Abstract
Bio-engineered teeth that can grow and remodel in a manner similar to that of natural teeth have the potential to serve as permanent replacements to the currently used prosthetic teeth, such as dental implants. A major challenge in designing functional bio-engineered teeth is to mimic both the structural and anisotropic mechanical characteristics of the native tooth. Therefore, the field of dental and whole tooth regeneration has advanced towards the molecular and nanoscale design of bio-active, biomimetic systems, using biomaterials, drug delivery systems and stem cells. The focus of this review is to discuss recent advances in tooth tissue engineering, using biomimetic scaffolds that provide proper architectural cues, exhibit the capacity to support dental stem cell proliferation and differentiation and sequester and release bio-active agents, such as growth factors and nucleic acids, in a spatiotemporal controlled manner. Although many in vitro and in vivo studies on tooth regeneration appear promising, before tooth tissue engineering becomes a reality for humans, additional research is needed to perfect methods that use adult human dental stem cells, as opposed to embryonic dental stem cells, and to devise the means to generate bio-engineered teeth of predetermined size and shape.
Keywords: teeth, scaffold, stem cells, drug delivery systems, bio-active agents, tissue engineering
1. Introduction
Tooth loss can result from a variety of genetic disorders or microbial diseases, iatrogenic, traumatic, or therapeutic insults, patient negligence and poor oral hygiene, or dental prosthesis failure (Cooper, 2009; Young et al., 2002). Currently, due to a variety of options for dental treatments, there are many choices to restore the function, integrity and morphology of missing tooth structure, or even tooth loss. However, despite advancements in dental therapies, tooth loss remains an individual concern, a professional responsibility and a prominent public health issue (Cooper, 2009). It is estimated that approximately 150 million adults currently suffer from tooth loss, and that > 10 million new cases of edentulism will arise during this decade (Cooper, 2009). Dental clinical procedures, such as root canal treatment, tooth allotransplantation, autotransplantation and dental implants have been used as tooth therapies (Chai and Slavkin, 2003; Park et al., 2010; Ravindran and George, 2015). Dental therapies have improved significantly, based on innovations from basic and translational research, materials science and clinical techniques (Chai and Slavkin, 2003). However, root canal therapy makes the tooth lose its sensitivity and vitality and become a dead tooth; hence, the tooth cannot respond immunologically to subsequent infections (Ravindran and George, 2015). Harvesting procedures required for autologous tooth autotransplantation can add significant morbidity to the surgical procedure, namely blood loss and postoperative pain. Dental implants, such as titanium implants, are not equivalent in either function or aesthetics to natural teeth (Yen and Yelick, 2011). Moreover, dental implants function through osseointegration, the direct connection between the implant and the surrounding alveolar bone, and lack periodontal and cementum tissues present in naturally formed teeth, which function to cushion and modulate the mechanical stress of mastication (Yen and Yelick, 2011). These disadvantages have prompted an ongoing search for alternative methods that would overcome the need for root canal treatment, autologous tooth harvest for autotransplanation and dental implants.
Scientific advances in stem cell biology and tissue-engineering technology have shown the potential use of dental stem cells, combined with biodegradable scaffolds supplied with bio-active agents, such as growth factors (GF), to control the spatial and temporal organization of dental progenitor cell proliferation, differentiation and function (Chai and Slavkin, 2003; Eap et al., 2014; Otsu et al., 2014). Any attempt to regenerate a missing tooth structure or even a whole tooth has to take into consideration guided tissue formation, the surrounding tissues (periodontium) and revascularization (Chou et al., 2006; Eap etal., 2014; Ravindran and George, 2015). Tooth engineering involving epithelial-mesenchymal interactions could be a model system to evaluate not only tooth development but also the revascularization and reinnervation of the bio-engineered tooth (Nakahara, 2011). Therefore, tissue engineering (TE) efforts have been focused on the development of new culture systems, part or whole tooth regeneration and vascularization and periodontium regeneration (Chen et al., 2011; Nakahara, 2011). New culture systems may establish an appropriate ex vivo culture environment without the use of an animal body (in vivo culture). Part or whole tooth regeneration aims to produce a bio-engineered tissue (root or tooth) in place of dental implants (Nakahara, 2011). The periodontium, consisting of gingiva, periodontal ligament, cementum and alveolar bone, is crucial for establishing a stable bone-tooth interface (Chou et al., 2006). Revascularization is one of the primary challenges associated with tooth regeneration. Therefore, the ability of biomimetic scaffolds to induce tooth formation, vascularization and periodontium regeneration is highly beneficial toward clinical translation of this approach.
We believe that dental TE will soon emerge as the preferred solution to replace missing tooth structures and whole teeth. Here, we summarize recent progress, perspectives and potential roles for tissue-engineering approaches for tooth regeneration, highlighting the importance of, and challenges associated with, the proper delivery of bio-active agents in an orderly temporal and spatial manner, in order to recapitulate natural tooth development.
2. Tooth development and function
Tooth development is the result of a complex and intricate cascade of gene expression patterns that direct cells to proper locations and differentiation pathways (Nanci and Cate, 2003). As for all organs, tooth formation is regulated by epithelial-mesenchymal interactions. The dental mesenchyme is derived from the neural crest, while the dental epithelium is derived from the ectoderm (Jernvall and Thesleff, 2012). Figure 1 shows the principal stages of tooth morphogenesis. To fully understand tooth development, one must consider the molecular signals that control cell migration, growth and differentiation (Chai and Slavkin, 2003; Lai et al., 2014; Nanci and Cate, 2003).
Figure 1.
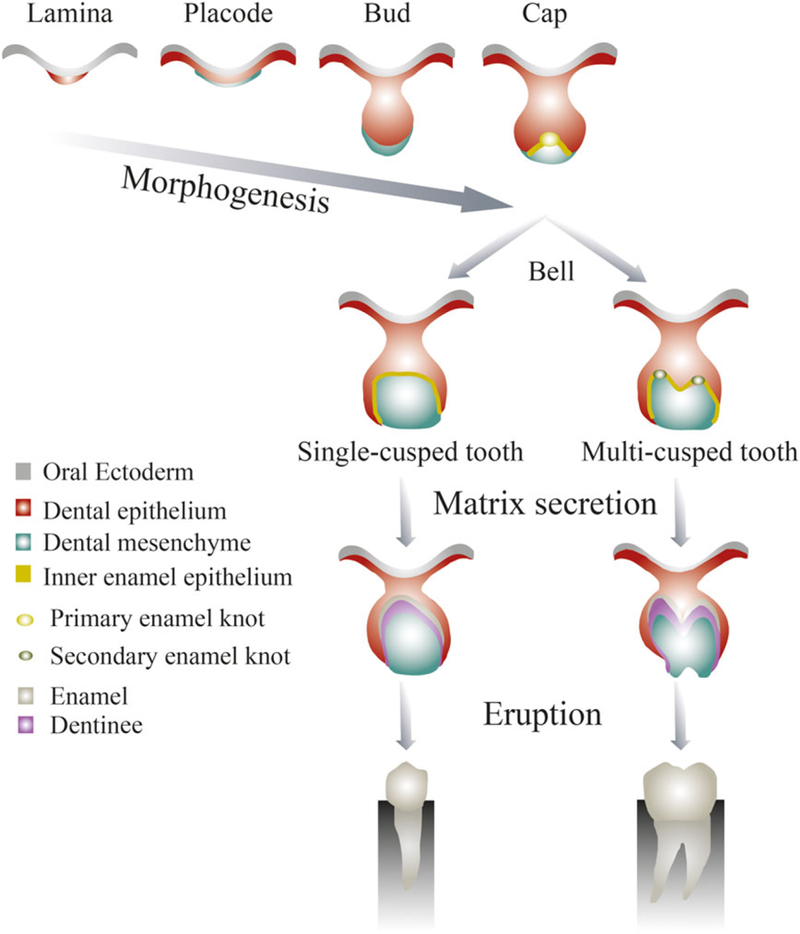
Principal stages of tooth formation. Adapted from Jernvall and Thesleff (2012). [Colour figure can be viewed at wileyonlinelibrary.com]
Tooth development is initiated by the formation of the dental lamina within the dental epithelium. Specific dental laminar domains, called placodes, exhibit localized proliferative activities leading to the formation of a series of epithelial outgrowths into the ectomesenchyme (Nanci and Cate, 2003). Tooth development then proceeds in three stages: the bud, cap and bell stages. In the bud stage, the dental epithelium invaginates into the dental mesenchyme (Jernvall and Thesleff, 2012). In the cap stage, the epithelium extends further into the dental mesenchymal tissue encompassing the condensing mesenchyme. In the bell stage, species-specific cusp patterns emerge: (a) in a single-cusped tooth, a primary enamel knot gives rise to the tooth crown; (b) in multicusped teeth, secondary and tertiary enamel knots form at future cusp locations. The bell stage is followed by the differentiation stage, where final growth and matrix secretion occur as the inner enamel epithelium differentiates into ameloblasts, producing enamel, while adjacent dental mesenchymal cells differentiate into odontoblasts, producing dentine (Jernvall and Thesleff, 2012; Lai et al., 2014).
Enamel, the most highly mineralized tissue in the body, consists of >96% hydroxy-apatite and a complex crystalline lattice organization (Jernvall and Thesleff, 2012; Nanci and Cate, 2003). The structure and composition of enamel provides sufficient mechanical strength to withstand the strong forces of mastication and attacks by acids from food and bacterial sources. Ameloblasts, which cover the entire surface of the enamel as it forms, undergo apoptosis prior to the time of tooth eruption, making enamel a non-vital and insensitive tissue that, when destroyed, cannot be replaced or regenerated (Nanci and Cate, 2003). Dentine is a resilient (approximately 70% mineralized) and elastic mineralized tissue that forms the bulk of the tooth and supports the enamel, compensating for its brittleness. Characteristic features of dentine are tubules that traverse its entire thickness, and which contain odontoblast cytoplasmic extensions. Dentine is a sensitive tissue that is capable of repair, because odontoblasts or dental mesenchymal cells in the pulp can be stimulated to deposit more dentine in response to mechanical injury (Nanci and Cate, 2003). Dental pulp, the soft connective tissue enclosed by the dentine (the central pulp chamber), has a variety of functions, including: (a) to produce dentine; (b) to nourish the avascular dentine; and (c) to support nerves that provide sensitivity. The tooth is tethered to the jaw by specialized supporting tissues, consisting of the periodontal ligament (PDL), the alveolar bone and the cementum, all of which are protected by the gingiva (see Figure 2) (Nanci and Cate, 2003).
Figure 2.
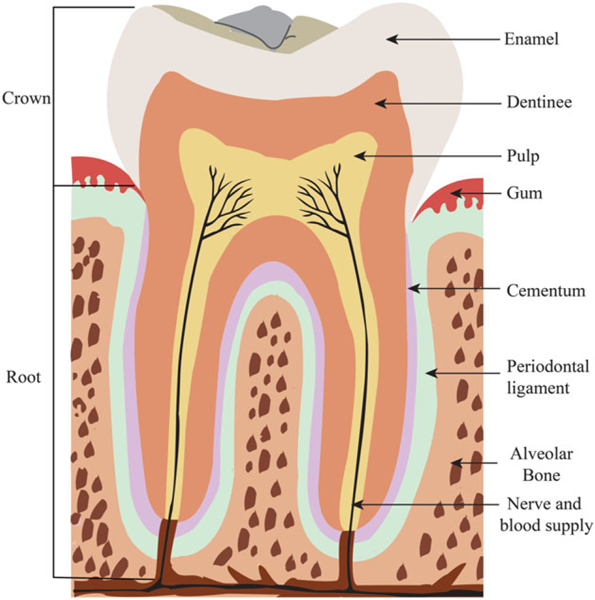
Adult human tooth morphology. [Colour figure can be viewed at wileyonlinelibrary.com]
Humans exhibit a dyphyodont dentition pattern, consisting of bilateral tooth types including two incisors, one canine, two premolars and three molars (Takahashi et al., 2013). Teeth constitute approximately 20% of the surface area of the mouth and serve several functions, including mastication, speech and aesthetics (Nanci and Cate, 2003). In the animal kingdom, teeth have important roles as weapons of attack and for defence, and missing teeth therefore result in reduced quality of life (Lai et al., 2014). When teeth are lost, the preferred solution is natural tooth replacement, generated from the successional dental lamina at the lingual side of the preceding tooth (Jernvall and Thesleff, 2012). In contrast to mammals, which have a limited capacity for tooth renewal, many vertebrates, e.g. fish and reptiles, continuously replace teeth throughout their lives (Jernvall and Thesleff, 2012; Lai et al., 2014). In humans, successional teeth accommodate the growth of the face and jaws as a child’s deciduous teeth are replaced by more numerous and larger adult teeth (Nanci and Cate, 2003). Although enamel is resistant to abrasion and therefore prolongs the life of a tooth, tooth wear over time can result in a gradual loss of crown morphology, ultimately leading to tooth loss. Adult human teeth cannot be regenerated or regrown. Thus, to solve the problem of limited tooth renewal in humans, the development of technologies that allow for tooth regeneration and repair has become a major goal of dental TE and regenerative medicine in recent years.
3. Tooth-regeneration approaches
Natural tooth formation is a highly complicated molecular process, where the spatiotemporal expression and interactions of growth factors, cytokines and transcription factors direct macromorphological (crown size, tooth length) and micromorphological (cusp number and position, root formation) tooth development. Proper control of these interactions is needed in order to generate bio-engineered teeth of specified size and shape (Lai et al., 2014). Several approaches to engineer entire biological teeth have been proposed, including dental tissue-engineering scaffolds, stimulation of third dentition formation, cell-tissue recombination, chimeric tooth TE and gene-manipulated tooth regeneration (Hirayama et al., 2013; Lai et al., 2014; Li et al., 2014; Takahashi et al., 2013). However, the two major approaches currently used for tooth regeneration are cell-tissue recombination and tissue-engineering scaffold approaches (Honda et al., 2008; Nakahara and Ide, 2007; Otsu et al., 2014).
3.1. Dental cell-tissue recombination approach
This approach (Figure 3) relies on replicating the natural processes of tooth development, where a cultured progenitor stem cell-tissue construct is directly implanted into the defect site (Otsu et al., 2014). Two sources of stem cells, embryonic and postnatal/adult stem cells, have been used to regenerate bio-teeth (Duailibi et al., 2004; Honda et al., 2008; Iglesias-Linares et al., 2013; Young et al., 2002). Stem cells can be totipotent, pluripotent and multipotent. Totipotent stem cells can generate all types of differentiated cells in an organism. Pluripotent stem cells, also called embryonic stem cells (ESCs), derived from the blastocyst inner cell mass, have the capacity to form each of the three germ layers-endoderm, ectoderm and mesoderm. Multipotent stem cells, often called postnatal or adult stem cells, can give rise to a restricted number of differentiated cell types (Mitalipov and Wolf, 2009).
Figure 3.

Cell tissue recombination approach. Adapted from Nakao et al. (2007). [Colour figure can be viewed at wileyonlinelibrary.com]
Several studies have shown that functional teeth can be formed from embryonic tooth germ cells cultured in vitro and/or implanted in vivo (Hirayama et al., 2013; Nakao et al., 2007; Ohazama et al., 2004; Oshima et al., 2012). Ohazama et al. (2004) used this approach to replicate natural tooth development; in this study, E10 embryonic oral epithelium was shown to stimulate an odontogenic response in cultured non-dental mesenchyme (neural and bone marrow stem cells). Bone marrow stem cell-derived recombinants formed tooth crown structures comprised of enamel, dentine and pulp in three of 35 explants. In contrast, recombinants derived from ESCs or neural stem cells did not form teeth, but expressed odontogenesis-related genes. Implantation of these embryonic tooth primordia into the adult jaw resulted in complete tooth development. In another study (Nakao et al., 2007), a three-dimensional (3D) organ-germ culture method was used to bio-engineer tooth organs. High cell density suspensions generated from E14.5 incisor epithelial and mesenchymal tissues, either cultured in vitro or transplanted in a subrenal capsule, formed bio-engineered teeth when implanted in the extraction socket of a rat mandibular incisor. Although these studies demonstrated the potential of using ESCs for tooth regeneration, several major concerns included possible tumourigenesis when transplanted; ethical issues regarding the use of human embryos and allogeneic immune rejection (Otsu et al., 2014; Zhang et al., 2010). The development of induced pluripotent stem cells (iPSCs) for tooth TE could overcome many of these issues (Takahashi and Yamanaka, 2006; Takahashi et al., 2007). iPSCs can be artificially induced from a patient’s cells and developed into cells required for treatment (Nakahara, 2011). As truly defined stem cells, iPSCs have shown pluripotency, with several characteristics related to differentiation potentials and self-renewal, similar to natural pluripotent ESCs. They are now expected to solve bioethical problems facing conventional ESC research and become beneficial for regenerative therapies using stem cells and tissue-engineering approaches based on a combination of biomaterials and signalling molecules. For further details about iPSCs for tooth regeneration, the reader is directed to a recent review that cites the significant contributions made in this field and future perspectives (Otsu et al., 2014).
3.2. Tissue-engineering approach
With advancements in the field of TE, tooth regeneration has become a realistic and attractive option for replacing lost or damaged teeth. Examples of TE strategies for tooth repair and regeneration are described in Table 1. TE is an inter-and multi-disciplinary field whose goal is to develop biological substitutes for the repair, restoration or regeneration of tissue function (Langer and Vacanti, 1993; Ma, 2008). TE approaches employ three basic components, cells, scaffolds and bio-active agents, to create tissues and organs similar to those of native human tissues and organs. Here we describe a variety of scaffold materials, designs, techniques and cell sources currently being used for tooth regeneration.
Table 1.
Examples of tissue engineering strategies for tooth repair and regeneration
| Material | Cell type | Results | Reference |
|---|---|---|---|
| PGA-PLLA | Pig tooth bud cells | This study demonstrated the first successful use of adult dental stem cells for the generation of tooth crowns containing dentine and enamel and suggested the presence of dental epithelial and mesenchymal stem cells in pig third molar tooth tissues | Young et al., 2002 |
| Alginate hydrogels | DPCs | TGFβ1 containing alginate hydrogels upregulated dentine matrix secretion and induced odontoblast-like cell differentiation, with subsequent secretion of regular tubular dentine matrix on cut pulpal surfaces | Dobie et al., 2002 |
| Collagen | – | Collagen scaffold induced successful regeneration of the periodontal tissues in a short period of time in dogs. Epithelial downgrowth and root resorption occurred and the defects were filled with connective tissue | Nakahara et al., 2003 |
| PGA and PLGA | Pig/rat tooth bud cells | Cell-seeded PGA or PLGA scaffolds were grown in the omenta of adult rat hosts for 12 weeks. Bioengineered pig tooth crowns containing dentine, pulp, and enamel formed in 25–30 weeks. Rat tooth crowns formed in just 12 weeks | Duailibi et al., 2004 |
| PGA-PLLA | Pig tooth bud cells | 3D computer modelling demonstrated a spatial organization of enamel, dentine and pulp resembling that of natural teeth | Young et al., 2005b |
| PGA/PLGA | Pig tooth bud cells and BMSCs | Tooth implants were generated from pig third molar tooth bud cells seeded onto PGA and PLGA scaffolds, and grown for 4 weeks in the omenta of adult rat hosts. Bone implants were generated from osteoblasts induced from bone marrow progenitor cells obtained from the same pig, seeded onto PLGA fused wafer scaffolds. The results showed that tooth development is not dependent on bone formation | Young et al., 2005a |
| Collagen and PGA | Pig molar tooth cells | Comparison of collagen and PGA scaffolds seeded with pig third molar tooth cells in vitro and in vivo. The in vivo results showed that the collagen scaffold allows tooth production with a higher degree of success than PGA scaffolds | Sumita et al., 2006 |
| Chitosan | Osteoblast | Chitosan monomer promoted tissue regeneration on dental pulp wounds | Matsunaga et al., 2006 |
| PGA | Embryonic tooth germs | Embryonic day 14 (E14) mice tooth germs were seeded into a PGA 3D scaffold and implanted under a kidney capsule in adult mice. The initial cell-proliferation patterns of the tissue-engineered teeth were similar to that at the cap and early bell stages in natural teeth | Iwatsuki et al., 2006 |
| PGA | Canine tooth bud | Cells were harvested from canine first molar tooth buds and the heterogeneous cell population was seeded on a PGA scaffolds and implanted into the same sockets after extracting the tooth buds. The biological results showed formation of dentine and bone, but enamel tissue and dental root formation were not observed | Honda et al., 2006 |
| Collagen | Pig dental epithelium and mesenchyme cell |
Mesenchymal cells were seeded onto the surface of the scaffold and epithelial cells were seeded on top to allow the direct contact of the two cell types. The results showed that the tooth morphology in vivo was similar to that of natural tooth, and only one tooth structure formed in each scaffold | Honda et al., 2007 |
| Chitosan-coral | Human PDLC | Chitosan-coral composites combined with plasmid encoding platelet-derived growth factor B were evaluated in vitro and in vivo in athymic mice. Results demonstrated the potential of the scaffold as a good substrate candidate in periodontal tissue regeneration | Zhang et al., 2007 |
| PLLA | SHED | SHED seeded in PLLA scaffolds prepared within human tooth slices were transplanted into immunodeficient mice. The resulting tissue presented architecture and cellularity that closely resemble those of a physiological dental pulp | Cordeiro et al., 2008 |
| Peptides | SHED and DPSC | SHED and DPSCs were cultured in peptide-amphiphile hydrogel scaffolds. The results showed that both cells proliferate and differentiate within the scaffolds, but distinct differences could be observed. The hydrogel scaffolds were easy to handle and could be introduced into small defects | Galler et al., 2008 |
| PGA-PLLAPLGA | Rat tooth bud cells | Comparison of bioengineered dental tissues grown in the mandible vs the omentum revealed both similarities and differences. Both implant sites supported the formation of bioengineered dentine, enamel, pulp and periodontal tissues. However, omental implant dental tissues seemed more organized than those grown in the mandible | Duailibi et al., 2008 |
| Collagen | DPSCs | Triad of DPSCs, collagen scaffold and DMP1 induced an organized matrix formation similar to that of pulpal tissue in vivo | Prescott et al., 2008 |
| Collagen | DPCs | Dental pulp stem cells (DPCs) collagen scaffolds can completely restore human mandible bone defects | d’Aquino et al., 2009 |
| PGA-PLLAPLGA | Pig BMSC, DMCs, DECs | Tooth and bone constructs were prepared from third molar tooth tissue and iliac crest bone marrow-derived osteoblasts isolated from, and implanted back into, the same pig as an autologous reconstruction. Small tooth structures were identified and consisted of organized dentine, enamel, pulp and periodontal ligament tissues, surrounded by new bone | Abukawa et al., 2009 |
| P(EMA), P(HEA), P (EMA-HEA)-silica | – | The tubular porous scaffolds resembled natural dentine with regard to its structure and properties and induced the precipitation of apatite on their surfaces in vitro | Lluch et al., 2009 |
| PGA-PLLA | Epithelial and mesenchymal DSCs | In vivo results showed the formation of small tooth-like structures consisting of organized dentine, enamel, pulp, cementum and periodontal ligament and surrounded by regenerated alveolar bone, suggesting the possibility of tooth regeneration and associated alveolar bone in a single procedure | Zhang et al., 2009 |
| HA-coated P(EMA-HEA)-silica | – | Scaffolds were implanted subcutaneously into immunocompromised nude mice for 4, 6 and 8 weeks. The result showed that the scaffold ultrastructural pattern imitates dentinal histological structure | Valles et al., 2010 |
| PGA-PLLA | Epithelial and mesenchymal DSCs | Co-cultured 3D DE-DM constructs maintained their predetermined shape and size. The data analyses suggested that DE and DM cells expressed proper dental tissue markers under both in vitro and in vivo culture conditions | Zhang et al., 2010 |
| Hyaluronic acid | Odontoblastic | Hyaluronic acid scaffold showed an appropriate structure, biocompatibility and biodegradation for dental pulp regeneration | Inuyama et al., 2010 |
| HA-coated P(EMA-HEA)-silica | – | Scaffolds were implanted subcutaneously into immunocompromised nude mice for 4, 6 and 8 weeks. The materials allowed cell colonization and neoangiogenesis. Such materials seem promising in tissue-engineering strategies for dentine regeneration | Valles-Lluch et al., 2010 |
| PCL-gelatin-HA | DPSCs | The performance of dental pulp stem cells on nanofibre PCL-gelatin-HA scaffolds was investigated in vitro and in vivo. The biological results showed that all implants were surrounded by a thin fibrous tissue capsule without any adverse effects and incorporation of nano-HA in nanofibres enhanced DPSCs differentiation towards an odontoblast-like phenotype | Yang et al., 2010 |
| Collagen and chitosan | Epithelial and mesenchymal stem cells | A 3D multilayered co-culture system was developed to study epithelial- mesenchymal interactions that occur during tooth morphogenesis. This system influenced the migration, proliferation and differentiation properties of the epithelial and mesenchymal cells in vitro and also permitted neovascularization in vivo | Ravindran et al., 2010 |
| HA-PCL | – | Dual GFs delivered from a 3D bioprinting scaffold with 200 μm diameter interconnecting microchannels. In vivo implantation results showed the regeneration of tooth-like structures and periodontal integration by cell homing, new bone formation and vessel formation | Kim et al., 2010c |
| Silk | Human DPCs | Silk porous (500 and 1000 μm diameter) scaffolds, with either RGD or DMP-1 peptide protein modification, were systematically investigated in vitro and in vivo. The biological results demonstrated that all silk scaffolds were well tolerated by host animals, whether they were cell-seeded or not | Zhang et al., 2011 |
| Chitosan | Osteoblast | Alendronate-loaded chitosan scaffolds achieved the dual functions of improvement in osteoblast functions and inhibition of osteoclast differentiation | Kim et al., 2012 |
| Decellularized scaffold |
Porcine DMCs | Decellularized porcine tooth bud scaffolds were reseeded with porcine tooth bud matrices. The results indicated that more dense patches of collagen were detected in areas of the reseeded scaffold when compared with unseeded scaffolds. This means that the dental cells established residence in the decellularized scaffold and remodelled the matrix | Traphagen et al., 2012 |
| Alginate hydrogel | PDLSCs and GMSCs | It was developed an injectable and biodegradable scaffold based on oxidized alginate microbeads encapsulating PDLSCs and GMSCs. The biological results showed that alginate is a promising candidate as a non-toxic scaffold for PDLSCs and GMSCs | Moshaverinia et al., 2012 |
| Fibrin | Tooth bud cells | Tooth bud cells were suspended in fibrin glue and the scaffold was autografted back into the original alveolar sockets of a pig. The pig developed a complete tooth with crown, root, pulp, enamel, dentine, odontoblast, cementum, blood vessels and periodontal ligaments in indiscriminate shape | Yang et al., 2012b |
| PLGA microspheres | Tooth pulp cells | The effect of the controlled release of FGF-2 and TGF/S1 from microspheres on permanent tooth pulp cell proliferation and migration was investigated. The results showed the usefulness of growth factor-controlled release in investigating the early events of pulp/dentine regeneration | Mathieu etal., 2013 |
| Collagen | iPS | The hanging drop method on a collagen scaffold combined with BMP-4-induced mouse iPS cells to form odontoblast-like cells without epithelialmesenchymal interaction | Ozeki et al., 2013 |
| HA-PLGA nanofibres | Epithelial and mesenchymal DSCs | The effects of fibre alignment and HA content in biodegradable electrospun PLGA scaffolds were investigated in vitro. Scaffold porosity was sufficient to allow migration of mesenchymal cells. HA incorporation did not have a positive effect on cell proliferation, especially of epithelial cells, but seemed to promote differentiation | van Manen et al., 2014 |
| Alginate hydrogel | DPSC | DPSCs immobilized in alginate hydrogels exhibit enhanced osteogenic potential while maintaining high cell viability | Kanafi et al., 2014 |
| Decellularized scaffold |
DPSCs and PDLSC | Subcutaneous implantation of the decellularized scaffolds containing DPSCs formed dental pulp-like tissue with cells expressing dentine sialoprotein and dentine phosphophoryn | Ravindran et al., 2014 |
PGA-PLLA, polyglycolate-poly-L-lactate; PLGA, poly-L-lactate-coglycolate; PDLSCs, periodontal ligament stem cells; GMSCs, gingival mesenchymal stem cells; DPCs, dental pulp stem/progenitor cells; DPSCs, dental pulp stem cells; TGFβ1, transforming growth factor-β1; FGF-2, fibroblast growth factor; P(EMA-HEA), polyethyl methacrylate-co-hydroxyethyl acrylate; SHED, stem cells from human exfoliated deciduous teeth; DMCs, dental mesenchymal cells; DECs, dental epithelial cells; PCL-TCP, polycaprolactone-tricalcium phosphate; DMP1, dentine matrix protein 1; HA, hydroxyapatite.
3.2.1. Adult stem cells
In some TE applications it is preferable to use autologous primary cells, obtained from the patient, which can be used directly by injection or in combination with scaffolds, without any in vitro cell culture. However, when a patient’s cells are in a diseased state, or would require excessively invasive cell-collection procedures, alternative stem cell sources are required (Howard et al., 2008). Indeed, much attention has been focused on the use of ESCs and postnatal/adult stem cells for TE applications. As was described above, the successful generation of a single bio-engineered tooth using embryonic mouse tooth bud cells is quite notable. However, it is difficult, if not impossible, to obtain autologous embryonic human ESCs for tooth regeneration (Zhang et al., 2010), in addition to other concerns, including ethical issues, tumourigenesis and potential allogeneic immune rejection. Therefore, autologous, multipotent adult stem cells remain the standard for use in TE and regenerative medicine, including bone marrow mesenchymal stem cells (BMSCs), umbilical cord-derived mesenchymal stem cells (UC-MSCs) and dental stem cells (Iglesias-Linares et al., 2013).
MSCs are a relatively new stem cell population that have been isolated from various dental tissues (Saito et al., 2015; Yang et al., 2014). Postnatal dental pulp stem cells (DPSCs) were the first type of MSCs of dental origin to be described (Gronthos et al., 2000). DPSCs produced only sporadic, but densely calcified nodules, and did not exhibit the capacity to form adipocytes, as compared to BMSCs that routinely calcified throughout the adherent cell layer with clusters of lipid-laden adipocytes. Moreover, DPSCs transplanted into immunocompromised mice generated a dentine-like structure, lined with human odontoblast-like cells that surrounded a pulp-like interstitial tissue. In contrast, BMSCs formed lamellar bone, containing osteocytes and surface-lining osteoblasts, surrounding a fibrous vascular tissue with active haematopoiesis and adipocytes (Gronthos et al., 2000). Periodontal ligament stem cells (PDLSCs) can differentiate into cementoblast-like cells, adipocytes and collagen-forming cells, and have the capacity to generate a cementum-PDL-like structure and contribute to periodontal tissue repair (Seo et al., 2004). Stem cells from human exfoliated deciduous teeth (SHEDs) were identified as a population of highly proliferative, clonogenic cells capable of differentiating into a variety of cell types, including neural cells, adipocytes and odontoblasts (Miura et al., 2003). SHEDs were found to be able to induce bone formation, generate dentine and survive in mouse brain after in vivo transplantation. SHEDs are a quite promising type of stem cell, in that they are not only derived from a very accessible tissue resource (autologous baby teeth) but are also capable of providing enough cells for potential clinical applications (Miura et al., 2003). Dental follicle progenitor stem cells (DFPCs) expressed higher amounts of insulin-like growth factor-2 (IGF-2) transcripts than hBMSCs and, after in vivo transplantation in immunocompromised mice, DFPCs expressed osteocalcin (OCN) and bone sialoprotein (BS), but without any sign of cementum or bone formation (Morsczeck et al., 2005). Stem cells from the apical papilla (SCAPs) proliferate two-to three-fold more than those obtained from the pulp organ, and are as potent in osteo/dentinogenic differentiation as BMSCs but are weaker in adipogenic potential (Sonoyama et al., 2008). Immunophenotypically, SCAPs are similar to DPSCs with respect to osteo/dentinogenic and growth factor receptor gene profiles, and they also express STRO-1 and dentinogenic markers, such as bone sialophosphoprotein, osteocalcin and the growth factor receptors FGFR1 and TGFbRI. Moreover, SCAPs express a wide variety of neurogenic markers, such as nestin and neurofilament M, upon stimulation with a neurogenic medium (Sonoyama et al., 2008). Based on these promising characteristics, tooth TE efforts are being focused on creating bio-teeth and supporting structures using SCAP populations combined with non-dental stem cells (i.e. BMSCs and UC-MSCs).
To date, two dental cell types, DPSCs and SHEDs, have been reported to differentiate into odontoblasts (Lee et al., 2011; Miura et al., 2003). The tooth pulp contains adult DPSCs, which are undifferentiated ectomesenchymal cells that can differentiate into odontoblasts (Eslaminejad et al., 2010). Various factors, such as rat incisor tooth germ cells and acid-soluble tooth proteins, regulate the fate of DPSCs (Lee et al., 2011). Also, odontoblast differentiation is regulated by various factors, such as DMP-4, nerve growth factor and BMP-2 (Lee et al., 2011). It has been suggested that hDPSCs attached to the glass substrate in response to odontogenic medium (Jones et al., 2015). Moreover, a 3D culture system using a scaffold improved the odontogenic differentiation of DPSCs (Eslaminejad et al., 2013; Ravindran et al., 2014). Despite all these studies using dental stem cells for TE, the best method to differentiate and select the most appropriate MSC type for regenerating dental tissues is yet to be defined. Although dental stem cells present a common marker profile, they differ in their clonogenicity, proliferative ability and differentiation potential in vitro and in vivo. This suggests that, although there are MSC-related specific surface markers associated with hierarchical commitment to differentiation pathways of dental stem cells, these properties are related to the microenvironments of origin of each cell lineage (Saito et al., 2015). Moreover, even in the same population of MSCs, there are heterogeneous cell subpopulations with distinct differentiation potentials (Okamoto et al., 2002).
Thus, an understanding of the regulation of MSCs during differentiation and dental development is required in order to develop approaches for dental tissue regeneration with predictable outcomes (Saito et al., 2015). Some fundamental issues must be determined to better understand odontoblast differentiation by dental epithelial factors. For instance, the developmental stage of dental epithelial cells that secretes the most effective factors for the odontogenic differentiation of DPSCs must be identified. These fundamental issues are important to understand normal odontoblast differentiation but also to repair dentine defects.
3.2.2. Natural and synthetic polymeric scaffolds
Direct delivery of cells to in vivo locations exposes the cells to a host of survival challenges. Immune issues related to inflammation and auto-immunity are among the first pathways of assault and destruction of therapeutically delivered cells. Transplanted cells must survive the initial immune response onslaught and, at the same time, interact with the adaptive immunity cascade to avoid being targeted for elimination (Petrie Aronin et al., 2011). In a typical TE methodology, progenitor cells are seeded in or onto a scaffold prior to transplantation in order to repopulate a defect and/or restore function (Dvir et al., 2011). Therefore, a scaffold provides a physical support for the development of new tissues in a manner that mimics the function of the natural ECM. TE strategies based on the combination of scaffolds, cells and bio-active agents have grown remarkably in recent years, leading to significant advances in the field of tooth regeneration (Yuan et al., 2011; Zhanga et al., 2013). It is of extreme importance to consider the physical aspects and composition of the biomaterial scaffold for successful tissue regeneration (Zhanga et al., 2013). The scaffolds must be designed to ensure mechanical integrity and functionality, and the scaffold surface needs to have appropriate properties for proper cell adhesion, proliferation and differentiation. Indeed, cell fate is strongly influenced by scaffold substrate rigidity, which can promote enhanced cell spreading (Fu et al., 2010). Thus, the selection of biomaterials is a critical factor to consider when determining the suitability of a scaffold to mimic the ECM.
Natural and synthetic biomaterials have been examined for their utility for bio-engineered tooth regeneration (Galler et al., 2010; Lai et al., 2014; Yuan et al., 2011; Zhanga et al., 2013). The foremost requirements of the biomaterials used as TE scaffolding are that they must be inherently biodegradable and biocompatible. Moreover, the biomaterial scaffolds must be porous, to allow cell colonization and migration, and mechanically stable, allowing for their manipulation, adaptation and integration in the defect site. Scaffolds are made in different forms, such as fibres, foam and gel. The major advantage of using natural-based vs synthetic scaffolds is the ability to tune their degradation rate, which can be readily achieved by varying the concentration of the polymer and/or crosslinking agents. The most commonly used natural biomaterials in TE scaffolding for tooth regeneration include collagen, alginate, fibrin, chitosan, gelatin, silk, peptides and hyaluronic acid (Table 1). Synthetic scaffolds also have been investigated, mainly because of their processing flexibility and ability to be manufactured in many desired shapes and sizes and with predefined architecture and structural parameters (Zhanga et al., 2013). The most commonly used synthetic polymers for TE scaffolding include Polylactic acid (PLA), Polyglycolic acid (PGA), Poly-L-lactide (PLLA), Poly (lactic-co-glycolic acid) (PLGA) and Polycaprolactone (PCL). Inorganic materials, such as calcium phosphate ceramics, bio-active glass and ceramic-polymer composites have been especially developed for tooth and bone TE applications (Polini et al., 2013; Sowmya et al., 2013). The manufacturing methods for fabricating polymeric 3D scaffold for TE applications have been widely reviewed in the literature (Ma, 2008). The conventional methods include solvent casting, fibre bonding, melt moulding, phase separation, particulate leaching, gas foaming, high-pressure processing, electrospinning and rapid prototyping (Chung and Park, 2007; Ma, 2008). Some TE scaffolds have been also produced using nanofabrication techniques that create porous and nanometer-sized features that influence cell fate, allowing for the regulation of specific gene and protein expression patterns (Gillette et al., 2011; Kelleher and Vacanti, 2010). Surface-patterning techniques involve a wide range of fabrication methods, including electron-beam lithography, nano-imprint lithography, photolithography combined with micro-electrical mechanical systems, nano-contact printing, micromachining and 3D printing (Kelleher and Vacanti, 2010).
Hydrogels, made of natural/synthetic biomaterials, are a particular class of scaffolds exhibiting huge potential for applications in tooth regeneration, as smart and stimuli responsive systems (Bidarra et al., 2014; Dobie et al., 2002). They are extremely versatile and adaptable biomaterials. A variety of strategies have been developed to decorate them with biofunctional moieties and to modulate their biophysical properties (Bidarra et al., 2014). Moreover, they can be used as injectable materials, which offer several advantages, such as: easy incorporation of therapeutic agents, such as cells, under mild conditions; minimally invasive local delivery; and high contourability, which is essential for filling in irregular defects (Bidarra et al., 2014; Galler et al., 2008).
Tooth scaffolds can be combined with appropriate cells and bio-active agents to initiate the formation of new tissue or tissues that can integrate with the surrounding tissues. Recently, our group has been focusing on ways to establish proper dental epithelial (DE)-mesenchymal (DM) cell interactions, using a variety of scaffold materials (Duailibi et al., 2004; Young et al., 2002; Zhang et al., 2011). Our published results showed that dental cells obtained from dissociated porcine or rat tooth buds were capable of generating multiple, small, organized tooth crowns (Duailibi et al., 2004; Young et al., 2002, 2005a). In these studies, cultured DE and DM cells were combined, seeded onto biodegradable polyester scaffolds, transplanted and grown in the omentum of immunodeficient rat hosts. These studies showed that instead of forming one tooth of size and shape similar to the scaffold, small tooth crowns were formed throughout the implant. Similarly, multiple teeth of normal and abnormal morphology were generated when mixed populations of DE and DM pig tooth bud cells were seeded onto collagen sponge scaffolds, which also exhibited tooth root-like structures (Sumita et al., 2006). The tooth scaffolds used in our prior studies lacked the ECM molecule gradients that are present in naturally formed teeth, and which provide essential cues for proper tooth development, periodontal tissues and surrounding alveolar bone (Zhang et al., 2011). Thus, our ongoing work will exploit the effect of bio-active agents that mimic natural ECM gradients, combined with scaffolds and dental stem cells, to promote tooth regeneration.
3.2.3. Bio-active agents for tooth regeneration
The molecular signals that control odontogenesis are still not entirely clear, and fully deciphering the network of regulatory events may be quite challenging (Nanci and Cate, 2003). However, with the scientific advances in embryonic, neonatal and adult stem cell biology, these molecular signals are increasingly being better understood, and this information is being used as the basis for approaches to bio-engineer biological replacement teeth (Sartaj and Sharpe, 2006). Odontogenesis involves the expression of different bioactive agents, including growth and transcription factors from several signalling families, throughout all stages of tooth development (Chai and Slavkin, 2003). At least 12 transcription factors are expressed in odontogenic mesenchyme (Nanci and Cate, 2003) and >200 genes have been identified that are expressed in the oral epithelium, dental epithelium and dental mesenchyme during the initiation of tooth development (Takahashi et al., 2013). Therefore, controlled release of selected bio-active agents from biodegradable scaffolds can be used to enhance the efficacy of tooth TE approaches (Lai et al., 2014). TE scaffolds can be used as bio-active agent reservoirs combined with the delivery cells, providing a multitude of advantages, such as a safe delivery profile, protection of bio-active agents from biodegradation and the ability to deliver the bio-active agents locally where the cells are attached (Kulkarni et al., 2010). This type of multifunctionalized system can be used to create a highly regulated network of signals able to orchestrate dental cell migration, proliferation and differentiation, directing the development of a fully functional tooth. In the next section, we describe the bio-active agents (growth factors and genes) that have been combined with scaffold for tooth regeneration.
3.2.3.1. Growth factors.
The most commonly used bio-active agents are growth factors (GFs). They are critical to the development, maturation, maintenance and repair of craniofacial and dental tissues, because they establish the communication between cells and tissues (Nanci and Cate, 2003). GFs are proteins known for their roles in cell migration, differentiation, proliferation, gene expression and organization of functional tissues (Chen et al., 2011; Stevens and George, 2005). Important for the use of stem cells in TE strategies is understanding which of the GFs resident in the dental stem cell niche provide cues that control their fate. For instance, dentine retains its regenerative capacity to a certain degree throughout adulthood, which is thought to be attributed to the production of certain GFs by dental stem cells, thus maintaining their proliferation and differentiation potential (Nanci and Cate, 2003). The dental mesenchymal cells in the tooth pulp are stimulated to differentiate into odontoblasts that deposit dentine. The pulp reacts to this aggression by producing tertiary dentine in a centripetal direction. The dentine does not regenerate when the pulp tissue is lost due to caries. Therefore, GFs can be incorporated into TE scaffolds to regulate their exposure to stem cells. The GFs act by binding to the extracellular domain of a target GF receptor, which in turn activates intracellular signal transduction pathways (Chen et al., 2011). Many GFs, such as bone morphogenetic protein (BMP), transforming growth factors (TGF), fibroblast growth factor (FGF) and vascular enthothelial growth factor (VEGF), have been found to be expressed during tooth formation and repair (Chen et al., 1996; D’Souza et al., 1990; Lai et al., 2014). Therefore, tooth regeneration may be facilitated by incorporating a broad spectrum of GFs into scaffolds to act locally as regulators of the most basic cellular functions. Long-term stability, dosage and proper regulation of multiple GF gradients in a specific spatiotemporal pattern are the main challenges in dental TE (Ravindran and George, 2015). In this section, we describe some of the growth factors that are closely associated with the odontogenic potential during tooth development and has been attracted extensive attention (Lai et al., 2014).
BMP4 has been shown to be able to substitute for the inductive functions of the dental epithelium, including the induction of morphological changes in the dental mesenchyme, and to rescue tooth defects in Msx1 mutant mice (Chen et al., 1996). Ohazama et al. (2005) showed that the addition of BMP4 to intact second pharyngeal arch explants resulted in the development of organized structures containing layers of cells that express marker genes of tooth-specific cells, odontoblasts and ameloblasts. Although whole tooth formation did not occur, BMP4 had the ability to stimulate organized differentiation of dental epithelial and mesenchymal cells from non-dental primordia. In vivo transplantation of BMP2-treated pellet cultures onto amputated pulp was found to direct pulp progenitor/stem cell differentiation into odontoblasts, and to stimulate dentine formation (Iohara et al., 2004). Comparison of DPSCs cultured on PLLA nanofibre scaffolds in medium containing dexamethasone, or BMP7 plus dexamethasone, both resulted in DPSCs differentiation into odontoblast-like cells (Wang et al., 2010). PLLA nanofibre scaffolds combined with odontogenic inductive factors may provide an excellent environment for DPSCs to regenerate dental pulp and dentine. Prescott et al. (2008) showed that dentine matrix protein 1 (DMP1) combined with DPSCs and a collagen scaffold induced organized matrix formation similar to that of pulpal tissue in vivo. In another study, stromal-derived factor-1 (SDF1) and BMP7 were delivered from 3D bioprinted tooth scaffolds made of PCL and hydroxy-apatite with 200 μm diameter interconnecting microchannels (Kim et al., 2010c). SDF1-and BMP7-loaded scaffolds were implanted both orthotopically at the rat incisor root and ectopically into the dorsum of rats. The biological results showed that, after 9 weeks, a putative periodontal ligament and new bone regenerated at the interface of the rat incisor scaffold with native alveolar bone. Kim et al. (2010b) reported complete filling of dental pulp-like tissue in the entire root canal from the root apex to the pulp chamber, including tissue integration to the dentinal wall, upon delivery of basic fibroblast growth factor (bFGF), VEGF, or platelet-derived growth factor (PDGF) with a basal set of nerve growth factors (NGFs) (Kim et al., 2010b). These studies suggest that anatomically correct dental pulp and dentine regeneration could be accomplished by using scaffolds loaded with GFs alone, and without the need for additional cells, as an alternative approach to cell delivery. However, this strategy needs further experimental investigations to explore its feasibility and efficacy (Mao et al., 2010).
TGFβ1 combined with transfilters was demonstrated to induce odontoblast differentiation and dentine formation (Li et al., 2011). This study indicates that the substrate for the inductive signals that initiate dental stem cell differentiation should be taken into consideration when designing optimized scaffold materials for dentine TE. In another study, acid-treated alginate hydrogels combined with either exogenous or endogenous TGFβ1 were used to regenerate the dentine-pulp complex (Dobie et al., 2002). The results showed that TGFβ1 can induce odontoblast-like cell differentiation, as well as upregulation of dentine matrix secretion in the human dentine-pulp complex. Therefore, alginate hydrogels provide a suitable matrix in which dental regeneration can take place. Moreover, this system could be useful for delivery of GFs to enhance the natural regenerative capacity of the dental pulp (Dobie et al., 2002).
The role of VEGF in tooth development and repair has been reviewed (Lai et al., 2014). VEGF is an angiogenetic factor that promotes vascularization, osteoblastic differentiation and bone regeneration (Lai et al., 2014). It is expressed predominately in dental epithelial cells in bud-and cap-stage tooth development. During the bell stage, VEGF is highly expressed in pre-ameloblasts and pre-odontoblasts, and later localizes mainly in differentiated ameloblasts and odontoblasts. In root formation-stage teeth, VEGF is expressed along the root dentine of the periodontal ligament (Lai et al., 2014). SHEDs alone, when seeded in tooth slice/scaffolds and implanted subcutaneously into immunodeficient mice, differentiated into functional odontoblasts and vascular endothelial cells (Sakai et al., 2010). When supplemented with VEGF, SHEDs differentiated into VEGF receptor-2-positive and CD31-positive endothelial cells in vitro (Bento et al., 2013; Sakai et al., 2010). Moreover, VEGF receptor 1-silenced SHEDs showed a reduction in human CD31-positive blood vessels in vivo. These studies demonstrate that the VEGF signalling pathway is a key regulator of endothelial cell-controlled differentiation of DPSCs. Scaffold biomaterials could be used to control the delivery of GFs such as VEGF for tooth TE (Lai et al., 2014).
During tooth development, FGF2 stimulates proliferation in the dental mesenchyme during the bud and cap stages in mouse tooth germ (Tsuboi et al., 2003). FGF2 localizes to dentine and enamel extracellular matrix in higher levels than in the enamel matrix or dental epithelial cells. FGF2 is thought to be responsible for the differentiation of the inner enamel epithelial cells into ameloblasts, which subsequently secrete amelogenin to form the enamel matrix (Tsuboi et al., 2003). For more information about the roles of the FGF family in tissue regeneration, the reader is directed to reviews by Yun et al. (2010) and Sonmez and Castelnuovo (2014). It has been demonstrated that FGF2 alone can significantly affect DPSCs proliferation in vitro, whereas TGFβ1 alone and FGF2 plus TGFβ1 stimulated odontoblastic differentiation (He et al., 2008). FGF has been immobilized in different scaffold materials (DeLong et al., 2005; Kim et al., 2010d). For example, Kim et al. (2010d) reversibly immobilized bFGF via conjugated heparin on fibrous matrices made of PCL and gelatin crosslinked with genipin. The sustained release of bFGF from these fibrous matrices enhanced the proliferation of human umbilical vein endothelial cells (HUVECs) and induced the formation of blood vessels in vivo (Kim et al., 2010d). Functionalized bFGF was also covalently immobilized in PEG-RGD hydrogel scaffolds by reaction with acryloyl-PEG-NHS (DeLong et al., 2005). In vitro analyses revealed that vascular smooth muscle cells aligned on these hydrogels in the direction of increased tethered bFGF concentration. In another study, immobilization of bFGF at the surface of PLGA scaffolds via plasma treatment resulted in increased cell adhesion and proliferation (Shen et al., 2008). Together, these systems reveal methods for the spatiotemporally controlled delivery of bio-active agents for tooth regeneration.
3.2.3.2. Gene delivery.
The signalling pathways and genes that control tooth development are highly conserved among different species (Jussila et al., 2014). The study of several model systems with continuous tooth replacement, such as zebrafish, snake, lizard, alligator and ferret, and of supernumerary teeth in human, have helped to identify signalling pathways and genes regulating tooth development and regeneration, which may hold promise for successful tooth regeneration strategies in humans (Jussila et al., 2014; Takahashi et al., 2013; Wu et al., 2013). The earliest dental mesenchymal markers for tooth formation are the transcription factors Lhx6 and Lhx7 (Grigoriou et al., 1998). It has been demonstrated that Fgf8 can induce the expression of Lhx6 and Lhx7 in vitro (Grigoriou et al., 1998). It has also been demonstrated that Lhx6 acts as a transcriptional repressor and represses the expression of several odontogenic genes, such as Pitx2 (Zhang et al., 2013). Lhx6-null mice were found to exhibit specific defects in incisor, molar, mandible, bone, tooth root development and late stage enamel formation, suggesting that Lhx6 may regulate cell proliferation and promote cell differentiation in the anterior region of the incisor (Zhang et al., 2013). The Pax9 transcription factor gene, which co-localizes to sites where the tooth germs appear, is induced by Fgf8 and repressed by BMP2 and BMP4 (Nanci and Cate, 2003). Aberg et al. (2004) reported that the transcription factor Runx2 is one of the mesenchymal factors that influences tooth morphogenesis and the subsequent differentiation of ameloblasts and odontoblasts. Mice missing the Runx2 gene lack bone and tooth development, developing teeth fail to advance beyond the bud stage and mandibular molar tooth organs were more severely affected than maxillary molar tooth organs (Aberg et al., 2004). In another study, it was reported that Runx2 prevents the formation of Shh-expressing successional tooth bud formation (Wang et al., 2005). In a study using ferret as model, Pitx2 and Foxi3 were found to be expressed in the interdental lamina, indicating that morphogenesis of the interdental lamina and the primary teeth are intimately linked (Jussila et al., 2014). Also, Bmp4 was co-expressed with the stem cell factor Sox2, suggesting that the interdental lamina may retain competence for tooth initiation. The Wnt pathway was activated in the budding successional lamina and adjacent mesenchyme when tooth replacement was initiated, but Fgf or Eda signalling was not activated. Moreover, the transcription factor Runx2 was mostly expressed in the mesenchyme around the successional lamina (Jussila et al., 2014). Together, these studies show that tooth development is controlled by a variety of signalling mechanisms and genes. However, the precise molecular mechanisms by which tooth regeneration is controlled are still to be precisely defined.
The expression of specific genes results in the production of specific GFs (Nanci and Cate, 2003). Therefore, GFs have an important role in TE and regenerative medicine. However, the use of GFs to stimulate stem cell differentiation in vivo has some limitations, including: short half-lives; denaturation during the encapsulation processes; their use can be time consuming and expensive, require supraphysiological doses, require long periods of time to obtain the differentiated cells and effectively require the use of a combination of GFs; and difficulty in differentiating cells into one specific lineage (Chen et al., 2011; Storrie and Mooney, 2006; Yau et al., 2012). Gene therapy has been proposed as a means to overcome these limitations and to control stem cell differentiation. The principle of gene therapy is the insertion of a gene, containing a sequence that encodes for a specific protein, into the host cell genome to replace a hereditary genetic defect or to provide a new function in a cell, such as overexpressing GFs or killing cancer cells (Wang et al., 2004; Winn et al., 2005). Therefore, the DNA has to enter into the nucleus of the host cell to be transcribed into messenger RNA (mRNA) and ultimately translated in the cytoplasm (Wang et al., 2004). The use of gene therapy for tooth regeneration is an attractive concept (Takahashi et al., 2013). This strategy relies on activating or repressing endogenous dental cell gene expression. Gene delivery can be performed either by an ex vivo approach or by direct cell targeting. In the ex vivo approach, autologous cells recovered from the patient’s body are generally transfected with viral vectors containing recombinant genes, and subsequently transplanted into the target tissue (Winn et al., 2005). Efficient gene transfection to neural crest cell (NCC)-derived dental mesenchymal cells has recently been reported (Takahashi et al., 1998). Gene therapies involving transcription factors can offer advantages over strategies that require the delivery of DNA encoding sets of proteins (Monteiro et al., 2014c). Transcription factors, which drive gene expression and native protein production, would ensure that the expression of all natural splice variants occurs in a coordinated time and sequence, and may regulate a cascade of multiple genes, all of which may be advantageous for tooth regeneration. Thus, transcription factor gene delivery approaches may exhibit utility for tooth regeneration.
A relatively new way to induce stem cell differentiation is through the delivery of interference RNA (RNAi) (Yau et al., 2012). RNAi is a specific gene-silencing mechanism mediated by the delivery of chemically synthesized small interfering RNA (siRNA), small hairpin RNA (shRNA) or micro-RNA (miRNA) (Gomes-da-Silva et al., 2012; Yau et al., 2012). Concisely, plasmid DNAs can be delivered to continuously transcribe shRNAs, which are then spliced by endogenous dicer proteins to release the corresponding siRNA (Scholz and Wagner, 2012; Yau et al., 2012). RNAi acts by binding to nucleic acids, inhibiting gene transcription and translation (Gomes-da-Silva et al., 2012; Storrie and Mooney, 2006; Yau et al., 2012). Delivery of RNAi has been widely exploited in cancer research (Gomes-da-Silva et al., 2012). The main advantage of this approach is that the RNAi induces the silencing of targeted genes without integration into the host genome. However, delivery of RNAi also faces challenges, such as apparent differences in size of the siRNA (only about 21–23 bp), stability of the formed nucleic acid complexes and the location and mechanism of action (Scholz and Wagner, 2012).
TE scaffold systems can be used to deliver genes in a controlled and spatially localized manner (Monteiro et al., 2014b). This strategy has been widely used for tendon, ligament, skin, nerve, muscle, bone, cartilage and periodontal regeneration (Bonadio, 2000; Chen et al., 2011; Storrie and Mooney, 2006). For instance, porous chitosan-coral composites were combined with a plasmid encoding the PDGFB gene, seeded with human PDLCs and subcutaneously implanted into mice (Zhang et al., 2007). The results showed that the PDLCs proliferated better on the gene-loaded scaffolds than on the pure scaffolds, in the presence of increased expression of PDGFB in vivo. In another study, chitosan-collagen scaffolds were loaded with an adenoviral vector encoding human TGFβ1 and seeded with human PDLCs (Zhang et al., 2006). In vitro analyses showed that adenoviral vector TGFβ1-loaded scaffold exhibited the highest PDLC proliferation rate. After implantation, transfected human PDLCs not only proliferated but also recruited surrounding tissue to grow in the scaffold (Zhang et al., 2006). In another study, a thermosensitive chitosan hydrogel was used as an siRNA reservoir to silence RANK signalling for PDL regeneration (Ma et al., 2014). The cumulative in vitro release of siRNA from the hydrogel was 50% over 14 days, and high PDLC viability was observed for cells seeded on the siRNA-loaded scaffold. In vivo studies showed that the fluorescent signal from siRNA within the hydrogel remained for up to 14 days when subcutaneously implanted in mice (Ma et al., 2014). Cao et al. (2010) encapsulated siRNA into PCL nanofibres, and controlled release of siRNA was achieved for 28 days under physiological conditions. Seeded human embryonic kidney 293 cells were successfully transfected with the siRNA released from fibrous scaffolds, demonstrating that the encapsulated siRNA remained bio-active. Moreover, silencing efficiency of 61–81% was observed when compared to conventional siRNA transfection (Cao et al., 2010). All these reports demonstrate the potential of TE scaffolds to deliver DNA and siRNA, and provide strategies that could be used in tooth regeneration.
3.2.4. Bio-active agent-loaded particles
A fine tuning of bio-active agent delivery from scaffolds is required for successful TE results (Monteiro et al., 2014b; Santo et al., 2012c). Thus, the modality of bio-active agent presentation to cells has been recognized as a fundamental element in many TE applications. TE scaffolds can be designed to physically and chemically control the release of incorporated bio-active agents (Kulkarni et al., 2010). Bio-active agents are available to cells that come into contact with the matrix, providing a highly localized signal to control cell fate. The delivery of bio-active agents from scaffolds to promote tissue regeneration is often challenging, due to the complex fabrication processes required. For example, bio-active agents can be incorporated into the matrix or encapsulated into hollow nanofibres (Cao et al., 2010). A bio-active agent incorporated in the matrix has a strong burst release within the first few hours (Thakur et al., 2008), while a bio-active agent encapsulated into hollow nanofibres has better control of the release profile (Sirc et al., 2012). Various techniques to conjugate bio-active agents to biomaterial scaffolds have been developed ( Figure 4) (Eap et al., 2014; Santo et al., 2012a). Bio-active agents can be physically adsorbed or covalently immobilized to biomaterial scaffolds. Covalent immobilization of bio-active agents offers more control over the spatiotemporal distribution than does physical adsorption, in that immobilized bio-active agents can be released upon degradation of the matrix, or by hydrolysis of degradable links (Santo et al., 2012a, 2012b). Eap et al. (2014) functionalized PCL membrane nanofibres with neural growth factor, using the layer-by-layer technique, for bone and tooth regeneration. This technique allows the deposition of stable and low amounts of bio-active agents on a scaffold. The results showed that, along with the dental germ, tooth innervation was observed, which allows complete functionality and tissue homeostasis of the tooth, such as dentinal sensitivity, odontoblast function, masticatory forces and blood flow.
Figure 4.
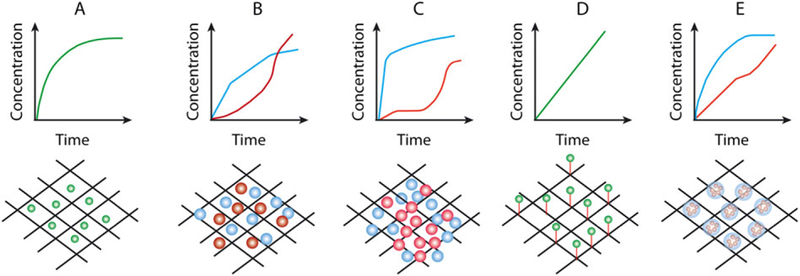
Strategies to incorporate and to promote the release of bio-active agents from biomaterial scaffolds. (A) Physical immobilization of bio-active agents leads to uncontrolled and rapid drug delivery kinetics. (B) Simultaneous or (C) sequential presentation of bio-active agents encapsulated into micro-and nanoparticles incorporated into scaffolds. (D) Bio-active agents or particulate carriers can be covalently bound to the scaffolds, resulting in a more controlled release mechanism. (E) Nanoparticles incorporated inside microparticles within the scaffold, promoting multiple release systems with distinct delivery rates. Adapted from Santo et al. (2012a). [Colour figure can be viewed at wileyonlinelibrary.com]
Another way to entrap bio-active agents into scaffolds is through the incorporation of micro-and nanoparticles. Research in the area of controlled drug delivery has been made tremendous progress due to the advancement of micro-and nanotechnologies (Shi et al., 2010). New biomaterials and formulations are enabling the production of particles for diagnostics, imaging, site-specific targeting and the controlled release of bio-active agents (Love et al., 2010; Monteiro et al., 2013; Sridharan et al., 2014). Smaller particles are more effective in promoting the release of bioactive agents than large particles, because they are closer to each other, reducing the distances required for diffusion (Santo et al., 2012a). Nanoparticles refer to particles with sizes in the range 1–100 nm (Kim et al., 2010a), but they also include sub-micron particles with sizes <1000 nm (Liu et al., 2008; Shi et al., 2010). There are many different types of particle delivery system, such as polymeric-based (i.e. nanocapsules, nanospheres, micelles and dendrimers) and lipid-based (i.e. liposomes and micelles) nanoparticles. The nanoscale nature of nanoparticles enables tuning of release kinetics, improved transport properties, diffusivity, solubility, regulated bio-distribution, minimization of toxic side effects and enhanced therapeutic index of bio-active agents (Santo et al., 2012a, 2012b). Also, nanoscale particles dispersed in the matrix can enhance the mechanical properties of the scaffold, providing better injectable and mouldability, and interactions with native nanoscale components (Liu et al., 2008; Yang and Webster, 2009). Other relevant properties of nanoparticles include the possibility of developing stimulus-responsive release systems, based on pH, temperature, ionic strength and light, to trigger the release of bio-active agents (Motornov et al., 2010). The combination of nanotechnology and stem cell biology is promising in regenerative medicine. However, the application of nanoparticle delivery systems for tooth regeneration has yet to be explored. The combination of TE scaffolds with nanoparticle delivery systems with stem cells allows for the creation of multifunctionalized systems that could be used in tooth regeneration. Proof of concept of these multifunctionalized systems approaches has been demonstrated in studies of osteogenic and chondrogenic differentiation of MSCs for bone and cartilage regeneration (Monteiro et al., 2014a, 2014c; Santo et al., 2012c).
In particular, a gene delivery system for stem cell transfection must take into consideration the negative charge of the cell membrane, the specific cell to be targeted, the stability of the DNA in the cytoplasm, DNA transport into the cell nucleus and the induced expression of the target gene. Since DNA and RNAi are poorly taken up by the host cells, and are subjected to degradation in the presence of blood proteins (Podesta and Kostarelos, 2009), viral and non-viral vectors are being used as carriers to transfect cells (Jang et al., 2004). Viral gene delivery methods (adenovirus, lentivirus, and retrovirus) are commonly used to transfer genes to stem cells, because of their high transgene expression and transduction efficiency. Indeed, these methods are preferred when the intention is to correct a genetic pathology by expressing the therapeutic gene for the duration of a patient’s life (Santos et al., 2011). In the case of adenoviruses, they are suitable for short-term gene delivery because the infection is generally non-toxic and self-limiting, and adenoviruses have been used in TE to transfect stem cells over the course of several weeks (Takahashi et al., 2013). Although the viral method is more efficient, viral vectors possess size limitations on the inserted transgene, safety issues including mutagenesis, high production cost, and raise concerns regarding immunogenicity of the virus proteins, lack of desired tissue selectivity and the possibility of generating infectious viruses due to recombination (Kawakami et al., 2008). Therefore, a delivery system is need that not only protects the DNA/RNAi and facilitate its cellular uptake but also enhances the potential for targeted and effective delivery (Podesta and Kostarelos, 2009; Santos et al., 2011). Conversely, non-viral vectors, such as nanoparticles, are preferred when stem cells are intended to treat non-inherited diseases and are only required to express the therapeutic gene for a short period of time (Monteiro et al., 2014c; Santos et al., 2011). Moreover, nanoparticles have been proposed due to their safety, easy production, higher plasmid size encapsulation and flexibility in formulation design, which can be tailored to interact with the nuclei acid cargo and target ligands that specify the route of vector administration and enhance the delivery to specific tissues or cells (Kawakami et al., 2008; Storrie and Mooney, 2006; Yau et al., 2012). However, in spite of all the advantages of using nanoparticles for bioactive agent delivery, significant challenges remain, such as stability, agglomeration, issues of toxicity for high concentrations, short half-life in the bloodstream, shortterm expression and low transfection efficiency (Kulkarni et al., 2010; Monopoli et al., 2013). Thus, gene transfection using electric fields (electroporation) has been proposed to deliver genes into the cells (Takahashi et al., 2013). However, this method involves the concern that the electric pulse causes tissue damage. Osawa et al. have proposed the use of ultra-fine needle as an effective alternative for gene transfer for tissue regeneration (Osawa et al., 2011). Coating nanoparticles with derivatized proteins during the fabrication process, prior to incorporation within scaffolds, may address some of these issues (Kulkarni et al., 2010; Monopoli et al., 2013). Indeed, the delivery of bio-active agents loaded into nanoparticles and incorporated into a biomaterials scaffold may reduce their exposure to immune cells, enhance cellular uptake, provide well-controlled and sustained delivery and induce localized gene expression of tissue-inductive factors (Jang et al., 2004; Kulkarni et al., 2010; Nelson et al., 2012; Rujitanaroj et al., 2011; Yau et al., 2012). Biomaterials, nanotechnologies and stem cell bio-engineering methodologies are developing at a rapid pace and will eventually be in standard use, along with established cell-based therapies. Intracellular delivery, cell imaging and organ culture are among some of the relevant state-of-the-art updates for tooth cell-based therapy and TE.
3.2.5. Decellularized scaffolds
The field of TE has made extensive breakthroughs during the past three decades. However, the field still faces challenges, such as identifying and optimizing scaffold materials that are biodegradable, biocompatible and able to provide mechanical, structural and biological cues for cell adhesion, proliferation and differentiation (Patnaik et al., 2013). Natural scaffold approaches face limitations, such as lack of mechanical properties and processing. Synthetic scaffold approaches face limitations concerning degradation rates, degradation products and biocompatibility. Current efforts in tooth TE focus on identifying methods to accurately control bio-engineered tooth size and shape and to create functional tooth roots in vivo (Traphagen et al., 2012). With the advancements in tissue decellularization processes, acellular tissue-derived scaffolds have been proposed as an alternative to synthetic scaffolds. Decellularization approaches have formed an area of research additional to the natural and synthetic scaffold approaches (Patnaik et al., 2013; Traphagen et al., 2012). Decellularization is a process that removes cellular components from tissues/organs in order to reduce the foreign body reaction, inflammation and potential immune rejection, and to generate instructive ECM templates. Decellularized scaffolds are intended to preserve the structure, shape compatibility, mechanical integrity and bio-active molecule gradients to facilitate cell interaction, adhesion and ECM formation (Patnaik et al., 2013; Tapias and Ott, 2014). There are different methods of decellularization, including chemical, enzymatic, physical and combinations of these methods. For more information about decellularization methods, the sterilization of decellularized scaffolds and their applications in TE, the reader is directed to the reviews by Patnaik et al. (2013) and Zhao et al. (2013). During the process of decellularization, the cell membrane is disrupted and the cellular contents are released and washed away. Various decellularization methods have different effects on the extent of cell removal and ECM composition and structure (Traphagen et al., 2012; Zhao et al., 2013). Therefore, optimized decellularization methods should be chosen according to the characteristics of the tissues and particular methods to achieve the ideal results. Figure 5 shows the tooth decellularized scaffold approach.
Figure 5.
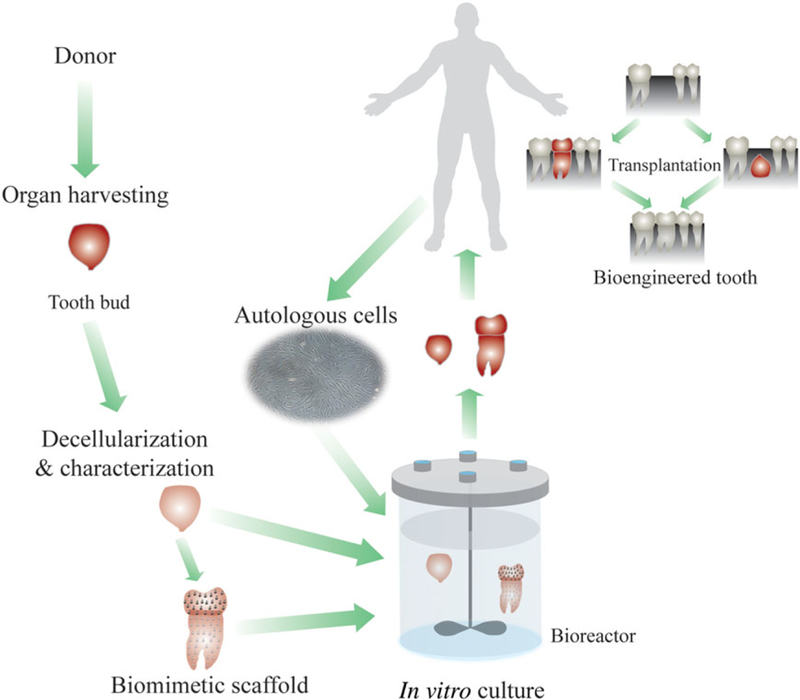
Tooth decellularized scaffold approach. [Colour figure can be viewed at wileyonlinelibrary.com]
We and others believe that detailed characterizations of ECM composition and organization in natural tooth development could facilitate human tooth TE efforts (Ravindran et al., 2014; Traphagen et al., 2012). Characterizations of tooth-expressed ECM molecules, including their respective developmental and spatial organizations, may facilitate the design of effective scaffolds for tooth regeneration (Figure 5). We have described three methods to effectively decellularize and demineralize porcine molar tooth buds and, at the same time, preserve natural ECM protein gradients (Traphagen et al., 2012). The results indicated that the collagen I, fibronectin, collagen IV and laminin gradients present in natural tooth tissues were retained in decellularized samples. Second harmonic generation image analysis and 3D reconstructions showed that natural tooth tissue exhibited higher collagen fibre density and less orientated and less organized collagen fibres, as compared to decellularized tooth tissue. Moreover, reseeded dental cells established residence in the decellularized scaffold and remodelled the matrix. We are now focusing on detailed 3D characterizations of tooth pulp tissue, to examine collagen fibre destruction and remodelling as a consequence of the decellularization and reseeding processes. Ravindran et al. (2014) demonstrated the odontogenic differentiation of human DPSCs and PDSCs when cultured on decellularized 3D scaffolds made of collagen-chitosan, without the need for exogenous addition of growth factors (Ravindran et al., 2014). Subcutaneous implantation of the reseeded decellularized 3D scaffolds showed the formation of dental pulp-like tissue with cells expressing dentine sialoprotein and dentine phosphophoryn. These studies demonstrate the potential for natural decellularized tooth scaffolds to instruct dental cell matrix synthesis, indicating potential utility as biomimetic scaffolds for dental TE applications.
3.2.6. Cell sheets for tooth regeneration
During the last two decades, many advances in cell sheet engineering have been reported (Okano, 2014; Owaki et al., 2014). A variety of techniques can be used to detach cell sheets from cell culture plates, including the use of temperature-responsive polymer coatings, cell scrapers, magnetic force, ionic solution, electrochemical polarization, pH decrease and light (Ito et al., 2005; Owaki et al., 2014; Zhou et al., 2012); for a more detailed description and comparison of cell sheets fabricated by these different techniques, the reader is directed to the recent review by Owaki et al. (2014). Specifically, temperature-responsive polymer techniques involve culturing cells until confluence on a thermoresponsive polymer (PIPAAm)-immobilized substrate that is hydrophobic at 37°C, and harvesting contiguous cell sheets at 20°C, at which temperature the substrate is hydrophilic. This technique allows for the preservation of the extracellular matrix formed by the cells, including adhesive proteins such as fibronectin. Also, the single cells or cell sheets can adhere to the host tissue without any biological glue or suture, increasing their survival following implantation (Okano, 2014). Indeed, the low survival rate of implanted stem cells is one of the major challenges in regenerative medicine (Okano, 2014). Moreover, it is also possible to make layers of several cell sheets, creating functional 3D tissues with perfusable blood vessels (Okano, 2014; Pandula et al., 2014; Sekine et al., 2013). Currently, cell sheets have been successfully used to regenerate cornea, heart, skin, bone, cartilage and bio-root and periodontal tissues (Okano, 2014; Pirraco et al., 2014; Wei et al., 2013; Zhou et al., 2012).
It is well known that dental epithelial-mesenchymal (DE–DM) cell interactions provide critical functions in tooth development. Thus, cell sheet engineering technology can be used to study DE–DM cell interactions. Moreover, the combined use of TE scaffolds with dental stem cell sheets may be a good approach to regenerate teeth with normal physiological functions. Yang et al. (2012a) reported successful bio-root regeneration using treated dentine matrix as a scaffold combined with human dental follicle cell (DFCS) sheets. After subcutaneous implantation into the dorsum of mice, it was observed that the scaffold could induce and support DFCSs to develop new dentine-pulp-like tissues and cementum–periodontal complexes. In another study (Zhou et al., 2012), tooth roots were wrapped with autologous PDL fibroblast multilayer cell sheets and replanted back to the prior socket. The PDL cell sheets group showed 88% healing compared to the 5% healing of the control group without PDL cell sheets. Wei et al. (2013) reported a root-shaped hydroxy-apatite scaffold containing dental pulp stem cells covered with a PDE stem cell sheet and implanted into a newly generated jaw bone implant socket; the results showed that the regenerated bio-root presented characteristics of a normal tooth after 6 months, including dentinal tubule-like and functional periodontal ligament-like structures. Regeneration of dental pulp tissue is crucial for treatment of dental pulp disease, and also for fully functional tooth engineering. A 3D stem cell sheet-derived pellet (CSDP) was designed by Na et al. (2013), using SCAPs. In vitro analyses showed that the gene expression was higher in CSDPs than in cell sheets, indicating that CSDPs have greater odontogenic and osteogenic potential. Subcutaneous implantation of human treated dentine matrix fragments combined with CSDPs and cell sheets in the backs of immunodeficient mice showed that the root space with CSDPs was entirely filled with a dental pulp-like tissue with well-established vascularity. Moreover, a layer of newly formed odontoblast-like cells with overt polarized morphology lining the mineralized dentine-like tissue was observed (Na et al., 2013).
4. Concluding remarks
Tooth loss remains the most common organ failure. Although clinical prosthetic dental procedures, such as dental implants, have been used for tooth replacement, they present many disadvantages. Many studies have demonstrated the potential of using ESCs for tooth regeneration, but the many concerns raised by the use of ESCs, such as possible tumourigenesis, ethical issues regarding the use of embryos and allogeneic immune rejection, make this approach problematic. The use of iPSCs may overcome many of these issues, but this technology is still in its infancy.
In this review, we have highlighted the application of TE for tooth regeneration. Scaffold biomaterials made of natural or synthetic polymers can act as carriers for bioactive agents and stem cells. They have been used in TE and regenerative medicine for more than five decades for the repair, restoration or regeneration of damaged tissue. By combining scaffolds and adult dental stem cells, it has been possible to form multiple anatomically correct tooth crowns within a scaffold, but they were very small and exhibited a variety of shapes as compared to natural tooth crowns. Indeed, the tooth is a complex biological organ, whose formation requires an intricate cascade of molecular signals and gene expression. Understanding the biological processes and interactions of various growth factors and gene expression underlying tooth development will be critical for tooth regeneration. Importantly, the successful differentiation of dental stem cells into specific lineages is one of the main challenges in stem cell research. Current methods to induce stem cells to differentiate into specific lineages relies on the use of bio-active agents, such as cocktails of growth factors and delivery of nucleotides (i.e. pDNA and RNAi). The interactions of scaffolds and bio-active agent-loaded delivery systems (i.e. nano-and microparticles) is an interesting option for future directions. The efficacy of biomaterials scaffolds combined with delivery systems is clear from the literature for connective tissue regeneration, indicating that similar approaches could be used to promote tooth regeneration. Another promising approach for tooth engineering is the use of decellularized tooth bud scaffolds. However, detailed characterizations of ECM composition, tooth pulp tissue, collagen fibre destruction and remodelling as a consequence of decellularization and reseeding are needed in order to identify the instructive signals and gradients needed. Also, the use of cells sheets, alone or in combination with scaffolds, may be a useful strategy to regenerate teeth with normal physiological functions. Together, these strategies may eventually meet the challenge of generating bio-engineered teeth of predetermined size and shape.
With respect to human clinical trials, autologous progenitor cells obtained from periodontal ligament have been used to treat intrabony defects (Feng et al., 2010). Also, the cultivation of periodontal ligament cells on titanium pins that were subsequently implanted in patients has been reported (Gault et al., 2010). Clinical evaluation of the implants showed satisfactory mechanical function, and radiographs revealed bone filling and formation of a lamina dura around the implants. These studies indicate that cell therapy using dental stem cells may be a feasible clinical approach in the near future. Therefore, to apply dental stem cell therapies and tissue-engineering approaches in dental treatment, strategic activities, including patenting, quality control and cooperation among governments, clinicians, researchers and companies, have to be established.
Acknowledgements
This study was supported by the National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR; Grant No. R01DE016132, to P.C.Y.).
Footnotes
Conflict of Interest
The authors have declared no conflict of interest.
References
- Aberg T, Cavender A, Gaikwad JS et al. 2004; Phenotypic changes in dentition of Runx2 homozygote-null mutant mice. J Histochem Cytochem 52: 131–139. [DOI] [PubMed] [Google Scholar]
- Abukawa H, Zhang W, Young CS et al. 2009; Reconstructing mandibular defects using autologous tissue-engineered tooth and bone constructs. J Oral Maxillofac Surg 67: 335–347. [DOI] [PubMed] [Google Scholar]
- Bento LW, Zhang Z, Imai A et al. 2013; Endothelial differentiation of SHED requires MEK1/ERK signaling. J Dent Res 92: 51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidarra SJ, Barrias CC, Granja PL. 2014; Injectable alginate hydrogels for cell delivery in tissue engineering. Acta Biomater 10: 1646–1662. [DOI] [PubMed] [Google Scholar]
- Bonadio J 2000; Tissue engineering via local gene delivery: update and future prospects for enhancing the technology. Adv Drug Deliv Rev 44: 185–194. [DOI] [PubMed] [Google Scholar]
- Cao H, Jiang X, Chai C et al. 2010; RNA interference by nanofiber-based siRNA delivery system. J Control Release 144: 203–212. [DOI] [PubMed] [Google Scholar]
- Chai Y, Slavkin HC. 2003; Prospects for tooth regeneration in the 21st century: a perspective. Microsc Res Tech 60: 469–479. [DOI] [PubMed] [Google Scholar]
- Chen FM, An Y, Zhang R et al. 2011; New insights into and novel applications of release technology for periodontal reconstructive therapies. J Control Release 149: 92–110. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I et al. 1996; Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development 122: 3035–3044. [DOI] [PubMed] [Google Scholar]
- Chou AM, Sae-Lim V, Hutmacher DW et al. 2006; Tissue engineering of a periodontal ligament-alveolar bone graft construct. Int J Oral Maxillofac Implants 21: 526–534. [PubMed] [Google Scholar]
- Chung HJ, Park TG. 2007; Surface engineered and drug releasing prefabricated scaffolds for tissue engineering. Adv Drug Deliv Rev 59: 249–262. [DOI] [PubMed] [Google Scholar]
- Cooper LF. 2009; The current and future treatment of edentulism. J Prosthodont 18: 116–122. [DOI] [PubMed] [Google Scholar]
- Cordeiro MM, Dong Z, Kaneko T et al. 2008; Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34: 962–969. [DOI] [PubMed] [Google Scholar]
- d’Aquino R, De Rosa A, Lanza V et al. 2009; Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater 18: 75–83. [DOI] [PubMed] [Google Scholar]
- D’Souza RN, Happonen RP, Ritter NM et al. 1990; Temporal and spatial patterns of transforming growth factor-β1 expression in developing rat molars. Arch Oral Biol 35: 957–965. [DOI] [PubMed] [Google Scholar]
- DeLong SA, Moon JJ, West JL. 2005; Covalently immobilized gradients of bFGF on hydrogel scaffolds for directed cell migration. Biomaterials 26: 3227–3234. [DOI] [PubMed] [Google Scholar]
- Dobie K, Smith G, Sloan AJ et al. 2002; Effects of alginate hydrogels and TGF-β1 on human dental pulp repair in vitro. Connect Tissue Res 43: 387–390. [DOI] [PubMed] [Google Scholar]
- Duailibi MT, Duailibi SE, Young CS et al. 2004; Bioengineered teeth from cultured rat tooth bud cells. J Dent Res 83: 523–528. [DOI] [PubMed] [Google Scholar]
- Duailibi SE, Duailibi MT, Zhang W et al. 2008; Bioengineered dental tissues grown in the rat jaw. J Dent Res 87: 745–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir T, Timko BP, Kohane DS et al. 2011; Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol 6: 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eap S, Bécavin T, Keller L et al. 2014; Nanofibers implant functionalized by neural growth factor as a strategy to innervate a bioengineered tooth. Adv Healthcare Mater 3: 386–391. [DOI] [PubMed] [Google Scholar]
- Eslaminejad MB, Bordbar S, Nazarian H. 2013; Odontogenic differentiation of dental pulp-derived stem cells on tricalcium phosphate scaffolds. J Dent Sci 8: 306–313. [Google Scholar]
- Eslaminejad MB, Vahabi S, Shariati M et al. 2010; In vitro growth and characterization of stem cells from human dental pulp of deciduous vs permanent teeth. J Dent (Tehran, Iran) 7: 185–195. [PMC free article] [PubMed] [Google Scholar]
- Feng F, Akiyama K, Liu Y et al. 2010; Utility of PDL progenitors for in vivo tissue regeneration: a report of three cases. Oral Dis 16: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Wang YK, Yang MT et al. 2010; Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nat Methods 7: 733–U795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galler KM, Cavender A, Yuwono V et al. 2008; Self-assembling peptide amphiphile nanofibers as a scaffold for dental stem cells. Tissue Eng Part A 14: 2051–2058. [DOI] [PubMed] [Google Scholar]
- Galler KM, D’Souza RN, Hartgerink JD. 2010; Biomaterials and their potential applications for dental tissue engineering. J Mater Chem 20: 8730–8746. [Google Scholar]
- Gault P, Black A, Romette JL et al. 2010; Tissue-engineered ligament: implant constructs for tooth replacement. J Clin Periodontol 37: 750–758. [DOI] [PubMed] [Google Scholar]
- Gillette BM, Rossen NS, Das N et al. 2011; Engineering extracellular matrix structure in 3D multiphase tissues. Biomaterials 32: 8067–8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-da-Silva LC, Fonseca NA, Moura V et al. 2012; Lipid-based nanoparticles for siRNA delivery in cancer therapy: paradigms and challenges. Acc Chem Res 45: 1163–1171. [DOI] [PubMed] [Google Scholar]
- Grigoriou M, Tucker AS, Sharpe PT et al. 1998; Expression and regulation of Lhx6 and Lhx7, a novel subfamily of LIM homeodomain encoding genes, suggests a role in mammalian head development. Development 125: 2063–2074. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J et al. 2000; Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97: 13625–13630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He HX, Yu JH, Liu Y et al. 2008; Effects of FGF2 and TGF-β1 on the differentiation of human dental pulp stem cells in vitro. Cell Biol Int 32: 827–834. [DOI] [PubMed] [Google Scholar]
- Hirayama M, Oshima M, Tsuji T. 2013; Development and prospects of organ replacement regenerative therapy. Cornea 32: S13–21. [DOI] [PubMed] [Google Scholar]
- Honda MJ, Fong H, Iwatsuki S et al. 2008; Tooth-forming potential in embryonic and postnatal tooth bud cells. Med Mol Morphol 41: 183–192. [DOI] [PubMed] [Google Scholar]
- Honda MJ, Ohara T, Sumita Y et al. 2006; Preliminary study of tissue-engineered odontogenesis in the canine jaw. J Oral Maxillofac Surg 64: 283–289. [DOI] [PubMed] [Google Scholar]
- Honda MJ, Tsuchiya S, Sumita Y et al. 2007; The sequential seeding of epithelial and mesenchymal cells for tissue-engineered tooth regeneration. Biomaterials 28: 680–689. [DOI] [PubMed] [Google Scholar]
- Howard D, Buttery LD, Shakesheff KM, et al. 2008; Tissue engineering: strategies, stem cells and scaffolds. J Anat 213: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Linares A, Yanez-Vico RM, Sanchez-Borrego E et al. 2013; Stem cells in current paediatric dentistry practice. Arch Oral Biol 58: 227–238. [DOI] [PubMed] [Google Scholar]
- Inuyama Y, Kitamura C, Nishihara T et al. 2010; Effects of hyaluronic acid sponge as a scaffold on odontoblastic cell line and amputated dental pulp. J Biomed Mater Res B 92B: 120–128. [DOI] [PubMed] [Google Scholar]
- Iohara K, Nakashima M, Ito M et al. 2004; Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res 83: 590–595. [DOI] [PubMed] [Google Scholar]
- Ito A, Hibino E, Kobayashi C et al. 2005; Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets, using magnetite nanoparticles and magnetic force. Tissue Eng 11: 489–496. [DOI] [PubMed] [Google Scholar]
- Iwatsuki S, Honda MJ, Harada H et al. 2006; Cell proliferation in teeth reconstructed from dispersed cells of embryonic tooth germs in a three-dimensional scaffold. Eur J Oral Sci 114: 310–317. [DOI] [PubMed] [Google Scholar]
- Jang JH, Houchin TL, Shea LD. 2004; Gene delivery from polymer scaffolds for tissue engineering. Expert Rev Med Devices 1: 127–138. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. 2012; Tooth shape formation and tooth renewal: evolving with the same signals. Development 139: 3487–3497. [DOI] [PubMed] [Google Scholar]
- Jones TD, Naimipour H, Sun S et al. 2015; Mechanical changes in human dental pulp stem cells during early odontogenic differentiation. J Endod 41: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussila M, Yanezl XC, Thesleff I. 2014; Initiation of teeth from the dental lamina in the ferret. Differentiation 87: 32–43. [DOI] [PubMed] [Google Scholar]
- Kanafi MM, Ramesh A, Gupta PK et al. 2014; Dental pulp stem cells immobilized in alginate microspheres for applications in bone tissue engineering. Int Endod J 47: 687–697. [DOI] [PubMed] [Google Scholar]
- Kawakami S, Higuchi Y, Hashida M. 2008; Nonviral approaches for targeted delivery of plasmid DNA and oligonucleotide. J Pharm Sci 97: 726–745. [DOI] [PubMed] [Google Scholar]
- Kelleher CM, Vacanti JP. 2010; Engineering extracellular matrix through nanotechnology. J R Soc Interface 7: S717–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BYS, Rutka JT, Chan WCW. 2010a; Nanomedicine. N Engl J Med 363: 2434–2443. [DOI] [PubMed] [Google Scholar]
- Kim JY, Xin X, Moioli EK et al. 2010b; Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue Eng Part A 16: 3023–3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Lee CH, Kim BK et al. 2010c; Anatomically shaped tooth and periodontal regeneration by cell homing. J Dent Res 89: 842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Bhang SH, Yang HS et al. 2010d; Development of functional fibrous matrices for the controlled release of basic fibroblast growth factor to improve therapeutic angiogenesis. Tissue Eng Part A 16: 2999–3010. [DOI] [PubMed] [Google Scholar]
- Kim SE, Suh DH, Yun YP et al. 2012; Local delivery of alendronate eluting chitosan scaffold can effectively increase osteoblast functions and inhibit osteoclast differentiation. J Mater Sci Mater Med 23: 2739–2749. [DOI] [PubMed] [Google Scholar]
- Kulkarni M, Greiser U, O’Brien T et al. 2010; Liposomal gene delivery mediated by tissue-engineered scaffolds. Trends Biotechnol 28: 28–36. [DOI] [PubMed] [Google Scholar]
- Lai WF, Lee JM, Jung HS. 2014; Molecular and engineering approaches to regenerate and repair teeth in mammals. Cell Mol Life Sci 71: 1691–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer R, Vacanti JP. 1993; Tissue engineering. Science 260: 920–926. [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee DS, Choung HW et al. 2011; Odontogenic differentiation of human dental pulp stem cells induced by preameloblast-derived factors. Biomaterials 32: 9696–9706. [DOI] [PubMed] [Google Scholar]
- Li G, Zhang T, Li M et al. 2014; Electrospun fibers for dental and craniofacial applications. Curr Stem Cell Res Ther 9: 187–195. [DOI] [PubMed] [Google Scholar]
- Li Y, Lu X, Sun X et al. 2011; Odontoblast-like cell differentiation and dentin formation induced with TGF-β1. Arch Oral Biol 56: 1221–1229. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jiao Y, Wang Y et al. 2008; Polysaccharides-based nanoparticles as drug delivery systems. Adv Drug Deliv Rev 60: 1650–1662. [DOI] [PubMed] [Google Scholar]
- Lluch AV, Fernandez AC, Ferrer GG et al. 2009; Bioactive scaffolds mimicking natural dentin structure. J Biomed Mater Res B 90B: 182–194. [DOI] [PubMed] [Google Scholar]
- Love KT, Mahon KP, Levins CG et al. 2010; Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci U S A 107: 1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma PX. 2008; Biomimetic materials for tissue engineering. Adv Drug Deliv Rev 60: 184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Yang C, Song W et al. 2014; Chitosan Hydrogel as siRNA vector for prolonged gene silencing. J Nanobiotechnology 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JJ, Stosich MS, Moioli EK et al. 2010; Facial reconstruction by biosurgery: cell transplantation versus cell homing. Tissue Eng Part B Rev 16: 257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu S, Jeanneau C, Sheibat-Othman N et al. 2013; Usefulness of controlled release of growth factors in investigating the early events of dentin-pulp regeneration. J Endod 39: 228–235. [DOI] [PubMed] [Google Scholar]
- Matsunaga T, Yanagiguchi K, Yamada S et al. 2006; Chitosan monomer promotes tissue regeneration on dental pulp wounds. J Biomed Mater Res A 76A: 711–720. [DOI] [PubMed] [Google Scholar]
- Mitalipov S, Wolf D. 2009; Totipotency, Pluripotency and nuclear reprogramming. In Engineering of Stem Cells, Martin U (ed.). 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Gronthos S, Zhao MR et al. 2003; SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100: 5807–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monopoli MP, Pitek AS, Lynch I et al. 2013; Formation and characterization of the nanoparticle-protein corona. Methods Mol Biol (Clifton NJ) 1025: 137–155. [DOI] [PubMed] [Google Scholar]
- Monteiro N, Martins A, Pires R et al. 2014a; Immobilization of bioactive factor-loaded liposomes at the surface of electrospun nanofibers targeting tissue engineering. Biomater Sci 2: 1195–1209 [DOI] [PubMed] [Google Scholar]
- Monteiro N, Martins A, Reis RL et al. 2014b; Liposomes in tissue engineering and regenerative medicine. J R Soc Interface 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro N, Martins A, Ribeiro D et al. 2013; On the use of dexamethasone-loaded liposomes to induce the osteogenic differentiation of human mesenchymal stem cells. J Tissue Eng Regen Med 7. [DOI] [PubMed] [Google Scholar]
- Monteiro N, Ribeiro D, Martins A et al. 2014c; Instructive nanofibrous scaffold comprising Runt-related transcription factor 2 gene delivery for bone tissue engineering. ACS Nano 8: 8082–8094. [DOI] [PubMed] [Google Scholar]
- Morsczeck C, Gotz W, Schierholz J et al. 2005; Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix Biol 24: 155–165. [DOI] [PubMed] [Google Scholar]
- Moshaverinia A, Chen C, Akiyama K et al. 2012; Alginate hydrogel as a promising scaffold for dental-derived stem cells: an in vitro study. J Mater Sci Mater Med 23: 3041–3051. [DOI] [PubMed] [Google Scholar]
- Motornov M, Roiter Y, Tokarev I et al. 2010; Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog Polym Sci 35: 174–211. [Google Scholar]
- Na S, Zhang H, Huang F et al. 2013; Regeneration of dental pulp/dentine complex with a three-dimensional and scaffold-free stem cell sheet-derived pellet. J Tissue Eng Regen Med. [DOI] [PubMed] [Google Scholar]
- Nakahara T 2011; Potential feasibility of dental stem cells for regenerative therapies: stem cell transplantation and whole-tooth engineering. Odontology 99: 105–111. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Ide Y. 2007; Tooth regeneration: implications for the use of bioengineered organs in first-wave organ replacement. Hum Cell 20, 63–70. [DOI] [PubMed] [Google Scholar]
- Nakahara T, Nakamura T, Kobayashi E et al. 2003; Novel approach to regeneration of periodontal tissues based on in situ tissue engineering: effects of controlled release of basic fibroblast growth factor from a sandwich membrane. Tissue Eng 9: 153–162. [DOI] [PubMed] [Google Scholar]
- Nakao K, Morita R, Saji Y et al. 2007; The development of a bioengineered organ germ method. Nat Methods 4: 227–230. [DOI] [PubMed] [Google Scholar]
- Nanci A, Cate ART. 2003; Ten Cate’s Oral Histology: Development, Structure, and Function. Mosby. [Google Scholar]
- Nelson CE, Gupta MK, Adolph EJ et al. 2012; Sustained local delivery of siRNA from an injectable scaffold. Biomaterials 33: 1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohazama A, Modino SAC, Miletich I et al. 2004; Stem-cell-based tissue engineering of murine teeth. J Dent Res 83: 518–522. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Tucker A, Sharpe PT. 2005; Organized tooth-specific cellular differentiation stimulated by BMP4. J Dent Res 84: 603–606. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Aoyama T, Nakayama T et al. 2002; Clonal heterogeneity in differentiation potential of immortalized human mesenchymal stem cells. Biochem Biophys Res Commun 295: 354–361. [DOI] [PubMed] [Google Scholar]
- Okano T 2014; Current progress of cell sheet tissue engineering and future perspective. Tissue Eng Part A 20, 1353–1354. [Google Scholar]
- Osawa K, Okubo Y, Nakao K et al. 2011; Feasibility of BMP-2 gene therapy using an ultra-fine needle. [Google Scholar]
- Oshima M, Ogawa M, Yasukawa M et al. 2012; Generation of a bioengineered tooth by using a three-dimensional cell manipulation method (organ germ method). Methods Mol Biol (Clifton NJ) 887: 149–165. [DOI] [PubMed] [Google Scholar]
- Otsu K, Kumakami-Sakano M, Fujiwara N et al. 2014; Stem cell sources for tooth regeneration: current status and future prospects. Front Physiol 5: 36–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owaki T, Shimizu T, Yamato M et al. 2014; Cell sheet engineering for regenerative medicine: current challenges and strategies. Biotechnol J 9: 904–914. [DOI] [PubMed] [Google Scholar]
- Ozeki N, Mogi M, Kawai R et al. 2013; Mouse-induced pluripotent stem cells differentiate into odontoblast-like cells with induction of altered adhesive and migratory phenotype of integrin. PLoS One 8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pandula PKC, Samaranayake LP, Jin LJ et al. 2014; Human umbilical vein endothelial cells synergize osteo/odontogenic differentiation of periodontal ligament stem cells in 3D cell sheets. J Periodontal Res 49: 299–306. [DOI] [PubMed] [Google Scholar]
- Park JH, Tai K, Hayashi D. 2010; Tooth autotransplantation as a treatment option: a review. J Clin Pediatr Dent 35: 129–135. [DOI] [PubMed] [Google Scholar]
- Patnaik SS, Wang B, Weed B et al. 2013; Decellularized scaffolds: concepts, methodologies, and applications in cardiac tissue engineering and whole-organ regeneration In Tissue Regeneration: Where Nanostructure Meets Biology, Liu Q (ed.). World Scientific: 77–124. [Google Scholar]
- Petrie Aronin CE, Kuhn NZ, Tuan RS. 2011; Tissue engineering and selection of cells In Comprehensive Biomaterials, Paul D (ed.). Elsevier: Oxford; 81–93. [Google Scholar]
- Pirraco RP, Iwata T, Yoshida T et al. 2014; Endothelial cells enhance the in vivo bone-forming ability of osteogenic cell sheets. Lab Invest 94: 663–673. [DOI] [PubMed] [Google Scholar]
- Podesta JE, Kostarelos K. 2009; Engineering cationic liposome: sirna complexes for in vitro and in vivo delivery. Methods Enzymol 464: 343–354. [DOI] [PubMed] [Google Scholar]
- Polini A, Bai H, Tomsia AP. 2013; Dental applications of nanostructured bioactive glass and its composites. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5: 399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott RS, Alsanea R, Tayad MI et al. 2008; In vivo generation of dental pulp-like tissue by using dental pulp stem cells, dentin matrix protein 1 transplantation in mice. J Endod 34: 421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran S, George A. 2015; Biomimetic extracellular matrix mediated somatic stem cell differentiation: applications in dental pulp tissue regeneration. Front Physiol 6: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran S, Song Y, George A. 2010; Development of three-dimensional biomimetic scaffold to study epithelial-mesenchymal interactions. Tissue Eng Part A 16: 327–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindran S, Zhang Y, Huang CC et al. 2014; Odontogenic induction of dental stem cells by extracellular matrix-inspired three-dimensional scaffold. Tissue Eng Part A 20: 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rujitanaroj PO, Wang YC, Wang J et al. 2011; Nanofiber-mediated controlled release of siRNA complexes for long term gene-silencing applications. Biomaterials 32: 5915–5923. [DOI] [PubMed] [Google Scholar]
- Saito MT, Silverio KG, Casati MZ et al. 2015; Tooth-derived stem cells: update and perspectives. World J Stem Cells 7: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai VT, Zhang Z, Dong Z et al. 2010; SHED differentiate into functional odontoblasts and endothelium. J Dent Res 89: 791–796. [DOI] [PubMed] [Google Scholar]
- Santo VE, Gomes ME, Mano JF et al. 2012a; Controlled release strategies for bone, cartilage and osteochondral engineering. Part I: recapitulation of tissue healing and variables for the design of delivery systems. Tissue Eng Part B Rev 19: 308–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo VE, Gomes ME, Mano JF et al. 2012b; Controlled release strategies for bone, cartilage and osteochondral engineering. Part II: challenges on the evolution from single towards multiple bioactive factor delivery. Tissue Eng Part B Rev 19: 327–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santo VE, Sato K, Ratanavaraporn J et al. 2012c; Enhanced orthotopic bone regeneration promoted by intracellular delivery of dexamethasone. J Tissue Eng Regen Med 6: 330–330. [Google Scholar]
- Santos JL, Pandita D, Rodrigues J et al. 2011; Non-viral gene delivery to mesenchymal stem cells: methods, strategies and application in bone tissue engineering and regeneration. Curr Gene Ther 11: 46–57. [DOI] [PubMed] [Google Scholar]
- Sartaj R, Sharpe P. 2006; Biological tooth replacement. J Anat 209: 503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz C, Wagner E. 2012; Therapeutic plasmid DNA versus siRNA delivery: common and different tasks for synthetic carriers. J Control Release 161: 554–565. [DOI] [PubMed] [Google Scholar]
- Sekine H, Shimizu T, Sakaguchi K et al. 2013; In vitro fabrication of functional threedimensional tissues with perfusable blood vessels. Nat Commun 4: 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BM, Miura M, Gronthos S et al. 2004; Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364: 149–155. [DOI] [PubMed] [Google Scholar]
- Shen H, Hu XX, Bei JZ et al. 2008; The immobilization of basic fibroblast growth factor on plasma-treated poly(lactide-co-glycolide). Biomaterials 29: 2388–2399. [DOI] [PubMed] [Google Scholar]
- Shi J, Votruba AR, Farokhzad OC et al. 2010; Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett 10: 3223–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirc J, Kubinova S, Hobzova R et al. 2012; Controlled gentamicin release from multi-layered electrospun nanofibrous structures of various thicknesses. Int J Nanomedicine 7: 5315–5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonmez AB, Castelnuovo J. 2014; Applications of basic fibroblastic growth factor (FGF-2, bFGF) in dentistry. Dent Traumatol 30: 107–111. [DOI] [PubMed] [Google Scholar]
- Sonoyama W, Liu Y, Yamaza T et al. 2008; Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34: 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowmya S, Bumgardener JD, Chennazhi KP et al. 2013; Role of nanostructured biopolymers and bioceramics in enamel, dentin and periodontal tissue regeneration. Prog Polym Sci 38: 1748–1772. [Google Scholar]
- Sridharan R, Karp JM, Zhao W. 2014; Bioengineering tools to elucidate and control the fate of transplanted stem cells. Biochem Soc Trans 42: 679–687. [DOI] [PubMed] [Google Scholar]
- Stevens MM, George JH. 2005; Exploring and engineering the cell surface interface. Science 310: 1135–1138. [DOI] [PubMed] [Google Scholar]
- Storrie H, Mooney DJ. 2006; Sustained delivery of plasmid DNA from polymeric scaffolds for tissue engineering. Adv Drug Deliv Rev 58: 500–514. [DOI] [PubMed] [Google Scholar]
- Sumita Y, Honda MJ, Ohara T et al. 2006; Performance of collagen sponge as a 3D scaffold for tooth-tissue engineering. Biomaterials 27: 3238–3248. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kiso H, Saito K et al. 2013; Feasibility of gene therapy for tooth regeneration by stimulation of a third dentition. [Google Scholar]
- Takahashi K, Nuckolls GH, Tanaka O et al. 1998; Adenovirus-mediated ectopic expression of Msx2 in even-numbered rhombomeres induces apoptotic elimination of cranial neural crest cells in ovo. Development 125: 1627–1635. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M et al. 2007; Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. 2006; Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676. [DOI] [PubMed] [Google Scholar]
- Tapias LF, Ott HC. 2014; Decellularized scaffolds as a platform for bioengineered organs. Curr Opin Organ Transplant 19: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur RA, Florek CA, Kohn J et al. 2008; Electrospun nanofibrous polymeric scaffold with targeted drug release profiles for potential application as wound dressing. Int J Pharm 364: 87–93. [DOI] [PubMed] [Google Scholar]
- Traphagen SB, Fourligas N, Xylas JF et al. 2012; Characterization of natural, decellularized and reseeded porcine tooth bud matrices. Biomaterials 33: 5287–5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi T, Mizutani S, Nakano M et al. 2003; FGF-2 regulates enamel and dentine formation in mouse tooth germ. Calcif Tissue Int 73: 496–501. [DOI] [PubMed] [Google Scholar]
- Valles-Lluch A, Novella-Maestre E, Sancho-Tello M et al. 2010; Mimicking natural dentin using bioactive nanohybrid scaffolds for dentinal tissue engineering. Tissue Eng Part A 16: 2783–2793. [DOI] [PubMed] [Google Scholar]
- Valles A, Novella E, Sancho M, et al. 2010; Bioactive nanohybrid scaffolds mimicking natural dentin xenotransplanted in immunodeficient mice. [Google Scholar]
- van Manen EH, Zhang W, Walboomers XF et al. 2014; The influence of electrospun fibre scaffold orientation and nano-hydroxyapatite content on the development of tooth bud stem cells in vitro. Odontology 102: 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu X, Jin X et al. 2010; The odontogenic differentiation of human dental pulp stem cells on nanofibrous poly(L-lactic acid) scaffolds in vitro and in vivo. Acta Biomater 6: 3856–3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XP, Aberg T, James MJ et al. 2005; Runx2 (Cbfa1) inhibits Shh signaling in the lower but not upper molars of mouse embryos and prevents the budding of putative successional teeth. J Dent Res 84: 138–143. [DOI] [PubMed] [Google Scholar]
- Wang Z, Troilo PJ, Wang X et al. 2004; Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther 11: 711–721. [DOI] [PubMed] [Google Scholar]
- Wei F, Song T, Ding G et al. 2013; Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells Dev 22: 1752–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn SR, Chen JC, Gong X et al. 2005; Non-viral-mediated gene therapy approaches for bone repair. Orthod Craniofac Res 8: 183–190. [DOI] [PubMed] [Google Scholar]
- Wu P, Wu X, Jiang TX et al. 2013; Specialized stem cell niche enables repetitive renewal of alligator teeth. Proc Natl Acad Sci U S A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Chen G, Li J et al. 2012a; Tooth root regeneration using dental follicle cell sheets in combination with a dentin matrix-based scaffold. Biomaterials 33: 2449–2461. [DOI] [PubMed] [Google Scholar]
- Yang KC, Wang CH, Chang HH et al. 2012b; Fibrin glue mixed with platelet-rich fibrin as a scaffold seeded with dental bud cells for tooth regeneration. J Tissue Eng Regen Med 6: 777–785. [DOI] [PubMed] [Google Scholar]
- Yang L, Webster TJ. 2009; Nanotechnology controlled drug delivery for treating bone diseases. Expert Opin Drug Deliv 6: 851–864. [DOI] [PubMed] [Google Scholar]
- Yang MB, Zhang HM, Gangolli R. 2014; Advances of mesenchymal stem cells derived from bone marrow and dental tissue in craniofacial tissue engineering. Curr Stem Cell Res Ther 9: 150–161. [DOI] [PubMed] [Google Scholar]
- Yang X, Yang F, Walboomers XF et al. 2010; The performance of dental pulp stem cells on nanofibrous PCL/gelatin/ nHA scaffolds. J Biomed Mater Res A 93A: 247–257. [DOI] [PubMed] [Google Scholar]
- Yau WWY, Rujitanaroj PO, Lam L et al. 2012; Directing stem cell fate by controlled RNA interference. Biomaterials 33: 2608–2628. [DOI] [PubMed] [Google Scholar]
- Yen AH, Yelick PC. 2011; Dental tissue regeneration-a mini-review. Gerontology 57: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young CS, Abukawa H, Asrican R et al. 2005a; Tissue-engineered hybrid tooth and bone. Tissue Eng 11: 1599–1610. [DOI] [PubMed] [Google Scholar]
- Young CS, Kim SW, Qin C et al. 2005b; Developmental analysis and computer modelling of bioengineered teeth. Arch Oral Biol 50: 259–265. [DOI] [PubMed] [Google Scholar]
- Young CS, Terada S, Vacanti JP et al. 2002; Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res 81: 695–700. [DOI] [PubMed] [Google Scholar]
- Yuan ZL, Nie HM, Wang S et al. 2011; Biomaterial selection for tooth regeneration. Tissue Eng Part B Rev 17: 373–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun YR, Won JE, Jeon E et al. 2010; Fibroblast growth factors: biology, function, and application for tissue regeneration. J Tissue Eng 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Abukawa H, Troulis MJ et al. 2009; Tissue engineered hybrid tooth-bone constructs. Methods 47: 122–128. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ahluwalia IP, Literman R et al. 2011; Human dental pulp progenitor cell behavior on aqueous and hexafluoroisopropanol-based silk scaffolds. J Biomed Mater Res A 97: 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Ahluwalia IP, Yelick PC. 2010; Three-dimensional dental epithelial-mesenchymal constructs of predetermined size and shape for tooth regeneration. Biomaterials 31: 7995–8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cheng X, Wang J et al. 2006; Novel chitosan/collagen scaffold containing transforming growth factor-β1 DNA for periodontal tissue engineering. Biochem Biophys Res Commun 344: 362–369. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Shi B et al. 2007; A platelet-derived growth factor releasing chitosan/coral composite scaffold for periodontal tissue engineering. Biomaterials 28: 1515–1522. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Gutierrez D, Li X et al. 2013; The LIM homeodomain transcription factor lhx6, a transcriptional repressor that interacts with pituitary homeobox 2 (pitx2) to regulate odontogenesis. J Biol Chem 288: 2485–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhanga L, Morsib Y, Wanga Y et al. 2013; Review scaffold design and stem cells for tooth regeneration. Jap Dent Sci Rev 49: 14–26. [Google Scholar]
- Zhao Y, Yu M, Bai S. 2013; Research progress of decellularization and application in tissue engineering. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 27: 950–954. [PubMed] [Google Scholar]
- Zhou Y, Li Y, Mao L et al. 2012; Periodontal healing by periodontal ligament cell sheets in a teeth replantation model. Arch Oral Biol 57: 169–176. [DOI] [PubMed] [Google Scholar]


