This meta-analysis examines whether differences in biomarkers, such as programmed cell death ligand 1 (PD-L1) expression and tumor mutation burden, are associated with improved response to immunotherapy and survival in patients with advanced non–small cell lung cancer (NSCLC).
Key Points
Question
Is immunotherapy associated with beneficial outcomes for advanced non–small cell lung cancer and how can combination strategies and biomarkers be deployed to produce the best benefit?
Findings
In this meta-analysis including 14 395 patients and individual patient–level analysis including 1833 patients, immunotherapy was associated with improved survival, and pembrolizumab with platinum-based chemotherapy was identified as the best first-line checkpoint blockade strategy. Programmed cell death ligand 1 expression and tumor mutation burden jointly exhibited promising predictive and prognostic associations, and CD8+ T-cell tumor-infiltrating lymphocytes score further synergized with these 2 biomarkers.
Meaning
Integrated tumor microenvironment-based biomarkers may be an effective means to predict responsiveness to immunotherapy for patients with advanced non–small cell lung cancer.
Abstract
Importance
The beneficial role of immunotherapy and the clinical relevance of current biomarkers in non–small cell lung cancer (NSCLC) remain inconclusive; thus, appropriate strategies and reliable predictors need further definition.
Objectives
To evaluate the association of clinical outcomes with immune checkpoint inhibitors, tumor vaccines, and cellular immunotherapy in patients with advanced NSCLC and to explore appropriate strategies, candidates, and predictors.
Data Sources
The PubMed, EMBASE, and Cochrane Central Register of Controlled Trials databases were searched from inception to June 2018, using relevant search keywords and Medical Subject Headings (MeSH) terms, including tumor vaccine, cellular immunotherapy, immune checkpoint inhibitor, cytotoxic T-lymphocyte-associated protein 4, programmed death-ligand 1, programmed death receptor 1, and non-small cell lung carcinoma. Systematic reviews, meta-analyses, references, and conference proceedings were manually searched.
Study Selection
English-language randomized clinical trials with available data that measured overall survival (OS), progression-free survival (PFS), or objective response rate comparing immune checkpoint inhibitors, tumor vaccines, or cellular immunotherapy with conventional therapy for patients with advanced or metastatic NSCLC were included. Thirty-one immunotherapy randomized clinical trials were included, and multicohort data included next-generation sequencing data from patients with advanced NCSLC.
Data Extraction and Synthesis
Hazard ratios and 95% CIs were pooled to estimate the survival increases in OS and PFS. Dichotomous data, such as object response rate data, were analyzed using the risk ratio. Mantel-Haenszel random-effects model was used. I2 was used to assess the heterogeneity between trials; an I2 value exceeding 50% indicated the existence of substantial heterogeneity. Analyses took place from February 1, 2018, to August 31, 2018.
Main Outcomes and Measures
Primary outcomes were OS and PFS.
Results
In total, 14 395 patients (9500 [66.0%] men) were included in the meta-analysis, and 1833 patients (mean [SD], 65.2 [9.9] years; 1063 [58.0%] men) were included in the individual patient–level study. Compared with conventional therapy, immunotherapy was associated with significantly longer OS (hazard ratio, 0.76; 95% CI, 0.71-0.82; P < .001) and PFS (hazard ratio, 0.76; 95% CI, 0.70-0.83; P < .001). The best checkpoint blockade strategy was first-line pembrolizumab with platinum-based chemotherapy. The combined predictive utility of programmed cell death ligand 1 (PD-L1) expression and tumor mutation burden (TMB) was associated with predictive prognosis (whole-exome sequencing: 1-year PFS area under the receiver operating characteristic curve [AUC], 0.829; 3-year PFS AUC, 0.839; targeted next-generation sequencing: 1-year PFS AUC, 0.826; 3-year PFS AUC, 0.948). Moreover, the addition of CD8+ T-cell tumor-infiltrating lymphocytes was associated with improved prognosis predictions for OS (3-year OS AUC, 0.659; 5-year OS AUC, 0.665). RYR1 or MGAM mutations were significantly associated with concomitantly increased durable clinical benefits (RYR1: durable clinical benefit [DCB], 12 of 51 patients [24%]; no durable benefit [NDB], 2 of 55 patients [4%]; P < .001; MGAM: DCB, 12 of 51 patients [24%]; NDB, 0 patients; P < .001), a higher TMB (RYRI: high TMB, 12 of 53 patients [23%]; low TMB, 2 of 53 patients [38%]; P < .001; MGAM: high TMB, 9 of 53 patients [17%]; low TMB, 0 patients; P < .001), and higher PD-L1 expression (RYRI: high PD-L1 expression, 8 of 30 patients [27%]; low PD-L1 expression, 6 of 85 [7.1%]; P < .001; MGAM: high PD-L1 expression, 6 of 30 patients [20%]; low PD-L1 expression, 5 of 85 patients [6%]; P < .001).
Conclusions and Relevance
Immunotherapies showed promising clinical outcomes for patients with NSCLC. Pembrolizumab with platinum-based chemotherapy was found to be the most appropriate first-line immune checkpoint inhibitor regimen for advanced NSCLC, and the combined use of PD-L1 expression and TMB was found to be a promising biomarker to evaluate patients’ survival and response to precision immunotherapy. The further combination of CD8+ T-cell tumor-infiltrating lymphocytes, PD-L1 expression, and TMB was associated with reliable prognosis. The predictive value of that combination needs to be prospectively validated in large-scale studies.
Introduction
Advances in immuno-oncology are changing the standard of care for non–small cell lung cancer (NSCLC) through immunotherapies, including tumor vaccines, cellular immunotherapies, and immune checkpoint inhibitors (ICIs), that aim to establish or enhance effective immune responses toward a tumor.1,2 However, immunotherapy has produced inconsistent results in previous randomized clinical trials (RCTs). In the KEYNOTE-024,3 CheckMate-057,4 and TIME5 trials and the study by Li et al,6 immunotherapies were found to significantly improve overall survival (OS) and progression-free survival (PFS) rates compared with chemotherapy in patients with advanced NSCLC, but inconsistent survival outcomes were shown in the CheckMate-026 trial7 and the studies by Takayama et al8 and Wu et al.9 Additionally, important questions remain regarding which immunotherapeutic strategy can be deployed to the best benefit.
Moreover, independent immune-related biomarkers that are currently used, such as programmed cell death ligand 1 (PD-L1)10,11 and tumor mutation burden (TMB),12 have achieved clinical relevance for a selection of patients to some extent, but to our knowledge, they are still far from clear and established,13,14 which warrants the development of an integrated tumor microenvironment–based signature with multiple parameters to maximize treatment effects and guide suitable strategies. In this meta-analysis and individual patient–level analysis, we describe the largest series to date, to our knowledge, to evaluate clinical outcomes, treatment strategies, exploratory patient subtypes, and predictive biomarkers in patients with NSCLC.
Methods
A systematic literature search of the PubMed, EMBASE, and Cochrane Central Register of Controlled Trials databases was performed to identify relevant RCTs published from inception to June 2018, using search keywords and Medical Subject Headings (MeSH) terms pertinent to the intervention of interest, such as tumor vaccine, cellular immunotherapy, immune checkpoint inhibitor, cytotoxic T-lymphocyte-associated protein 4, programmed death-ligand 1, programmed death receptor 1, and non–small cell lung carcinoma. Furthermore, we manually searched and checked references of systematic reviews, meta-analyses, and conference proceedings of the American Society of Clinical Oncology, the European Society for Medical Oncology, the American Association for Cancer Research, and the World Conference on Lung Cancer. Database searches were conducted in January 2018 and updated in July 2018. The inclusion criteria were (1) RCTs comparing ICIs, tumor vaccines, or cellular immunotherapy with conventional therapy for patients with advanced or metastatic NSCLC; (2) RCTs with reported available data that measured OS, PFS, or objective response rate (ORR); and (3) RCTs published in English. The primary outcomes were OS and PFS; the secondary outcome was the ORR and durable clinical benefit (DCB). This study is reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (eFigure 1 in the Supplement).
Statistical Analysis
For the meta-analysis, hazard ratios (HRs) and 95% CIs were pooled to estimate the survival increases in OS and PFS. Dichotomous data, such as ORR data, were analyzed using the risk ratio. The Mantel-Haenszel random-effects model was used. Two-sided P values less than .05 were regarded as statistically significant. We used I2 to assess the heterogeneity between trials; an I2 value exceeding 50% indicated substantial heterogeneity. The differences in treatment effect between subgroups were measured by P value for interaction. In addition, we conducted network meta-analyses to compare the OS and PFS of different ICI strategies using the random-effects Bayesian model.
The GRADE (Grading of Recommendations Assessment, Development, and Evaluation) method was used to categorize the quality of the evidence as high, moderate, low, or very low. Randomized clinical trials were initially considered high-quality evidence but could be rated lower because of a risk of bias, imprecision, inconsistency, indirectness, and publication bias.
For individual patient–level analysis, aggregated OS and PFS were computed using the Kaplan-Meier estimates method and compared with the log-rank test. Hazard ratios and 95% CIs were calculated by using the Cox regression model. Treatment effects between 2 groups were calculated using the difference in restricted mean survival time. Categorical variables were compared with χ2 or Fisher exact tests, and continuous variables were compared with Wilcoxon rank sum tests for 2-group comparisons or the Kruskal-Wallis exact test for multiple comparisons. We categorized PD-L1, TMB, and the neoantigen burden (NAB) into high-value and low-value groups with the optimal cutoff values defined by the R statistical software version 3.4.1 ggsurvimier package (R Project for Statistical Computing). Tumor mutation burden was defined as the number of nonsynonymous single-nucleotide variants or insertion or deletion variants. Spearman rank correlation coefficients were used to estimate the correlations. Receiver operating characteristic curves were generated to assess the sensitivity and specificity of continuous variables with the area under the receiver operating characteristic curve (AUC), and a P value less than .05 was considered statistically significant. Analyses took place from February 1, 2018 to August 31, 2018. All statistical analyses were performed with R statistical software version 3.4.1. Full details of the methods are described in the eAppendix in the Supplement.
Results
Our search found 31 relevant RCTs3,4,5,6,7,8,9,10,11,12,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35 for meta-analyses of ICIs, tumor vaccines, or cellular immunotherapy, including 14 395 patients (9500 [66.0%] men) with advanced NSCLC. Next, we conducted an individual patient–level analysis by examining next-generation sequencing data for 1833 patients with NSCLC (mean [SD], 65.2 [9.9] years; 1063 [58.0%] men) in multicohort trials. A total of 825 patients who received ICIs were analyzed, including 349 patients in cohort 1 from 3 studies (the KEYNOTE-001 trial,36 the CheckMate-012 trial,37 and the study by Rizvi et al38), 56 patients in cohort 2 for OS (from cBioPortal for Cancer Genetics39), and 420 patients from the OAK trial.10 Additionally, 1008 patients were included from the Cancer Genome Atlas.40 The characteristics of the RCTs are summarized in eTable 1 in the Supplement, and characteristics of the ICI cohorts are summarized in eTable 2 in the Supplement. The results of the methodological quality analysis of the RCTs are summarized in eFigure 2 and eFigure 3 in the Supplement, and publication bias analyses of the RCTs are summarized eFigure 4 in the Supplement.
Association of Immunotherapy With OS and PFS
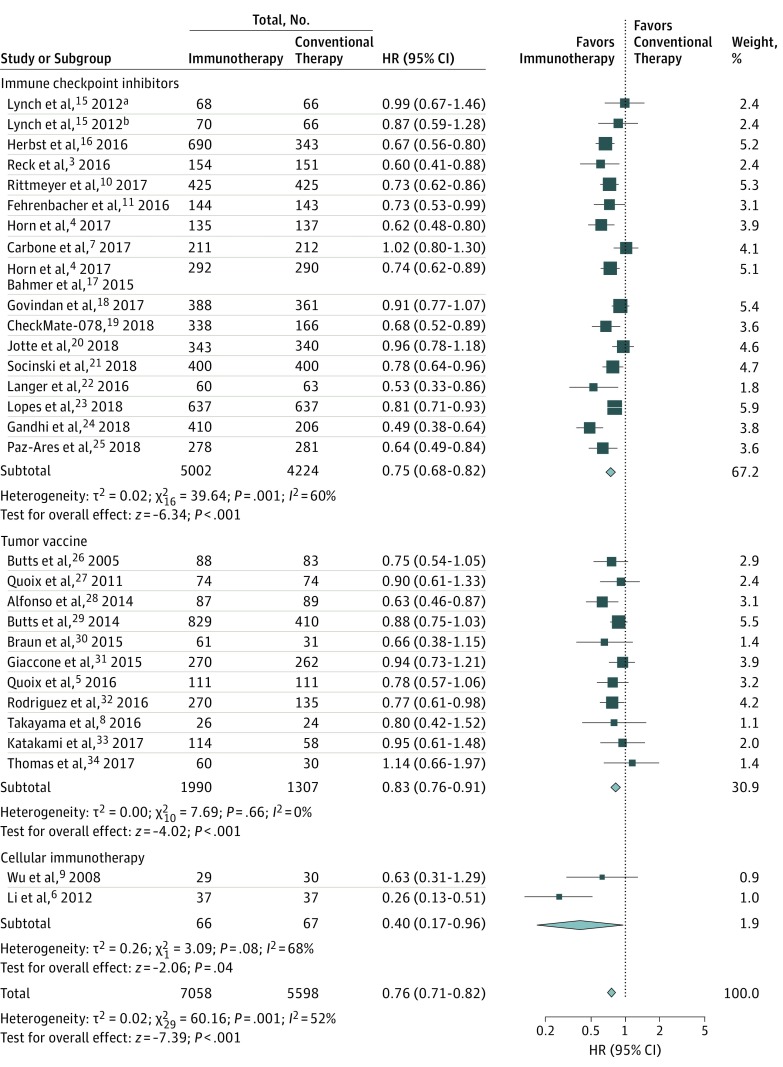
Overall, compared with conventional therapy, immunotherapy was associated with significantly longer OS (HR, 0.76; 95% CI, 0.71-0.82; P < .001; ratio of the medians, 1.29; 95% CI, 1.14-1.46; P < .001) (Figure 1; eFigure 5 in the Supplement) and PFS (HR, 0.76; 95% CI, 0.71-0.82; P < .001; ratio of the medians, 1.15; 95% CI, 1.01-1.32; P = .04) (eFigure 6 and eFigure 7 in the Supplement). When classified by treatment, ICIs (HR, 0.75; 95% CI, 0.68-0.82; P < .001), tumor vaccines (HR, 0.83; 95% CI, 0.76-0.91; P < .001), and cellular immunotherapy (HR, 0.40; 95% CI, 0.17-0.96; P = .04) were associated with improved OS, while a significant improvement in PFS was recorded for ICIs (HR, 0.76; 95% CI, 0.68-0.84; P < .001) and tumor vaccines (HR, 0.86; 95% CI, 0.78-0.94; P = .001) (Figure 1; eFigure 6 in the Supplement). The GRADE evidence ranged from moderate to high quality.
Figure 1. Pooled Hazard Ratios (HRs) for Overall Survival With Immunotherapy vs Conventional Therapy.
Size of boxes indicates proportional weight of each trial. Diamonds indicate point estimates and 95% CIs of the combined result.
aPatients were treated by phased regimen.
bPatients were treated by concurrent regimen.
Across the ICI RCTs, moderate-quality to high-quality evidence revealed significant improvements in PFS in first-line dual ICIs vs chemotherapy (HR, 0.83; 95% CI, 0.72-0.96; P = .01), first-line ICIs with chemotherapy vs chemotherapy alone (HR, 0.68; 95% CI, 0.58-0.80; P < .001), first-line ICIs with anti–vascular endothelial growth factor receptor therapy and chemotherapy vs anti–vascular endothelial growth factor receptor therapy with chemotherapy (HR, 0.61; 95% CI, 0.52-0.72; P < .001), first-line maintenance ICIs vs chemotherapy (HR, 0.52; 95% CI, 0.42-0.65; P < .001), and ICIs vs chemotherapy in patients who had been previously treated (HR, 0.85; 95% CI, 0.77-0.94; P = .002) (eTable 3 in the Supplement). Similar survival benefits were also recorded for OS (eTable 4 in the Supplement). Furthermore, comprehensive network meta-analysis showed that first-line pembrolizumab with platinum-based chemotherapy was superior to nivolumab, atezolizumab with platinum-based chemotherapy, and ipilimumab with platinum-based chemotherapy in OS and PFS. There were no survival differences among atezolizumab, nivolumab, and pembrolizumab in patients who had been previously treated (eFigures 8-14 in the Supplement).
In tumor vaccine trials, moderate-quality to high-quality evidence indicated that, compared with chemotherapy, first-line tumor vaccine immunotherapy with chemotherapy was associated with improved PFS (HR, 0.74; 95% CI, 0.60-0.91; P = .005). Compared with no vaccine treatment, first-line maintenance tumor vaccine immunotherapy was associated with improved OS (HR, 0.89; 95% CI, 0.81-0.99; P = .001) and PFS (HR, 0.83; 95% CI, 0.74-0.92; P = .02). More results are shown in eTable 3 and eTable 4 in the Supplement.
Association of Immunotherapy With Response
The ORR was higher with immunotherapy than with conventional therapy (risk ratio, 1.33; 95% CI, 1.18-1.51; P < .001), and the benefit of objective response with ICIs was statistically significant (risk ratio, 1.47; 95% CI, 1.25-1.73; P < .001) (eFigure 15 in the Supplement). Furthermore, we performed trial sequential analysis on the ORR results and found a 20% relative risk increment in the response with immunotherapy (eFigure 16A in the Supplement) and specifically with ICIs (eFigure 16B in the Supplement). There was moderate-quality to high-quality evidence for a response benefit of first-line or second-line ICIs and first-line or maintenance tumor vaccines compared with conventional therapy (eTable 5 in the Supplement).
Association of PD-L1 Expression, TMB, and NAB With OS and PFS Among Patients Treated With ICIs
The subgroup analysis of meta-analysis showed that PFS was significantly longer with ICIs than with conventional therapy among patients with a high TMB but not among those with a low TMB. In addition, OS and PFS benefits from ICIs increased with increasing PD-L1 expression compared with conditional therapy. By comparing the HR of OS for ICIs alone vs chemotherapy alone (HR, 0.70; 95% CI, 0.61-0.79) and that of ICIs with chemotherapy vs chemotherapy alone (HR, 0.50; 95% CI, 0.38-0.66), we found that ICIs with chemotherapy were associated with significantly longer OS compared with ICIs alone in patients with PD-L1 expression scores of 1, 2, or 3 for tumor cells (TCs) (defined as the number of TCs expressing PD-L1 as a percentage of total TCs; TC1, ≥1% to <5%; TC2, ≥5% to <50%; TC3, ≥50%) or for tumor-infiltrating immune cells (ICs) (defined as the number of tumor-infiltrating ICs as a percentage of tumor area; IC1, ≥1% to <5%; IC2, ≥5% to <10%; IC3, ≥10%) (P for interaction = .03). However, the OS benefit of ICIs with chemotherapy compared with ICIs alone did not differ in patients with PD-L1 expression scores of TC3 or IC3 or for patients with PD-L1 expression scores TC2 or IC2. More results of subgroup analyses and the GRADE evidence are presented in eFigure 17, eFigure 18, eTable 6, and eTable 7 in the Supplement.
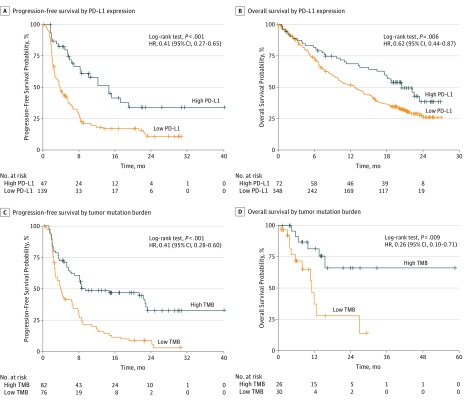
Individual patient–level analysis in the ICI cohorts found that PD-L1 expression, TMB, and the NAB were significantly greater in the patients with a complete response (defined as all target lesions have disappeared and any target or nontarget pathological lymph nodes have been reduced to <10 mm in the short axis) or partial response (defined as ≥30% decreased sum of diameters of target lesions compared with the baseline sum diameters) compared with patients with stable disease (defined as neither sufficient shrinkage to qualify for partial response nor sufficient increase to qualify for progressive disease, using the smallest sum diameters during the study as the reference) or progressive disease (defined as ≥20% increase in the sum diameters of target lesions, using the smallest sum during the study as reference, and the sum diameters demonstrate an absolute increase of ≥5 mm)41 (eFigure 19 in the Supplement). Likewise, these biomarkers were also greater in the patients with DCB than in the patients with no durable benefit (NDB) (eFigure 19 in the Supplement). Additionally, PFS was higher in the patients with high PD-L1 expression than in those with low PD-L1 expression (HR, 0.41; 95% CI, 0.27-0.65; P < .001) (Figure 2A). Similar results were observed when the patients were stratified into high TMB vs low TMB groups (HR, 0.41; 95% CI, 0.28-0.60; P < .001) (Figure 2C) as well as high NAB vs low NAB groups (HR, 0.34; 95% CI, 0.18-0.66; P < .001) (eFigure 20 in the Supplement). We found significantly improved OS associated with patients with high PD-L1 expression (HR, 0.62; 95% CI, 0.44-0.81; P = .006) or high TMB (HR, 0.26; 95% CI, 0.10-0.71; P = .009) (Figure 2B-D). The OS results were further confirmed by calculating the restricted mean survival time, which showed that the mean months of life gained through 24 months for high vs low PD-L1 expression was 3.36 (95% CI, 1.17-5.54) months (P = .003); for high vs low TMB, restricted mean survival time was 6.78 (95% CI, 1.75-11.81) months (P = .008). We also characterized PD-L1 expression, TMB, and the NAB in the Cancer Genome Atlas40 cohort and found consistent results (eFigure 21 in the Supplement).
Figure 2. Survival Analysis of Patients Stratified by Programmed Cell Death Ligand 1 (PD-L1) Expression or Tumor Mutation Burden (TMB) .
A, Progression-free survival curves were plotted for patients stratified by PD-L1 expression in cohort 1. B, Overall survival curves were plotted for patients stratified by PD-L1 expression in the OAK trial.10 C, Progression-free survival curves were plotted for patients stratified by tumor mutation burden in cohort 1. D, Overall survival curves were plotted for patients stratified by tumor mutation burden in cohort 2. Crosses indicate censoring of data. HR indicates hazard ratio.
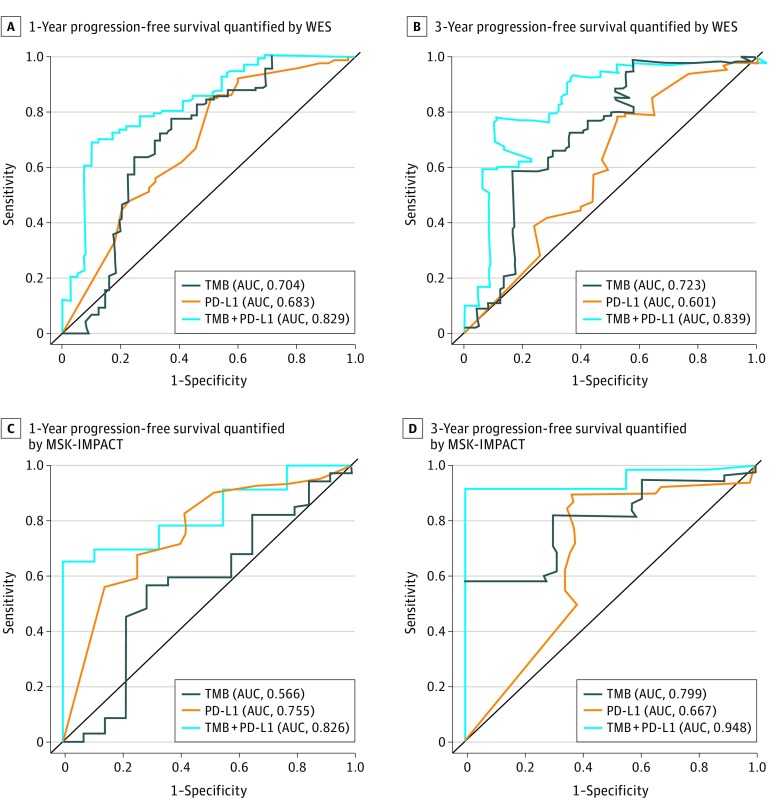
There was an association of increased PD-L1 expression or an increased TMB with an increased rate of 1-year PFS (PD-L1: AUC, 0.683; TMB: AUC, 0.704), 3-year PFS (PD-L1: AUC, 0.601; TMB: AUC, 0.723), complete response or partial response (PD-L1: AUC, 0.643; TMB: AUC, 0.727), and DCB (PD-L1: AUC, 0.621; TMB: AUC, 0.679) in cohort 1 (Figure 3A and B; eFigure 22 in the Supplement).
Figure 3. Joint Association of Programmed Cell Death Ligand 1 (PD-L1) Expression and Tumor Mutation Burden (TMB) With Survival in Checkpoint Blockade.
A and B, Patient tumor tissues were quantified by whole-exome sequencing (WES) and receiver operating characteristic curves were plotted for sensitivity vs 1 − specificity of 1-year progression-free survival (A) and 3-year progression-free survival (B). C and D, Patient tumor tissues were quantified by Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT) sequencing and receiver operating characteristic curves were plotted for sensitivity vs 1 − specificity of 1-year progression-free survival (C) and 3-year progression-free survival (D). AUC indicates area under the receiver operating characteristic curve.
Association of Genomic Biomarker Combinations With ICI Response
We next evaluated the joint use of the 2 biomarkers in predicting response and PFS. A strong correlation was found between TMB and NAB (Spearman ρ = 0.78; P < .001). No correlation was found between PD-L1 expression and TMB (eFigure 23 in the Supplement), which suggests that PD-L1 expression and TMB were independent predictive measures of immunotherapy benefit. Compared with patients with only 1 high variable or no high variables, patients with concomitantly high TMB and PD-L1 expression had improved PFS (HR, 0.42; 95% CI, 0.30-0.58; P < .001) (eFigure 24 in the Supplement) and a higher complete response or partial response rate (high TMB or high PD-L1, 62.5%; high TMB and low PD-L1, 43.9%; low TMB and high PD-L1, 27.3%; low TMB and low PD-L1, 8.0%; eFigure 25A in the Supplement) or DCB rate (high TMB or high PD-L1, 77.3%; high TMB and low PD-L1, 57.1%; low TMB and high PD-L1, 63.6%; low TMB and low PD-L1, 25.9%) (eFigure 25B in the Supplement). Among patients with NSCLC who had undergone whole-exome sequencing, the clinical use of TMB combined with PD-L1 expression had a higher value of prediction with PFS, complete response, partial response, or DCB than TMB or PD-L1 expression alone (1-year PFS: AUC, 0.829; 3-year PFS: AUC, 0.839; ORR: AUC, 0.803; DCB: AUC, 0.740) (Figure 3A and B; eFigure 22 in the Supplement). The predictability of TMB quantified by targeted next-generation sequencing (Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets) combined with PD-L1 expression was further validated in tumor tissues (1-year PFS: AUC, 0.826; 3-year PFS: AUC, 0.948) (Figure 3C and D). We also assessed its prognostic use in the Cancer Genome Atlas40 cohort (eFigure 26 in the Supplement).
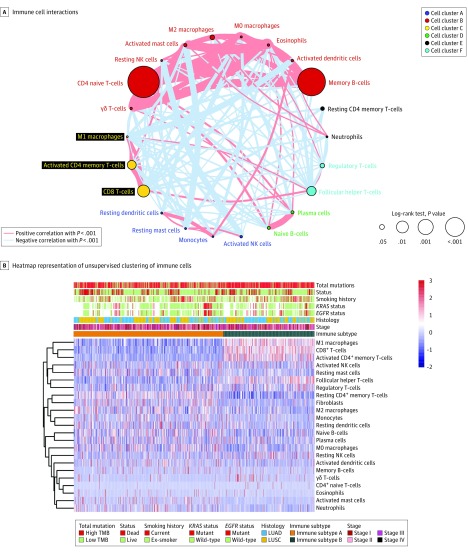
Further, a network of ICs that represented a comprehensive landscape of IC interactions was established (Figure 4A). Through unsupervised consensus matrix analyses (eFigure 27 in the Supplement), we developed an immune signature consisting of 2 immune subtypes (Figure 4B). Tumor mutation burden was significantly greater in the patients with immune subtype B compared with those with immune subtype A (eFigure 28A in the Supplement), and the immune subtype B group was associated with a higher OS (HR, 0.71; 95% CI, 0.55-0.90; P = .006) (eFigure 28B in the Supplement). Patients with immune subtype B had a higher proportion of high CD8+ T-cell tumor-infiltrating lymphocytes (TILs) compared with patients with immune subtype A (319 of 353 [90.4%] vs 187 of 588 [31.8%]) (eFigure 28C in the Supplement). Kaplan-Meier analysis found a significant difference in OS between the patients with high-CD8+ T-cell TILs vs those with low CD8+ T-cell TILs (HR, 0.67; 95% CI, 0.53-0.85; P < .001) (eFigure 28D in the Supplement). Random forest analysis consistently confirmed that the infiltration of CD8+ T-cell TILs was the most important variable in our clustering (eFigure 29 in the Supplement).
Figure 4. Landscape of the Immune Cells in Non–Small Cell Lung Cancer.
A, Cellular interaction of immune cell types. B, The heatmap representation of the unsupervised clustering of immune cells. EGFR indicates epidermal growth factor receptor; KRAS, Kirsten rat sarcoma viral oncogene homologue; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; NK, natural killer; and TMB, tumor mutation burden.
Next, we demonstrated that CD8+ T-cell TILs could further synergize with PD-L1 expression and TMB to have a prognostic association in the Cancer Genome Atlas40 cohort (3-year OS: AUC, 0.659; 5-year OS: AUC, 0.665) (eFigure 26 in the Supplement). Small correlations were identified between CD8+ T-cell TILs and PD-L1 expression (Spearman ρ = 0.21), between TMB and CD8+ T-cell TILs (Spearman ρ = 0.20), and between PD-L1 expression and TMB (Spearman ρ = 0.06) (eFigure 30 in the Supplement).
Association of Individual Gene Alterations With Response and Resistance to ICI Therapy
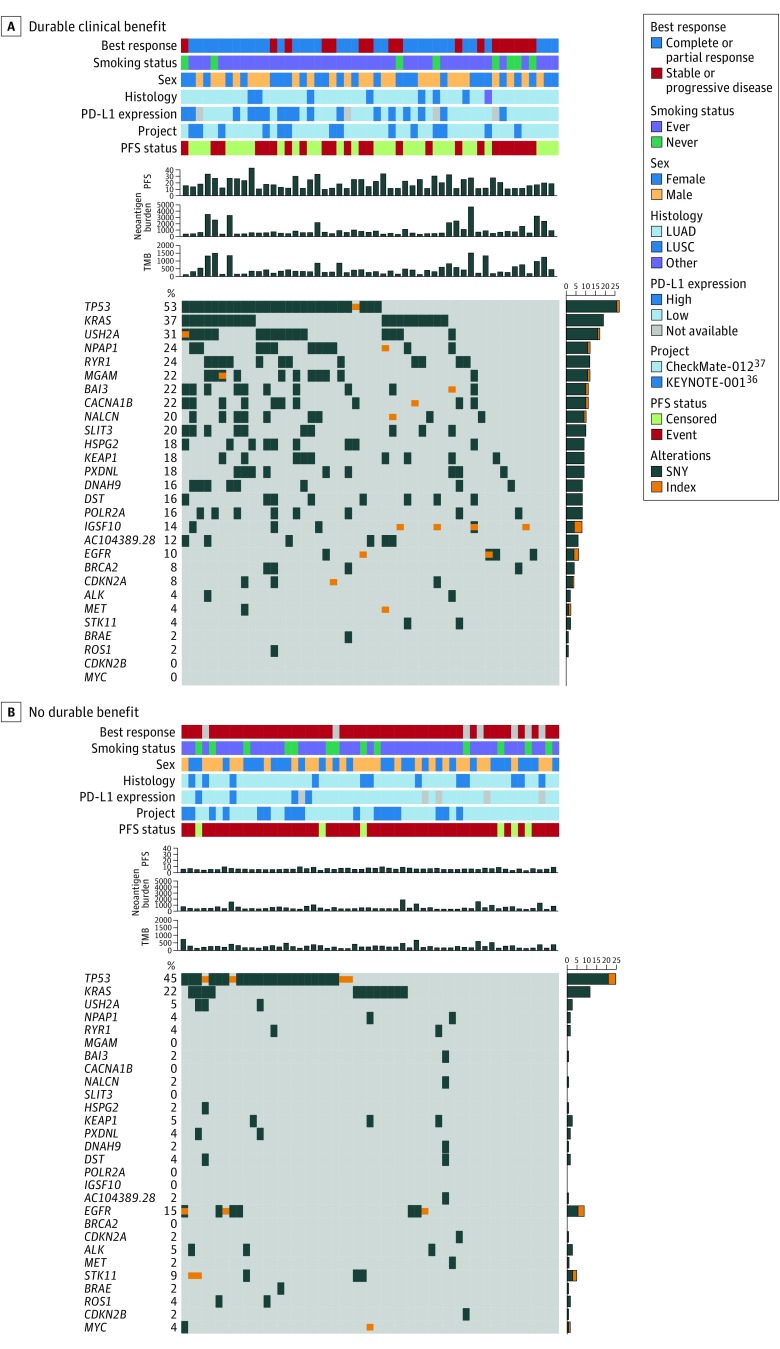
Within the KEYNOTE-00136 and CheckMate-01237 cohorts, we examined the associations of the frequencies of common NSCLC carcinogenic driver mutations with the clinical benefit derived from checkpoint blockade (Figure 5; eFigure 31A in the Supplement). Gene alterations were mostly enriched in the DCB group. Mutations in TP53 (DCB, 27 of 51 [53%]; NDB, 25 of 55 [45%]) and KRAS (DCB, 19 of 51 [37%]; NDB, 12 of 55 [22%]) were common and associated with increased responsiveness but did not reach statistical significance. Additionally, we identified other potential genes associated with responsiveness, such as USH2A (DCB, 17 of 51 [33%]; NDB, 3 of 55 [5%]; P < .001), NPAP1 (DCB, 12 of 51 [24%]; NDB, 2 of 55 [4%]; P < .001), RYR1 (DCB, 12 of 51 [24%]; NDB, 2 of 55 [4%]; P < .001), and MGAM (DCB, 12 of 51 [24%]; NDB, 0 of 55; P < .001). However, the frequencies of mutations in EGFR, ALK, ROS1, STK11, and BRAF were not significantly different between the DCB group and the NDB group (EGFR: DCB, 6 of 51 [10%]; NDB, 9 of 55 [16%]; ALK: DCB, 2 of 51 [4%]; NDB, 3 of 55 [5%]; ROS1: DCB, 1 of 51 [2%]; NDB, 2 of 55 [4%]; STK11: DCB, 2 of 51 [4%]; NDB, 5 of 55 [9%]; and BRAF: DCB, 1 of 51 [2%]; NDB, 1 of 55 [2%]). We also compared the frequencies of altered genes in the high TMB group with those in the low TMB group and those in the high PD-L1 group with those in the low PD-L1 group. Notably, RYR1 or MGAM mutations were associated with concomitantly increased DCB (RYR1: DCB, 12 of 51 [24%]; NDB, 2 of 55 [4%]; P < .001; MGAM: DCB, 12 of 51 [24%]; NDB, 0 of 55; P < .001), a higher TMB (RYR1: high TMB, 12 of 53 [23%]; low TMB, 2 of 53 [38%]; P < .001; MGAM: high TMB, 9 of 53 [17%]; low TMB, 0 of 53; P < .001), and higher PD-L1 expression (RYR1: high PD-L1, 8 of 30 [27%]; low PD-L1, 6 of 85 [7%]; P < .001; MGAM: high PD-L1, 6 of 30 [20%]; low PD-L1, 5 of 85 [6%]; P < .001) (eFigure 31B and C in the Supplement).
Figure 5. Individual Gene Alterations Associated With Response and Molecular Features in Checkpoint Blockade.
LUAD indicates lung adenocarcinoma; LUSC, lung squamous cell carcinoma; PD-L1, programmed cell death ligand 1; PFS, progression-free survival; SNV, single-nucleotide variants; and TMB, tumor mutation burden.
Discussion
Our analysis revealed that immunotherapies, including ICIs, tumor vaccines, and cellular immunotherapy, were associated with improved OS and PFS in patients with advanced NSCLC. Our results indicated that first-line pembrolizumab with platinum-based chemotherapy was superior to other ICI regimens and therefore should be the preferred choice for patients with advanced NSCLC. Other strategies, such as first-line tumor vaccines with chemotherapy and first-line tumor vaccine maintenance therapy, also showed promising results with high-quality evidence. Additionally, increasing PD-L1 expression and TMB were associated with increasing improvements in OS and PFS in patients with NSCLC treated with ICIs. We further found improved OS and PFS associated with using a single ICI for patients in the TC3 or IC3 and TC2/3 or IC2/3 groups, whereas ICIs with chemotherapy might be more suitable for patients in the TC1 or IC1 groups. The combined predictive relevance of PD-L1 expression and TMB was found to be a promising biomarker for patient survival and response to precision immunotherapy. We also demonstrated the potential to jointly use CD8+ T-cell TILs with PD-L1 expression and TMB in candidate identification.
There is an increasing immunotherapeutic trend in dual immunotherapy, which has shown to be effective in treating melanoma42 and renal cell carcinoma43 in RCTs. The results of a 2017 phase 3 RCT42 with 945 patients with advanced melanoma showed that nivolumab with ipilimumab as a first-line treatment was associated with longer OS than nivolumab or ipilimumab alone. The combination of ICIs and tumor vaccines, such as sargramostim, with ipilimumab showed significantly longer OS and lower toxic effects than ipilimumab alone in treating melanoma.44 In addition, the efficacy of chimeric antigen receptor T-cells in treating NSCLC is being evaluated in a randomized phase 1/2 trial (ClinicalTrials.gov identifier: NCT03525782), although RCTs concerning the combination of chimeric antigen receptor T-cells and immunotherapy remain restricted to hematologic malignancies, to our knowledge.45 Overall, the evidence of the beneficial role of various dual therapies that combine ICIs with tumor vaccines or cellular immunotherapy for the treatment of NSCLC remains too preliminary, and these combinations need to be further explored and established.
Independent biomarkers, such as PD-L1 expression and TMB, are probably inadequately predictive in intratumoral immune microenvironments with heterogeneous features, and an integrated multiparameter evaluation will aid efforts to overcome within-tumor heterogeneity and identify patients who could derive the greatest therapeutic benefit.46 Based on the data from a 2018 pan-tumor study,47 the highest ORR and longest PFS were observed in patients with high PD-L1 expression and a high TMB. This finding is also supported in our study, and we further demonstrated that the joint use of PD-L1 expression and TMB represented greater predictive and prognostic relevance for immunotherapy survival and response than the PD-L1 expression or TMB alone, which merits future clinical investigation on the combined use of PD-L1 expression and TMB as a predictor.
Characterizing the immune infiltrate could facilitate evaluating the treatment effect of immunotherapy; immune-inflamed tumors, which have a higher density of CD8+ T-cell TILs than immune-desert tumors, can elicit a strong immune response.48,49,50 The IMpassion130 RCT,51 a phase III study that evaluated the predictive effect of ICs on atezolizumab with nab-paclitaxel therapy in patients with triple-negative breast cancer, showed that high intratumoral CD8+ expression was significantly associated with PD-L1 expression and improvements in OS and PFS. According to our findings, using CD8+ T-cell TILs, PD-L1 expression, and TMB as an integrated variable was associated with improved OS and PFS compared with using a single biomarker or a combination of 2 of these 3 biomarkers. Future development of an optimized, integrated predictive model for immunotherapy should consider the integration of multiple approaches involving biomarkers associated with the T cell–inflamed tumor microenvironment, such as PD-L1 expression, ICs, and those associated with tumor neoepitope burden.
The examination of oncogenic driver mutations is becoming a novel approach to identify appropriate patients for immunotherapy. However, previous small-scale cohort studies showed inconsistent results concerning the associations of oncogenic alterations with immunotherapeutic outcomes. A study by Dong et al52 demonstrated that KRAS or TP53 mutations were statistically significantly associated with an increased clinical benefit of immunotherapy in NSCLC, but these findings were not confirmed in later studies.53,54 In our study, we used the largest data series to date, to our knowledge, and found that the differences between the DCB group and the NDB group were not statistically significant when the patients were stratified by TP53 or KRAS status. Mutated genes in patients with NSCLC probably represent diverse molecular functions; thus, cooccurring patterns of mutations could describe different patient immune subsets that are associated with distinct clinical benefits,55 which may also explain the observed contradictory findings. A 2018 cohort study53 of lung adenocarcinoma showed that KRAS mutation was not associated with enhanced response or survival among patients with TP53, STK11, EGFR, or wild-type NSCLC. In agreement, our nonsignificant results for KRAS may be owing to most of the patients in our included cohorts exhibiting the TP53, STK11, EGFR, or wild-type status. Of note, we further identified novel genes, such as RYR1 and MGAM, in which mutations were significantly associated with increased response and higher PD-L1 expression and TMB, indicating that these genes may contribute to IC infiltration and may be important components of the immunogenetic landscape. Future prospective sequencing tools that examine predictors for immunotherapy should be able to expand the landscape of immuno-oncological genes to more fully realize the potential for precision immunotherapy.
Limitations
This study has several limitations. First, we could not perform further subgroup analyses and examine distinct molecular features among the patients who received tumor vaccines and cellular immunotherapy owing to limited data. Additional trials are needed to identify the candidates who would most benefit from treatment with tumor vaccines and cellular immunotherapy. Second, patient data were limited regarding dual immunotherapy vs a single agent. The beneficial role of dual immunotherapy in NSCLC remained unclear. Third, the results about the biomarkers are somewhat scattered, as there were patients who did not receive quantification of both PD-L1 and TMB. Fourth, the interpretation in the ICI cohorts was restricted to an inadequate type of data owing to mainly using exome data; therefore, an integrated analysis based on multidimensional data comprising genomics, transcriptomics, proteomics, and signaling pathways might represent a powerful explanation of our findings.
Conclusions
In this study, overall, ICIs, tumor vaccines, and cellular immunotherapy showed promising clinical outcomes in patients with NSCLC. We recommend pembrolizumab with platinum-based chemotherapy as the most appropriate first-line ICI regimen for advanced NSCLC and suggest the combined use of PD-L1 expression and TMB to evaluate patients’ survivals and responses to precision immunotherapy. Moreover, the combination of CD8+ T-cell TILs, PD-L1 expression, and TMB were associated with reliable prognostic relevance. The predictive value of that combination needs to be prospectively validated in large-scale studies.
eAppendix. Methods
eTable 1. Characteristics of the Included Patients and Randomized Clinical Trials
eTable 2. Characteristics and Clinical Outcomes of the Individual Patients in Checkpoint Inhibitor Cohorts
eTable 3. Summary of the Pooled Estimates and Grading of Recommendations, Assessment, Development, and Evaluation Evidence of Progression-Free Survival
eTable 4. Summary of the Pooled Estimates and Grading of Recommendations, Assessment, Development, and Evaluation Evidence of Overall Survival
eTable 5. Summary of the Pooled Estimates and Grading of Recommendations, Assessment, Development, and Evaluation Evidence of Objective Response Rate
eTable 6. Summary of the Estimates and Grading of Recommendations, Assessment, Development, and Evaluation Evidence in the Subgroup Analysis of Clinical Outcomes
eTable 7. Summary of the Estimates Stratified by Programmed Cell Death Ligand 1 Expression and Treatment Strategy
eFigure 1. PRISMA Flow Diagram for the Meta-analysis
eFigure 2. Risk of Bias Summary of the Randomized Clinical Trials Included in the Meta-analysis
eFigure 3. Risk of Bias Graph for the Randomized Clinical Trials Included in the Meta-analysis
eFigure 4. Analysis of Publication Bias in the Meta-analyses of Immunotherapy Versus Conventional Therapy
eFigure 5. Pooled Analysis of the Ratio of the Median Overall Survival With Immunotherapy vs Conventional Therapy
eFigure 6. Pooled Hazard Ratios for Progression-Free Survival With Immunotherapy vs Conventional Therapy
eFigure 7. Pooled Analysis of the Ratio of the Median Progression-Free Survival With Immunotherapy vs Conventional Therapy
eFigure 8. Network Diagram of Studies Comparing Clinical Outcomes of Different Immune Checkpoint Inhibitors Strategies for Advanced Non–Small Cell Lung Cancer
eFigure 9. Network Meta-analysis of Immune Checkpoint Inhibitors in Terms of Progression-Free Survival
eFigure 10. Network Meta-analysis of Immune Checkpoint Inhibitors in Terms of Overall Survival
eFigure 11. Network Meta-analysis of Immune Checkpoint Inhibitors as a First-line Therapy in Terms of Progression-Free Survival
eFigure 12. Network Meta-analysis of Immune Checkpoint Inhibitors as a First-line Therapy in Terms of Overall Survival
eFigure 13. Network Meta-analysis of Immune Checkpoint Inhibitors in Terms of Progression-Free Survival in Previously Treated Patients
eFigure 14. Network Meta-analysis of Immune Checkpoint Inhibitors in Terms of Overall Survival in Previously Treated Patients
eFigure 15. Pooled Analysis of the Objective Response Rate With Immunotherapy vs Conventional Therapy
eFigure 16. Trial Sequential Analyses of Trials Comparing Immunotherapy With Conventional Therapy
eFigure 17. Subgroup Analyses of Progression-Free Survival in Patients Receiving Immune Checkpoint Inhibitor Therapy
eFigure 18. Subgroup Analyses of Overall Survival in Patients Receiving Immune Checkpoint Inhibitor Therapy
eFigure 19. Response and Clinical Benefit to Checkpoint Inhibitor Relative to Molecular Features in Cohort 1
eFigure 20. Progression-Free Survival Analysis Stratified by Neoantigen Burden in Cohort 1
eFigure 21. Overall Survival Analysis Stratified by Molecular Features in the Cancer Genome Atlas Cohort
eFigure 22. Receiver Operating Characteristic Curves Correlating Molecular Features With Clinical Outcomes in Cohort 1
eFigure 23. Scatterplots of Molecular Features in Cohort 1
eFigure 24. Progression-Free Survival Analysis Stratified by Programmed Cell Death Ligand 1 Expression and Tumor Mutation Burden in Cohort 1
eFigure 25. Response and Clinical Benefit to Checkpoint Inhibitor Stratified by Programmed Cell Death Ligand 1 and Tumor Mutation Burden
eFigure 26. Receiver Operating Characteristic Curves Correlating Molecular Features With Survival in the Cancer Genome Atlas Cohort
eFigure 27. Unsupervised Consensus Clustering of Immune Subtypes in the Cancer Genome Atlas Cohort
eFigure 28. Molecular Features and Survival Stratified by Immune Subtype in the Cancer Genome Atlas Cohort
eFigure 29. Identification of the Most Important Immune Feature Using Random Forest Method
eFigure 30. Scatterplots of Molecular Features in the Cancer Genome Atlas Cohort
eFigure 31. Individual Gene Alterations Associated With Checkpoint Blockade Benefits and Molecular Features
eReferences.
References
- 1.Tan WL, Jain A, Takano A, et al. Novel therapeutic targets on the horizon for lung cancer. Lancet Oncol. 2016;17(8):-. doi: 10.1016/S1470-2045(16)30123-1 [DOI] [PubMed] [Google Scholar]
- 2.Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299-311. doi: 10.1016/S0140-6736(16)30958-8 [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 4.Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol. 2017;35(35):3924-3933. doi: 10.1200/JCO.2017.74.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quoix E, Lena H, Losonczy G, et al. TG4010 immunotherapy and first-line chemotherapy for advanced non-small-cell lung cancer (TIME): results from the phase 2b part of a randomised, double-blind, placebo-controlled, phase 2b/3 trial. Lancet Oncol. 2016;17(2):212-223. doi: 10.1016/S1470-2045(15)00483-0 [DOI] [PubMed] [Google Scholar]
- 6.Li R, Wang C, Liu L, et al. Autologous cytokine-induced killer cell immunotherapy in lung cancer: a phase II clinical study. Cancer Immunol Immunother. 2012;61(11):2125-2133. doi: 10.1007/s00262-012-1260-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carbone DP, Reck M, Paz-Ares L, et al. ; CheckMate 026 Investigators . First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. doi: 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takayama K, Sugawara S, Saijo Y, et al. Randomized phase II study of docetaxel plus personalized peptide vaccination versus docetaxel plus placebo for patients with previously treated advanced wild type EGFR non-small-cell lung cancer. J Immunol Res. 2016;2016:1745108. doi: 10.1155/2016/1745108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu C, Jiang J, Shi L, Xu N. Prospective study of chemotherapy in combination with cytokine-induced killer cells in patients suffering from advanced non-small cell lung cancer. Anticancer Res. 2008;28(6B):3997-4002. [PubMed] [Google Scholar]
- 10.Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group . Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fehrenbacher L, Spira A, Ballinger M, et al. ; POPLAR Study Group . Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-1846. doi: 10.1016/S0140-6736(16)00587-0 [DOI] [PubMed] [Google Scholar]
- 12.Hellmann MD, Ciuleanu TE, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093-2104. doi: 10.1056/NEJMoa1801946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. 2019;30(1):44-56. doi: 10.1093/annonc/mdy495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dempke WCM, Fenchel K, Dale SP. Programmed cell death ligand-1 (PD-L1) as a biomarker for non-small cell lung cancer (NSCLC) treatment: are we barking up the wrong tree? Transl Lung Cancer Res. 2018;7(suppl 3):S275-S279. doi: 10.21037/tlcr.2018.04.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046-2054. doi: 10.1200/JCO.2011.38.4032 [DOI] [PubMed] [Google Scholar]
- 16.Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. doi: 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 17.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Govindan R, Szczesna A, Ahn MJ, et al. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449-3457. doi: 10.1200/JCO.2016.71.7629 [DOI] [PubMed] [Google Scholar]
- 19.Wu YL, Lu S, Cheng Y, et al. Nivolumab versus docetaxel in a predominantly Chinese patient population with previously treated advanced NSCLC: CheckMate 078 randomized phase III clinical trial. J Thorac Oncol. 2019;14(5):867-875. doi: 10.1016/j.jtho.2019.01.006 [DOI] [PubMed] [Google Scholar]
- 20.Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol. 2018;36(18)(suppl):LBA9000. doi: 10.1200/JCO.2018.36.18_suppl.LBA9000 [DOI] [Google Scholar]
- 21.Socinski MA, Jotte RM, Cappuzzo F, et al. ; IMpower150 Study Group . Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. doi: 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 22.Langer CJ, Gadgeel SM, Borghaei H, et al. ; KEYNOTE-021 Investigators . Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17(11):1497-1508. doi: 10.1016/S1470-2045(16)30498-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopes G, Wu Y-L, Kudaba I, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: open-label, phase 3 KEYNOTE-042 study. J Clin Oncol. 2018;36(18)(suppl):LBA4. doi: 10.1200/JCO.2018.36.18_suppl.LBA4 [DOI] [Google Scholar]
- 24.Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators . Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. doi: 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 25.Paz-Ares LG, Luft A, Tafreshi A, et al. Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non-small cell lung cancer (NSCLC). J Clin Oncol. 2018;36(15)(suppl):105. doi: 10.1200/JCO.2018.36.15_suppl.105 [DOI] [Google Scholar]
- 26.Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23(27):6674-6681. doi: 10.1200/JCO.2005.13.011 [DOI] [PubMed] [Google Scholar]
- 27.Quoix E, Ramlau R, Westeel V, et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 2011;12(12):1125-1133. doi: 10.1016/S1470-2045(11)70259-5 [DOI] [PubMed] [Google Scholar]
- 28.Alfonso S, Valdés-Zayas A, Santiesteban ER, et al. A randomized, multicenter, placebo-controlled clinical trial of racotumomab-alum vaccine as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2014;20(14):3660-3671. doi: 10.1158/1078-0432.CCR-13-1674 [DOI] [PubMed] [Google Scholar]
- 29.Butts C, Socinski MA, Mitchell PL, et al. ; START Trial Team . Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(1):59-68. doi: 10.1016/S1470-2045(13)70510-2 [DOI] [PubMed] [Google Scholar]
- 30.Braun A, Engel-Riedel W, Schneller F, et al. 112P: efficacy and safety of imprime PGG, a novel innate immune modulator, in combination with bevacizumab (BEV), carboplatin and paclitaxel for the 1st-line treatment of stage IV NSCLC. Ann Oncol. 2015;26(suppl 1):i35. doi: 10.1093/annonc/mdv050.16 [DOI] [Google Scholar]
- 31.Giaccone G, Bazhenova LA, Nemunaitis J, et al. A phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancer. Eur J Cancer. 2015;51(16):2321-2329. doi: 10.1016/j.ejca.2015.07.035 [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez PC, Popa X, Martínez O, et al. A phase III clinical trial of the epidermal growth factor vaccine CIMAvax-EGF as switch maintenance therapy in advanced non-small cell lung cancer patients. Clin Cancer Res. 2016;22(15):3782-3790. doi: 10.1158/1078-0432.CCR-15-0855 [DOI] [PubMed] [Google Scholar]
- 33.Katakami N, Hida T, Nokihara H, et al. Phase I/II study of tecemotide as immunotherapy in Japanese patients with unresectable stage III non-small cell lung cancer. Lung Cancer. 2017;105:23-30. doi: 10.1016/j.lungcan.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 34.Thomas M, Sadjadian P, Kollmeier J, et al. A randomized, open-label, multicenter, phase II study evaluating the efficacy and safety of BTH1677 (1,3-1,6 beta glucan; Imprime PGG) in combination with cetuximab and chemotherapy in patients with advanced non-small cell lung cancer. Invest New Drugs. 2017;35(3):345-358. doi: 10.1007/s10637-017-0450-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators . Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919-1929. doi: 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 36.Shaverdian N, Lisberg AE, Bornazyan K, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18(7):895-903. doi: 10.1016/S1470-2045(17)30380-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hellmann MD, Rizvi NA, Goldman JW, et al. Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): results of an open-label, phase 1, multicohort study. Lancet Oncol. 2017;18(1):31-41. doi: 10.1016/S1470-2045(16)30624-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizvi H, Sanchez-Vega F, La K, et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J Clin Oncol. 2018;36(7):633-641. doi: 10.1200/JCO.2017.75.3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.cBioPortal for Cancer Genetics Whole exome sequencing molecular profiling of tumors across multiple cancer types including melanoma, non-small cell lung cancer, head and neck cancer, and bladder cancer. http://www.cbioportal.org/study?id=mixed_allen_2018&tab=summary. Accessed May 29, 2019.
- 40.Weinstein JN, Collisson EA, Mills GB, et al. The Cancer Genome Atlas pan-cancer analysis project. Nat Genet. 2013;45(10):1113-1120. doi: 10.1038/ng.2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. [DOI] [PubMed] [Google Scholar]
- 42.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med. 2017;377(14):1345-1356. doi: 10.1056/NEJMoa1709684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Motzer RJ, Tannir NM, McDermott DF, et al. ; CheckMate 214 Investigators . Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodi FS, Lee S, McDermott DF, et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA. 2014;312(17):1744-1753. doi: 10.1001/jama.2014.13943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359(6382):1361-1365. doi: 10.1126/science.aar6711 [DOI] [PubMed] [Google Scholar]
- 46.Jia Q, Wu W, Wang Y, et al. Local mutational diversity drives intratumoral immune heterogeneity in non-small cell lung cancer. Nat Commun. 2018;9(1):5361. doi: 10.1038/s41467-018-07767-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593.Medline:30309915 doi: 10.1126/science.aar3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22(8):1865-1874. doi: 10.1158/1078-0432.CCR-15-1507 [DOI] [PubMed] [Google Scholar]
- 49.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541(7637):321-330. doi: 10.1038/nature21349 [DOI] [PubMed] [Google Scholar]
- 50.Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35-44. doi: 10.1016/j.cell.2016.02.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emens LA, Loi S, Rugo H, et al. IMpassion130: efficacy in immune biomarker subgroups from phase III study of atezolizumab + nab-paclitaxel in patients with treatment-naïve, locally advanced or metastatic TNBC. Cancer Res. 2019;79(4 Suppl):GS1-04. [Google Scholar]
- 52.Dong ZY, Zhong WZ, Zhang XC, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res. 2017;23(12):3012-3024. doi: 10.1158/1078-0432.CCR-16-2554 [DOI] [PubMed] [Google Scholar]
- 53.Biton J, Mansuet-Lupo A, Pécuchet N, et al. TP53, STK11, and EGFR mutations predict tumor immune profile and the response to anti-PD-1 in lung adenocarcinoma. Clin Cancer Res. 2018;24(22):5710-5723. doi: 10.1158/1078-0432.CCR-18-0163 [DOI] [PubMed] [Google Scholar]
- 54.Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):210-216. doi: 10.1001/jamaoncol.2017.4427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arbour KC, Jordan E, Kim HR, et al. Effects of co-occurring genomic alterations on outcomes in patients with KRAS-mutant non-small cell lung cancer. Clin Cancer Res. 2018;24(2):334-340. doi: 10.1158/1078-0432.CCR-17-1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods
eTable 1. Characteristics of the Included Patients and Randomized Clinical Trials
eTable 2. Characteristics and Clinical Outcomes of the Individual Patients in Checkpoint Inhibitor Cohorts
eTable 3. Summary of the Pooled Estimates and Grading of Recommendations, Assessment, Development, and Evaluation Evidence of Progression-Free Survival
eTable 4. Summary of the Pooled Estimates and Grading of Recommendations, Assessment, Development, and Evaluation Evidence of Overall Survival
eTable 5. Summary of the Pooled Estimates and Grading of Recommendations, Assessment, Development, and Evaluation Evidence of Objective Response Rate
eTable 6. Summary of the Estimates and Grading of Recommendations, Assessment, Development, and Evaluation Evidence in the Subgroup Analysis of Clinical Outcomes
eTable 7. Summary of the Estimates Stratified by Programmed Cell Death Ligand 1 Expression and Treatment Strategy
eFigure 1. PRISMA Flow Diagram for the Meta-analysis
eFigure 2. Risk of Bias Summary of the Randomized Clinical Trials Included in the Meta-analysis
eFigure 3. Risk of Bias Graph for the Randomized Clinical Trials Included in the Meta-analysis
eFigure 4. Analysis of Publication Bias in the Meta-analyses of Immunotherapy Versus Conventional Therapy
eFigure 5. Pooled Analysis of the Ratio of the Median Overall Survival With Immunotherapy vs Conventional Therapy
eFigure 6. Pooled Hazard Ratios for Progression-Free Survival With Immunotherapy vs Conventional Therapy
eFigure 7. Pooled Analysis of the Ratio of the Median Progression-Free Survival With Immunotherapy vs Conventional Therapy
eFigure 8. Network Diagram of Studies Comparing Clinical Outcomes of Different Immune Checkpoint Inhibitors Strategies for Advanced Non–Small Cell Lung Cancer
eFigure 9. Network Meta-analysis of Immune Checkpoint Inhibitors in Terms of Progression-Free Survival
eFigure 10. Network Meta-analysis of Immune Checkpoint Inhibitors in Terms of Overall Survival
eFigure 11. Network Meta-analysis of Immune Checkpoint Inhibitors as a First-line Therapy in Terms of Progression-Free Survival
eFigure 12. Network Meta-analysis of Immune Checkpoint Inhibitors as a First-line Therapy in Terms of Overall Survival
eFigure 13. Network Meta-analysis of Immune Checkpoint Inhibitors in Terms of Progression-Free Survival in Previously Treated Patients
eFigure 14. Network Meta-analysis of Immune Checkpoint Inhibitors in Terms of Overall Survival in Previously Treated Patients
eFigure 15. Pooled Analysis of the Objective Response Rate With Immunotherapy vs Conventional Therapy
eFigure 16. Trial Sequential Analyses of Trials Comparing Immunotherapy With Conventional Therapy
eFigure 17. Subgroup Analyses of Progression-Free Survival in Patients Receiving Immune Checkpoint Inhibitor Therapy
eFigure 18. Subgroup Analyses of Overall Survival in Patients Receiving Immune Checkpoint Inhibitor Therapy
eFigure 19. Response and Clinical Benefit to Checkpoint Inhibitor Relative to Molecular Features in Cohort 1
eFigure 20. Progression-Free Survival Analysis Stratified by Neoantigen Burden in Cohort 1
eFigure 21. Overall Survival Analysis Stratified by Molecular Features in the Cancer Genome Atlas Cohort
eFigure 22. Receiver Operating Characteristic Curves Correlating Molecular Features With Clinical Outcomes in Cohort 1
eFigure 23. Scatterplots of Molecular Features in Cohort 1
eFigure 24. Progression-Free Survival Analysis Stratified by Programmed Cell Death Ligand 1 Expression and Tumor Mutation Burden in Cohort 1
eFigure 25. Response and Clinical Benefit to Checkpoint Inhibitor Stratified by Programmed Cell Death Ligand 1 and Tumor Mutation Burden
eFigure 26. Receiver Operating Characteristic Curves Correlating Molecular Features With Survival in the Cancer Genome Atlas Cohort
eFigure 27. Unsupervised Consensus Clustering of Immune Subtypes in the Cancer Genome Atlas Cohort
eFigure 28. Molecular Features and Survival Stratified by Immune Subtype in the Cancer Genome Atlas Cohort
eFigure 29. Identification of the Most Important Immune Feature Using Random Forest Method
eFigure 30. Scatterplots of Molecular Features in the Cancer Genome Atlas Cohort
eFigure 31. Individual Gene Alterations Associated With Checkpoint Blockade Benefits and Molecular Features
eReferences.