Abstract
Lipid storage droplet protein 5 (LSDP5) is specifically expressed in tissues with high oxidative metabolism such as liver and heart. The present study aimed to explored the role of LSDP5 in sodium palmitate-induced lipotoxicity in LO2 normal human liver cells. LO2 cells were treated with various concentrations of sodium palmitate (25, 50, 75, 100, 125 and 150 µmol/l) for 12, 24 and 48 h, and cell viability was determined by Cell Counting Kit-8. Subsequently, LO2 cells were exposed to 100 µmol/l sodium palmitate for 48 h to induce lipotoxicity (Model). Lipotoxicity Model LO2 cells were also transfected with pCMV5-LSDP5 overexpression vector, and reactive oxygen species (ROS) production, mitochondrial membrane potential (MMP) and apoptotic rates were measured. The contents of non-esterified fatty acid (NEFA), malondialdehyde (MDA) and superoxide dismutase (SOD) were also measured. The expression levels of LSDP5, and apoptosis-, mitochondrial-, lipid metabolism-related factors were detected using reverse transcription-quantitative polymerase chain reaction and western blot assays. The results indicated that sodium palmitate exposure inhibited cell viability and induced lipotoxicity in LO2 cells. LSDP5 overexpression decreased ROS and apoptotic rates, and reduced NEFA and MDA content. LSDP5 transfection rescued the loss of MMP and elevated SOD content in lipotoxicity Model LO2 cells. In addition, LSDP5 upregulated the expression levels of B-cell lymphoma-2, acetyl-CoA carboxylase1/2 and fatty acid synthase (Fas), whereas the expression levels of activated-caspase-3, Bcl-2-associated X protein, cytochrome c, cytochrome c oxidase subunits IV, carnitine palmitoyltransferase 1a and peroxisome proliferator-activated receptors α levels were downregulated. LSDP5 may produce a protective effect on sodium palmitate-induced lipotoxicity in LO2 cells by regulating lipid metabolism-related factors.
Keywords: lipid storage droplet protein 5, sodium palmitate, lipotoxicity, human normal liver cells
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a clinicopathological syndrome that is characterized by fatty deposits in the liver cells; it is caused by genetic, environmental and metabolic stress-associated factors (1). In China, the incidence of NAFLD is increasing, and NAFLD is one of the major diseases that seriously threatens public health (2); NAFLD is able to cause hepatocellular carcinoma (3,4). The prevalence of NAFLD is 17–33% in the Americas and the prevalence of nonalcoholic steatohepatitis (NASH), a type of NAFLD, accounts for ~10–20%. Noticeably, the incidence rate of cirrhosis among patients with NASH, within 10–15 years, reaches as high as 15–25% (5,6). At present, it is believed that the core factor leading to NASH is lipotoxicity (7).
A number of studies have demonstrated that free fatty acid (FFA) produce a toxic effect, which contributed to liver cell dysfunction and apoptosis (8–10). When the level of FFA exceeds the oxidative and metabolic capacity of the liver, lipids accumulate in the liver and inflammatory factors are released, subsequently initiating apoptotic signals, increasing the sensitivity of hepatocytes to inflammatory reactions and various injury factors. This damage will ultimately lead to hepatocellular dysfunction or death that is called lipotoxicity (11,12). In liver cells, excessive amounts of fatty acids are mainly stored in lipid droplets (LD) (13); therefore, the abnormal metabolism of LD is closely related to lipotoxicity.
LD are a major storage site for neutral lipids in cells, and are widely found in bacteria, fungi, plants and animals (14,15). LD is considered a simple structure of energy storage; however, it has been reported that LD serves a role in diseases, including obesity and fatty liver (16). Structurally, LD is composed of neutral lipid and phospholipid, which contains many LD-related proteins embedded in the phospholipid monolayer; according to previous studies, these proteins serve a vital role in maintaining the stability and metabolic processes of LD (17,18). Lipid storage droplet protein 5 (LSDP5; also known as myocardial lipid droplet protein or oxidative tissue-enriched PAT protein) is mainly distributed in tissues with high oxidative metabolism, such as liver, heart and skeletal muscle (19–21). In addition, LSDP5 is a member in PAT family of lipid droplet proteins (19). A number of previous studies have reported PAT family proteins serve a regulatory role in lipid droplet metabolism and in maintaining lipid balance in cells (22,23). However, the specific function and mechanism of LSDP5 in hepatocellular lipid metabolism is still not fully understood.
In the present study, it was hypothesized that LSDP5 may produce a protective effect on the liver cells with lipotoxicity by regulating lipid metabolism. An in vitro model of lipotoxicity was established by treating LO2 normal human liver cells with sodium palmitate. The effects of LSDP5 on lipid metabolism, oxidative stress, apoptosis and mitochondrial function in sodium palmitate-induced LO2 cells were examined.
Materials and methods
Cell culture
LO2 normal human liver cells were purchased from Procell Life Technology Co., Ltd. (Wuhan, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Huayueyang Biology, Beijing, China) that contained 10% foetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA) and 100 U/ml streptomycin and 100 µg/ml penicillin (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) in a Forma CO2 incubators (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 5% CO2 at 37°C.
Preparation of sodium palmitate solution
Solid sodium palmitate was purchased from Heowns Biochemical Technology Co., Ltd. (Tianjin, China). The sodium palmitate was dissolved in BSA solution, which was subsequently diluted in DMEM; the final concentrations of sodium palmitate were 25, 50, 75, 100, 125 and 150 µmol/l.
Cell transfection and treatment
The pCMV5-LSDP5 and pCMV5-NC plasmids were obtained from Genewiz Inc. (Suzhou, China). LO2 cells (1×105 ml) were transfected with 2 µg of pCMV5-LSDP5 or pCMV5-NC plasmid using Lipofectamine® 2000 transfection reagent (Thermo Fisher Scientific, Inc.). The experiments comprised four groups: i) Control group, which was treated with 0.1% PBS; ii) Model group, which was treated with 100 µmol/l sodium palmitate; iii) Model + negative control (M + NC) group, which was treated with 100 µmol/l sodium palmitate transfected with pCMV5-NC plasmid and; and iv) Model + LSDP5 group (M + LSDP5), which was transfected with pCMV5-LSDP5 plasmid and treated with 100 µmol/l sodium palmitate at 37°C for 24 h.
Cell Counting Kit-8 (CCK-8) viability assay
CCK-8 (Beijing Saichi Biological Technology Co., Ltd., Beijing, China) was used to detect cell viability, following the manufacturer's protocol. Briefly, cells (3.5×103 cells/well) were cultured in 96-well plates in a humidified incubator with 5% CO2 at 37°C. Following a protocol from a previous study (24), cells were treated with sodium palmitate at 25, 50, 75, 100, 125 or 150 µmol/l and incubated for 12, 24 or 48 h. Following incubation, CCK-8 reagent was added into each well and the cells were incubated in humidified environment with 5% CO2 at 37°C for 2 h. Absorbance was measured at 450 nm using a SpectraMax iD3 microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Non-esterified fatty acid (NEFA) assay
The content of NEFA was measured using a NEFA Detection kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan), following the manufacturer's protocol. Briefly, cells were treated with drugs and grouped as aforementioned. Following treatments, 22 µM [9,10-3H]oleate was added to cells for 4 h. Cells were subsequently washed two times with PBS and cultured in DMEM for 4 h. The cells were digested with 0.25% EDTA-trypsin (Beijing Solarbio Science & Technology Co., Ltd.), resuspended in DMEM and centrifuged with 1,000 × g for 10 min. The supernatant was transferred into a clean centrifuge tube. Lipid was extracted from supernatant using N-hexane: Isopropanol solution (3:2) and a thin layer chromatography silica gel plate (25). The silica gel of containing NEFA fraction was scraped from the silica gel plate and dissolved in N-hexane: Isopropanol solution (3:2). Scintillation solution was added and the 3H-labeled NEFA protein content in the cells was measured using a L6500 liquid scintillation counter (Beckman Coulter, Inc., Brea, CA, USA). NEFA protein concentration was detected using a Bradford protein assay kit (Beyotime Institute of Biotechnology, Haimen, China).
Cell apoptosis assay
An Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis Detection kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was used to determine the rates of cell apoptosis, following the manufacturer's protocol. Briefly, cells (5×104 cells/well) were cultured overnight in 6-well plates in a humidified incubator with 5% CO2 at 37°C. Cells were treated with drugs and grouped as aforementioned. The cells were washed two times with cold PBS, centrifuged at 300 × g at 4°C for 5 min and resuspended in 1X Binding Buffer. Subsequently, the cells were stained with Annexin V-FITC/PI staining solution in the dark at room temperature for 10–15 min. Apoptotic cells were detected using an A28999 flow cytometer (Thermo Fisher Scientific, Inc.) and analyzed with BD CellQuest™ Pro Software version 1.2; BD Biosciences, San Jose, CA, USA).
Reactive oxygen species (ROS) assay
ROS activity was determined with a ROS assay kit (Beyotime Institute of Biotechnology), following the manufacturer's protocol. Briefly, cells were treated with drugs and grouped, as aforementioned. Subsequently, the cells were incubated with 2′,7′-dichlorofluorescein diacetate at 37°C for 25 min. The cells were washed three times with PBS, and ROS was detected using a flow cytometer and analyzed by Summit Software V4.3 (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA).
Mitochondrial membrane potential (MMP) assay
MMP was measured using a JC-1 kit (Beyotime Institute of Biotechnology), following the manufacturer's protocol. Briefly, cells were treated with drugs and grouped, as aforementioned. Following treatments, cells were digested with 0.25% EDTA-trypsin, re-suspended in DMEM and stained with JC-1 solution at 37°C for 20 min. Subsequently, cells were centrifuged at 600 × g at 4°C for 3 min. The supernatant was discarded and cells were washed two times with PBS. MMP was detected by a flow cytometer with analysis software version 1.2 (BD CellQuest™ Pro Software; BD Biosciences).
ELISA
Levels of oxidative stress-related factors were respectively measured using an MDA Elisa kit (cat. no. E-EL-0060c, Elabscience, Wuhan, China) and an SOD ELISA kit (cat. no. S0109; Beyotime Institute of Biotechnology), following the protocols of the manufacturer. Briefly, cells were treated with drugs and grouped, as aforementioned. Following treatments, cells were digested with 0.25% EDTA-trypsin, centrifuged at 800 × g for 6 min. The cells (1×106 ml) were re-suspended in DMEM and added to wells at 37°C for 24 h. A total of 100 µl biotinylated antibody (included in kits) was added and the cells were incubated at 37°C for 30 min. Cells were washed three times with PBS, followed by the addition of the termination solution into wells. Absorbance was measured at 450 nm using a microplate reader (SkanIt software V3.1, Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Cells were treated with drugs and grouped, as aforementioned. Total RNA was extracted using TRIzol® Reagent (Takara Biotechnology Co., Ltd., Dalian, China). RNA (1 µg) was used to synthesize cDNA using an RT Master Mix kit (Takara Biotechnology Co., Ltd.) and the following parameters: 85°C for 15 min, followed by 4°C for 10 min. SYBR Premix Taq II kit (Takara Biotechnology Co., Ltd.) was used for amplifying cDNA, and the 50 µl reactions were set up as follows: 25 µl SYBR-Green Mix, 1 µl forward/reverse primers (Table I), 19 µl ddH2O and 4 µl cDNA. PCR thermocycling conditions were: 85°C for 15 min; followed by 30 cycles at 85°C for 20 sec and 65°C for 45 sec; and 85°C for 20 sec, at 37°C for 2 min. β-actin was used as a loading control for the normalization of expression. The formula 2−ΔΔCq was used to calculate relative mRNA expression levels (26).
Table I.
Sequences of forward and reverse primers used for reverse transcription-quantitative polymerase chain reaction.
| Gene | Primer sequence (5′→3′) |
|---|---|
| LSDP5 | F: GTGATCAGACAGCTCAGGACCCT |
| R: CGATTCACCACATTCTGCTG | |
| Active-caspase-3 | F: TGCCCAAGTGACTGACATCA |
| R: CATCCCCATTGACTGTGCAG | |
| Bax | F: GACCCGGTGCCTCAGGATGC |
| R: AGGTCAGCTCATCATGCTTG | |
| Bcl-2 | F: GTGGAGGAGCTCTTCAGGGA |
| R: GTCTGTGTCCACGGCGGCAA | |
| Cytc | F: CCAAATCTCCACGGTCTGTTC |
| R: ATCAGGGTATCCTCTCCCCAG | |
| Cox IV | F: CGGCGTGACTACCCCTTG |
| R: TGAGGGATGGGGCCATACA | |
| CPT1a | F: TGTCCAAGTATCTGGCAGTCG |
| R: CATAGCCGTCATCAGCAACC | |
| ACC1 | F: TTTGTTTGGTCGTGACTG |
| R: CCCAGCACTCACATAACC | |
| ACC2 | F: GACGCCCGAGGATCTGAAG |
| R: GGGACAGGGACGTACTGATC | |
| Fas | F: ATGACAGGAGATGGAAGG |
| R: CTGACTTCCACCAGCAGC | |
| PPARα | F: CGGCGTGACTACCCCTTG |
| R: TGAGGGATGGGGCCATACA | |
| β-actin | F: GACTCCTATGTGGGTGACGA |
| R: ACGGTTGGCCTTAGGGTTCA |
ACC, acetyl-co A carboxylase; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; Cox IV, cytochrome c oxidase subunits IV; CPT1a, carnitine palmitoyltransferase 1a; Cytc, cytochrome c; F, forward; Fas, fatty acid synthase; LSDP5, lipid storage droplet protein 5; PPARα, peroxisome proliferator-activated receptors α; R, reverse.
Western blot assay
Cells were treatment as aforementioned and subsequently washed twice with PBS, lysed in Radioimmunoprecipitation Assay Buffer (high) (Beijing Solarbio Science & Technology Co., Ltd.) to obtain protein extracts. Protein concentrations were detected by a Bradford protein assay kit. Proteins (20 µg/lane) were separated by 10% SDS-PAGE. Proteins were transferred to polyvinylidene fluoride membranes (Hangzhou Renomem Technology Co., Ltd., Hangzhou, China). Subsequently, the membranes were blocked in 5% non-fat milk for 2 h at room temperature. The membranes were incubated with primary antibodies against LSDP5 (1:1,000; cat. no. ab222811; Abcam, Cambridge, UK), active-caspase-3 (1:2,000; cat. no. AF-605-NA; R&D Systems, Inc., Minneapolis, MN, USA), B-cell lymphoma-2 (Bcl-2; 1:800; cat. no. AF810; R&D Systems, Inc.), Bcl-2-associated X protein (Bax; 1:1,000; cat. no. AF820; R&D Systems, Inc.,), cytochrome c (Cytc; 1:1,000; cat. no. MAB897; R&D Systems, Inc.), Cytc oxidase subunits IV (Cox IV; 1:1,200; cat. no. MAB6980; R&D Systems, Inc.), carnitine palmitoyltransferase 1a (CPT1a; 1:1,200; cat. no. ab83862; Abcam), acetyl-co A carboxylase1 (ACC1; 1:1,000; cat. no. 4190; Cell Signaling Technology, Inc., Danvers, MA, USA), ACC2 (1:1,000; cat. no. 8578; Cell Signaling Technology, Inc.), anti-Fas (1:1,000, cat. no. 3180; Cell Signaling Technology, Inc.), peroxisome proliferator-activated receptors α (PPARα; 1:100; cat. no. PP-H0723-00) and β-actin (1:2,000; cat. no. MAB8969) on the rocking table at 4°C overnight. Subsequently, the membranes were washed 2–3 times with PBS and 0.05% Tween-20 for 6 min, followed by incubation with the following horseradish peroxidase-conjugated secondary antibodies at 37°C for 60 min: Mouse anti-goat immunoglobulin G (1:7,000; cat. no. 31107; Invitrogen; Thermo Fisher Scientific, Inc.), anti-Mouse IgG Secondary Antibody (1:1,000; cat. no. HAF007; R&D Systems, Inc.) and mouse anti-rabbit (1:7,000; cat. no. 31213; Invitrogen; Thermo Fisher Scientific, Inc.). The membranes were washed 2–3 times with PBST, and the proteins were visualized using Enhanced Chemiluminescent Detection Reagent (Taixin Biotechnology, Beijing, China). β-actin was treated as a loading control for normalization. The blot density was analyzed by Quantity One software version 4.6.2 (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data were analysed by SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA); results were expressed as the mean ± standard deviation. The differences between groups were analysed using one-way analysis of variance followed by a Dunnett's post-test. Each experiment was independently repeated in triplicate. P<0.05 was considered to indicate a statistically significant difference.
Results
LSDP5 increases the viability of sodium palmitate-treated LO2
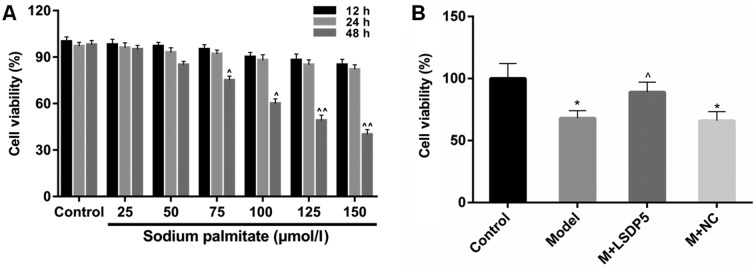
CCK-8 analysis was performed to examine cell viability to assess the lipotoxic effects of sodium palmitate on LO2 cells. The data revealed that viability remained relatively stable when LO2 cells were treated with sodium palmitate at low doses (25 and 50 µmol/l) for a short period of treatment (12 and 24 h; Fig. 1A). Viability was significantly inhibited in cells treated with 125 and 150 µmol/l of sodium palmitate for 48 h, compared with untreated control cells at the same time (P<0.01; Fig. 1A). Treatment with 100 µmol/l sodium palmitate for 48 h also significantly inhibited viability, compared with the respected Control cells (P<0.05; Fig. 1A), and this concentration and incubation time was used in subsequent experiments as the lipotoxicity Model.
Figure 1.
Sodium palmitate represses the viability of LO2 cells. (A) CCK-8 assay was performed to determine the effects of various concentrations of sodium palmitate (25, 50, 75, 100, 125 and 150 µmol/l) on LO2 human liver cells treated for 12, 24 and 48 h. ^P<0.05 and ^^P<0.01 vs. Control. (B) CCK-8 assay was used to measure the effect of LSDP5 on cell viability in LO2 cells treated with 100 µmol/l sodium palmitate for 48 h (Model). *P<0.05 vs. Control; ^P<0.05 vs. Model. LSDP5, lipid storage droplet protein 5; M, model; NC, negative control.
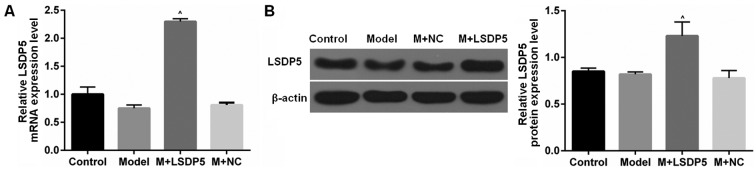
Model cells were transfected with LSDP5 overexpression vectors, which resulted in a significant increase in viability compared with untransfected Model cells (P<0.05; Fig. 1B). RT-qPCR and western blot assays were conducted to examine the LSDP5 mRNA and protein expressions levels, respectively, in LO2 Model cells transfected with pCMV5-LSDP5 plasmid (Fig. 2). The expression levels of LSDP5 mRNA and protein were significantly increased in cells transfected with pCMV5-LSDP5 (both P<0.05; Fig. 2A and B, respectively), which indicated successful transfection of the LSDP5 overexpression vector in LO2 cells. Notably, the expression levels of LSDP5 were slightly reduced in untransfected Model cells, compared with Control cells (Fig. 2).
Figure 2.
LSDP5 expression in LO2 cells. (A and B) Experiments were divided into Control group (0.1% PBS treatment), Model group (100 µmol/l sodium palmitate treatment), NC group (Model cells transfected with pCMV5-NC plasmid) and LSDP5 group (Model cells transfected with pCMV5-LSDP5 overexpression plasmid), and the (A) mRNA and (B) protein expression levels were determined reverse transcription-quantitative polymerase chain reaction and western blotting, respectively; β-actin served as an internal control and for normalization. ^P<0.05 vs. Model. LSDP5, lipid storage droplet protein 5; M, model; NC, negative control.
LSDP5 suppresses the activity of oxidative stress in LO2 lipotoxicity Model cells
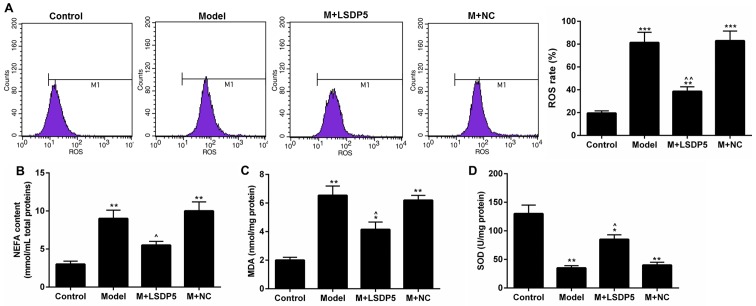
Compared with Control cells, Model cells exhibited a significantly increased rate of ROS production (P<0.001; Fig. 3A). Although the contents of NEFA and MDA were increased (P<0.001; Fig. 3B and C, respectively), SOD content was decreased (P<0.001; Fig. 3D) in Model cells compared with Control. Conversely, LSDP5 overexpression suppressed the oxidative stress of LO2 Model cells; it was also demonstrated that LSDP5 reduced the ROS level and NEFA and MDA content, and increased SOD, compared with the untransfected Model group (P<0.05; Fig. 3).
Figure 3.
LSDP5 suppresses the activity of oxidative stress in LO2 lipotoxicity Model cells. (A) The rate of ROS production was measured using an ROS detection kit. (B) NEFA content was detected by NEFA detection kit. (C and D) The contents of (C) MDA and (D) SOD were analysed by ELISA. *P<0.05, **P<0.01 and ***P<0.001 vs. Control; ^P<0.05 and ^^P<0.01 vs. Model. LSDP5, lipid storage droplet protein 5; M, model; MDA, malondialdehyde; NC, negative control; NEFA, non-esterified fatty acid; SOD, superoxide dismutase.
LSDP5 reduces apoptotic rates of LO2 Model cells
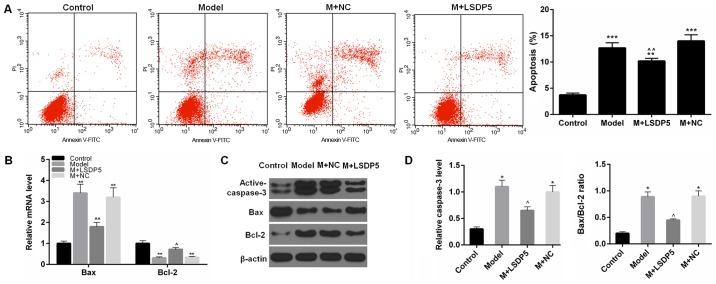
The results demonstrated that the rate of apoptosis was significantly increased (P<0.001; Fig. 4A), and the expression levels of active-caspase-3 and Bax were also significantly increased in Model cells compared with Control, whereas Bcl-2 expression levels were reduced compared with Control (P<0.05 or P<0.01; Fig. 4B-D). In LSDP5-transfected Model cells, apoptotic rates were significantly decreased, expression levels of activated-caspase-3 and Bax were reduced, and Bcl-2 expression levels were increased compared with the Model group (P<0.05 or P<0.01; Fig. 4).
Figure 4.
LSDP5 reduces apoptosis in LO2 lipotoxicity Model cells. (A) Apoptosis was detected by Annexin V-FITC/PI apoptosis detection kit. (B) mRNA expression levels of Bax and Bcl-2 were measured by reverse transcription-quantitative polymerase chain reaction. (C and D) Protein expression levels of active-caspase-3, Bax and Bcl-2 were measured by western blot assay; β-actin was used as a loading control and for normalization. *P<0.05, **P<0.01 and ***P<0.001 vs. Control; ^P<0.05 and ^^P<0.01 vs. Model. Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; FITC, fluorescein isothiocyanate; LSDP5, lipid storage droplet protein 5; M, model; NC, negative control; PI, propidium iodide.
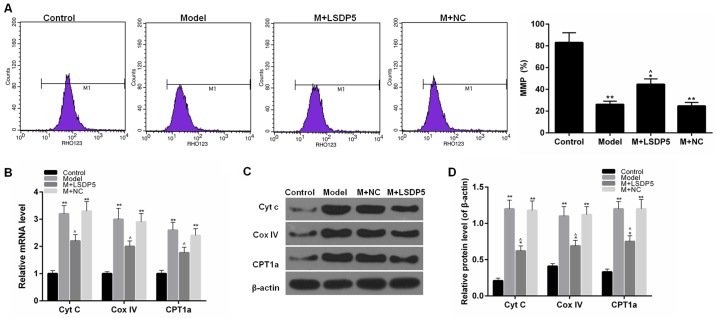
LSDP5 reduces mitochondrial damage in Model cells
To examine mitochondrial activity, the rate of MMP and the expression levels of Cytc, Cox IV and CPT1A. The data indicated that sodium palmitate reduced the MMP rate of LO2 cells and increased the expression levels of Cytc, Cox IV and CPT1A, compared with Control. However, LSDP5 overexpression increased the rate of MMP and reduced Cytc, Cox IV and CPT1A expression levels compared with the untransfected Model group (P<0.05; Fig. 5).
Figure 5.
LSDP5 reduces mitochondrial damage in LO2 lipotoxicity Model. (A) MMP rates were detected by JC-1 kit. (B) mRNA expression levels of Cytc, Cox IV and CPT1a were measured by reverse transcription-quantitative polymerase chain reaction. (C and D) Protein expression levels of Cytc, Cox IV and CPT1a were measured by western blot assay; β-actin was used as a loading control and for normalization. *P<0.05 and **P<0.01 vs. Control; ^P<0.05 vs. Model. Cox IV, cytochrome c oxidase subunit IV; CPT1a, carnitine palmitoyltransferase 1a; Cytc, cytochrome c; LSDP5, lipid storage droplet protein 5; M, model; MMP, mitochondrial membrane potential; NC, negative control.
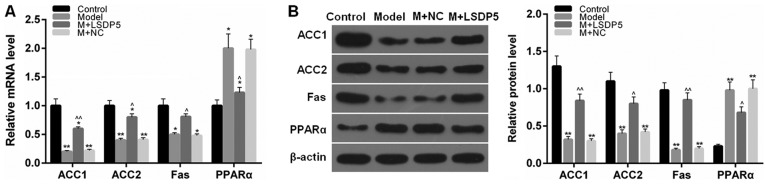
LSDP5 regulates lipid metabolism-related factors in LO2 Model cells
RT-qPCR and western blotting assays were performed to determine the mRNA and protein expression levels, respectively, of lipid metabolism-related factors. The data indicated that the expression levels of ACC1, ACC2 and Fas were significantly decreased, whereas PPARα expression levels were increased following sodium palmitate treatment, compared with Control cells (P<0.05 or P<0.01; Fig. 6). However, LSDP5 overexpression upregulated the expression levels of ACC1, ACC2 and Fas, and downregulated PPARα expression levels, compared with the untransfected Model group (P<0.05 or P<0.01; Fig. 6).
Figure 6.
LSDP5 regulates lipid metabolism-related factors in LO2 lipotoxicity Model cells. (A and B) ACC1, ACC2, Fas and PPARα (A) mRNA and (B) protein expression levels were assessed by reverse transcription-quantitative polymerase chain reaction and western blot analysis, respectively; β-actin served as an internal control and for normalization. *P<0.05 and **P<0.01 vs. Control; ^P<0.05 and ^^P<0.01 vs. Model. ACC, acetyl-co A carboxylase1; Fas, fatty acid synthase; LSDP5, lipid storage droplet protein 5; M, model; NC, negative control.
Discussion
In the clinic, increased FFA levels in patients with NASH have been associated with the severity of the disease (27). Previous studies have demonstrated that sodium palmitate inhibited the viability of cells and induced lipotoxicity, which led to lipoapoptosis in liver cells (24,28). In addition, oxidative stress produced by NEFA oxidation was reported to be the main mechanism of liver lipotoxicity (29,30). Mitochondrial damage is able to mediate apoptosis, which serves important roles in the pathogenesis of NASH (31). Based on these findings, the present study used sodium palmitate-treated LO2 cells to establish the lipotoxicity model. In this study, sodium palmitate was demonstrated to increase the NEFA content, and it contributed to the accumulation of oxidative stress and apoptosis, as well as mitochondrial damage in LO2 cells.
LSDP5 is a member of PAT family, which are specifically expressed in tissues with high oxidative metabolism. A previous report indicated that the overexpression of LSDP5 increased lipid accumulation, while that siRNA-LSDP5 promoted fatty acid oxidation in liver cells (32). Though LSDP5 deletion significantly reduces LD content, it increases ROS content in cardiomyocytes (33). Therefore, LSDP5 may produce a certain effect on fatty acid-induced lipotoxicity. LSDP5 was observed to be overexpressed in transfected with pCMV5-LSDP5 and treated with sodium palmitate LO2 cells; LSDP5 overexpression decreased NEFA content in Model LO2 cells. It was suggested that overexpression of LSDP5 may inhibit lipolysis and the accompanied liver injury. A previous study reported that the knockdown of LSDP5 may stimulate lipolysis and increase the level of fatty-acid β oxidation (32), which may be a primary cause of liver injury (7). These data suggested that knockdown of LSDP5 accelerated the lipolysis and induced liver injury, which was consistent with the present study results. However, although these previous studies reported that LSDP5 promoted lipid accumulation, the present study considered that it was relative to the lipolysis by LSDP5 knockdown. Taken together, the present study results indicated that LSDP5 reduced the effect of lipotoxicity by regulating lipid metabolism-related factors.
A previous study reported that ROS may attack unsaturated fatty acids in a biofilm and enable lipid peroxidation to form end products such as MDA (34), an important index of cellular oxidative damage. SOD is also an antioxidant substance (35). The present study results demonstrated that LSDP5 reduced ROS and MDA content, and increased SOD content, which indicated that LSDP5 may suppress oxidative stress in sodium palmitate-treated LO2 cells.
Hepatocellular apoptosis was also reported to serve a vital role in the development of NASH (36,37). It is known that apoptosis may cause the change of the apoptotic protein. Bcl-2 is an indispensable anti-apoptotic gene, and Bax belongs to the Bcl-2 gene family promoting apoptosis (38). Active-caspase-3 also contributes to and can stimulate apoptosis (39). The present data indicated that LSDP5 overexpression inhibited apoptosis in lipotoxicity Model cells, and upregulated Bcl-2 expression and downregulated the levels of activated-caspase-3 and Bax. In addition, LSDP5 increased the rate of MMP and increased the expression levels of CPT1a; however, the expression levels of Cytc and Cox IV were reduced. These results suggested that LSDP5 may reduce apoptotic rates and mitochondrial damage in lipotoxicity Model LO2 cells. The results were in support of a literature that the number of mitochondria increased in LSDP5-deficient cells (32).
A previous study reported that exogenous LSDP5 increased the content of lipid in oleic acid-induced COS7 and OP9 liver cells (40). Another study suggested that LSDP5 may function to regulate the interaction between lipase and LD, and such a function is similar to perilipin, which regulated the lipolytic activity of adipocyte triglyceride lipase (41). In addition, PPARα is a key regulator of lipid oxidation; LSDP5 is downstream of PPARα and the upregulation of PPARα may significantly increase LSDP5 expression in hepatocytes (18). ACC1, ACC2 and Fas are related to fatty acid synthesis (42). Results from the present study demonstrated that the overexpression of LSDP5 in LO2 Model cells upregulated the expression levels of ACC1, ACC2 and Fas, and downregulated PPARα expression levels; these data are consistent with a previous study, which reported that the PPARα activity was increased by small interfering (si)RNA-mediated LSDP5 knockdown (32). In addition, the expression levels of ACC1 and Fas were not altered by si-LSDP5 in that study. Considering these findings, it was hypothesized that the downregulation of ACC1 and Fas may be a stress response when the cells were stimulated with high concentration of sodium palmitate; in this condition, the fatty acid synthesis may be inhibited.
However, the present study data were acquired using only one cell line in vitro, which was a limitation. Thus, to investigate the role of LADP5 in other cell lines or in vivo may further confirm the results from this study.
To conclude, sodium palmitate induced lipotoxicity in LO2 cells. However, overexpression of LSDP5 in lipotoxicity Model LO2 cells reduced the activity of oxidative stress and mitochondrial damage, and inhibited apoptosis by regulating lipid metabolism-related factors. These results demonstrated that LSDP5 may produce a protective effect on sodium palmitate-induced lipotoxicity in LO2 cells. However, the specific signalling pathway(s) of LSDP5 regulation of lipid metabolism remains to be further examined. This study provided a molecular basis for understanding lipotoxicity in liver, as well as a indicated a possible candidate target for treating liver lipotoxicity.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analysed data sets generated during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XM and TZ made substantial contributions to the design of the present study. Data acquisition and interpretation was conducted by FC, KY and KJ. TZ and FC critically revised the manuscript for important intellectual content. All authors approved the final version of the manuscript. KY and KJ aggree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zheng X, Gong L, Luo R, Chen H, Peng B, Ren W, Wang Y. Serum uric acid and non-alcoholic fatty liver disease in non-obesity Chinese adults. Lipids Health Dis. 2017;16:202. doi: 10.1186/s12944-017-0531-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan JG, Farrell GC. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50:204–210. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Angulo P. GI epidemiology: Nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2007;25:883–889. doi: 10.1111/j.1365-2036.2007.03246.x. [DOI] [PubMed] [Google Scholar]
- 4.de Alwis NM, Day CP. Non-alcoholic fatty liver disease: The mist gradually clears. J Hepatol. 2008;48(Suppl 1):S104–S112. doi: 10.1016/j.jhep.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Fan JG, Saibara T, Chitturi S, Kim BI, Sung JJ, Chutaputti A, Asia-Pacific Working Party for NAFLD What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol. 2007;22:794–800. doi: 10.1111/j.1440-1746.2007.04952.x. [DOI] [PubMed] [Google Scholar]
- 6.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: From steatosis to cirrhosis. Hepatology. 2006;43(Suppl 1):S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 7.Garbarino J, Sturley SL. Saturated with fat: New perspectives on lipotoxicity. Curr Opin Clin Nutr Metab Care. 2009;12:110–116. doi: 10.1097/MCO.0b013e32832182ee. [DOI] [PubMed] [Google Scholar]
- 8.Weiss TS, Lupke M, Ibrahim S, Buechler C, Lorenz J, Ruemmele P, Hofmann U, Melter M, Dayoub R. Attenuated lipotoxicity and apoptosis is linked to exogenous and endogenous augmenter of liver regeneration by different pathways. PLoS One. 2017;12:e0184282. doi: 10.1371/journal.pone.0184282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapoor A, Sanyal AJ. Endoplasmic reticulum stress and the unfolded protein response. Clin Liver Dis. 2009;13:581–590. doi: 10.1016/j.cld.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Bellanti F, Mitarotonda D, Tamborra R, Blonda M, Iannelli G, Petrella A, Sanginario V, Iuliano L, Vendemiale G, Serviddio G. Oxysterols induce mitochondrial impairment and hepatocellular toxicity in non-alcoholic fatty liver disease. Free Radic Biol Med. 2014;75(Suppl 1):S16–S17. doi: 10.1016/j.freeradbiomed.2014.10.594. [DOI] [PubMed] [Google Scholar]
- 11.Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: Lipotoxicity. Apoptosis. 2009;14:1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 12.Unger RH, Orci L. Lipoapoptosis: Its mechanism and its diseases. Biochim Biophys Acta. 2002;1585:202–212. doi: 10.1016/S1388-1981(02)00342-6. [DOI] [PubMed] [Google Scholar]
- 13.McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease. Clin Liver Dis. 2004;8(viii):521–533. doi: 10.1016/j.cld.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Murphy DJ. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res. 2001;40:325–438. doi: 10.1016/S0163-7827(01)00013-3. [DOI] [PubMed] [Google Scholar]
- 15.Zehmer JK, Huang Y, Peng G, Pu J, Anderson RG, Liu P. A role for lipid droplets in inter-membrane lipid traffic. Proteomics. 2009;9:914–921. doi: 10.1002/pmic.200800584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng F, Guo D. Lipid droplets, potential biomarker and metabolic target in glioblastoma. Intern Med Rev (Wash DC) 2017 May 3; doi: 10.18103/imr.v3i5.443. (Epub ahead of print). doi: 10.18103/imr.v3i5.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bartz R, Zehmer JK, Zhu M, Chen Y, Serrero G, Zhao Y, Liu P. Dynamic activity of lipid droplets: Protein phosphorylation and GTP-mediated protein translocation. J Proteome Res. 2007;6:3256–3265. doi: 10.1021/pr070158j. [DOI] [PubMed] [Google Scholar]
- 18.Brasaemle DL. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: Stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, Nebb HI. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta. 2007;1771:210–227. doi: 10.1016/j.bbalip.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, Yao-Borengasser A, Rasouli N, Kern PA, et al. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes. 2006;55:3418–3428. doi: 10.2337/db06-0399. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi T, Matsushita S, Motojima K, Hirose F, Osumi T. MLDP, a novel PAT family protein localized to lipid droplets and enriched in the heart, is regulated by peroxisome proliferator-activated receptor alpha. J Biol Chem. 2006;281:14232–14240. doi: 10.1074/jbc.M601682200. [DOI] [PubMed] [Google Scholar]
- 22.Gandotra S, Le Dour C, Bottomley W, Cervera P, Giral P, Reznik Y, Charpentier G, Auclair M, Delépine M, Barroso I, et al. Perilipin deficiency and autosomal dominant partial lipodystrophy. N Engl J Med. 2011;364:740–748. doi: 10.1056/NEJMoa1007487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao J, Feng XX, Yao L, Ning B, Yang ZX, Fang DL, Shen W. Saturated free fatty acid sodium palmitate-induced lipoapoptosis by targeting glycogen synthase kinase-3β activation in human liver cells. Dig Dis Sci. 2014;59:346–357. doi: 10.1007/s10620-013-2896-2. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Gu Y, Dong W, Li H, Zhang L, Li N, Li W, Zhang L, Song Y, Jiang L, et al. Cell death-inducing DFF45-like effector, a lipid droplet-associated protein, might be involved in the differentiation of human adipocytes. FEBS J. 2010;277:4173–4183. doi: 10.1111/j.1742-4658.2010.07806.x. [DOI] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Bhala N, Younes R, Bugianesi E. Epidemiology and natural history of patients with NAFLD. Curr Pharm Des. 2013;19:5169–5176. doi: 10.2174/13816128113199990336. [DOI] [PubMed] [Google Scholar]
- 28.Cao J, Dai DL, Yao L, Yu HH, Ning B, Zhang Q, Chen J, Cheng WH, Shen W, Yang ZX. Saturated fatty acid induction of endoplasmic reticulum stress and apoptosis in human liver cells via the PERK/ATF4/CHOP signaling pathway. Mol Cell Biochem. 2012;364:115–129. doi: 10.1007/s11010-011-1211-9. [DOI] [PubMed] [Google Scholar]
- 29.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ning B, Bai M, Shen W. Reduced glutathione protects human hepatocytes from palmitate-mediated injury by suppressing endoplasmic reticulum stress response. Hepatogastroenterology. 2011;58:1670–1679. doi: 10.5754/hge10845. [DOI] [PubMed] [Google Scholar]
- 31.Cao SS, Kaufman RJ. Targeting endoplasmic reticulum stress in metabolic disease. Expert Opin Ther Targets. 2013;17:437–448. doi: 10.1517/14728222.2013.756471. [DOI] [PubMed] [Google Scholar]
- 32.Li H, Song Y, Zhang LJ, Gu Y, Li FF, Pan SY, Jiang LN, Liu F, Ye J, Li Q. LSDP5 enhances triglyceride storage in hepatocytes by influencing lipolysis and fatty acid β-oxidation of lipid droplets. PLoS One. 2012;7:e36712. doi: 10.1371/journal.pone.0036712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, et al. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem. 2012;287:23852–23863. doi: 10.1074/jbc.M111.328708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engin A. Non-alcoholic fatty liver disease. Adv Exp Med Biol. 2017;960:443–467. doi: 10.1007/978-3-319-48382-5_19. [DOI] [PubMed] [Google Scholar]
- 35.Kishikawa N, Kuroda N. Chemiluminescence assay for the investigation of reactive oxygen species generator. Yakugaku Zasshi. 2015;135:191–196. doi: 10.1248/yakushi.14-00213-1. (In Japanese) [DOI] [PubMed] [Google Scholar]
- 36.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, Gores GJ. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/S0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 37.Schattenberg JM, Galle PR, Schuchmann M. Apoptosis in liver disease. Liver Int. 2006;26:904–911. doi: 10.1111/j.1478-3231.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- 38.Cao Z, Zhang H, Cai X, Fang W, Chai D, Wen Y, Chen H, Chu F, Zhang Y. Luteolin promotes cell apoptosis by inducing autophagy in hepatocellular carcinoma. Cell Physiol Biochem. 2017;43:1803–1812. doi: 10.1159/000484066. [DOI] [PubMed] [Google Scholar]
- 39.Diamantis A, Magiorkinis E, Sakorafas GH, Androutsos G. A brief history of apoptosis: From ancient to modern times. Onkologie. 2008;31:702–706. doi: 10.1159/000165071. [DOI] [PubMed] [Google Scholar]
- 40.Granneman JG, Moore HP, Mottillo EP, Zhu Z. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J Biol Chem. 2009;284:3049–3057. doi: 10.1074/jbc.M808251200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Bell M, Sreenivasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, et al. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem. 2011;286:15707–15715. doi: 10.1074/jbc.M110.207779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansour M, Schwartz D, Judd R, Akingbemi B, Braden T, Morrison E, Dennis J, Bartol F, Hazi A, Napier I, Abdel-Mageed AB. Thiazolidinediones/PPARγ agonists and fatty acid synthase inhibitors as an experimental combination therapy for prostate cancer. Int J Oncol. 2011;38:537–546. doi: 10.3892/ijo.2010.877. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analysed data sets generated during the present study are available from the corresponding author on reasonable request.