with acute ischaemic stroke. Crit Care Resusc. 2012;14(1):14. [PubMed] [Google Scholar]
- 21.Rincon F, Kang J, Maltenfort M, Vibbert M, Urtecho J, Athar MK, et al. Association between hyperoxia and mortality after stroke: a multicenter cohort study. Crit Care Med. 2014;42(2):387–396. doi: 10.1097/CCM.0b013e3182a27732. [DOI] [PubMed] [Google Scholar]
- 22.Batool S, Garg R. Appropriate use of oxygen delivery devices. Open Anesthesiol J 2017;11(1).
- 23.Chen JZ, Katz IM, Pichelin M, Zhu K, Caillibotte G, Noga ML, et al. Comparison of pulsed versus continuous oxygen delivery using realistic adult nasal airway replicas. Int J Chron Obstruct Pulmon Dis. 2017;12:2559. doi: 10.2147/COPD.S141976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen JZ, Katz IM, Pichelin M, Zhu K, Caillibotte G, Finlay WH, et al. In vitro-in silico comparison of pulsed oxygen delivery from portable oxygen concentrators to continuous flow oxygen delivery. Respir Care. 2019;64(2):117–29. [DOI] [PubMed]
- 25.Burnell PKP, Asking L, Borgström L, Nichols SC, Olsson B, Prime D, et al. Studies of the human oropharyngeal airspaces using magnetic resonance imaging IV—the oropharyngeal retention effect for four inhalation delivery systems. J Aerosol Med 1 sept. 2007;20(3):269–281. doi: 10.1089/jam.2007.0566. [DOI] [PubMed] [Google Scholar]
- 26.Delvadia R, Hindle M, Longest PW, Byron PR. In vitro tests for aerosol deposition II: IVIVCs for different dry powder inhalers in Normal adults. J Aerosol Med Pulm Drug Deliv 4 sept 2012;26(3):138–144. [DOI] [PubMed]
- 27.Weers JG, Clark AR, Rao N, Ung K, Haynes A, Khindri SK, et al. In vitro–in vivo correlations observed with Indacaterol-based formulations delivered with the Breezhaler®. J Aerosol Med Pulm Drug Deliv 17 déc 2014;28(4):268–280. [DOI] [PubMed]
- 28.Zhang Yu, Gilbertson Kyle, Finlay Warren H. In Vivo–In Vitro Comparison of Deposition in Three Mouth–Throat Models with Qvar® and Turbuhaler® Inhalers. Journal of Aerosol Medicine. 2007;20(3):227–235. doi: 10.1089/jam.2007.0584. [DOI] [PubMed] [Google Scholar]
- 29.Kang M-Y, Katz I, Sapoval B. A new approach to the dynamics of oxygen capture by the human lung. Respir Physiol Neurobiol. 2015;205:109–119. doi: 10.1016/j.resp.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Katz I, Pichelin M, Montesantos S, Kang M-Y, Sapoval B, Zhu K, et al. An in silico analysis of oxygen uptake of a mild COPD patient during rest and exercise using a portable oxygen concentrator. Int J Chron Obstruct Pulmon Dis 29 sept 2016;11:2427–2434. [DOI] [PMC free article] [PubMed]
- 31.Golshahi L, Noga ML, Thompson RB, Finlay WH. In vitro deposition measurement of inhaled micrometer-sized particles in extrathoracic airways of children and adolescents during nose breathing. J Aerosol Sci. 2011;42(7):474–488. doi: 10.1016/j.jaerosci.2011.04.002. [DOI] [Google Scholar]
- 32.Zhou S, Chatburn RL. Effect of the anatomic reservoir on low-flow oxygen delivery via nasal cannula: constant flow versus pulse flow with portable oxygen concentrator. Respir Care. 6 mai 2014;respcare.02878. [DOI] [PubMed]
- 33.Bazuaye EA, Stone TN, Corris PA, Gibson GJ. Variability of inspired oxygen concentration with nasal cannulas. Thorax 1 août 1992;47(8):609–611. [DOI] [PMC free article] [PubMed]
- 34.Wettstein RB, Shelledy DC, Peters JI. Delivered oxygen concentrations using low-flow and high-flow nasal cannulas. Respir Care 1 mai 2005;50(5):604–609. [PubMed]
- 35.Sim MAB, Dean P, Kinsella J, Black R, Carter R, Hughes M. Performance of oxygen delivery devices when the breathing pattern of respiratory failure is simulated. Anaesthesia. 2008;63(9):938-40. [DOI] [PubMed]
- 36.Bateman NT, Leach RM. Acute oxygen therapy. Bmj. 1998;317(7161):798–801. doi: 10.1136/bmj.317.7161.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 4 juin 2015;372(23):2185–2196. [DOI] [PubMed]
- 38.SRLF Trial Group. Hypoxemia in the ICU: prevalence, treatment, and outcome. Ann Intensive Care 1 déc 2018;8(1):82. [DOI] [PMC free article] [PubMed]
- 39.Duprez F, Mashayekhi S, Cuvelier G, Legrand A, Reychler G. A new formula for predicting the fraction of delivered oxygen during low-flow oxygen therapy. Respir Care 1 déc 2018;63(12):1528–1534. [DOI] [PubMed]
- 40.Walsh BK, Smallwood CD. Pediatric oxygen therapy: a review and update. Respir Care. 2017;62(6):645–661. doi: 10.4187/respcare.05245. [DOI] [PubMed] [Google Scholar]
- 41.Jubran A. Pulse oximetry. Crit Care 16 juill 2015;19:272. [DOI] [PMC free article] [PubMed]
- 42.Seifi S, Khatony A, Moradi G, Abdi A, Najafi F. Accuracy of pulse oximetry in detection of oxygen saturation in patients admitted to the intensive care unit of heart surgery: comparison of finger, toe, forehead and earlobe probes. BMC Nurs 17 avr 2018;17:15. [DOI] [PMC free article] [PubMed]
- 43.Cole CH, Wright KW, Tarnow-Mordi W, Phelps DL. Resolving our uncertainty about oxygen therapy. Pediatrics 1 déc 2003;112(6):1415–9. [DOI] [PubMed]
- 44.Finer NN, Rich WD. Neonatal resuscitation: raising the bar. Curr Opin Pediatr. 2004;16.2:157-62. [DOI] [PubMed]
- 45.Tin W. Oxygen therapy: 50 years of uncertainty. Pediatrics. 2002;110(3):615–616. doi: 10.1542/peds.110.3.615. [DOI] [PubMed] [Google Scholar]
- 46.Anderson CG, Benitz WE, Madan A. Retinopathy of prematurity and pulse oximetry: a national survey of recent practices. J Perinatol. 2004;24(3):164. doi: 10.1038/sj.jp.7211067. [DOI] [PubMed] [Google Scholar]
- 47.Higgins RD, Bancalari E, Willinger M, Raju TN. Executive summary of the workshop on oxygen in neonatal therapies: controversies and opportunities for research. Pediatrics. 2007;119(4):790–796. doi: 10.1542/peds.2006-2200. [DOI] [PubMed] [Google Scholar]
- 48.Walsh M, Engle W, Laptook A, Kazzi SNJ, Buchter S, Rasmussen M, et al. Oxygen delivery through nasal cannulae to preterm infants: can practice be improved? Pediatrics. 2005;116(4):857–861. doi: 10.1542/peds.2004-2411. [DOI] [PubMed] [Google Scholar]
- 49.Sola A, Saldeno YP, Favareto V. Clinical practices in neonatal oxygenation: where have we failed? What can we do? J Perinatol. 2008;28(S1):S28. doi: 10.1038/jp.2008.47. [DOI] [PubMed] [Google Scholar]
- 50.Branson RD. Oxygen Therapy in COPD Respir Care 1 juin 2018;63(6):734–748. [DOI] [PubMed]
- 51.O’Driscoll BR, Howard LS, Earis J, Mak V. British Thoracic Society guideline for oxygen use in adults in healthcare and emergency settings. BMJ Open Respir Res. 2017;4(1):e000170. doi: 10.1136/bmjresp-2016-000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green R. Green’s Respiratory Therapy: A practical and essential tutorial on the core concepts of respiratory care. Chula Vista: Aventine Press; 2017.
- 53.Hansen EF, Hove JD, Bech CS, Jensen J-US, Kallemose T, Vestbo J. Automated oxygen control with O2matic® during admission with exacerbation of COPD. Int J Chron Obstruct Pulmon Dis 14 déc 2018;13:3997–4003. [DOI] [PMC free article] [PubMed]
- 54.West JB. Respiratory physiology: the essentials. Philadelphia: Lippincott Williams & Wilkins; 2012.
- 55.Ye L, Argha A, Yu H, Celler BG, Nguyen HT, Su S. Dynamic characteristics of oxygen consumption. Biomed Eng OnLine 23 avr 2018;17:44. [DOI] [PMC free article] [PubMed]
- 56.Rossiter HB. Exercise: Kinetic Considerations for Gas Exchange. In: Terjung R, éditeur. Comprehensive Physiology [Internet]. Hoboken, NJ, USA: Wiley; 2010. Disponible sur: http://doi.wiley.com/10.1002/cphy.c090010 [DOI] [PubMed]
- 57.Poole DC, Jones AM. Oxygen Uptake Kinetics. In: Comprehensive Physiology [Internet]. American Cancer Society; 2012. p. 933–96. Disponible sur: https://onlinelibrary.wiley.com/doi/abs/10.1002/cphy.c100072 [DOI] [PubMed]
- 58.Zoladz JA, Grassi B, Majerczak J, Szkutnik Z, Korostyński M, Grandys M, et al. Mechanisms responsible for the acceleration of pulmonary V̇o2 on-kinetics in humans after prolonged endurance training. Am J Physiol-Regul Integr Comp Physiol 27 août 2014;307(9):R1101–R1114. [DOI] [PubMed]
- 59.Haddad A, Zhang Y, Su S, Celler B, Nguyen H. Modelling and regulating of cardio-respiratory response for the enhancement of interval training. Biomed Eng OnLine 6 févr 2014;13:9. [DOI] [PMC free article] [PubMed]
- 60.Zhang Y, Haddad A, Su SW, Celler BG, Coutts AJ, Duffield R, et al. An equivalent circuit model for onset and offset exercise response. Biomed Eng Online 18 oct 2014;13:145. [DOI] [PMC free article] [PubMed]
- 61.Cao Z, Luo Z, Hou A, Nie Q, Xie B, An X, et al. Volume-targeted versus pressure-limited noninvasive ventilation in subjects with acute hypercapnic respiratory failure: a multicenter randomized controlled trial. Respir Care. 2016:respcare–04619. [DOI] [PubMed]
- 62.Couture MA. PaO2/FIO2 is not a Guesstimation. Respir Care. 2017;62(3):388–389. doi: 10.4187/respcare.05393. [DOI] [PubMed] [Google Scholar]
- 63.Luo Z, Cao Z. PaO2/FIO2 is not a Guestimation—reply. Respir Care 1 mars 2017;62(3):389–389. [DOI] [PubMed]
- 64.Paxman T, Noga M, Finlay WH, Martin AR. Experimental evaluation of pressure drop for flows of air and heliox through upper and central conducting airway replicas of 4- to 8-year-old children. J Biomech 3 janv 2019;82:134–141. [DOI] [PubMed]
- 65.Tavernini S, Church TK, Lewis DA, Noga M, Martin AR, Finlay WH. Deposition of micrometer-sized aerosol particles in neonatal nasal airway replicas. Aerosol Sci Technol 3 avr 2018;52(4):407–419.
- 66.Bachman TE, Newth CJL, Iyer NP, Ross PA, Khemani RG. Hypoxemic and hyperoxemic likelihood in pulse oximetry ranges: NICU observational study. Arch Dis Child Fetal Neonatal Ed 20 juin 2018; [DOI] [PubMed]
- 67.Helmerhorst HJF, Arts DL, Schultz MJ, van der Voort PHJ, Abu-Hanna A, de Jonge E, et al. Metrics of arterial Hyperoxia and associated outcomes in critical care. Crit Care Med févr 2017;45(2):187–195. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of experimental cases and results. (DOCX 53 kb)
Data Availability Statement
All supporting data is available on request.