Abstract
Background
An association between thrombocytopenia and retinopathy of prematurity (ROP) has been suggested but not been studied longitudinally. We sought to identify the time period in postnatal development during which thrombocytopenia and the subsequent development of severe ROP are associated.
Methods
This was a retrospective case–control study of 100 subjects who received laser photocoagulation for type 1 ROP between 2005 and 2009 and 100 controls with no ROP or only stage 1 ROP. The proportions of infants with thrombocytopenia, defined as a serum platelet level of <150,000/μL, among cases versus controls were compared on a weekly basis from birth through the time of laser during early (postmenstrual age [PMA] weeks 24–28), middle (PMA weeks 29–34), and late (PMA weeks 35–38) time periods. Main outcome measures were odds ratios for the association between thrombocytopenia and type 1 ROP from multivariate logistic regression model adjusted for gestational age, birth weight, culture-proven sepsis, and necrotizing enterocolitis.
Results
Thrombocytopenia was significantly associated with severe ROP during PMA weeks 24–28 (adjusted OR, 4.7; 95% CI, 2.0–1.1; P = 0.001) and 29–34 (adjusted OR, 2.8; 95% CI, 1.4–5.6; P = 0.006), but not during weeks 35–38 (adjusted OR, 2.0; 95% CI, 0.9–4.3; P = 0.10).
Conclusions
Thrombocytopenia from birth through 34 weeks’ PMA was associated with subsequent severe ROP. This time period corresponds to a period of poor retinal vascular growth, suggesting a possible proangiogenic effect of platelets in normal retinal vascular development in infants at risk for ROP.
The pathogenesis of retinopathy of prematurity (ROP) is currently thought to involve phases of initial hyperoxia, followed by retinal hypoxia and finally vascular proliferation.1 Disease development and progression are largely mediated by alterations in local vascular endothelial growth factor (VEGF) and systemic insulin-like growth factor 1 (IGF-1). Normally, hypoxia in the developing and increasingly metabolically active avascular retina induces VEGF secretion, which stimulates vessel growth.2 However, VEGF activity requires sufficient levels of serum IGF-1, a systemic growth hormone.2,3 In premature infants, lack of maternal sources and poor endogenous secretion result in low serum IGF-1 and poor retinal vessel growth despite hypoxia and upregulated local VEGF production. With time, endogenous serum IGF-1 levels rise4 and permit VEGF activity, resulting in a proliferative retinopathy.2
In addition to hemostasis, platelet functions may include both pro- and anti-angiogenic regulation.5,6 Platelets store, transport, and deliver key angiogenic factors,7 including VEGF5,6 and IGF-1,8 and therefore may influence the development of ROP. In 2010 Vinekar and colleagues9 found a relationship between thrombocytopenia and aggressive posterior ROP (AP-ROP). Based on a single serum platelet level prior to laser, they found lower mean serum platelet counts in 10 cases compared to 21 controls. We subsequently reported an association between thrombocytopenia immediately preceding laser and Early Treatment of ROP Study type 1 ROP in 91 cases versus 91 matched controls, again considering only a single serum platelet level prior to laser.10 In a subgroup analysis, the overall association seemed primarily attributable to zone I cases. However, it was unclear whether these findings reflected the timing of the platelet level being considered (earlier for zone I disease in our study and for AP-ROP in the Vinekar and colleagues9 study) or the anatomic location of the disease, because the association between thrombocytopenia and ROP has yet to been investigated longitudinally. Recently, Lundgren and colleagues11 found significantly lower serum platelet counts preceding aggressive-posterior ROP diagnosis among 9 infants who developed AP-ROP compared to 9 infants who developed ROP of stage 2 or less, although multivariable analysis was not performed to control for sepsis and NEC. We sought to evaluate the temporal course of the association between thrombocytopenia and subsequent development of severe retinopathy of prematurity (ROP). We hypothesized that there would be a time period in postnatal development during which the association would be strongest.
Subjects and Methods
This study was approved by the institutional review boards of the Children’s Hospital of Philadelphia and the Hospital of the University of Pennsylvania and complied with all requirements of the US Health Insurance Portability and Accountability Act of 1996 and the tenets of the Declaration of Helsinki. The medical records of premature infants at risk for ROP at both institutions between January 1, 2005, and December 31, 2009, were reviewed retrospectively. Cases were infants who received ROP laser photocoagulation surgery; decisions to treat were made using Early Treatment for ROP (ETROP) Study type 1 ROP criteria.12 Controls infant received inpatient ROP examinations at either hospital during the same time period and developed either no ROP or at worst stage 1 ROP prior to documentation of one of the following: mature retinal vasculature, immature vasculature in zone III without prior disease, and regressing or regressed stage 1 disease. Although some of these case and control infants were included in a prior report,10 only a single serum platelet level for each infant was considered; in the current study, new longitudinal data are collected and reported. In addition, the inclusion criteria for the prior and current studies differed. In the prior study cases had to have “matchable” controls with regard to birth weight (BW) and gestational age at birth (GA) and have platelet data available within 1 week prior to laser (cases) or within 2 weeks’ postmenstrual age (PMA) of matched (controls). Neither were required in the current study.
Data were collected from inpatient and outpatient medical records at the study hospitals, including outside hospital transfer summaries. All available serum platelet levels were abstracted from birth through day of laser treatment for cases and through 38 weeks’ PMA or hospital discharge for controls, whichever occurred first. Platelets were obtained as part of complete blood counts, which were drawn routinely at least weekly for the majority of patients at both study hospitals. For each ROP examination, stage, zone, and presence or absence of plus disease were recorded. BW, GA, episodes of culture-proven sepsis, and diagnosis of Bell’s stage IIA or greater necrotizing enterocolitis (NEC)13 were abstracted as potential confounders.
Data Analysis
Thrombocytopenia was defined as a serum platelet count of <150,000/μL.14,15 For all analyses, a subject was considered to have thrombocytopenia during a given week of PMA if any of the available serum platelet levels during that week were <150,000/μL. The proportion of cases with thrombocytopenia was compared to that of controls during each week PMA until the time of laser treatment using the Fisher exact test. A two-sided P value of <0.05 was considered statistically significant. The association between thrombocytopenia and type 1 ROP was evaluated during early (PMA weeks 24–28), middle (PMA weeks 29–34), and late (PMA weeks 35–38) time periods by using multivariate logistic regression with adjustment for BW, GA, sepsis, and NEC. These time periods were chosen to approximately correspond to the initial hyperoxic, middle hypoxic, and later neovascular periods thought to occur in ROP pathogenesis,1 although these definitions were known to be somewhat imprecise due to the varying GA of the infants studied. The study was designed to be an exploratory hypothesis-generating study; therefore, no corrections for multiple comparisons were made. The primary analysis considered all cases and controls. Subgroup analyses were performed by zone of disease, because prior studies suggested an association specifically for posterior disease. Consideration of confounding by sepsis and NEC depended upon the date of the platelet level being considered. Thrombocytopenia may precede other identifiable signs of neonatal sepsis or NEC up to 24 hours, continues until sepsis or NEC is controlled, and typically resolves over 1–2 weeks.16 Therefore, an episode of sepsis was considered as a potential confounder if it occurred within a period ranging from one week prior to one week following the serum platelet level being considered. We considered a given serum platelet level as potentially being affected by a diagnosis of NEC made any time prior to through two weeks after the date of the platelet level. All analyses were performed using SAS statistical software version 9.2 (SAS INC, Cary, NC).
Results
During the study period, 110 infants underwent ROP laser photocoagulation surgery. Ten treated infants, all of whom had been transferred from an outside hospital for laser, were excluded because platelet data were lacking. Baseline characteristics for the remaining 100 cases and 100 controls appear in Table 1. The mean PMA at laser for the cases was 37.3 ± 3.0 weeks (standard deviation).
Table 1.
Characteristics of 100 cases treated for retinopathy of prematurity and 100 control infants with no or stage 1 retinopathy of prematurity
| Characteristic | Cases (n = 100) | Controls (n = 100) | P value |
|---|---|---|---|
| Sex, no. (%) | |||
| Male | 51 (51) | 58 (58) | 0.394b |
| Birth weight, g | |||
| Mean ± SD | 750 ± 186 | 800 ± 192 | 0.031a |
| Median (range) | 713 (420–1305) | 748 (490–1306) | |
| GA, weeks | |||
| Mean (SD) | 25 (2) | 26 (2) | 0.014a |
| Median (range) | 25 (23–33) | 26 (23–33) | |
| Sepsis, no. (%) | 31 (31) | 35 (35) | 0.652b |
| Necrotizing enterocolitis, no. (%) | 19 (19) | 20 (20) | 1.000b |
| PMA at laser | |||
| Median (range) | |||
| Overall | 37 (31–45) | ||
| Zone I (n = 13) | 34 (31–36) | ||
| Zone II (n = 87) | 38 (33–45) |
GA, gestational age; PMA, postmenstrual age; SD, standard deviation.
Two-group t test.
Fisher exact test.
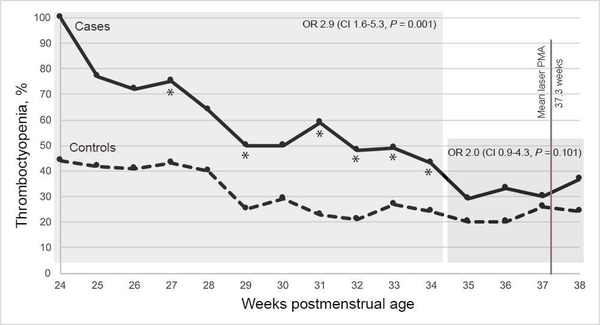
The proportions of cases and controls with thrombocytopenia for each PMA week from 24 to 38 appear in Table 2 and Figure 1. There was a significant association between thrombocytopenia and type 1 ROP during PMA weeks 24–28 (OR adjusted for BW, GA, sepsis, and NEC, 4.7; 95% CI, 2.0–1.1; P = 0.001), PMA weeks 29–34 (adjusted OR 2.8; 95% CI, 1.4–5.6; P = 0.006), and the combined period, PMA weeks 24–34 (adjusted OR 2.9; 95% CI, 1.6–5.3; P = 0.001). There was not a significant association during PMA weeks 35–38 (adjusted OR 2.0; 95% CI, 0.9–4.3; P = 0.10).
Table 2.
Infants with thrombocytopenia among cases and controls, by postmenstrual age (PMA) at platelet level
| PMA at platelet level | Cases, n/N (%) | Controls, n/N (%) | P value |
|---|---|---|---|
| 24 | 4/4 (100) | 4/9 (44) | 0.105 |
| 25 | 10/13 (77) | 8/19 (42) | 0.075 |
| 26 | 13/18 (72) | 12/29 (41) | 0.070 |
| 27 | 15/20 (75) | 18/42 (43) | 0.029a |
| 28 | 14/22 (64) | 20/50 (40) | 0.077 |
| 29 | 13/26 (50) | 13/53 (25) | 0.040a |
| 30 | 15/30 (50) | 17/59 (29) | 0.063 |
| 31 | 20/34 (59) | 14/62 (23) | <0.001a |
| 32 | 20/42 (48) | 13/61 (21) | 0.009a |
| 33 | 19/39 (49) | 18/67 (27) | 0.034a |
| 34 | 20/47 (43) | 16/66 (24) | 0.044a |
| 35 | 13/45 (29) | 14/70 (20) | 0.367 |
| 36 | 16/48 (33) | 14/70 (20) | 0.132 |
| 37 | 13/44 (30) | 18/69 (26) | 0.829 |
| 38 | 13/35 (37) | 14/58 (24) | 0.239 |
P < 0.05.
FIG 1.
Proportions of all cases and all controls with thrombocytopenia by weeks postmenstrual age (PMA). OR, odds ratio; CI, confidence interval; *P < 0.05 (Fisher exact test).
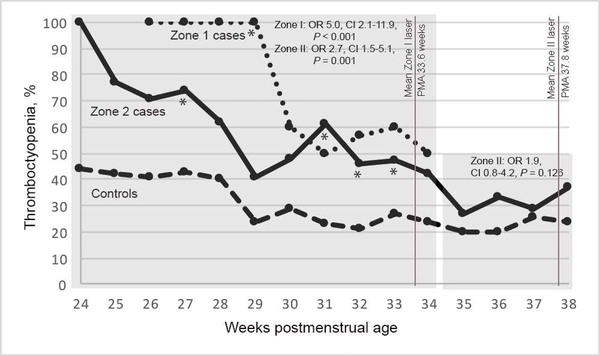
Thirteen cases had zone I disease, and 87 cases had zone II disease. The mean PMA at laser was 33.6 ± 1.7 weeks for zone I cases and 37.8 ± 2.7 weeks for zone II cases. The association between thrombocytopenia and type 1 ROP for zone I cases versus controls was significant for PMA weeks 24–34 (adjusted OR 5.0; 95% CI, 2.1–11.9; P < 0.001). See Figure 2. Only 3 infants had laser for zone I disease after 34 weeks’ PMA; thus an odds ratio was not calculated for the late time window. The association between thrombocytopenia and type 1 ROP among zone II cases versus controls was significant for PMA weeks 24–34 (adjusted OR 2.7; 95% CI, 1.5–5.1; P = 0.001) but not significant for PMA weeks 35–38 (OR 1.9; 0.8–4.2; P = 0.13).
FIG 2.
Proportions of zone I cases, zone II cases, and all controls with thrombocytopenia by weeks postmenstrual age (PMA). *P < 0.05, Fischer exact test.
Discussion
We found a significant association between thrombocytopenia and type 1 ROP in earlier (PMA weeks 24–34) but not later (PMA weeks 35–38) periods in postnatal development. The proportions of thrombocytopenia among cases (43% to 100%) were double or nearly double those of controls (21% to 44%) for each PMA week between 24 and 34 (Table 2, Figure 1). These differences were either significant or showed a trend toward significance (P < 0.001 to P = 0.10). We posit that the failure to meet the chosen significance level of 0.05 in some of the individual weeks is due to the small number of infants with platelet measurements during those weeks, because there was consistency in the proportions of infants with thrombocytopenia in each group across weeks and the P values were not higher than 0.10. Generally, there were fewer subjects during the earlier PMA weeks, reducing the statistical power for those weeks. During each PMA week from 35 to 38, the proportions of thrombocytopenia among cases (29% to 37%) and controls (20% to 26%) were more similar than in the two earlier time periods, and none of the comparisons were significant (P = 0.13 to P = 0.83).
The association between thrombocytopenia during PMA weeks 24–34 was independent of several potential confounders, including BW, GA, sepsis, and NEC. To be a confounder in the association between thrombocytopenia and ROP, a factor must be associated separately with each condition. Therefore, whereas many comorbidities of prematurity have been reported as risk factors for severe ROP, we specifically controlled for sepsis and NEC in our analysis because both are known independent risk factors for ROP, and the vast majority (>80%) of thrombocytopenia after the first 72 hours of life is associated with one of these two conditions.14,16 Controlling for other ROP risk factors that fall within the general health of the infant is not necessary, because they are not commonly associated with thrombocytopenia and therefore cannot confound the association between thrombocytopenia and ROP.
A previous analysis of the current cohort identified an association between thrombocytopenia at a single time point (immediately preceding laser) and the subsequent development of severe ROP.10 The current study examined the timing of that association. In addition, we wanted to clarify the results of our and other reports that suggested an association primarily among infants with posterior disease.9–11 In the current study, there appeared to be a significant association among both zone I cases and zone II cases during the earlier time windows (24–34 weeks’ PMA) but not the later time window (35–38 weeks’ PMA), so the association appears dependent upon the PMA and not the zone of disease. It is possible to reconcile this finding with the earlier studies by considering that in the prior studies, only a single platelet level immediately preceding laser was studied. Zone I cases were treated earlier than zone II cases; therefore, the serum platelet levels for zone I cases were measured at earlier PMAs than the serum platelet levels for zone II cases in those studies. The apparent difference between zone I and zone II disease was related to the timing of the platelet levels, with zone of disease acting as a confounding factor.
The study results also suggest a testable pathophysiological hypothesis. Platelets influence angiogenesis through the scavenging, expression, and delivery of mitogenic and angiogenic factors.5–8 Platelet alpha granules have been found to contain VEGF, IGF-1, IGF-binding protein 3 (the primary serum binding protein for IGF-1), platelet-derived growth factor, and other mitogens, as well as platelet factor 4, an inhibitor of angiogenesis. The mechanisms through which platelets endocytose, package, express, and release such factors, and therefore influence vascular development, have been studied.5–8 We hypothesize that one mechanism connecting thrombocytopenia and the development of severe ROP involves the delivery of IGF-1 by platelets. This is based on prior research and the following findings: (1) thrombocytopenia at earlier PMA weeks, during a period of poor vascular growth in the pathogenesis of ROP, is associated with subsequent type 1 disease; (2) low serum IGF-1 during this period is associated with the later development of severe ROP17–19; and (3) IGF-1 is necessary for VEGF-induced vessel growth.20 Our hypothesis requires evaluation in further clinical and laboratory studies.
Our study has several limitations. We collected retrospective data on platelet levels that were ordered for nonstudy reasons. However, during the time period studied most infants underwent weekly routine lab testing, which included serum platelet levels; therefore, bias is unlikely to have been introduced with regards to the indication for testing. These retrospective data were from approximately 10 years ago; subsequent changes in neonatal care practices might affect the possible association between thrombocytopenia and ROP. There were statistically significant differences in mean GA (about 50 g lower) and BW (about 1 week earlier) for cases versus controls, but we controlled for these differences in our analysis. We defined thrombocytopenia as <150,000, but another threshold level may prove to be more relevant with regard to ROP. We also did not consider platelet transfusions, cumulative platelet deficits, or the duration of thrombocytopenia. We classified a week with even a single low platelet level as a week with thrombocytopenia, which might overestimate the degree of thrombocytopenia. However, we applied this definition equally to both cases and controls, so differential information bias was unlikely to be introduced. Finally, we did not control for slow postnatal growth, a surrogate measure for low serum IGF-1 and a risk factor for the later development of severe ROP.21–25 Although we are unaware of a specific association between slow growth or low IGF-1 and thrombocytopenia, IGF-1 delivery is one hypothesized mechanism by which platelets may influence vasculogenesis, and serum IGF-1 levels may therefore modify the association between thrombocytopenia and type 1 ROP.
Our longitudinal case–control study found an association between thrombocytopenia occurring at 34 weeks’ PMA or earlier and the subsequent development of type 1 ROP. Future studies might identify a narrower time window during which the association is strongest, evaluate the effects of cumulative platelet deficits or the degree of thrombocytopenia, or explore in a laboratory setting the pathophysiological mechanisms by which platelets may influence retinal angiogenesis.
Acknowledgments
The authors would like to thank Haresh Kirpalani, MD, MSc, for his discussions with the authors about thrombocytopenia in premature infants.
Supported by the National Institutes of Health grants K12 EY015398, P30 EY01583-26, and L30 EY018451-03. The funding organization had no role in the design or conduct of this research.
Footnotes
Conflicts of interest: Gil Binenbaum, MD, participates as a site investigator in a retinopathy of prematurity treatment randomized controlled trial sponsored by Novartis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis 2007;10:133–40. [DOI] [PubMed] [Google Scholar]
- 2.Smith LE. Pathogenesis of retinopathy of prematurity. Growth Horm IGF Res 2004;14 Suppl A:S140–44. [DOI] [PubMed] [Google Scholar]
- 3.Hellström A, Ley D, Hansen-Pupp I, et al. New insights into the development of retinopathy of prematurity—importance of early weight gain. Acta Paediatr 2010;99:502–8. [DOI] [PubMed] [Google Scholar]
- 4.Smith WJ, Underwood LE, Keyes L, Clemmons DR. Use of insulin-like growth factor I (IGF-I) and IGF-binding protein measurements to monitor feeding of premature infants. J Clin Endocrinol Metab 1997;82:3982–8. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007;6:273–86. [DOI] [PubMed] [Google Scholar]
- 6.Italiano JE Jr, Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 2008;111:1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee M, Huang Z, Zhang W, et al. Distinct platelet packaging, release, and surface expression of proangiogenic and antiangiogenic factors on different platelet stimuli. Blood 2011;117:3907–11. [DOI] [PubMed] [Google Scholar]
- 8.Smyth SS, McEver RP, Weyrich AS, et al. ; 2009 Platelet Colloquium Participants. Platelet functions beyond hemostasis. J Thromb Haemost 2009;7:1759–66. [DOI] [PubMed] [Google Scholar]
- 9.Vinekar A, Hegde K, Gilbert C, et al. Do platelets have a role in the pathogenesis of aggressive posterior retinopathy of prematurity? Retina 2010;30:S20–S23. [DOI] [PubMed] [Google Scholar]
- 10.Jensen AK, Ying GS, Huang J, Karp K, Quinn GE, Binenbaum G. Thrombocytopenia and retinopathy of prematurity. J AAPOS 2011;15:e3–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lundgren P, Lundberg L, Hellgren G, et al. Aggressive posterior retinopathy of prematurity is associated with multiple infectious episodes and thrombocytopenia. Neonatology 2017;111:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol 2003;121:1684–94. [DOI] [PubMed] [Google Scholar]
- 13.Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr 1987;17:213–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts I, Stanworth S, Murray NA. Thrombocytopenia in the neonate. Blood Rev 2008;22:173–86. [DOI] [PubMed] [Google Scholar]
- 15.Sola-Visner M, Saxonhouse MA, Brown RE. Neonatal thrombocytopenia: what we do and don’t know. Early Hum Dev 2008;84:499–506. [DOI] [PubMed] [Google Scholar]
- 16.Chakravorty S, Murray N, Roberts I. Neonatal thrombocytopenia. Early Hum Dev 2005;81:35–41. [DOI] [PubMed] [Google Scholar]
- 17.Jensen AK, Ying GS, Huang J, Quinn GE, Binenbaum G. Postnatal serum insulin-like growth factor I and retinopathy of prematurity. Retina 2017;37:867–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellström A, Engström E, Hård AL, et al. Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth. Pediatrics 2003;112:1016–20. [DOI] [PubMed] [Google Scholar]
- 19.Pérez-Muñuzuri A, Fernández-Lorenzo J, Couce-Pico M, Blanco-Tijeiro MJ, Fraga-Bermúdez JM. Serum levels of IGF1 are a useful predictor of retinopathy of prematurity. Acta Paediatr 2010;99:519–25. [DOI] [PubMed] [Google Scholar]
- 20.Smith LE, Shen W, Perruzzi C, et al. Regulation of vascular endothelial growth factor-dependent retinal neovascularization by insulin-like growth factor-1 receptor. Nat Med 1999;5:1390–95. [DOI] [PubMed] [Google Scholar]
- 21.Binenbaum G, Ying GS, Quinn GE, et al. The CHOP postnatal weight gain, birth weight, and gestational age retinopathy of prematurity risk model. Arch Ophthalmol 2012;130:1560–65. [DOI] [PubMed] [Google Scholar]
- 22.Hellström A, Hård AL, Engström E, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics 2009;123:e638–45. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Löfqvist C, Smith LE, VanderVeen DK, Hellström A; WINROP Consortium. Importance of early postnatal weight gain for normal retinal angiogenesis in very preterm infants: a multicenter study analyzing weight velocity deviations for the prediction of retinopathy of prematurity. Arch Ophthalmol 2012;130:992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Binenbaum G, Ying GS, Quinn GE, et al. ; Premature Infants in Need of Transfusion Study Group. A clinical prediction model to stratify retinopathy of prematurity risk using postnatal weight gain. Pediatrics 2011;127:e607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eckert GU, Fortes Filho JB, Maia M, Procianoy RS. A predictive score for retinopathy of prematurity in very low birth weight preterm infants. Eye (Lond) 2012;26:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]