Abstract
Background
Cutis laxa (CL) is a group of rare connective tissue disorders mainly characterized by wrinkled, redundant, inelastic, and sagging skin. Besides skin anomalies, in most CL forms multiple organs are involved, leading to severe multisystem disorders involving skeletal, cardiovascular, pulmonary, and central nervous systems. CL might be challenging to diagnose because of its different inheritance patterns, extensive phenotypic variability, and genetic heterogeneity. Herein, we report the clinical and molecular characterization of an 18‐month‐old infant with signs suggestive of recessive cutis laxa type 1C (ARCL1C), although with a relatively mild presentation.
Methods
To confirm the clinical suspicion, mutational screening of all the exons and intron‐flanking regions of the latent transforming growth factor‐beta binding protein 4 gene (LTBP4) was performed by Sanger sequencing on an ABI3130XL Genetic Analyzer.
Results
Apart from the presence of the dermatological hallmark, the reported patient did not show pulmonary emphysema, which is the most common and discriminative finding of ARCL1C together with gastrointestinal and urinary involvement. Indeed, pulmonary involvement only included episodes of respiratory distress and diaphragmatic eventration; intestinal dilation and tortuosity and hydronephrosis were also present. Molecular analysis disclosed the novel homozygous c.1450del (p.Arg484Glyfs*290) pathogenic variant in exon 12 of LTBP4, thus leading to the diagnosis of ARCL1C.
Conclusion
Our findings expand both the knowledge of the clinical phenotype and the allelic repertoire of ARCL1C. The comparison of the patient's features with those of the other patients reported up to now offers future perspectives for clinical research in this field.
Keywords: ARCL1C, autosomal recessive cutis laxa type 1C, latent transforming growth factor‐beta binding protein 4, LTBP4
1. INTRODUCTION
Cutis laxa (CL) refers to a heterogeneous group of rare connective tissue disorders characterized by wrinkled, redundant, inelastic, and sagging skin. Both hereditary and acquired forms exist. The latter appear secondary to infections, administration of medications or as a paraneoplasm, whereas inherited CL is caused by structural abnormalities of the extracellular matrix (ECM). Different inborn metabolic errors have also been found to be associated with CL (Berk, Bentley, Bayliss, Lind, & Urban, 2012; Gardeitchik & Morava, 2013; Mohamed, Voet, Gardeitchik, & Morava, 2014). The inheritance can be autosomal dominant, autosomal recessive and X‐linked recessive, and 13 causative genes have been identified yet (Mohamed et al., 2014; Van Damme et al., 2017).
Among the different hereditary forms (incidence 1/400,000), the autosomal recessive form (ARCL) is the most prevalent and heterogeneous type (Gardeitchik & Morava, 2013; Morava, Guillard, Lefeber, & Wevers, 2009). Indeed, ARCL is divided into two major types and several subtypes based on the variable phenotypes and underlying defects in different genes. Given the phenotypic overlap among the different forms, proper differential diagnosis might be challenging as well as predicting the causative gene according to the clinical picture of patients, mainly due to the lack of detailed comparative phenotype data (Berk et al., 2012; Gardeitchik & Morava, 2013; Morava et al., 2009). ARCL1 type 1 is subdivided in ARCL1A (MIM #219100), which is caused by pathogenic variants in the fibulin‐5 gene (FBLN5, MIM *604,580) (Elahi et al., 2006; Hu et al., 2006; Loeys et al., 2002; Tekedereli et al., 2019), ARCL1B (MIM #614437) caused by mutations in the EGF‐containing fibulin‐like extracellular matrix protein 2 gene or fibulin‐4 gene (EFEMP2 or FBLN4; MIM *604,633) (Dasouki et al., 2007; Hucthagowder et al., 2006; Letard et al., 2018), and ARCL1C (MIM #613177), a.k.a. Urban‐Rifkin‐Davis syndrome, which is due to biallelic variants in the latent transforming growth factor‐beta binding protein 4 gene (LTBP4, MIM *604,710) (Callewaert et al., 2013; Su et al., 2015; Urban et al., 2009). ARCL type 2 is separated into ARCL2A (MIM #219200), caused by mutations in the gene encoding for the H+ transporting α2 subunit of the vesicular ATPase complex (ATP6V0A2, MIM *611,716) (Fischer et al., 2012; Hucthagowder et al., 2009; Kornak et al., 2008; Ritelli et al., 2014), ARCL2B (MIM #612940) that results from mutations in the pyrroline‐5‐carboxylate reductase 1 gene (PYCR1; MIM *179,035) (Dimopoulou et al., 2013; Reversade et al., 2009; Ritelli et al., 2017), and ARCL2C (MIM #612940) and ARCL2D (MIM #617403) that are due to biallelic variants in the ATPase H+ transporting V1 subunits E1 (ATP6V1E1; MIM *108,746) and A (ATP6V1A, MIM *607,027), respectively (Alazami et al., 2016; Van Damme et al., 2017). De Barsy syndrome (DBS), previously known as ARCL3A (ARCL3A; MIM #219150), forms a phenotypic continuum with ARCL2 and patients with DBS have been characterized for mutations in the aldehyde dehydrogenase 18 gene (ALDH18A1, MIM *138,250) (Guernsey et al., 2009; Skidmore et al., 2011) as well as in ATP6V0A2 and PYCR1 (Leao‐Teles, Quelhas, Vilarinho, & Jaeken, 2010; Zampatti et al., 2012). Additional recessive conditions with CL‐like phenotypes include “macrocephaly‐alopecia‐cutis laxa‐scoliosis syndrome” (MACS, MIM #613075), which is caused by mutations in the RAS and RAB interactor 2 gene (RIN2; MIM *610,222) (Aslanger et al., 2014; Basel‐Vanagaite et al., 2009), and geroderma osteodysplasticum (GO; MIM #231070) caused by mutations in the Golgi, RAB6‐interacting gene (GORAB, MIM *607,983) (Hennies et al., 2008).
Besides skin anomalies, in most ARCL forms multiple organs are involved, leading to severe multisystem disorders involving skeletal, cardiovascular, pulmonary, and central nervous systems (Gardeitchik & Morava, 2013; Mohamed et al., 2014). In particular, ARCL1 patients fall within the severe end of the phenotypic spectrum (Callewaert & Urban, 2016; Loeys, Paepe, & Urban, 2001; Van Maldergem & Loeys, 2009). Inguinal/umbilical hernias, vesicourinary and gastroesophageal reflux and/or diverticula are present in all patients and bladder/intestinal diverticula and/or pyloric stenosis together with CL are considered pathognomonic (Callewaert & Urban, 2016; Loeys et al., 2001; Mohamed et al., 2014; Van Maldergem & Loeys, 2009). Likewise, severe pulmonary emphysema is the most common and discriminative finding, since it has been described in all ARCL1 subtypes. Cardiac involvement might be variable and includes peripheral pulmonary artery or supravalvular aortic stenoses. The disease course depends on the cardiovascular and pulmonary involvement. Lung emphysema, recurrent pulmonary infections and cardiac failure determine the long‐term survival and most children die in early childhood (Callewaert & Urban, 2016; Loeys et al., 2001; Van Maldergem & Loeys, 2009). Differential diagnosis between different ARCL1 subtypes relies on the presence/absence of gastrointestinal and urinary involvement (less gastrointestinal involvement in EFEMP2 compared to FBLN5, urinary diverticula in EFEMP2, and severe involvement of both systems in LTBP4). EFEMP2‐related CL patients have severe arterial tortuosity with predisposition for aneurysms/dissections, which is rare in the other subtypes (Callewaert & Urban, 2016; Loeys et al., 2001; Mohamed et al., 2014; Van Maldergem & Loeys, 2009).
Herein, we report on an 18‐month‐old Venezuelan female with signs suggestive of ARCL1 and compare the patient's features with those of the other individuals with LTPB4‐related CL reported up to now.
2. PATIENT AND METHODS
2.1. Ethical compliance
This study follows the Helsinki Declaration's principles and was carried out from routine diagnostic activity; formal ethics review was therefore not requested. The patient's parents provided written informed consent for and publication of clinical data and photographs. The patient was evaluated at the Unit of Medical Genetics (Department of Pediatrics) of the University Hospital of Mérida in Venezuela. Genetic testing was performed at the Division of Biology and Genetics (Department of Molecular and Translational Medicine) of the University of Brescia in Italy.
2.2. Molecular analysis
After informed consent was obtained from the patient's parents, molecular characterization was performed on genomic DNA purified from peripheral blood leukocytes using standard procedures. All of the exons and intron‐flanking regions of the LTBP4 gene (reference sequences: NG_021201.1 NM_003573.2, NP_003564.2) were PCR amplified by using optimized genomic primers (available upon request) that were analyzed for the absence of known variants using the GnomAD database (https://gnomad.broadinstitute.org/). PCR products were purified with ExoSAP‐IT (USB Corporation) followed by bidirectional sequencing with the BigDye Terminator v1.1 Cycle Sequencing kit on an ABI3130XL Genetic Analyzer (Applied Biosystems). The sequences were analyzed with the Sequencher 5.0 software (www.genecodes.com) and variants were annotated according to the Human Genome Variation Society (HGVS) nomenclature by using the Alamut Visual software version 2.11 (www.interactive-biosoftware.com). The novel pathogenetic LTBP4 variant identified in the patient was submitted to the Leiden Open Variation Database (LOVD).
3. RESULTS
The patient was born at 39 weeks of gestation from consanguineous (cousins) unaffected Venezuelan parents via an uneventful, spontaneous vaginal delivery. At birth, her weight was 3.6 kg (1.2 SD) and length 50 cm (0.6 SD). Clinical history was remarkable for perinatal respiratory distress and neonatal hypotonia. Delayed anterior fontanel closure and postnatal growth retardation were also reported. At 9 months, a clinical diagnosis of CL was given for the presence of the dermatological hallmark, that is, loose, wrinkled, sagging, and redundant skin (Figure 1A a,b). At 10 months, heart ultrasound revealed a small interatrial septal defect without hemodynamic repercussion and renal ultrasound right pyelocalicial ectasia and hydronephrosis. On examination at 13 months, several craniofacial features were observed, that is, narrow forehead, down slanting palpebral fissures, periorbital fullness, epicanthus, hypertelorism, long philtrum, fat midface, depressed nasal bridge, anteverted nares, posteriorly rotated ears, micro‐retrognathia, and short neck. The skin was inelastic, sagging, and redundant on cheeks (with a prematurely aged appearance), neck, axillae, arms, abdomen, glutei, and limbs (Figure 1A c). An umbilical hernia was present. Delayed psychomotor development, hypotonia, and hypermobility of small joints were also observed. Thorax/abdomen radiography showed discreetly prominent aortic arch with mild tortuosity, diaphragmatic eventration, normal pulmonary parenchyma with atelectasis in the left lung, and elongated gastrointestinal tract with dilatation and tortuosity (Figure 1A d). At 18 months, she was hospitalized for pneumonia with significant respiratory distress, successfully treated with antibiotics and oxygen supplementation.
Figure 1.
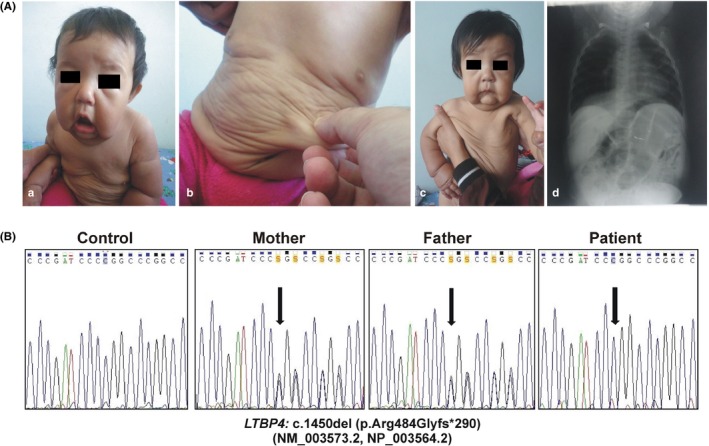
(A) Clinical appearance of the patient. At 9 months of age (a, b), a diagnosis of CL was given for the presence of loose, wrinkled, sagging, and redundant skin, and several craniofacial features. On examination at 13 months of age (c), normocephaly (between 25th and 50th percentile), dysmorphisms, that is, narrow forehead, down slanting palpebral fissures, periorbital fullness, epicanthus, hypertelorism, long philtrum, fat midface, depressed nasal bridge, anteverted nares, micro‐retrognathia, and short neck were observed. Cutis laxa was evident on cheeks (with a prematurely aged appearance), neck, axillae, arms, abdomen, glutei, and limbs. Thorax and abdomen radiography, performed at 10 months of age (d), showed discreetly prominent aortic arch with mild tortuosity, diaphragmatic eventration, normal pulmonary parenchyma with atelectasis in the left lung, and elongated gastrointestinal tract with dilatation and tortuosity. (B) Molecular analysis. Sequence chromatograms showing the position of the c.1450del (p.Arg484Glyfs*290) variant (arrows) identified in the patient in homozygosity in exon 12 of the LTBP4 gene. Both healthy parents were heterozygous carriers. Mutation is annotated according to HGVS nomenclature (http://www.hgvs.org/mutnomen; NM_003573.2, NP_003564.2)
Considering the patient's cutaneous and craniofacial features, the presence of respiratory distress, diaphragmatic eventration, and hydronephrosis, and the absence of major vascular, skeletal and central nervous systems' involvement, ARCL1C was supposed. Sanger sequencing of the LTBP4 gene (NM_003573.2, NP_003564.2) confirmed the clinical suspicion disclosing the homozygous c.1450del variant leading to frameshift and formation of a premature termination codon (PTC) (p.Arg484Glyfs*290). Both parents were heterozygous carriers (Figure 1b). The variant (hg19/GRCh37:g.41114443del) was not found in population and disease databases including gnomAD (https://gnomad.broadinstitute.org/), Bravo (https://bravo.sph.umich.edu/freeze5/hg38/), ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), and LOVD (https://www.lovd.nl/), and was, therefore, submitted to the gene‐specific LOVD database (https://databases.lovd.nl/shared/variants/LTBP4/; DB‐ID: LTBP4_000036).
4. DISCUSSION
Our report highlights the importance of clinical expertise to address targeted molecular analysis, which, in turn, allows a definite diagnosis, in patients suggestive of ARCL1C, also considering the absence of formal diagnostic criteria due to the limited number of reported patients. Indeed, until now only 18 patients (Callewaert et al., 2013; Su et al., 2015; Urban et al., 2009; this work) from 14 families are described (Table 1). Cutis laxa and dysmorphism were evident from birth in all patients (18/18). The most frequently observed craniofacial features were depressed nasal bridge with anteverted nares (9/10), narrow forehead (9/11), hypertelorism (7/9), periorbital swelling (6/9), and long philtrum (9/12). Consistently, our patient presented severe cutis laxa, mainly localized to face, thorax, and abdomen, resulting in a coarse/aged appearance, and all the abovementioned dysmorphism. Severe pulmonary emphysema was present in all patients (13/13), except ours, and represented the most common cause of death (10/12). Indeed, the overall prognosis is poor, with a mortality rate of 72% (13/18). Mean age at death was 2.4 years (median age 6 months). The five surviving patients were all female (ages 1.5–23 years). In addition to pulmonary emphysema, brain abscess and gastric perforation were each reported once as a cause of death.
Table 1.
Summary of clinical features of all patients with LTBP4 pathogenic variants
| Citations | Our Patient | Urban et al. (2009) | Callewaert et al. (2013) | Su et al. (2015) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | P12 | P13 | P14 | P15 | P16 | P17 | P18 | |
| Sex | F | M | M | F | F | F | M | F | M | M | M | F | F | M | M | F | F | M |
| Age | 18 month | 9 month | 4 month | 7 year | 26 month | 23 years | 4 weeks | 3 months | 2 years | 10 years | 6 months | 6 months | 13 years | 6 weeks | 15 months | 14 years | 20 years | 6 weeks |
| Cause of death | − | PE | PE | − | PE | ‐ | PE | PE | PE | PE | PE PHrT | PHrT GP | BA | PE | NA | − | − | BD |
| Skin | ||||||||||||||||||
| Cutis laxa | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Craniofacial | ||||||||||||||||||
| Long philtrum | + | + | + | + | NA | − | NA | NA | + | NA | NA | + | + | − | − | + | + | NA |
| Fat midface | + | + | + | + | NA | − | NA | NA | − | NA | NA | − | − | − | − | NA | NA | NA |
| Narrow forehead | + | + | + | NA | + | − | NA | NA | + | NA | NA | + | + | + | − | + | NA | NA |
| Periorbital swelling | + | + | + | NA | NA | − | NA | NA | + | NA | NA | + | − | − | + | NA | NA | NA |
| Hypertelorism | + | + | + | NA | NA | − | NA | NA | + | NA | NA | + | + | − | + | NA | NA | NA |
| Depressed nasal bridge anteverted nares | + | + | + | NA | NA | + | NA | NA | + | NA | NA | + | − | + | + | + | NA | NA |
| Retrognathia, micrognathia or mandibular hypoplasia | + | + | + | + | + | − | NA | NA | − | NA | NA | − | − | − | − | NA | NA | NA |
| Wide suture or fontanels | + | + | + | NA | − | − | − | − | − | − | − | − | − | + | − | NA | NA | NA |
| Pulmonary | ||||||||||||||||||
| Tachypnea, respiratory distress | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | NA | NA |
| Pneumonia | + | + | − | − | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | + | NA | + |
| Laryngomalacia Tracheomalacia Bronchomalacia | − | − | + | NA | + | − | + | − | − | − | − | − | − | + | − | NA | NA | NA |
| Diaphragmatic hernia or eventration | + | − | + | + | + | + | − | − | + | + | − | + | − | + | + | NA | NA | NA |
| Emphysema | − | + | + | NA | + | + | + | + | + | + | + | + | + | + | + | NA | NA | NA |
| Gastrointestinal | ||||||||||||||||||
| Diverticula | − | − | + | + | NA | − | − | − | − | − | − | + | − | − | + | NA | NA | + |
| Intestinal dilation, tortuosity | + | + | + | NA | − | − | − | − | − | − | + | − | − | − | + | NA | NA | NA |
| Umbilical hernia | + | + | − | − | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Gastrointestinal reflux | − | − | − | + | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Rectal prolapse | − | − | − | + | + | − | − | − | − | + | − | − | − | − | NA | NA | NA | NA |
| Pyloric stenosis | − | − | + | − | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Genitourinary | ||||||||||||||||||
| Bladder diverticula | − | + | + | + | NA | + | − | + | − | + | − | − | + | + | + | NA | − | + |
| Hydronephrosis | + | + | + | − | NA | − | + | − | − | − | − | − | − | + | NA | NA | NA | NA |
| Inguinal hernia | − | + | + | − | − | − | − | − | − | + | − | − | − | − | + | NA | NA | NA |
| Cardiovascular | ||||||||||||||||||
| Peripheral pulmonary artery stenosis | − | + | − | NA | + | + | + | − | + | − | − | + | + | − | + | + | + | + |
| Atrial septal defect or aneurysms | + | − | − | NA | − | − | − | − | − | + | − | + | − | − | − | − | + | + |
| Cardiac valve insufficiency | − | − | − | NA | − | − | − | − | + | − | + | − | + | + | + | − | − | |
| Pulmonary or aortic valve stenosis | − | − | − | NA | + | − | − | − | − | − | − | − | − | − | + | − | − | − |
| Pulmonary hypertension | − | + | − | NA | + | − | − | − | − | − | + | + | + | + | + | − | + | − |
| Patent foramen ovale | − | + | + | − | NA | − | − | − | − | − | − | − | − | + | + | − | − | − |
| Other | ||||||||||||||||||
| Joint laxity | + | + | + | + | + | + | − | − | − | − | − | − | − | + | NA | + | + | + |
| Muscular hypotonia | + | + | + | NA | + | − | + | − | + | − | − | + | − | + | + | NA | NA | + |
| Foot deformity | − | + | + | NA | + | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | + | + | NA |
Abbreviations: BD, breathing difficulties; Ba, brain abscesses; GP, gastric perforation; PE, pulmonary emphysema; PHrT, pulmonary hypertension; NA, not available.
Diaphragmatic hernia or eventration was also common (10/15) as well as bladder (10/16) and gastrointestinal diverticula (5/15), intestinal dilatation/tortuosity (5/14), and hydronephrosis (5/13). Up to the moment of evaluation, our patient only presented episodes of respiratory distress, diaphragmatic eventration, intestinal dilation and tortuosity, and hydronephrosis. However, multidisciplinary evaluations are planned including immunizations against respiratory infections and periodic assessment of pulmonary function and imaging of gastrointestinal and urinary tracts. The absence of pulmonary emphysema in our patient is noteworthy but it is not easy to explain. In our opinion, it is most likely due to clinical variability rather than to the specific type of pathogenic variant or the young age of the patient. Indeed, the patient's variant should represent a null allele, as the majority of the mutations reported so far (Table 2), and fatal pulmonary emphysema was described in as many as eight patients who were younger than ours (Table 1).
Table 2.
LTBP4 pathogenic variants
| Patient | Cons. | Status | Exon | cDNA | Protein | Type | Domains | References |
|---|---|---|---|---|---|---|---|---|
| P1 | + | Homozygous | 12 | c.1450del | p.(Arg484Glyfs*290) | Frameshift‐PTC | ‐ | This report |
| P2 | + | Homozygous | 28 | c.3554del | p.(Gln1185Argfs*27) | Frameshift‐PTC | Second 8‐Cys domain | Urban et al. (2009) |
| P3 | − | Compound Heterozygous | 9 | c.791del | p.(Pro264Argfs*37) | Frameshift‐PTC | Hybrid domain | |
| 22 | c.2570_2571delGCinsAA | p.(Cys857*) | Frameshift‐PTC | Eleventh EGF‐like domain | ||||
| P4 | + | Homozygous | 9 | c.820T>G | p.(Cys274Gly) | Missense | Hybrid domain | |
| P5 | − | Compound Heterozygous | 22 | c.2570_2571delGCinsAA | p.(Cys857*) | Frameshift‐PTC | Eleventh EGF‐like domain | |
| 33 | c.4127dup | p.(Arg1377Alafs*27) | Frameshift‐PTC | Third 8‐Cys domain | ||||
| P6 | − | Compound Heterozygous | 11 | c.1342C>T | p.(Arg448*) | Nonsense | First 8‐Cys domain | Callewaert et al. (2013) |
| 31 | c.4115dup | p.(Tyr1373Ilefs*2) | Frameshift‐PTC | Third 8‐Cys domain | ||||
| P7 | + | Homozygous | 19 | c.2408C>A | p.(Ser803*) | Nonsense | Seventh EGF‐like domain | |
| P8 | − | Compound Heterozygous | 28 | c.3661C>T | p.(Gln1221*) | Nonsense | Second 8‐Cys domain | |
| 29 | c.3886C>T | p.(Gln1296*) | Nonsense | Fourteenth EGF‐like domain | ||||
| P9 | Homozygous | 6 | c.780+2T>G | ‐ | Splicing | ‐ | ||
| P10 | + | Homozygous | 11 | c.1263del | p.(Cys422Alafs*352) | Frameshift‐PTC | First 8‐Cys domain | |
| P11 | + | Homozygous | 15 | c.1851C>A | p.(Cys617*) | Nonsense | Second EGF‐like domain | |
| P12 | + | Homozygous | 31 | c.4127dup | p.(Arg1377Alafs*27) | Frameshift‐PTC | Third 8‐Cys domain | |
| P13 | + | Homozygous | 31 | c.4128C>T | p.(Arg1377*) | Nonsense | Third 8‐Cys domain | |
| P14 | + | Homozygous | 26 | c.3556T>C | p.(Cys1186Arg) | Missense | Second 8‐Cys domain | |
| P15 | − | Compound Heterozygous | 7 | c.883+1G>T | ‐ | Splicing | Hybrid domain | Su et al. (2015) |
| 17 | c.2161C>T | p.(Arg721*) | Nonsense | Eighth EGF‐like domain | ||||
| P16 | − | Compound Heterozygous | 18 | c.2377_2378insA | p.(Gly793Glufs*5) | Frameshift‐PTC | Ninth EGF‐like domain | |
| 29 | c.3856T>A | p.(Cys1286Ser) | Missense | Eighteenth EGF‐like domain | ||||
| P17 | − | Compound Heterozygous | 20 | c.2632G>T | p.(Gly878*) | Nonsense | Eleventh EGF‐like domain | |
| 31 | c.4113dup | p.(Ala1372Argfs*3) | Frameshift‐PTC | Third 8‐Cys domain | ||||
| P18 | + | Homozygous | 5 | c.341−1G>C | ‐ | Splicing | First EGF‐like domain |
Exons and mutations numbering are based on transcript NM_003573.2, NP_003564.2; Cons, consanguinity; PTC, premature termination codon.
Concerning the cardiovascular phenotype, peripheral pulmonary artery stenosis (11/17) and pulmonary hypertension (8/17) are common features in addition to arterial septal defect (5/17) and cardiac valve insufficiency (5/17). Other prevalent findings are muscular hypotonia (10/15) and joint hypermobility, usually of small joints (10/17) (Table 1).
LTBP4 encodes a member of the latent transforming growth factor‐beta (TGFβ) binding proteins (LTBPs) that are structurally related to fibrillins. LTBP4 binds the small latent complex (SLC) consisting of TGFβ1 and its latency‐associated peptide. This interaction allows LTBP4 to sequester TGFβ1 and control its activation. LTBP4 also enhances elastogenesis by regulating the incorporation of elastin‐fibulin‐5 complexes into the microfibrillar bundles to form elastic fibers (Callewaert et al., 2013). In addition, LTBP4 stabilizes the TGFβ receptors and loss of LTBP4 results in diminished TGFβ signaling (Callewaert & Urban, 2016; Su et al., 2015). The majority (19/23) of the currently described pathogenic variants (Table 2) is frameshift (8/23), nonsense (8/23), and splice variants (3/23) resulting in a PTC and activation of the nonsense‐mediated mRNA decay (NMD), as demonstrated by qPCR analysis (Callewaert et al., 2013). In the absence of LBTP4 protein, fibulin‐5‐elastin complexes fail to target the microfibrils, resulting in severely impaired elastic fiber formation and altered TGFβ signaling (Callewaert et al., 2013; Dabovic et al., 2015; Urban et al., 2009). One exception, the recurrent c.4127dup variant, was described to result in a C‐terminal truncated LTPB4 protein (p.Arg1377Alafs*27) with a presumed gain‐of‐function mechanism (Callewaert et al., 2013). Furthermore, few missense substitutions (3/23) are reported, which cause the loss of one of the highly conserved cysteine residues located in a TGFβ‐binding (TB) domain or hybrid domain that are implicated in binding of the SLC (Table 2). Loss of these cysteine residues was shown to interfere with the conformation and function both in LTBPs and fibrillin (Jensen, Iqbal, Lowe, Redfield, & Handford, 2009; Lack et al., 2003). The novel pathogenic frameshift variant c.1450del (p.Arg484Glyfs*290) identified in the present study is predicted, with a high degree of confidence, to activate the NMD; however, the real functional outcome was not investigated, since patient's fibroblasts were not available.
5. CONCLUSIONS
Our findings expand both the knowledge of the clinical phenotype and the allelic repertoire of ARCL1C. Further reports are needed to better characterize the LTBP4‐related phenotype and define specific clinical criteria that might facilitate the differential with other ARCL1 subtypes, delineate genotype–phenotype correlations, and collect natural history data for prognostication.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL APPROVAL
The patient's parents provided written informed consent for genetic testing and publication of clinical data and photographs. This study follows the Helsinki Declaration's principles and was carried out from routine diagnostic activity; formal ethics review was therefore not requested.
ACKNOWLEDGMENTS
The authors thank the patient's parents for their cooperation during the diagnostic process and the Fazzo Cusan family for its generous support.
Ritelli M, Cammarata‐Scalisi F, Cinquina V, Colombi M. Clinical and molecular characterization of an 18‐month‐old infant with autosomal recessive cutis laxa type 1C due to a novel LTBP4 pathogenic variant, and literature review. Mol Genet Genomic Med. 2019;7:e735 10.1002/mgg3.735
Marco Ritelli and Francisco Cammarata‐Scalisi are contributed equally to this work.
REFERENCES
- Alazami, A. M. , Al‐Qattan, S. M. , Faqeih, E. , Alhashem, A. , Alshammari, M. , Alzahrani, F. , … Alkuraya, F. S. (2016). Expanding the clinical and genetic heterogeneity of hereditary disorders of connective tissue. Human Genetics, 135(5), 525–540. 10.1007/s00439-016-1660-z [DOI] [PubMed] [Google Scholar]
- Aslanger, A. D. , Altunoglu, U. , Aslanger, E. , Satkin, B. N. , Uyguner, Z. O. , & Kayserili, H. (2014). Newly described clinical features in two siblings with MACS syndrome and a novel mutation in RIN2. American Journal of Medical Genetics. Part A, 164A(2), 484–489. 10.1002/ajmg.a.36277 [DOI] [PubMed] [Google Scholar]
- Basel‐Vanagaite, L. , Sarig, O. , Hershkovitz, D. , Fuchs‐Telem, D. , Rapaport, D. , Gat, A. , … Sprecher, E. (2009). RIN2 deficiency results in macrocephaly, alopecia, cutis laxa, and scoliosis: MACS syndrome. American Journal of Human Genetics, 85(2), 254–263. 10.1016/j.ajhg.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk, D. R. , Bentley, D. D. , Bayliss, S. J. , Lind, A. , & Urban, Z. (2012). Cutis laxa: A review. Journal of the American Academy of Dermatology, 66(5), 842.e1–842.e17. 10.1016/j.jaad.2011.01.004 [DOI] [PubMed] [Google Scholar]
- Callewaert, B. , Su, C.‐T. , Van Damme, T. , Vlummens, P. , Malfait, F. , Vanakker, O. , … De Paepe, A. (2013). Comprehensive clinical and molecular analysis of 12 families with type 1 recessive cutis laxa. Human Mutation, 34(1), 111–121. 10.1002/humu.22165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callewaert, B. L. , & Urban, Z. (2016). LTBP4‐related cutis laxa In: Adam M. P., Ardinger H. H., Pagon R. A., Wallace S. E., Bean L. J. H., Stephens K., & Amemiya A. GeneReviews® (pp. 1993–2018). Seattle, WA: University of Washington, Seattle. [PubMed] [Google Scholar]
- Dabovic, B. , Robertson, I. B. , Zilberberg, L. , Vassallo, M. , Davis, E. C. , & Rifkin, D. B. (2015). Function of latent TGFβ binding protein 4 and fibulin 5 in elastogenesis and lung development. Journal of Cellular Physiology, 230(1), 226–236. 10.1002/jcp.24704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasouki, M. , Markova, D. , Garola, R. , Sasaki, T. , Charbonneau, N. L. , Sakai, L. Y. , & Chu, M.‐L. (2007). Compound heterozygous mutations in fibulin‐4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. American Journal of Medical Genetics. Part A, 143A(22), 2635–2641. 10.1002/ajmg.a.31980 [DOI] [PubMed] [Google Scholar]
- Dimopoulou, A. , Fischer, B. , Gardeitchik, T. , Schröter, P. , Kayserili, H. , Schlack, C. , … Kornak, U. (2013). Genotype‐phenotype spectrum of PYCR9‐related autosomal recessive cutis laxa. Molecular Genetics and Metabolism, 110(3), 352–361. 10.1016/j.ymgme.2013.08.009 [DOI] [PubMed] [Google Scholar]
- Elahi, E. , Kalhor, R. , Banihosseini, S. S. , Torabi, N. , Pour‐Jafari, H. , Houshmand, M. , … Loeys, B. (2006). Homozygous missense mutation in fibulin‐5 in an Iranian autosomal recessive cutis laxa pedigree and associated haplotype. The Journal of Investigative Dermatology, 126(7), 1506–1509. 10.1038/sj.jid.5700247 [DOI] [PubMed] [Google Scholar]
- Fischer, B. , Dimopoulou, A. , Egerer, J. , Gardeitchik, T. , Kidd, A. , Jost, D. , … Kornak, U. (2012). Further characterization of ATP6V0A2‐related autosomal recessive cutis laxa. Human Genetics, 131(11), 1761–1773. 10.1007/s00439-012-1197-8 [DOI] [PubMed] [Google Scholar]
- Gardeitchik, T. , & Morava-Kozicz, E. (2013). Cutis laxa In Brenner's encyclopedia of genetics (2nd edn, pp. 254–257). Cambridge, MA: Elsevier Inc; 10.1016/B978-0-12-374984-0.00367-3 [DOI] [Google Scholar]
- Guernsey, D. L. , Jiang, H. , Evans, S. C. , Ferguson, M. , Matsuoka, M. , Nightingale, M. , … Samuels, M. E. (2009). Mutation in pyrroline‐5‐carboxylatereductase 1 gene in families with cutis laxa type 2. American Journal of Human Genetics, 85(1), 120–129. 10.1016/j.ajhg.2009.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennies, H. C. , Kornak, U. , Zhang, H. , Egerer, J. , Zhang, X. , Seifert, W. , … Mundlos, S. (2008). Gerodermia osteodysplastica is caused by mutations in SCYL1BP1, a Rab‐6 interacting golgin. Nature Genetics, 40(12), 1410–1412. 10.1038/ng.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Q. , Loeys, B. L. , Coucke, P. J. , De Paepe, A. , Mecham, R. P. , Choi, J. , … Urban, Z. (2006). Fibulin‐5 mutations: Mechanisms of impaired elastic fiber formation in recessive cutis laxa. Human Molecular Genetics, 15(23), 3379–3386. 10.1093/hmg/ddl414 [DOI] [PubMed] [Google Scholar]
- Hucthagowder, V. , Morava, E. , Kornak, U. , Lefeber, D. J. , Fischer, B. , Dimopoulou, A. , … Urban, Z. (2009). Loss‐of function mutations in ATP6V0A2 impair vesicular trafficking, tropoelastin secretion and cell survival. Human Molecular Genetics, 18(12), 2149–2165. 10.1093/hmg/ddp148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hucthagowder, V. , Sausgruber, N. , Kim, K. H. , Angle, B. , Marmorstein, L. Y. , & Urban, Z. (2006). Fibulin‐4: A novel gene for an autosomal recessive cutis laxa syndrome. American Journal of Human Genetics, 78(6), 1075–1080. 10.1086/504304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, S. A. , Iqbal, S. , Lowe, E. D. , Redfield, C. , & Handford, P. A. (2009). Structure and interdomain interactions of a hybrid domain: A disulphide‐rich module of the fibrillin/LTBP superfamily of matrix proteins. Structure, 17(5), 759–768. 10.1016/j.str.2009.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornak, U. , Reynders, E. , Dimopoulou, A. , van Reeuwijk, J. , Fischer, B. , Rajab, A. , … Mundlos, S. (2008). Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+‐ATPase subunit ATP6V0A2. Nature Genetics, 40(1), 32–34. 10.1038/ng.2007.45 [DOI] [PubMed] [Google Scholar]
- Lack, J. , O'Leary, J. M. , Knott, V. , Yuan, X. , Rifkin, D. B. , … Downing, A. K. (2003). Solution structure of the third TB domain from LTBP1 provides insight into assembly of the large latent complex that sequesters latent TGF‐beta. Journal of Molecular Biology, 334(2), 281–291. [DOI] [PubMed] [Google Scholar]
- Leao‐Teles, E. , Quelhas, D. , Vilarinho, L. , & Jaeken, J. (2010). De Barsy syndrome and ATP6V0A2‐CDG. European Journal of Human Genetics, 18(5), 526 10.1038/ejhg.2009.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letard, P. , Schepers, D. , Albuisson, J. , Bruneval, P. , Spaggiari, E. , … Guimiot, F. (2018). Severe phenotype of cutis laxa type 1B with antenatal signs due to a novel homozygous nonsense mutation in EFEMP2. Molecular Syndromology, 9(4), 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeys, B. , De Paepe, A. , & Urban, Z. (2001). EFEMP2‐related cutis laxa In Adam M. P., Ardinger H. H., Pagon R. A., Wallace S. E., Bean L. J. H., Stephens K., & Amemiya A. (Eds.), GeneReviews® (pp. 1993–2019). Seattle, WA: University of Washington. [PubMed] [Google Scholar]
- Loeys, B. , Van Maldergem, L. , Mortier, G. , Coucke, P. , Gerniers, S. , … De Paepe, A. (2002). Homozygosity for a missense mutation in fibulin‐5 (FBLN5) results in a severe form of cutis laxa. Human Molecular Genetics, 11(18), 2113–2118. 10.1093/hmg/11.18.2113 [DOI] [PubMed] [Google Scholar]
- Mohamed, M. , Voet, M. , Gardeitchik, T. , & Morava, E. (2014). Cutis laxa. Advances in Experimental Medicine and Biology, 802, 161–184. [DOI] [PubMed] [Google Scholar]
- Morava, E. , Guillard, M. , Lefeber, D. J. , & Wevers, R. A. (2009). Autosomal recessive cutis laxa syndrome revisited. European Journal of Human Genetics, 17(9), 1099–1110. 10.1038/ejhg.2009.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reversade, B. , Escande‐Beillard, N. , Dimopoulou, A. , Fischer, B. , Chng, S. C. , Li, Y. , … Kornak, U. (2009). Mutations in PYCR27 cause cutis laxa with progeroid features. Nature Genetics, 41(9), 1016–1021. 10.1038/ng.413 [DOI] [PubMed] [Google Scholar]
- Ritelli, M. , Chiarelli, N. , Quinzani, S. , Dordoni, C. , Venturini, M. , Pezzani, L. , … Colombi, M. (2014). Identification of two novel ATP6V0A2 mutations in an infant with cutis laxa by exome sequencing. Journal Dermatological Science, 75(1), 66–68. 10.1016/j.jdermsci.2014.04.004 [DOI] [PubMed] [Google Scholar]
- Ritelli, M. , Palit, A. , Giacopuzzi, E. , Inamadar, A. C. , Dordoni, C. , Mujja, A. , … Colombi, M. (2017). Clinical and molecular characterization of a 13‐year‐old Indian boy with cutis laxa type 2B: Identification of two novel PYCR29 mutations by amplicon‐based semiconductor exome sequencing. Journal Dermatological Science, 88(1), 141–143. 10.1016/j.jdermsci.2017.04.010 [DOI] [PubMed] [Google Scholar]
- Skidmore, D. L. , Chitayat, D. , Morgan, T. , Hinek, A. , Fischer, B. , … Robertson, S. P. (2011). Further expansion of the phenotypic spectrum associated with mutations in ALDH18A1, encoding Delta(1)‐pyrroline‐5‐carboxylate synthase (P5CS). American Journal of Medical Genetics. Part A, 155A(8), 1848–1856. [DOI] [PubMed] [Google Scholar]
- Su, C.‐T. , Huang, J.‐W. , Chiang, C.‐K. , Lawrence, E. C. , Levine, K. L. , Dabovic, B. , … Urban, Z. (2015). Latent transforming growth factor binding protein 4 regulates transforming growth factor beta receptor stability. Human Molecular Genetics, 24, 4024–4036. 10.1093/hmg/ddv139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tekedereli, I. , Demiral, E. , Gokce, I. K. , Esener, Z. , Camtosun, E. , & Akinci, A. (2019). Autosomal recessive cutis laxa: A novel mutation in the FBLN5 gene in a family. Clinical Dysmorphology, 28(2), 63–65. 10.1097/MCD.0000000000000258 [DOI] [PubMed] [Google Scholar]
- Urban, Z. , Hucthagowder, V. , Schürmann, N. , Todorovic, V. , Zilberberg, L. , Choi, J. , … Davis, E. C. (2009). Mutations in LTBP4 cause a syndrome of impaired pulmonary, gastrointestinal, genitourinary, musculoskeletal, and dermal development. American Journal of Human Genetics, 85, 593–605. 10.1016/j.ajhg.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme, T. , Gardeitchik, T. , Mohamed, M. , Guerrero‐Castillo, S. , Freisinger, P. , Guillemyn, B. , … Wevers, R. A. (2017). Mutations in ATP6V1E1 or ATP6V1A cause autosomal‐recessive Cutis laxa. American Journal of Human Genetics, 100(2), 216–227. 10.1016/j.ajhg.2016.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Maldergem, L. , & Loeys, B. (2009). FBLN5‐related cutis laxa InAdam M. P., Ardinger H. H., Pagon R. A., Wallace S. E., Bean L. J. H., Stephens K., & Amemiya A. (Eds.), GeneReviews® (pp. 1993–2019). Seattle, WA: University of Washington. [PubMed] [Google Scholar]
- Zampatti, S. , Castori, M. , Fischer, B. , Ferrari, P. , Garavelli, L. , Dionisi‐Vici, C. , … Brancati, F. (2012). De Barsy syndrome: A genetically heterogeneous autosomal recessive cutis laxa syndrome related to P5CS and PYCR36 dysfunction. American Journal of Medical Genetics. Part A, 158A(4), 927–931. 10.1002/ajmg.a.35231 [DOI] [PubMed] [Google Scholar]


