Abstract
Vascular remodeling induced by long-term hyperglycaemia is the main pathological process in diabetic vascular complications. Thus, vascular remodeling may be a potential therapeutic target in diabetes mellitus (DM) with macrovascular disease. The present study aimed to investigate the effect of RING finger protein 10 (RNF10) on vascular remodeling under conditions of chronic hyperglycaemia stimulation. We found that overexpression of RNF10 clearly decreased intimal thickness and attenuated vascular remodeling in DM. TUNEL staining showed that apoptosis was clearly inhibited, an effect that may be mediated by decreases in Bcl-2 protein expression. Quantitative analysis demonstrated that overexpression of RNF10 could suppress inflammation by reducing the levels of TNF-α, and MCP-1 mRNA and NF-κB protein. Meanwhile, overexpression of RNF10 prevented vascular smooth muscle cell (VSMC) hyperproliferation through the downregulation of cyclin D1 and CDK4 proteins. Notably, short hairpin RNF10 (shRNF10) greatly aggravated the pathological responses of diabetic vascular remodeling. These outcomes revealed that the differential expression of RNF10 had a completely opposite effect on vascular damage under hyperglycaemia, further displaying the core function of RNF10 in regulating vascular remodeling induced by diabetes. Consequently, RNF10 could be a novel target for the treatment of diabetic vascular complications.
Keywords: RING finger protein 10, vascular remodeling, diabetes, inflammation, hyperproliferation, apoptosis
Introduction
Diabetes mellitus (DM) is a serious public health concern worldwide due to its gradually increasing prevalence (1). Diabetes is also an independent risk factor for many cardiovascular diseases, leading to the increased incidence of vascular remodeling among vascular diabetic complications. The pathogeneses of vascular remodeling induced by vascular smooth muscle cell (VSMC) dysfunction involve excessive inflammation and overactivation of proliferation, accompanied by increased amounts of extracellular matrix secretion, ultimately contributing to the thickening of arterial walls (2–5). Although vascular remodeling in diabetes is known to be associated with the combination of these abnormal pathological processes, the specific mechanism is still unknown.
The ubiquitin-proteasome system (UPS), the major mechanism by which proteins are degraded in cells, can degrade 80–90% of ubiquitinated proteins related to almost all biological activities in the organism, such as apoptosis, inflammation and cell cycle progression (6,7). The UPS has been considered a potential drug target in diabetes and other cardiovascular diseases (8,9). In the entire UPS, the E3 ubiquitin ligase is the most critical, as it identifies and promotes the degradation of substrate proteins by determining the time and specificity of ubiquitination (7,10). Thus, targeting a proper E3 ubiquitin ligase that participates in the regulation of crucial proteins in vascular remodeling could represent a new strategy for the treatment of diabetic vascular complications.
Ring finger protein 10 (RNF10), located on the long arm of chromosome 12, is a member of the RING finger family of E3 ubiquitin ligases, with the function of recruiting ubiquitin ligase E2 and substrate proteins (11,12). A clinical study demonstrated that RNF10 mRNA expression is significantly enhanced in obese patients and greatly increases the risk of diabetes (13). In our previous study, overexpression of RNF10 was shown to significantly prevent neointimal formation by promoting apoptosis reactions and inhibiting Bcl-2 protein activation in metabolic syndrome (14). The aim of the present study was to investigate the effect of RNF10 on vascular remodeling and the main pathological processes in diabetic rats after balloon injury, including inflammation, proliferation and apoptosis responses.
Materials and methods
Animal models
All animal experiments were approved by the Ethics Committee of Chongqing Medical University (Chongqing, China) and were performed according to the ARRIVE guidelines and the UK Animals (Science Procedures) Act, 1986 (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/116843/aspa-draft-guidance.pdf), and its associated guidelines. All experimental male Sprague-Dawley rats (4 weeks of age) were purchased from the Experimental Animal Centre of Chongqing Medical University (Chongqing, China). After one week of acclimation, the rats were randomly divided into control (n=12) and DM (n=60) groups. For a total of 12 weeks, the control group was fed a standard diet that included 13% fat, 61% carbohydrates, and 26% protein, while the DM group was fed a high-fat diet with 60% fat, 21% carbohydrates, and 19% protein. The animal experiment was performed in a temperature-controlled room maintained at 22±2°C and a 12 h light/dark cycle was maintained. Groups of 5 rats were housed in cages with free access to food and water.
The diabetic model was induced by feeding rats a high-fat diet resulting in hyperglycaemia and insulin resistance. All SD rats were fasted overnight (16–18 h) after receiving a standard diet or a high-fat diet for 12 weeks. Then, blood samples were collected from the retro-orbital plexus into a capillary tube to measure the diabetic indices. Plasma was frozen and stored at −80°C after being separated by centrifugation at 1,000 × g for 30 min at −4°C. Fasting blood glucose was quantified using a portable blood glucose meter (Sinocare Inc., Changsha, Hunan, China). An intraperitoneal glucose tolerance test (IPGTT) was used to detect insulin resistance and impaired glucose tolerance.
Carotid artery balloon injury (BI) and adenovirus (Ad) infection
Within the DM group on a high-fat (HF) diet, all rats were classified into 5 groups based on whether the balloon injury operation and adenovirus infection occurred. Eventually, all the SD rats were divided into the following 6 groups: i) the control group, ii) the DM+Sham group, iii) the DM+BI group, iv) the DM+BI+Ad-GFP group, v) the DM+BI+Ad-RNF10 group, and vi) the DM+BI+Ad-shRNF10 group.
Balloon injury was performed and measured as described previously (15); the balloon catheter (Medtronic Inc., Minneapolis, MN, USA) was introduced from the left external carotid artery into the thoracic aorta. The left common carotid artery was damaged by repeatedly pushing and pulling the inflated balloon three times. After washing the damaged artery with phosphate-buffered saline (PBS), 50 µl of adenovirus encoding RNF10, shRNF10 or GFP (2×1010 pfu/ml, Obio Technology Corp. Ltd., Shanghai, China) was injected into the injured artery to incubate for 30 min. After 14 days, the rats were sacrificed by high-dose injection of sodium pentobarbital (≥90 mg/kg), and the carotid arteries were harvested.
Histological and morphometric analysis
To examine the establishment of the vascular remodeling model, the obtained carotid tissue sections were stained using haematoxylin and eosin (H&E) as previously described (16). The carotid arteries of the rats were fixed in 4% paraformaldehyde for 24 h and then embedded in paraffin. Next, the sections were prepared for haematoxylin staining at room temperature for 15 min and eosin staining at room temperature for 5 min. Finally, the sections were stained with picrosirius red for 30 min at room temperature. Photomicrographs (×100 magnification) were captured with a routine light microscope (cat. no. 4.10.00, Nikon Co., Ltd., Tokyo, Japan).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
To determine the proportion of apoptotic carotid artery cells in the diabetic rats, a TUNEL assay was performed using an In Situ Cell Death Detection Kit-POD (Roche, Basel, Switzerland). First, carotid tissue sections were sequentially incubated with proteinase K for 30 min. Then, these sections were incubated with membrane disruption solution (Service Bio Inc., Woburn, MA, USA) for 20 min at room temperature, followed by incubation with a mixture of reagent 1 (TdT) and reagent 2 (dUTP) from the TUNEL kit for 2 h. Next, the sections were incubated with reagent 3 (converter-POD) for 30 min after incubation with hydrogen peroxide and ethanol for 15 min. Finally, the sections were mounted and observed under a routine light microscope (at magnification, ×400, cat. no. 4.10.00, Nikon Co., Ltd., Tokyo, Japan) after 3,3′-diaminobenzidine (DAB) (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) was added. The cells with brown and yellow nuclei were TUNEL-positive cells (17). The apoptosis index = number of apoptosis-positive cells × total number of intimal cells.
Quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from the carotid arteries of rats with TRIzol reagent (Takara Bio Inc., Kusatsu, Japan), and up to 1 µg of total RNA was reverse transcribed into cDNA following the manufacturer's instructions (Takara Bio Inc.). The reaction protocol was: Polymerase activation for 10 min at 95°C and then 40 amplification cycles of 60 sec at 60°C. The cycle threshold values were normalized to the level of β-actin, and the relative gene expression was measured by the 2−ΔΔCq method (18). The primers included those for β-actin (5′-CCCATCTATGAGGGTTACGC-3′ and 5′-TTTAATGTCACGCACGATTTC-3′), RNF10 (5′-ATTTTAGCAACCAGTCCCGTCG-3′ and 5′-CCTCATCCCGTCTTCCACCAT-3′), TNF-α (5′-CTACTGAACTTCGGGGTGATC-3′ and 5′-GGTCTGGGCCATAGAACTGA-3′) and MCP-1 (5′-GCTGACCCCAAGAAGGAATG-3′ and 5′-GTGCTTGAGGTGGTTGTGGA-3′).
Western blot analysis
The common carotid total protein was extracted using 10 µl/µg RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) and quantified via a BCA Protein Assay Kit (Beyotime Institute of Biotechnology). Protein (40 µg) was separated on 10–12% SDS-polyacrylamide gels (Beyotime Institute of Biotechnology) and transferred to PVDF membranes. The membranes were blocked in 5% non-fat dry milk for approximately 2 h and incubated overnight at 4°C with primary antibodies, including antibodies for Bcl-2 (1:2,000; cat. no. 12789; ProteinTech, Wuhan, Hubei, China), Bax (1:2,000; cat. no. 50599; ProteinTech), NF-κB (1:1,000; cat. no. 14220; ProteinTech), Cyclin D1 (1:1,000; cat. no. 60186; ProteinTech), CDK4 (1:1,000; cat. no. 11026; ProteinTech), RNF10 (1:500; cat. no. abs127972; Absin Bioscience Inc., Shanghai, China) and β-actin (1:5,000; cat. no. 20536; ProteinTech). Enhanced chemiluminescence development methods (Advansta Inc., Menlo Park, CA, USA) were used to visualize the immunoblotting bands (19). The bands were analyzed with ImageLab 3.0 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All the data are expressed as the means ± standard deviation (SD), and statistical analysis was performed using SPSS 19.0 (IBM Corp., Armonk, NY, USA). One-way ANOVA and LSD was used to analyse the differences among groups; P<0.05 was considered to indicate statistical significance.
Results
Differentially expressed RNF10 affects the neointimal apoptosis index in diabetic rats
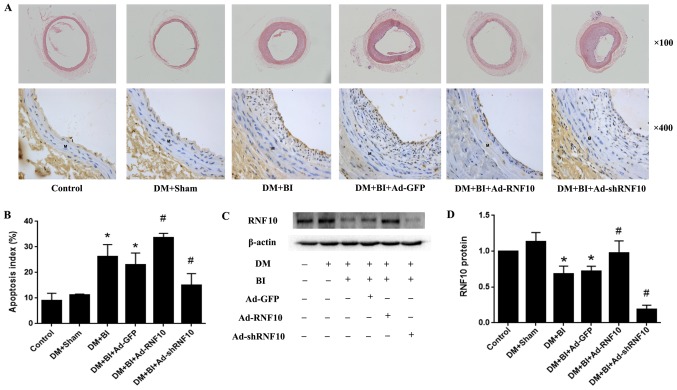
To evaluate whether vascular remodeling was induced by balloon injury, H&E staining was performed to detect neointimal formation. In general, we observed that the balloon-injured arteries had clearly increased intimal thickness compared with that of the DM+Sham group arteries. Compared with that in the Ad-GFP-treated group, the intimal thickness in the Ad-RNF10 group was markedly reduced. However, the intimal thickness was clearly increased in the Ad-shRNF10-treated group (Fig. 1A).
Figure 1.
Effects of RNF10 on apoptosis in the carotid artery. (A) H&E staining (original magnification, ×100) (top panels) and TUNEL staining (original magnification, ×400) (bottom panels) on cross-sections of the carotid arteries. (B) Quantification of the apoptosis index, defined as the ratio of TUNEL-positive cell neointimal numbers to the total neointimal numbers (n≥3). (C and D) Representative western blot analysis of RNF10 protein and quantitative analysis of RNF10 protein level (n≥3). *P<0.05 vs. the DM+Sham group; #P<0.05 vs. the DM+BI+Ad-GFP group. DM, diabetes mellitus; RNF10, RING finger protein 10; shRNF10, short hairpin RNF10; BI, balloon injury; Ad, adenovirus; GFP, green fluorescent protein; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; H&E, haematoxylin and eosin.
To assess whether the balloon-injured arteries were successfully transduced with the corresponding RNF10 gene, western blotting was performed to detect RNF10 protein expression. Compared with that of the arteries in the DM+Sham group, the RNF10 protein expression of the balloon-injured arteries was significantly decreased (P<0.001). Among the rats with balloon-injured arteries that were infected with different adenoviruses, RNF10 protein expression was significantly increased in the group receiving Ad-RNF10 (P<0.05) and was markedly decreased in the Ad-shRNF10 group (P<0.001) (Fig. 1C and D).
To examine whether RNF10 affected the apoptotic response in the model of diabetic vascular remodeling, we used TUNEL staining to determine the proportion of cells that were apoptotic in the neointima of the carotid artery. Very few apoptosis-positive cells were observed in the control group and the DM+Sham group, but the apoptotic index was significantly higher in the balloon-injured groups than in the DM+Sham groups (24.34±5.15 vs. 10.10±5.22%, P<0.001). Compared with that in the Ad-GFP group, the proportion of apoptotic cells was significantly increased in the Ad-RNF10 group (32.52±3.77 vs. 24.18±5.56%, P<0.05), but the apoptosis index was clearly decreased in the group infected with Ad-shRNF10 (14.59±4.29 vs. 24.18±5.56%, P<0.01) (Fig. 1A and B).
Overexpression of RNF10 attenuates apoptosis by decreasing the expression of marker protein Bcl-2
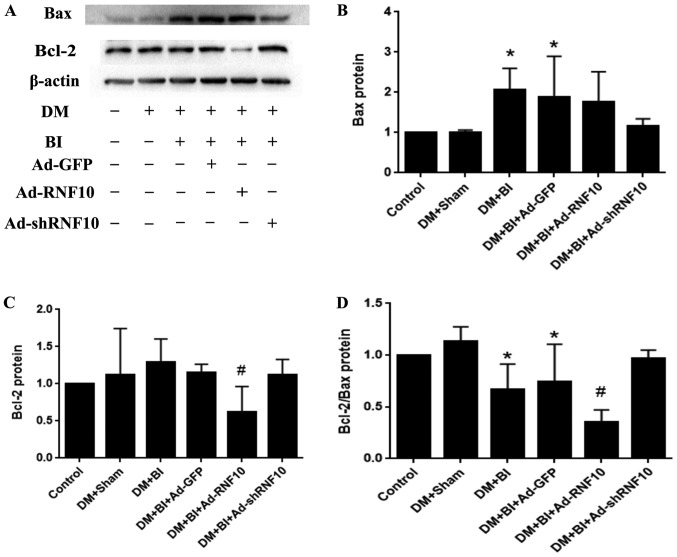
To verify the expression of apoptotic proteins in carotid vessels, western blotting was performed to detect the levels of the Bax and Bcl-2 proteins. Bax protein expression was significantly increased in the DM+BI group compared to the DM+Sham group (P<0.05), but there were no significant differences due to infection with Ad-GFP, Ad-RNF10 or Ad-shRNF10 (P>0.05) (Fig. 2A and B). Compared with that in the arteries of the Ad-GFP-transduced rats, the Bcl-2 protein expression in the arteries of the group receiving Ad-RNF10 group was significantly reduced (P<0.05), while the expression was not significantly changed in the group infected with Ad-shRNF10. In addition, the ratio of Bcl-2 to Bax was significantly decreased in the Ad-RNF10 group (P<0.05) and showed a trend towards a slight increase in the group infected with Ad-shRNF10, but there was no significant difference (P>0.05) (Fig. 2A, C and D).
Figure 2.
Effect of RNF10 on apoptotic marker proteins of Bax and Bcl-2 in the carotid artery. (A) Representative western blot analysis of Bax and Bcl-2 protein. Quantification of Bax (B), Bcl-2 (C) and Bcl-2/Bax (D) protein levels was analyzed by western blot analysis (n≥3). *P<0.05 vs. the DM+Sham group; #P<0.05 vs. the DM+BI+Ad-GFP group. DM, diabetes mellitus; RNF10, RING finger protein 10; shRNF10, short hairpin RNF10; BI, balloon injury; Ad, adenovirus; GFP, green fluorescent protein; Bax, B-cell CLL/lymphoma 2 associated X protein; Bcl-2, B-cell CLL/lymphoma 2.
Overexpression of RNF10 suppresses VSMC inflammation levels by inhibiting the marker protein NF-κB in diabetic rats
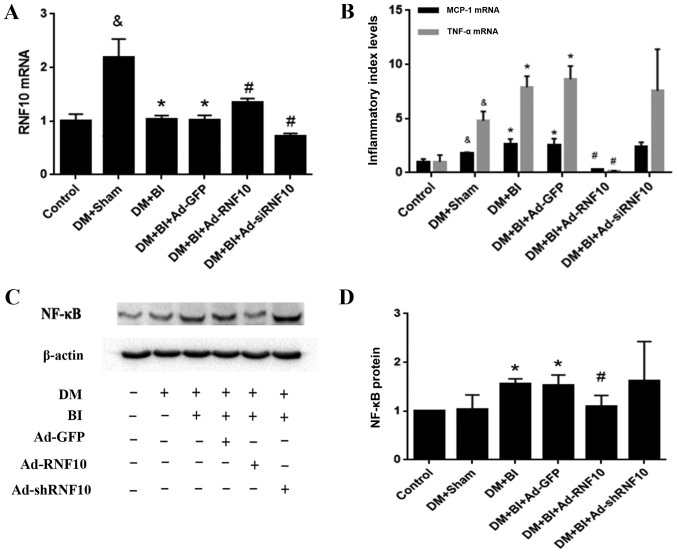
To determine whether the RNF10 gene was successfully transduced into carotid arteries after balloon injury, qPCR was used to detect RNF10 mRNA expression. Initially, the transcription level of RNF10 in the DM +Sham group was higher than that in the control group (P<0.001). After balloon injury, this elevation in RNF10 mRNA was clearly reversed (P<0.001). Compared with that in the Ad-GFP group, the expression of RNF10 mRNA in the group receiving Ad-RNF10 was significantly enhanced (P<0.05), and the expression in the group receiving Ad-shRNF10 was markedly reduced (P<0.001) (Fig. 3A).
Figure 3.
Effects of RNF10 on the inflammatory responses in carotid artery injury. (A) RNF10, (B) MCP-1 and TNF-α mRNA expression levels were analyzed with qPCR (n≥3). (C) Representative western blot of NF-κB protein. (D) Quantification of NF-κB protein level as determined by western blot analysis (n≥3). &P<0.05 vs. the corresponding control group; *P<0.05 vs. the corresponding DM+Sham group; #P<0.05 vs. the corresponding DM+BI+Ad-GFP group. DM, diabetes mellitus; RNF10, RING finger protein 10; shRNF10, RNF10 short hairpin RNA; BI, balloon injury; Ad, adenovirus; GFP, green fluorescent protein; qPCR, quantitative polymerase chain reaction; TNF-α, tumor necrosis factor α; MCP-1, monocyte chemotactic protein 1; NF-κB, nuclear factor-κ-gene binding.
To detect whether the RNF10 gene was involved in the inflammatory response of vascular remodeling in diabetic rats, we evaluated the mRNA levels of the inflammatory indicators MCP-1 and TNF-α, and analysed the expression of the inflammatory marker protein NF-κB. Compared with those in the control group, the levels of MCP-1 and TNF-α mRNA in the DM+Sham group were markedly increased (P<0.05). In addition, the expression of these inflammatory indicators in the DM+BI group was higher than that in the DM+Sham group (P<0.05). Importantly, the MCP-1 and TNF-α transcription levels were decreased in the group receiving Ad-RNF10 compared with the group receiving Ad-GFP (P<0.001). Concerning the inflammatory marker protein NF-κB, NF-κB levels were significantly higher in groups with balloon injury than in the DM+Sham group (P<0.05). Furthermore, balloon injured arteries infected with Ad-RNF10 displayed a significant reduction in NF-κB protein compared with carotid arteries infected with Ad-GFP (P<0.05). However, the mRNA expression of the inflammatory indicators MCP-1 and TNF-α mRNA, and the protein expression of NF-κB were not significantly changed by Ad-shRNF10 infection (P>0.05) (Fig. 3B-D).
Differentially expressed RNF10 regulates vascular hyperproliferation in diabetic rats
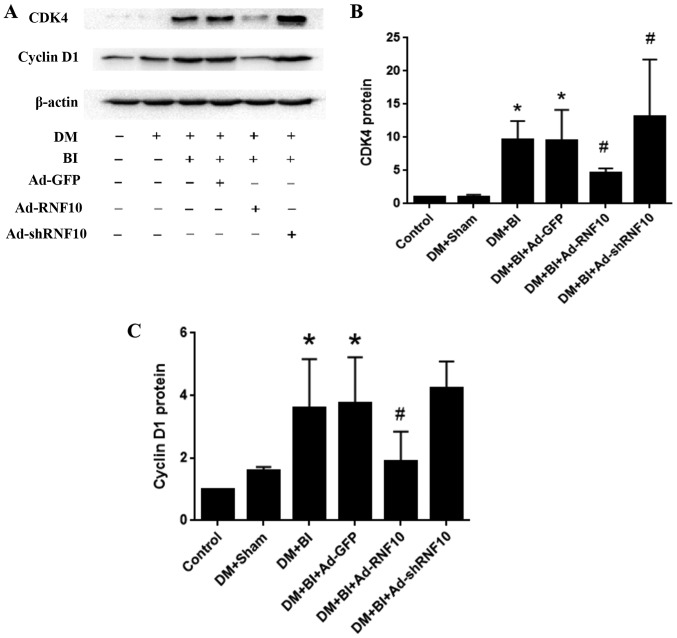
To assess whether RNF10 regulated the hyperproliferation of VSMCs in diabetic rats after balloon injury, we detected the protein levels of cyclin D1 and CDK4 in the carotid tissue. After balloon injury in DM rats, the protein levels of cyclin D1 and CDK4 were markedly higher than those in the DM+Sham group (P<0.05). In comparison to the Ad-GFP group, the Ad-RNF10 group showed significantly inhibited cyclin D1 protein expression (P<0.05). While shRNF10 slightly promoted cyclin D1 expression, there was no significant difference between the Ad-RNF10-treated group and the Ad-GFP-treated group (P>0.05). In addition, CDK4 protein expression was significantly decreased in the Ad-RNF10 group and significantly increased in the Ad-shRNF10 group compared with the Ad-GFP group (P<0.05) (Fig. 4A-C).
Figure 4.
Effects of RNF10 on proliferation in the carotid artery. (A) Representative western blot of CDK4 and cyclin D1 protein. (B and C) Quantification of CDK4 protein and cyclin D1 levels was assessed by western blot analysis (n≥3). *P<0.05 vs. the DM+Sham group; #P<0.05 vs. DM+BI+Ad-GFP. DM, diabetes mellitus; RNF10, RING finger protein 10; shRNF10, short hairpin RNF10; BI, balloon injury; Ad, adenovirus; GFP, green fluorescent protein; CDK4, cyclin dependent kinase 4.
Discussion
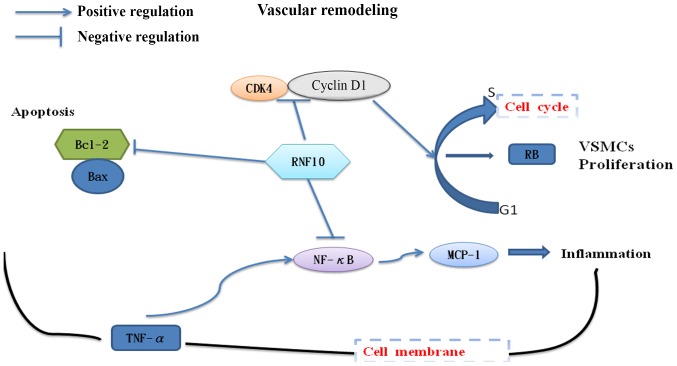
In the present study, we found that overexpression of RING finger protein 10 (RNF10) promoted an apoptotic reaction, inhibited the inflammatory response and repressed vascular smooth muscle cell (VSMC) hyperproliferation in carotid arteries, all of which showed that RNF10 exerts a protective influence in diabetic vascular remodeling. In addition, shRNF10 had an opposite impact on hyperproliferation and apoptosis, further destroying arterial function and structure in diabetes. Taken together, the results of this study demonstrated that changes in RNF10 expression affected vascular remodeling, indicating that the RNF10 gene plays a central role in diabetic rats with vascular complications (Fig. 5).
Figure 5.
Effects of RNF10 on vascular remodeling in diabetic rats with carotid artery injury. RNF10 as a key factor attenuated vascular remodeling via suppressing inflammatory responses, hyperproliferation and inhibitory apoptosis. RNF10, RING finger protein 10; VSMCs, vascular smooth muscle cells; TNF-α, tumor necrosis factor α; MCP-1, monocyte chemotactic protein 1; NF-κB, nuclear factor-κ-gene binding; Bax, B-cell CLL/lymphoma 2 associated X protein; Bcl-2, B-cell CLL/lymphoma 2; CDK4, cyclin dependent kinase 4.
A series of studies have revealed the relationship between the ubiquitin-proteasome system (UPS) and vascular remodeling, demonstrating that ubiquitin protease can inhibit vascular intimal proliferation in a dose-dependent manner, attenuate VSMC inflammation and promote apoptosis by decreasing the activity of the NF-κB signalling pathway (9,20). RNF10, an E3 ubiquitin ligase, is widely expressed in mammalian tissues and actively participates in the UPS. Previous studies have shown that RNF10 not only affects the signal transduction of insulin, but also regulates nearly all physiological functions, including apoptosis and the cell cycle (11,21). Consistent with the results described above, our present study found that the level of RNF10 mRNA was elevated in the DM group, but this phenomenon was reversed following the balloon injury of carotid arteries. This finding indicated that RNF10 may be inhibited after balloon injury and may function in diabetic vascular remodeling. Overall, this study further investigated the comprehensive effects of RNF10 on vascular remodeling in a diabetic model, including the role of RNF10 in anti-inflammation, anti-proliferation and pro-apoptosis reactions.
Many clinical trials have demonstrated that chronic hyperglycaemia is a pathological condition that accelerates the development of multiple cardiovascular diseases and is clearly correlated with microvascular and macrovascular complications in diabetes (22). In diabetic patients, hyperglycaemia-induced activation of the NF-κB signalling pathway causes the overproduction of a large number of cytokines, which is a crucial aspect of the process of diabetic vascular remodeling. These activated cytokines, such as TNF-α and MCP-1, trigger the aggregation and infiltration of inflammatory cells, affecting the permeability and structure of blood vessels, and contributing to the development of diabetic vascular disease (23–26). Our study confirmed that overexpression of RNF10 might inhibit the mRNA expression of the inflammatory markers TNF-α and MCP-1 via the NF-κB signalling pathway. However, shRNF10 could not change the levels of these inflammatory indicators, probably due to compensatory mechanisms in the organisms. Therefore, RNF10 might be a crucial target to regulate inflammation reactions in diabetic vascular remodeling.
VSMC hyperproliferation is also an important part of the vascular remodeling process and is affected by the cell cycle regulatory proteins cyclin D1 and CDK4. The regulation of the cell cycle depends on complexes of cyclin and CDK, which are core materials driving the smooth progression of the cell cycle (27,28). A previous study demonstrated that RNF10 could cause a 1.95-fold activation of the P21 promoter. P21, an important cell cycle regulatory protein, effectively inhibits pRb phosphorylation mediated by the cyclin D1-CDK4 complex, thereby slowing the cell cycle transition from the G1 phase to the S phase (21,29). Based on these findings, we suggest that the inhibition of VSMC hyperproliferation may be ascribed to the modulation of P21 expression and the cyclin D1-CDK4 complex. Furthermore, shRNF10 also significantly aggravated hyperproliferation by increasing CDK4 protein levels. However, as a result of insufficient sample size, the levels of cyclin D1 were not significantly altered. Therefore, RNF10 might be a central factor that regulates VSMC hyperproliferation in diabetes.
Furthermore, the apoptosis reaction is also involved in diabetic rats with microvascular and macrovascular complications. The inhibition of apoptosis in VSMCs exposed to high glucose determines the proliferation level in vascular remodeling and is also regulated by the anti-apoptosis gene Bcl-2 and the pro-apoptosis gene Bax (5). Both Bcl-2 and Bax are crucial members of the Bcl-2 family that widely regulate apoptosis, and their ratio always reflects the apoptosis level in cells (30). In our previous study, we observed that RNF10 promoted VSMC apoptosis and downregulated the anti-apoptotic Bcl-2 protein to prevent neointimal hyperplasia in metabolic syndrome (14). Although the Bcl-2/Bax protein ratio was slightly increased without a significant difference, the increase may have resulted from insufficient sample size. In this study, overexpression of RNF10 was found to promote apoptosis to prevent vascular remodeling by decreasing the Bcl-2/Bax ratio, and shRNF10 was also found to inhibit the apoptosis response to aggravate neointimal formation in diabetic rats. Therefore, RNF10 may be considered a key factor in regulating apoptosis in vascular remodeling in diabetic rats.
In summary, RNF10 may be the central target for the regulation of diabetic vascular remodeling due to its pro-apoptosis, anti-inflammation and anti-hyperproliferation activity (Fig. 5). However, the specific mechanism of the signalling pathway remains unclear and will be investigated in future studies.
Acknowledgements
The authors would like to thank The First Affiliated Hospital of Chongqing Medical University for the experimental platform assistance.
Glossary
Abbreviations
- DM
diabetes mellitus
- RNF10
RING finger protein 10
- shRNF10
short hairpin RNF10
- VSMCs
vascular smooth muscle cells
- UPS
ubiquitin-proteasome system
- HF
high fat
- IPGTT
intraperitoneal glucose tolerance test
- BI
balloon injury
- PBS
phosphate-buffered saline
- Ad
adenovirus
- GFP
green fluorescent protein
- qPCR
quantitative polymerase chain reaction
- TNF-α
tumor necrosis factor α
- MCP-1
monocyte chemotactic protein 1
- NF-κB
nuclear factor-κ-gene binding
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- H&E
haematoxylin and eosin
- Bax
B-cell CLL/lymphoma 2 associated X protein
- Bcl-2
B-cell CLL/lymphoma 2
- CDK4
cyclin dependent kinase 4
Funding
This study was sponsored by the National Natural Science Foundation of China (grant no. 31501097).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
SL, GY, WH and RW performed the histological examination and other animal experiments. SL and MC analyzed and interpreted all the data. PP was a major contributor in designing the study and writing the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All animal experiments were approved by the Ethics Committee of Chongqing Medical University (Chongqing, China) and were performed according to the ARRIVE guidelines and the UK Animals (Science Procedures) Act, 1986 (https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/116843/aspa-draft-guidance.pdf), and its associated guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there have no competing interests.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Doran AC, Meller N, McNamara CA. Role of smooth muscle cells in the initiation and early progression of atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:812–819. doi: 10.1161/ATVBAHA.107.159327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang CN, Chan KC, Lin WT, Su SL, Peng CH. Hibiscus sabdariffa inhibits vascular smooth muscle cell proliferation and migration induced by high glucose-a mechanism involves connective tissue growth factor signals. J Agric Food Chem. 2009;57:3073–3079. doi: 10.1021/jf803911n. [DOI] [PubMed] [Google Scholar]
- 4.Hall JL, Matter CM, Wang X, Gibbons GH. Hyperglycemia inhibits vascular smooth muscle cell apoptosis through a protein kinase C-dependent pathway. Circ Res. 2000;87:574–580. doi: 10.1161/01.RES.87.7.574. [DOI] [PubMed] [Google Scholar]
- 5.Li H, Télémaque S, Miller RE, Marsh JD. High glucose inhibits apoptosis induced by serum deprivation in vascular smooth muscle cells via upregulation of Bcl-2 and Bcl-xl. Diabetes. 2005;54:540–545. doi: 10.2337/diabetes.54.2.540. [DOI] [PubMed] [Google Scholar]
- 6.Balasubramanyam M, Sampathkumar R, Mohan V. Is insulin signaling molecules misguided in diabetes for ubiquitin-proteasome mediated degradation? Mol Cell Biochem. 2005;275:117–125. doi: 10.1007/s11010-005-1083-y. [DOI] [PubMed] [Google Scholar]
- 7.Patterson C. Search and destroy: The role of protein quality control in maintaining cardiac function. J Mol Cell Cardio. 2006;40:438–441. doi: 10.1016/j.yjmcc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Drews O, Taegtmeyer H. Targeting the ubiquitin-proteasome system in heart disease: The basis for new therapeutic strategies. Antioxid Redox Signal. 2014;21:2322–2343. doi: 10.1089/ars.2013.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meiners S, Laule M, Rother W, Guenther C, Prauka I, Muschick P, Baumann G, Kloetzel PM, Stangl K. Ubiquitin-proteasome pathway as a new target for the prevention of restenosis. Circulation. 2002;105:483–489. doi: 10.1161/hc0402.102951. [DOI] [PubMed] [Google Scholar]
- 10.Herrmann J, Ciechanover A, Lerman LO, Lerman A. The ubiquitin-proteasome system in cardiovascular diseases-a hypothesis extended. Cardiovasc Res. 2004;61:11–21. doi: 10.1016/j.cardiores.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 11.Seki N, Hattori A, Sugano S, Muramatsu M, Saito T. cDNA cloning, expression profile, and genomic structure of human and mouse RNF10/Rnf 10 genes, encoding a novel RING finger protein. J Hum Genet. 2000;45:38–42. doi: 10.1007/s100380050007. [DOI] [PubMed] [Google Scholar]
- 12.Saurin AJ, Borden KL, Boddy MN, Freemont PS. Does this have a familiar RING? Trends Biochem Sci. 1996;21:208–214. doi: 10.1016/S0968-0004(96)80017-X. [DOI] [PubMed] [Google Scholar]
- 13.Huang K, Nair AK, Muller YL, Piaggi P, Bian L, Del Rosario M, Knowler WC, Kobes S, Hanson RL, Bogardus C, Baier LJ. Whole exome sequencing identifies variation in CYB5A and RNF10 associated with adiposity and type 2 diabetes. Obesity (Silver Spring) 2014;22:984–988. doi: 10.1002/oby.20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu G, Chen J, Li S, Pu P, Huang W, Zhao Y, Wang R, Lei H. RING finger protein 10 prevents neointimal hyperplasia by promoting apoptosis in vitro and in vivo. Life Sci. 2018;208:325–332. doi: 10.1016/j.lfs.2018.04.055. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Fan Z, Yang J, Ding J, Yang C, Chen L. MicroRNA-24 attenuates neointimal hyperplasia in the diabetic rat carotid artery injury model by inhibiting Wnt4 signaling pathway. Int J Mol Sci. 2016;17(pii):E765. doi: 10.3390/ijms17060765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan X, Zhang T, Yao F, Liao Y, Liu F, Ren Z, Han L, Diao L, Li Y, Zhou B, et al. THO complex-dependent posttranscriptional control contributes to vascular smooth muscle cell fate decision. Circ Res. 2018;123:538–549. doi: 10.1161/CIRCRESAHA.118.313527. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Xia G, Zhang Y, Liu J, Liu X, Li W, Lv Y, Wei S, Liu J, Quan J. Palmitate induces VSMC apoptosis via toll like receptor (TLR)4/ROS/p53 pathway. Atherosclerosis. 2017;263:74–81. doi: 10.1016/j.atherosclerosis.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Yang D, Sun C, Zhang J, Lin S, Zhao L, Wang L, Lin R, Lv J, Xin S. Proliferation of vascular smooth muscle cells under inflammation is regulated by NF-κB p65/microRNA-17/RB pathway activation. Int J Mol Med. 2018;41:43–50. doi: 10.3892/ijmm.2017.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan CH, Li PC, Chien YC, Yeh WT, Liaw CC, Sheu MJ, Wu CH. Suppressive activities and mechanisms of ugonin J on vascular smooth muscle cells and balloon angioplasty-induced neointimal hyperplasia. Phytother Res. 2018;32:312–320. doi: 10.1002/ptr.5979. [DOI] [PubMed] [Google Scholar]
- 20.Lecker SH, Solomon V, Price SR, Kwon YT, Mitch WE, Goldberg AL. Ubiquitin conjugation by the N-end rule pathway and mRNAs for its components increase in muscles of diabetic rats. J Clin Invest. 1999;104:1411–1420. doi: 10.1172/JCI7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin J, Friesen MT, Bocangel P, Cheung D, Rawszer K, Wigle JT. Characterization of mesenchyme homeobox 2 (MEOX2) transcription factor binding to RING finger protein 10. Mol Cell Biochem. 2005;275:75–84. doi: 10.1007/s11010-005-0823-3. [DOI] [PubMed] [Google Scholar]
- 22.Paolisso G, Rizzo MR, Barbieri M, Manzella D, Ragno E, Maugeri D. Cardiovascular risk in type 2 diabetics and pharmacological regulation of mealtime glucose excursions. Diabetes Metab. 2003;29:335–340. doi: 10.1016/S1262-3636(07)70044-7. [DOI] [PubMed] [Google Scholar]
- 23.Navarro-González Juan F, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol. 2008;19:433–442. doi: 10.1681/ASN.2007091048. [DOI] [PubMed] [Google Scholar]
- 24.Winkler G, Salamon F, Harmos G, Salamon D, Speer G, Szekers O, Hajós P, Kovács M, Simon K, Cseh K. Elevated serum tumor necrosis factor-alpha concentrations and bioactivity in type 2 diabetics and patients with android type obesity. Diabetes Res Clin Pract. 1998;42:169–174. doi: 10.1016/S0168-8227(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 25.Bayón Y, Alonso A, Hernández M, Nieto ML, Sánchez Crespo M. Mechanisms of cell signaling in immune-mediated inflammation. Cytokines Cell Mol Ther. 1998;4:275–286. [PubMed] [Google Scholar]
- 26.Brownlee M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 27.Sherr CJ, Roberts JM. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 28.Sylvester AM, Chen D, Krasinski K, Andrés V. Role of c-fos and E2F in the induction of cyclin A transcription and vascular smooth muscle cell proliferation. J Clin Invest. 1998;101:940–948. doi: 10.1172/JCI1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Z, Hunter T. Ubiquitylation and proteasomal degradation of the p21(Cip1), p27(Kip1) and p57(Kip2) CDK inhibitors. Cell Cycle. 2010;9:2342–2352. doi: 10.4161/cc.9.12.11988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59(Suppl 7):S1693–S1700. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.