Abstract
Hemothorax is an important complication of blunt trauma chest. The presentation may be delayed, especially in elderly patients with multiple rib fractures. Delayed presentation can be associated with retained hemothorax where a simple chest drain is often insufficient to evacuate the pleural cavity. Video-assisted thoracoscopy surgery is often used to manage such patients in a minimally invasive manner. Here, we demonstrate a novel application of flexi-rigid thoracoscopy with CryoProbe® for evacuation of retained hemothorax in an elderly woman through a subcentimeter incision.
KEY WORDS: Blunt trauma chest, cryoextraction, medical thoracoscopy, retained hemothorax
INTRODUCTION
Presence of blood in the thoracic cavity is known as hemothorax. It is most commonly secondary to thoracic trauma and, in rare cases, can be spontaneous.[1] Evacuation of the blood is essential for quantification of blood loss as well as allowing the lung to expand and have a tamponading effect over the bleeding surface. Relief is provided in emergency situation by simple chest tube drainage.[2] A significant proportion of patients can have incomplete drainage and may require additional intervention.[3] Medical thoracoscopy (MT) has revolutionized the diagnosis of pleural diseases and, after the introduction of semi-rigid thoracoscope, the utilization of this intervention by pulmonologists has increased exponentially. CryoProbe® (CP) is an important bronchoscopic accessory which works on the basis of rapid cooling due to expansion of compressed gas leading to crystallization of water in the tissue, leading to tissue adherence. However, there have been no reports till date about the use of CP for the extraction of retained hemothorax through a flexi-rigid thoracoscope. Here, we present the case of an elderly female patient presenting 8 days after sustaining blunt chest trauma with retained hemothorax which was successfully managed using a novel technique of cryoextraction through a semi-rigid pleuroscope.
CASE REPORT
An 82-year-old female patient presented to the emergency department with respiratory failure; on evaluation, she had a right-sided massive effusion [Figure 1a]. She had a history of fall about 8 days back and had sustained blunt trauma to the chest. Chest X-ray done at that time did not show any effusion, but over the next few days, she deteriorated to the current state. Computed tomography (CT) thorax done in the emergency department showed large right effusion, with suggestion of retained hemothorax over the diaphragmatic surface in the posterior aspect [Figure 1b]. In view of delayed presentation with radiological evidence of retained hemothorax, she was taken up for flexi-rigid MT under general anesthesia with single-lung ventilation using Coopdech endobronchial blocker. After entry into the thoracic cavity through an incision of <1 cm in length, about 1.45 L of blood was evacuated from her right pleural cavity. A large hematoma was seen densely adherent to the subpulmonic area and the superior area of the diaphragm in the posterior aspect [Figure 2a]. This was well organized and could not be evacuated by suction of the thoracoscope. A flexible CP (2.4-mm outer diameter and 900-mm length, ERBE Elektromedizin, Tubingen, Germany) was introduced through the channel of flexi-rigid thoracoscope so that the organized hematoma could be removed after freezing and withdrawing the probe and scope as a unit [Figure 2b]. The process was repeated until the entire pleural cavity was free of organized hematoma [Figure 3a and b]. The patient was extubated on table and immediately had relief of dyspnea, lung expansion was documented by day 1 postprocedure, and chest drain was removed on day 2 [Figure 4]. The patient was discharged on day 3.
Figure 1.
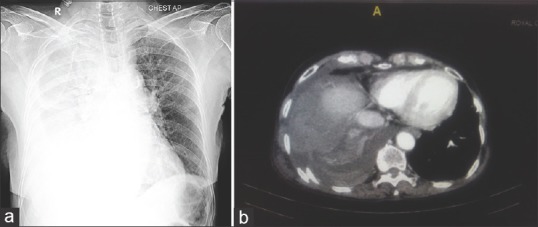
(a) Massive right-sided pleural effusion. (b) Computed tomography showing retained hemothorax in the posterior aspect
Figure 2.
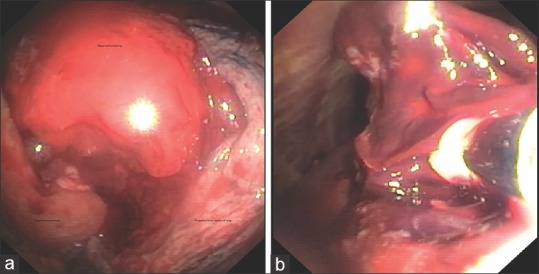
(a) Large densely adherent retained hemothorax. (b) Cryoextraction of retained hemothorax
Figure 3.
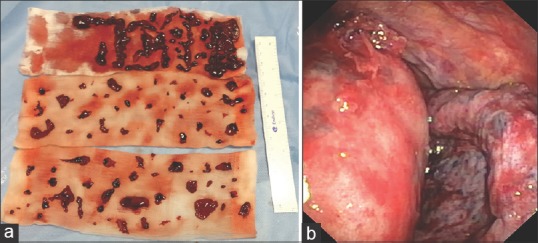
(a) Extracted clots. (b) Thoracoscopic view postextraction of retained hemothorax
Figure 4.
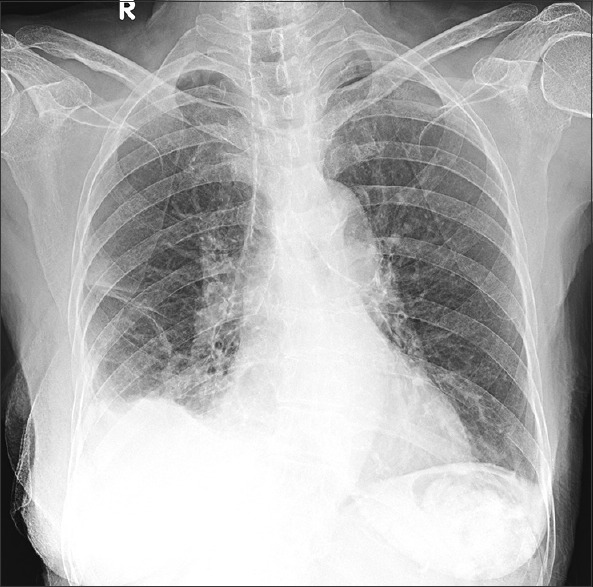
Postprocedure after chest drain removal
DISCUSSION
Retained hemothorax is a serious complication of bleeding into the pleural cavity and it can occur in as many as 18% of patients with blunt trauma to the chest.[4] Retained hemothorax has 26.8% chance of developing empyema, and these patients carry 94.3% risk of additional interventions for management, resulting in significant increase in hospital stay and morbidity; this was demonstrated in a large study involving over twenty trauma centers.[5] This can occur despite drainage of hemothorax with chest tube. Some patients with hemothorax may present late, as it may not have been obvious in the initial radiology due to slow accumulation of blood. Schweigert et al. demonstrated that in a subset of patients falling in the age group of over 80 years, the presentation after hemothorax is often delayed.[6] Incomplete drainage and delayed presentation pose considerable risk for complications including retained hemothorax, empyema, pleural thickening, trapped lung, and severe restrictive physiology as sequelae.[7] Although open thoracotomy was considered gold standard for the management of complications of hemothorax, recent studies show increasing trends toward the use of minimally invasive video-assisted thoracoscopy surgery (VATS) early in the course of illness to prevent complications and reduce morbidity.[8] Primary VATS in hemothorax without tube thoracostomy has also been explored in an attempt to reduce complications and reduce the duration of hospital stay.[9,10] There have been no reports of use of MT or CP for retained hemothorax till date.
The spectrum of interventions by MT has considerably increased with reports of its use for the management of multiloculated effusions and empyema.[11] Retained hemothorax is a type of loculated effusion, and therefore application of MT in its management is intuitive. CP has proved to be a valuable addition to the arsenal of interventional pulmonologists and has revolutionized transbronchial and endobronchial biopsies, providing samples comparable to those obtained by surgical means.[12,13] The unique freezing property of CP also has facilitated quick evacuation of large endobronchial clots using flexible bronchoscope in patients with respiratory failure.[14] CP has also demonstrated that larger specimens are obtained compared to those by forceps when used for taking parietal pleural biopsies.[15]
In this case, we faced a challenge of dealing with delayed presentation of an elderly female patient with multiple rib fractures presenting with respiratory failure. CT showed retained hemothorax and she was a high-risk candidate for open thoracotomy. This prompted us to explore the possibility of utilizing primary MT for evacuating hemothorax. Once the thorax was clear of free-flowing blood, the residual clot posed a difficulty, as it was resistant to suction evacuation. At this juncture, the options were to either make another port for additional instruments to be introduced or increase the size of the current port which was about 0.8 cm in length to accommodate instruments. We were reluctant to do either as we felt that would increase the postoperative pain and may increase the duration of hospital stay.[16,17]
As we use CP routinely for evacuating clots and foreign bodies from bronchial tree and its use is not new in the pleural cavity, we improvised the use of CP and used it to evacuate organized hematoma. Although several attempts were needed to completely evacuate the clot, it was a successful means to achieve the end result. The patient was extubated on table and shifted to the ward. As all the clots and most of the fluids were evacuated on table, she had minimal drain postprocedure, with chest X-ray showing complete expansion. We could remove the drain in 48 h and discharge her by 72 h. A word of caution while using CP is that once the target is frozen, it needs to be avulsed from the base and the operator has to ensure that the lung is not adherent as it can lead to parenchymal damage. Use of single-lung ventilation can ensure that the lung is fully deflated and falls away from the organized clot.
CONCLUSION
MT and use of CP is an intervention for evacuation of retained hemothorax in a minimally invasive manner. This technique can decrease the postoperative pain due to small incision size, ensure complete evacuation, reduce the duration of chest drain, and decrease the duration of hospital stay. This case demonstrates another potential use for two versatile equipments in a modern interventional pulmonology suite. This however cannot be recommended as the standard procedure for management without further studies. All precautions exercised while using CP in bronchoscopy and thoracoscopy for pleural biopsy need to be adhered to while attempting to manage retained hemothorax.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We thankfully acknowledge the staff members of the bronchoscopy suite (Mrs. Sathya Janani, Mr. Uma Maheshwaran, Mrs. Anitha, and Miss. Kalpana) for assisting in interventional procedures and collecting reliable and meaningful information about our study subject. We thank Mrs. Sai Chandini Krishnamoorthy for editing the script.
REFERENCES
- 1.Broderick SR. Hemothorax: Etiology, diagnosis, and management. Thorac Surg Clin. 2013;23:89. doi: 10.1016/j.thorsurg.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Mowery NT, Gunter OL, Collier BR, Diaz JJ, Jr, Haut E, Hildreth A, et al. Practice management guidelines for management of hemothorax and occult pneumothorax. J Trauma. 2011;70:510–8. doi: 10.1097/TA.0b013e31820b5c31. [DOI] [PubMed] [Google Scholar]
- 3.DuBose J, Inaba K, Demetriades D, Scalea TM, O’Connor J, Menaker J, et al. Management of post-traumatic retained hemothorax: A prospective, observational, multicenter AAST study. J Trauma Acute Care Surg. 2012;72:11–22. doi: 10.1097/TA.0b013e318242e368. [DOI] [PubMed] [Google Scholar]
- 4.Helling TS, Gyles NR, 3rd, Eisenstein CL, Soracco CA. Complications following blunt and penetrating injuries in 216 victims of chest trauma requiring tube thoracostomy. J Trauma. 1989;29:1367–70. doi: 10.1097/00005373-198910000-00013. [DOI] [PubMed] [Google Scholar]
- 5.DuBose J, Inaba K, Okoye O, Demetriades D, Scalea T, O’Connor J, et al. Development of posttraumatic empyema in patients with retained hemothorax: Results of a prospective, observational AAST study. J Trauma Acute Care Surg. 2012;73:752–7. doi: 10.1097/TA.0b013e31825c1616. [DOI] [PubMed] [Google Scholar]
- 6.Schweigert M, Beron M, Dubecz A, Stadlhuber R, Stein H. Video-assisted thoracoscopic surgery for posttraumatic hemothorax in the very elderly. Thorac Cardiovasc Surg. 2012;60:474–9. doi: 10.1055/s-0031-1298069. [DOI] [PubMed] [Google Scholar]
- 7.Chou YP, Lin HL, Wu TC. Video-assisted thoracoscopic surgery for retained hemothorax in blunt chest trauma. Curr Opin Pulm Med. 2015;21:393–8. doi: 10.1097/MCP.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyer DM, Jessen ME, Wait MA, Estrera AS. Early evacuation of traumatic retained hemothoraces using thoracoscopy: A prospective, randomized trial. Ann Thorac Surg. 1997;64:1396–400. doi: 10.1016/S0003-4975(97)00899-0. [DOI] [PubMed] [Google Scholar]
- 9.Huang WY, Lu IY, Yang C, Chou YP, Lin HL. Efficiency analysis of direct video-assisted thoracoscopic surgery in elderly patients with blunt traumatic hemothorax without an initial thoracostomy. Biomed Res Int. 2016;2016:3741426. doi: 10.1155/2016/3741426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oosthuizen GV, Clarke DL, Laing GL, Bruce J, Kong VY, Van Staden N, et al. Introducing video-assisted thoracoscopy for trauma into a South African township hospital. World J Surg. 2013;37:1652–5. doi: 10.1007/s00268-013-2026-5. [DOI] [PubMed] [Google Scholar]
- 11.Hardavella G, Papakonstantinou NA, Karampinis I, Papavasileiou G, Ajab S, Shafaat M, et al. Hippocrates quoted “If an empyema does not rupture, death will occur”: Is medical thoracoscopy able to make it rupture safely? J Bronchology Interv Pulmonol. 2017;24:15–20. doi: 10.1097/LBR.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 12.Hagmeyer L, Theegarten D, Treml M, Priegnitz C, Randerath W. Validation of transbronchial cryobiopsy in interstitial lung disease – Interim analysis of a prospective trial and critical review of the literature. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33:2–9. [PubMed] [Google Scholar]
- 13.Ehab A, Khairy El-Badrawy M, Abdelhamed Moawad A, El-Dosouky Abo-Shehata M. Cryobiopsy versus forceps biopsy in endobronchial lesions, diagnostic yield and safety. Adv Respir Med. 2017;85:301–6. doi: 10.5603/ARM.2017.0052. [DOI] [PubMed] [Google Scholar]
- 14.Sehgal IS, Dhooria S, Agarwal R, Behera D. Use of a flexible cryoprobe for removal of tracheobronchial blood clots. Respir Care. 2015;60:e128–31. doi: 10.4187/respcare.03861. [DOI] [PubMed] [Google Scholar]
- 15.Tousheed SZ, Manjunath PH, Chandrasekar S, Murali Mohan BV, Kumar H, Hibare KR, et al. Cryobiopsy of the pleura: An improved diagnostic tool. J Bronchology Interv Pulmonol. 2018;25:37–41. doi: 10.1097/LBR.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 16.Hao Z, Cai Y, Fu S, Zhang N, Fu X. Comparison study of post-operative pain and short-term quality of life between uniportal and three portal video-assisted thoracic surgery for radical lung cancer resection. Zhongguo Fei Ai Za Zhi. 2016;19:122–8. doi: 10.3779/j.issn.1009-3419.2016.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dhooria S, Singh N, Aggarwal AN, Gupta D, Agarwal R. A randomized trial comparing the diagnostic yield of rigid and semirigid thoracoscopy in undiagnosed pleural effusions. Respir Care. 2014;59:756–64. doi: 10.4187/respcare.02738. [DOI] [PubMed] [Google Scholar]


