Abstract
Introduction:
Pulmonary hypertension (PH) is a deadly enigmatic disease with increasing prevalence. Cellular pathologic hallmarks of PH are driven at least partly by metabolic rewiring, but details are just emerging. The discovery that vascular matrix stiffening can mechanically activate the glutaminase (GLS) enzyme and serve as a pathogenic mechanism of PH has advanced our understanding of the complex role of glutamine in PH. It has also offered a novel therapeutic target for development as a next-generation drug for this disease.
Area covered:
This review discusses the cellular contribution of glutamine metabolism to PH together with the possible therapeutic application of pharmacologic GLS inhibitors in this disease.
Expert opinion:
Despite advances in our understanding of glutamine metabolism in PH, questions remain unanswered regarding the development of therapies targeting glutamine in PH. The comprehensive mechanisms by which glutamine metabolism rewiring influences pulmonary vascular cell behavior to drive PH are incompletely understood. Because glutamine metabolism exhibits a variety of functions in organ repair and homeostasis, a better understanding of the overall risk-benefit ratio of these strategies with long-term follow-up is needed. This knowledge should pave the way for the design of new strategies to prevent and hopefully even regress PH.
Keywords: Anaplerosis, Glutamine, Pulmonary hypertension, vascular glutamine metabolism
1). Introduction
Pulmonary hypertension (PH) is a heterogeneous and progressive vascular disease stemming from myriad of molecular origins, which can lead to right heart failure as well as multi-organ dysfunction, and is associated with a poor prognosis [1–4]. With all of its multiple clinical manifestations, this disease and all of its subtypes is estimated to affect up to 100 million people worldwide [3]. At the cellular and molecular level, PH is characterized by a plethora of cellular dysfunctions, including, but not limited to, complex panvasculopathy involving excessive proliferation, dysregulation of multiple vascular cell types, and is accompanied by inflammation and fibrosis throughout the vasculature [1]. These events ultimately lead to increased pulmonary arterial pressure, accompanied by adverse pulmonary arteriolar, and often right ventricular (RV), remodeling.
Exogenous injuries, such as hypoxia, various infections, drugs and toxins, among others, as well as various secondary co-morbidities are linked to PH development [5]. A growing cadre of mutations, including those in the bone morphogenetic protein receptor 2 (BMPR2) gene, predispose to hereditary forms of PH [6,7]. The latest World Health Organization (WHO) classification includes 5 broad categories, based on histopathologic and hemodynamic characteristics and presumed etiologies [5]. Pulmonary arterial hypertension (PAH) or Group 1 is composed of heritable and idiopathic pulmonary arterial hypertension; drug- and toxin-induced PH; and PAH associated with connective tissue diseases (mostly systemic sclerosis in North America), portal hypertension, HIV infection, schistosomiasis, and congenital heart disease. Even in the contemporary era, Group 1 PAH carries a poor prognosis despite optimal medical therapy. Group 2 encompasses PH driven by pulmonary venous hypertension due to left-heart disease and includes the most populous group of PH patients in the developed world. PH related to significant parenchymal lung diseases or hypoxemia is included in Group 3, Group 4 encompasses chronic thromboembolic PH, and Group 5 PH includes diseases with unclear multifactorial mechanisms, such as sarcoidosis, mitochondrial disease, and sickle cell disease. Untreated or severe refractory PH, regardless of group, is often fatal. Before present-day therapies, the median survival with symptomatic idiopathic PAH was estimated at 2.8 years from the time of diagnosis. There exist over a dozen approved pulmonary vasodilatory therapies for PAH which provide symptomatic relief, and many prolong the time to clinical worsening. In the current age of such therapy, the median survival for idiopathic PAH and PAH associated with systemic sclerosis is 8 and 4 years, respectively [3,4,8]. Thus, while there has been an improvement in the PH patient experience, there is an obvious and critical need for novel therapies that target the molecular origins of disease beyond simply vasodilatation.
Despite PH class-distinct phenotypes, all categories of disease can engender a complex phenotype that recapitulates that observed in cancer. Fibrosis, resistance to apoptosis, excessive proliferation and inflammation due to significant vascular extracellular matrix remodeling, are all features of cancer noted in PH [9–11]. Recently, studies have proposed that this phenotype is partly explained by reprogramming of arterial and surrounding stromal cell metabolic processes [12–15]. Consistent with metabolic rewiring observed in cancer, a major tenet of the observed metabolic changes in PH is the shift from oxidative phosphorylation to glycolysis, known as the Warburg effect. This phenomenon is frequently observed in tumor tissue, but has also been reported in pulmonary vasculature cells and the failing right ventricle in PH patients [14,16,17].
Even so, this shift towards increased glycolysis is inadequate to provide for all the metabolic demands of proliferating cells. Carbon and nitrogen flux rewiring is a less commonly discussed, yet important aspect of vascular metabolism in PH. The tricarboxylic acid (TCA) cycle function requires constant replenishment of carbon intermediates, known as anaplerosis. TCA function is vital for the production of lipid precursors, amino acids, and nucleotides [18,19]. One of the most well-described anaplerotic pathways in cancer is glutaminase (GLS)-driven glutaminolysis (glutamine catabolism) [20,21]. Building from this observation, we and others have highlighted recently the involvement of glutamine metabolism and glutaminolysis in PH [22,23]. Nevertheless, its precise contribution to PH is still incompletely understood. This article aims to review the latest findings regarding the influence of glutamine metabolism on pulmonary vascular pathobiology across multiple PH subtypes, in large part guided by our advancing insights in cancer, and discuss the possible clinical application of pharmacologic GLS inhibitors in PH.
2. Glutamine metabolism
Glutamine metabolism is an essential aspect of cellular function given its capability to donate either its nitrogen or carbon into a wide variety of growth-promoting pathways (Figure 1). Given this indispensable nature, glutamine displays a spectrum of inter-organ dynamics, as organs can function either as glutamine produces or consumers [21], as well as being the most abundant amino acid in plasma [24]. The arteriovenous difference in plasma glutamine abundance provides important information about organ-specific metabolism of this amino acid. In healthy control subjects, the principal glutamine generator is skeletal muscle, producing the major plasma glutamine pool [25,26]. A minor, but still significant source of glutamine is adipose tissue in humans and rats [27,28]. Interestingly, although the liver can synthesize or catabolize glutamine, it is not a major contributor of glutamine to the plasma pool in either rats or humans [26,29]. Furthermore, lungs of both humans and rats have glutamine production capacity, in fact, in rats, glutamine production by the lungs is comparable to that released by skeletal muscle [30].
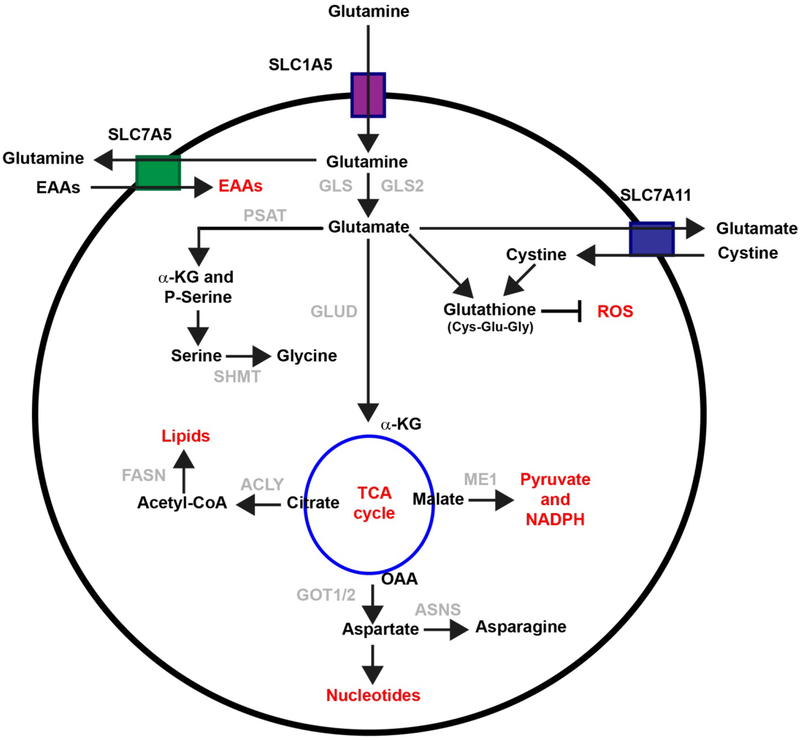
Figure 1: Overview of cellular glutamine metabolism:
After transporting into cytosol through SLC1A5 or LAT1 or other transporters, glutamine is converts to glutamate and ammonia by glutaminase (GLS). It then provides carbon and nitrogen for macromolecular synthesis. Briefly, glutamate is metabolized to alpha-ketoglutarate (α-KG) through the action of either glutamate dehydrogenase (GLUD) or transaminases. The tricarboxylic acid cycle (TCA) cycle metabolite malate can be exported out of the cytoplasm to generate nicotinamide adenine dinucleotide phosphate (NADPH) and pyruvate through the activity of the decarboxylating malate dehydrogenase enzyme (ME1). Oxaloacetate (OOA) can be converted back to aspartate, which supports asparagine generation, and nucleotide synthesis. Citrate can be exported out of the mitochondria for de novo fatty acid synthesis. ACLY: ATP-citrate synthase; ASNS: Asparagine Synthetase; FASN: Fatty Acid Synthase; EAAs: Essential amino acids; GOT1/2: Glutamic-oxaloacetic transaminase 1/2; PSAT: Phosphoserine aminotransferase; ROS: Reactive oxygen species; SHMT: Serine hydroxymethyltransferase.
During periods of rapid growth or other stresses, glutamine demand surpasses the body’s synthetic capabilities, and glutamine becomes essential [31]. This requirement for glutamine is particularly true in highly proliferative cells such as cancer cells or diseased vascular cells [20,32]. Consistently, diseased-lungs such as in PH patients have been found to significantly take up glutamine [23]. Correspondingly, similar observations have been made for cultured pulmonary arterial endothelial cells (PAECs) and pulmonary arterial smooth muscle cells (PASMCs) exposed to PH-relevant triggers [22]. To promote a favorable milieu for biosynthesis and proliferation, the maintenance and transport of high levels of glutamine in the blood is crucial. Transporters such as SLC1A5 bring glutamine into the cell to be used for biosynthesis. Furthermore, glutamine and its derivatives can also be exchanged for other amino acids such as leucine, through the L-type amino acid transporter 1 (LAT1, a heterodimer of SLC7A5 and SLC3A2) antiporter [33], or cystine, through the xCT antiporter (a heterodimer of SLC7A11 and SLC3A2). which is quickly reduced to cysteine inside the cell (Figure 1) [20,33,34].
2.1. Glutaminolysis
Glutaminolysis begins with glutaminase (GLS) catalyzed conversion from glutamine to glutamate. These reactions can release the amide nitrogen as ammonia, or donate it into downstream synthetic pathways. Many different GLS isozymes exist, and are coded for in genes GLS and GLS2 [35], although those encoded by gene GLS are of particular interest. These isozymes are correlated with progression and growth rate of tumors in both rat and mice models, and silencing of these enzymes either by genetic knockout or inhibition delay tumor proliferation [36–38]. However, the role of GLS2 seems to be regulated by factors that are not yet completely defined, and relatively context-specific [39].
In the lung vasculature, both isoform GLS and GLS2 are expressed. However, the messenger RNA transcript levels of GLS, but not GLS2, are induced by matrix stiffening in both PAECs and PASMCs and upregulated in rodent and human diseased lungs [22]. While both isozymes encoded by GLS (i.e., KGA and GAC) are induced by matrix stiffening and upregulated in PH/PAH lungs, the specific contribution of these two isozymes to overall glutamine metabolism in PH remains undefined [22].
2.2. Redox homeostasis
While at physiological levels, reactive oxygen species (ROS) are essential to maintain homeostasis in the pulmonary vasculature, excess ROS can be highly damaging to macromolecules [40,41].
Several modes of ROS formation have been reported, including superoxide (O2−) generation through electrons leaked from the mitochondrial electron transport chain [42]. Therefore, a connection between increased glutamine oxidation and ROS production may exist [32]. Notably, products of glutamine metabolic pathways are known to directly control ROS levels. Indeed, the rate-determining step of glutathione synthesis is glutamine input [33,41]. Glutathione is a short, three amino acid residue (Glu-Cys-Gly) neutralizer of peroxide-based free radicals, and, as shown in Figure 1, glutamine contributes to each amino acid component that together comprise glutathione’s structure. As glutathione levels are correlated with tumor progression, drug resistance in cancer, as well as PH/PAH severity, a better understanding of this pathway may contribute to more directed development of therapeutic targets for both cancer and PH. Glutamine also contributes to ROS equilibrium through the production of NADPH via the glutamate dehydrogenase (GLUD) pathway [43,44]. Furthermore, pyruvate/malate cycling provides reducing equivalents for glutathione through the oxidation of malate by malic enzymes and subsequent production of NADPH [45,46].
2.3. Nucleotide biosynthesis
The ability of glutamine to donate nitrogen or carbon toward cellular biomass makes it vital for cellular growth and function. Namely, amino acid and fatty acid synthesis, as well as purine and pyrimidine biosynthesis, rely on these carbons and nitrogens from glutamine respectively [47]. Glutamine’s utility as a nitrogen reserve is bolstered by the fact that cells lacking glutamine undergo cell cycle arrest that can be reversed by exogenous nucleotides but not TCA cycle intermediates [48]. In fact, nucleotide biosynthesis from exogenous glutamine has been observed in both cell culture and ex vivo primary human lung cancer samples.
Other glutamine-involving pathways are involved in nucleotide synthesis. Glutamine can be converted into aspartate, a key carbon source for nitrogenous bases, via the TCA cycle and subsequent transamination (Figure 1), and aspartate treatment suffices to rescue cell cycle arrest due to glutamine deprivation [49–53]. Moreover, enzymes that catalyze the consolidation of glutamine-derived nitrogen into pyrimidine precursors may be activated by glutamine-dependent mTOR signalling [54,55].
2.4. Amino acid biosynthesis
Glutamine is necessary to maintain the delicate balance of amino acid flux in the cell. Glutamine-derived non-essential amino acids make up at least half of all residues used by cultured cells in vitro for protein synthesis [20]. Proline, a major player in the production of extracellular collagen, can be produced by utilizing the carbon and nitrogen from glutamine-derived glutamate [9,60]. Likewise, the role of aspartate biosynthesis, which is closely related to glutamine flux through the TCA cycle and glutamate transamination, is crucial for cell survival. Transaminases or aminotransferases catalyze a transamination reaction between an α-keto acid and an amino acid. Aspartate appears to be a limiting amino acid for nucleotide biosynthesis in order to support cell proliferation [49,52,53], as discussed in greater detail in the context of PH below [22].
In sum, glutamine can directly donate its carbon and nitrogen, as well as secondarily produce reducing equivalents and stimulate signaling pathways, making it vital for proper fatty acid, amino acid, and nucleotide homeostasis.
2.5. Regulation of glutamine metabolism (Figure 2)
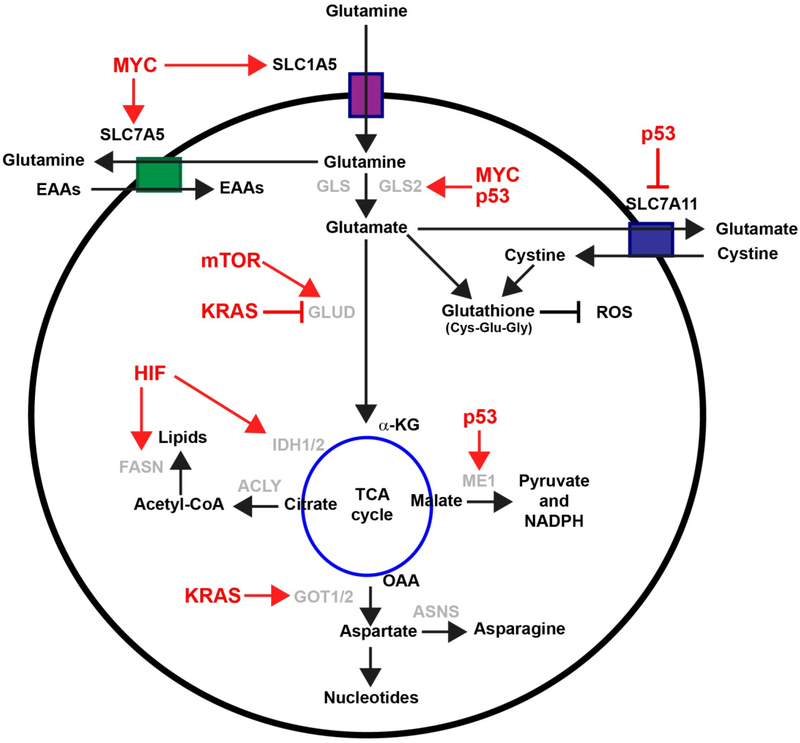
Figure 2: Regulation of glutamine metabolism:
Enzymes involved in glutaminolysis are regulated by both oncogenes and tumor suppressor genes (red font). α-KG: Alpha-ketoglutarate; ACLY: ATP-citrate synthase; ASNS: Asparagine Synthetase; EAAs: Essential amino acids; FASN: Fatty Acid Synthase; GLUD: glutamate dehydrogenase GLS: Glutaminase; GOT1/2: Glutamic-oxaloacetic transaminase 1/2; HIF: Hypoxia inducible factors; IDH1/2: Isocitrate dehydrogenase 1/2; ME1: malate dehydrogenase enzyme 1; NADPH: nicotinamide adenine dinucleotide phosphate; OAA: Oxaloacetate; PSAT: Phosphoserine aminotransferase; SHMT: Serine hydroxymethyltransferase; ROS: Reactive oxygen species.
Glutamine metabolism is modulated by multiple triggers as well as genetic mutations important in both normal cellular physiology and disease [20].
2.5.1. MYC
The actions of Myc, one of the most frequently amplified genes in human cancer [56], has been mostly associated with upregulated glutamine metabolism [57]. The mRNA and protein levels of glutamine transporters and GLS are all upregulated in response to Myc. In addition, it has been shown that Myc drives the glutamine-dependent fueling of the TCA cycle as well as glutathione production [58]. In Myc-mutated cells, glutamine can be used for de novo proline synthesis [59] or production of the oncometabolite 2-hydroxyglutarate in breast cancer [60]. At the mechanistic level, it has been proposed that Myc transcriptionally represses a microRNA family, miR-23 [36]. MicroRNAs are small (~22nt) non-coding RNAs that negatively regulate gene expression. Since the discovery of the first miRNA in C.elegans in 1993 [61], more than 2500 mature miRNAs have been annotated in the human genome (miRBase Release 22, March 2018; www.mirbase.org). miRNAs have been involved in the regulation of multiple physiological and pathophysiological processes, including differentiation, proliferation, metabolism, apoptosis, immune response, and response to cellular stresses [62–64]. Moreover the implication of miRNAs in the pathogenesis of a myriad of diseases including metabolic and cardiovascular diseases, and cancer has been reported [65–67]. By repressing miR-23a and miR-23b expression, Myc increases the expression of their target protein GLS leading to upregulation of glutamine catabolism, leading to both increased TCA cycle-dependent ATP production and increased substrate for glutathione synthesis. Glutamine metabolism is modified in response to Myc-dependent induction of oncogenic pathways; mTOR [68], and in the case of breast cancer, crosstalk with Her2 (Erbb2) and the estrogen receptor [69,70]. Altogether, data suggest glutaminolysis is an integral unit of Myc-driven oncogenesis, across multiple cancer types and contexts. With regard to these results, in PH, Myc is induced under hypoxia by a hypoxia inducible factor (HIF)-2α -dependent mechanism [71,72]. While the contribution of Myc to metabolic rewiring in pulmonary hypertension has not been elucidated, HIF-2α has been demonstrated to be a crucial driver of the endothelial pathophenotypes of PH in vivo, and separately, Myc activation has been found to sustain the proliferation of both PAECs and PASMCs [72,73]. Moreover, recombinant interleukin-6 (IL-6) treatment under hypoxia in rodent models leads to robust pulmonary vascular remodeling and ultimately a PH phenotype [74]. In addition, mice with IL-6 overexpression develop PH spontaneously, driven by an obliterative form of remodeling comparable to human disease. Conversely, mice with IL-6 knockout are PH development resistant, even under hypoxia [74–76]. IL-6-induced arteriopathic changes are accompanied by activation of pro-proliferative transcription factors c-Myc and Max, suggesting that IL-6 promotes the development and progression of pulmonary vascular remodeling and PAH through, at least in part, activation of c-Myc [74]. Further studies will be necessary to more precisely define the contribution of Myc-dependent rewiring to glutamine metabolism.
While certain miRNAs have been extensively studied in the context of PH [77], the role of the miR-23 family remains elusive and poorly understood. Expression level of miR-23a was found to be modulated in the circulating plasma of PH patients [78]. More recently, it has been proposed that miR-23a can play a role in the control of PASMC proliferation and migration through modulation of its target BMPR2 [79]. Although modulation of the miR-23 family has been reported in small cohort of PH patients and has yet to be confirmed, it is appealing to consider its candidacy as a metabolic target for therapeutic intervention in PH.
2.5.2. KRAS
Oncogenic KRAS-driven transformation also drives a pathologic dependence on glutamine metabolism [80,81]. Importantly, the effect varies, depending on the particular KRAS mutation. KRAS-G12C or G12D mutations are shown to induce dependence on glutamine-dependent nucleotide metabolism in lung cancer cells, whereas the KRAS-G12V variation does not; albeit this disparity has yet to be mechanistically explained [82]. Moreover, KRAS mutations can drive a downregulation of GLUD, increasing reliance on aminotransferases and ultimately increasing production of glutathione to affect ROS levels [83]. Correspondingly, a mechanistic interconnection was recently described among RAS mutations, lung cancer, and PH in both genetic models of lung cancer and patients [84]; but the role of glutamine metabolism remains here incompletely explored.
2.5.3. Tumor protein p53 (TP53; also known as p53)
p53 is one of the best known tumor suppressor proteins and plays important role in the induction and maintenance of senescence [85]. Increasing evidence suggests that p53-dependent metabolic changes underlie substantial cell-fate decisions and p53-mediated tumour suppression. In the context of transformed cells, p53 represses the TCA-associated malic enzymes ME1 and ME2 which are essential for NADPH production, lipogenesis, and, relevant to this topic, glutamine metabolism. Downregulation of ME1 and ME2 reciprocally activates p53 through AMP- and MDM2-activated protein kinase-mediated processes in a feed-forward manner, enhancing p53 activity [86]. In addition, p53-mediated transcriptional activation of GLS2, which promotes mitochondrial oxidative phosphorylation, enhances GSH synthesis to reduce cellular ROS levels [87,88].
Although p53 mutations have not yet been described in PH, p53−/− mice develop more severe PH under chronic hypoxia compared with normoxic controls [89]. Notably, direct pharmacological inactivation of p53 (pifithrin-α) promotes pulmonary vascular remodeling and/or aggravates monocrotaline-induced PH (MCT-PH) in rats [90]. Conversely, treatments that activate p53, such as Nitulin-3, an analog of cis-imidazoline, blunted PH progression in several murine models of PH by inducing PASMC growth arrest [91], but the role of glutamine metabolism here has yet to be described.
2.5.4. Hypoxia inducible factors (HIFs)
HIF-1α and HIF-2α are master transcription factors which become stabilized under conditions of low oxygen. These factors direct glutamine towards anaerobic processes [92,93]. HIF-α stabilization, as controlled primarily by a repression of HIF prolyl hydroxylation, ultimately upregulates shuttling of glucose away from the TCA cycle by accelerating its conversion to lactate. While glutamine can usually replenish the carbon intermediates in the TCA cycle, notably α-ketoglutarate [18,94], in certain cell types this α-ketoglutarate is used to produce citrate, acetyl-CoA, and lipids through reductive carboxylation [92,95]. In other cell types, such as human B cell lymphoma cells, this reductive carboxylation is minor, and TCA cycling drives glutamine metabolism under hypoxia [96]. HIF-α can also be stabilized under normoxia in tumors. Loss-of-function mutations in enzymes involved in HIF-α subunit degradation (for instance, the von Hippel-Lindau tumor suppressor (VHL)), activation of mTOR, or even glutamine itself can all promote accumulation of HIF-α [97,98]. The continued study of genes and tissues will likely shed further light onto the modulation of glutamine metabolism through both expected and surprising pathways.
The functions of HIF in the progression of hypoxemia-related pulmonary hypertension (WHO Group 3 PH) has been extensively characterized through rodent modelling [99]. Partial HIF deficiency of either HIF, HIF-1α (Hif1a+/− mice) or HIF-2α (Hif2a+/− mice), significantly mitigated the increase of pulmonary arterial pressure and RV hypertrophy that would be seen in wild-type mice exposed to chronic hypoxia [100–102]. Conversely, stabilization of HIF in VHL−/− mice resulted in development of spontaneous PH [103]. Mice harboring an endothelial-specific knockout of prolyl-4-hydroxylase 2 (PHD2) [104], implicating a connection with HIF-2α were particularly susceptible to severe PH with histologic features more akin to PAH, indicating the cell-type-specific importance of this HIF isoform in this disease and the overarching role of these molecules in multiple subtypes of PH, some of which are not directly driven by alveolar hypoxia. Mechanistically, it has been proposed that HIF stabilization induces rewiring of oxidative metabolism which contributes pathogenically, at least in part through activation of the hypoxia-induced microRNA-210 (miR-210) [105–107]. Indeed increasing appreciation has recently emerged regarding the importance of hypoxamiRs [107,108], miRNAs involved in the regulation of hypoxia response, in the pathogenesis of cardiovascular diseases such as PH [77] as well as in metabolic reprogramming [65]. In particular, miR-210 has been identified as an adaptive factor regulating iron-sulfur (Fe-S) biogenesis, mitochondrial metabolism, and cellular redox states through repressing the Fe-S cluster assembly proteins ISCU1/2 [106,109,110]. In the acute setting, this miRNA facilitates adaptation to hypoxia. However, via chronically repressing ISCU1/2 and Fe-S biogenesis, miR-210 acts as an intracellular pathogenic factor in pulmonary arterial cells to induce oxidative stress and to promote PH in vivo. In addition by controlling expression of several TCA cycle enzymes such as SDHD and NDUFA4, miR-210 indirectly regulates glutamine metabolism [108,111–114]. In turn, decreased TCA cycle activity can lead to an accumulation of TCA cycle intermediates, such as succinate, which in turn contribute to HIF stabilization by inhibiting prolyl hydroxylases.
3. Glutamine metabolism in PH (Figure 3)
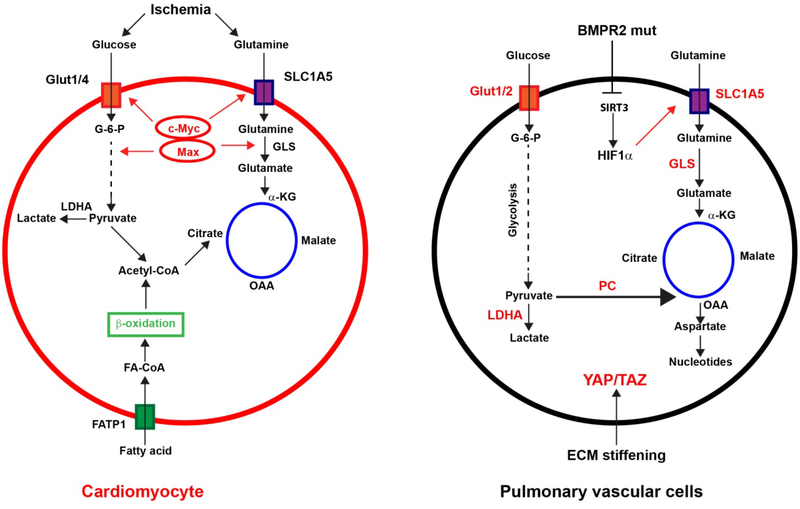
Figure 3: Dysregulated glutamine metabolism in pulmonary hypertension (PH):
Relevant for PH, glutamine metabolism has been shown to be modulated by either hypoxia or mechanical cues from the stiffened extracellular matrix (ECM). α-KG: Alpha-ketoglutarate; BMPR2: Bone Morphogenetic Protein Receptor Type 2; FA-CoA: Fatty acid coenzyme A; FATP1: Fatty acid transport protein 1; G-6-P: Glucose-6-phosphate; GLS: Glutaminase; HIF1α: Hypoxia inducible factor 1 alpha; LDHA: Lactate dehydrogenase A; OAA: Oxaloacetate; PC: Pyruvate carboxylase; SIRT3: Sirtuin 3.
While metabolic reprogramming and mitochondrial dysfunction has been proposed as a key event in PH, the importance of amino acid metabolism and especially glutamine metabolism still remains poorly appreciated. In part, these deficiencies in our understanding may stem from the fact that previous studies of PH have relied mainly on modelling this disease with hypoxia. However, this only scratches the surface of PH disease triggers. Genetic mutations, congenital heart disease, and human immunodeficiency virus infection - just a couple examples of other forms of PH – are also shown to have significant metabolic dysfunction, independent of hypoxic insult. Nevertheless, recent advances have pointed out the role of glutamine metabolism in PH, in addition to the direct influence of hypoxic injury.
3.1. Genetic mutation
Haploinsufficient loss-of-function mutations in the bone morphogenetic protein type 2 receptor (BMPR2) gene, a member of the transforming growth factor-beta (TGF-β) superfamily, account for at least 80% of hereditary and about 20% of idiopathic PAH cases [115,116]. Other TGF-β/bone morphogenetic protein (BMP) signaling pathway components have also been identified as pathogenic, such as the Activin A Receptor Like Type 1, ACVRL1), a type I membrane glycoprotein endoglin (ENG), the secreted ligand growth differentiation factor 2 (GDF2), the SMAD family member 9 (SMAD9), and the scaffolding protein caveolin 1 (CAV1), offering convincing verification for the pivotal role dysregulated BMP signaling has in the context of PAH disease progression. More recently, alterations in genes not immediately related to TGF-β/BMP signaling, including the potassium two pore domain channel subfamily K member 3, KCNK3 [117,118], the T-box transcription factor 4 (TBX4), the water channel aquaporin 1 (AQP1), the cation-transporting ATPase 13A3 (ATP13A3), the amino-sensing serine/threonine-protein kinase General Control Nonderepressible 2 (GCN2), and SOX17 have been linked to PAH [7,119]. Yet, the contribution of factors genetically associated with PAH to vascular cell metabolism has not been investigated in depth, and only recent evidence has emerged regarding the possible contribution of these factors to glutamine metabolism.
Indeed, abnormal glutamine metabolism in both plasma and pulmonary vascular tissue has been observed in genetically haploinsufficient PAH patients [23]. When comparing transpulmonary glutamine gradients among BMPR2-deficient WHO Group 1 PAH patients, WHO Group 3 patients, and healthy controls, WHO Group 1 PAH patients displayed substantial decline in glutamine levels across this transpulmonary vascular gradient compared with controls. This suggests that abnormal BMPR2 function may substantially affect pulmonary vascular glutamine metabolism. In the same study, cell culture models along with transgenic mice expressing PAH-causing BMPR2 mutations displayed appreciably more glutamine-derived carbon throughout the TCA cycle than wild-type endothelium. As such, upregulated glutamine metabolism was found to be crucial to sustaining the metabolic needs of diseased and presumably hyperproliferative PAECs.
Beyond BMPR2, the direct contribution of other factors genetically associated with PAH has not been fully investigated, but it is tempting to speculate that pathogenic mutations found in genes related to the TGF-β/BMP pathway can also affect glutamine metabolism in the lung vasculature [120–122]. Furthermore, a number of factors genetically linked to PAH may also carry relevance to glutamine biology, independent of BMP signaling. By leveraging advanced computational modelling, it was recently reported that the actions of a network of factors linked to heritable PAH converge upon the matrix stiffening-miR-130/301 molecular circuit (see Section 3.4) in order to remodel extracellular matrix (ECM) [123]. Given the predominant role of ECM remodeling and stiffening on glutamine metabolism in diverse contexts such as cancer [124] and PH [22], it is reasonable to suspect that these same factors genetically related to PAH could be involved in glutamine metabolism. Moreover, the GCN2 gene has been found to carry loss of function mutations that are associated with an increased risk of PAH. It encodes for a protein implicated in metabolic-stress sensing and modulating amino acid metabolism in response to nutrient deprivation through its crosstalk with the mTORC1 pathway [125,126]. Considering the importance of the mTORC1 pathway in the regulation of amino acid and notably glutamine metabolism, it is conceivable that mutations in GCN2 that predispose to PAH may also affects glutamine metabolism in the lung vasculature.
3.2. Hypoxia
WHO Group 3 PH due to hypoxia-driven lung diseases encompasses almost a quarter of all patients with PH [3,5]. In parallel, hypoxia has been recognized for decades as a potent driver of metabolic switching and glutamine metabolism (see Section 2.5) in both non-transformed and transformed cells [127,128]. Correspondingly, it was reported that the lung tissue of Group 3 PH patients exhibit a trend towards more robust consumption of glutamine than lungs of non-diseased patients [23]. Consistent with this result, an independent study demonstrated upregulation of GLS in the pulmonary vasculature of Group 3 PH patients [22]. Yet, it remains difficult to determine whether hypoxia alone is the primary trigger of GLS induction in Group 3 PH patients in vivo, as these individuals often present with notable vascular matrix stiffening [129], which in itself can also modulate glutamine metabolism (see Section 3.4 below).
3.3. Inflammation
Lately, more attention is being given to the perivascular inflammation that has been regularly observed across many types of PH, including both idiopathic and systemic autoimmune disease forms of PAH [12,130,131]. Inflammation is now known to be closely related to metabolic shifts and genetic susceptibility in vascular cells, which may have implications in the progression of PAH. Therefore, classifying genetic predispositions that impair inflammatory resolution, and determining how immune driven injury to the vasculature alters metabolic function and the presentation of PH cells is imperative. Dysregulated immunity linked to PH can arise from other genetic causes (such as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy, APECED) [132], viruses (such as human immunodeficiency virus, HIV), parasites (such as Schistosoma mansoni) or other generalized autoimmune processes (such as in connective-tissue diseases). As a result, recent evidence implicating glutamine metabolism to inflammatory processes correspond with the above insights linking the importance of glutamine directly to PH.
Tissue inflammation in general is characterized by significant changes in metabolic activity that are attributable to the activation of vascular cell proliferation [22,133], migration and contractility, but also to the recruitment of monocytes and neutrophils [14,134], in addition to locally-proliferating lymphocyte populations. Human studies of scleroderma-induced PAH [135] as well as systemic sclerosis [136] have begun to implicate alterations of glutamine metabolism via metabolomic studies in blood and urine. Similarly, infection with the parasite Schistosoma mansoni, a predominant cause of PAH in the developing world, has demonstrated dysregulated glutamine handling across multiple tissue compartments [137]. HIV infection, a predisposing risk factor for PAH, has also been linked to dysregulated glycolytic [138] and glutaminolytic [139] pathways in infected primary human CD4-positive lymphocytes. Interestingly, such alterations of glutamine metabolism with HIV infection have been noted in non-lymphocyte cells, such as in primary neural tissue, mediating numerous aspects of neuroinflammation [140]. Moreover, a number of studies have implicated dysregulated glutamine and glutamate metabolism in microglia [141–144] and macrophages relevant to HIV infection, particularly in the central nervous system. Given that inflammatory interstitial macrophages are highly active in PAH pathogenesis [130,131,145] in the lung, it is tempting to speculate that similar alterations of glutamine utilization may play a crucial role in how these innate immune cells interact with the pulmonary vasculature to result in disease.
3.4. Stiffness
The composition and quantity of ECM, as well as vascular tone, influence vascular stiffness. Matrix remodeling, perivascular fibrosis, and consequent ECM stiffening have recently emerged as early hallmarks of PH that are both cause and consequence of disease [129,146–149]. Various forms of PH report attenuated compliance of the vessel wall (i.e. stiffness), marking it as an important contributor and index of disease development. These changes are driven by activated vascular cells exhibiting metabolic alteration along with hyperproliferative, migratory, and invasive capabilities. Vascular stiffening was recently flagged as a predecessor to varieties of PH moderated by mechanoactivation of two transcriptional co-activators, YAP (Yes Associated Protein 1) and TAZ (Transcriptional Coactivator with PDZ-Binding Motif), known to regulate cell survival, proliferation, ECM, and organ size [150–153]. Downstream induction by YAP/TAZ of the microRNA-130/301 family further promotes collagen remodeling and matrix stiffening in PH [123,129,154,155]. In addition, it was found that YAP/TAZ-induced pulmonary vascular stiffness can control the metabolic shifts important in PH – namely, by inducing GLS and driving proliferation even during glycolysis, thus promoting PH in vivo [22]. Notably, the true breadth of metabolic influence of the ECM remodeling and stiffening across multiple cell types in PH may be even more extensive and awaits further delineation. Recent findings in cancer have implicated metabolic cooperation/competition involving glutamine metabolism within the tumor niche to drive cancer progression [156,157]. Given the importance of vascular cell crosstalk in PH [158], it is likely that similar metabolic cooperation and competition in the vascular niche also drives PH development.
3.5. Right ventricle.
Right-sided heart failure is the primary cause of death in PAH [159–161], and maladaptive right ventricular hypertrophy (RVH) occurs as a consequence of increased pulmonary arterial pressure and possibly other poorly described inherent or acquired triggers. PH prognosis is directly related to the performance of the right heart [160]. Recently, it has been proposed that right ventricular hypertrophy could also be a trigger of vascular alterations in PH itself [162,163]. Specifically, increasing evidence supports the notion that right heart dysfunction may reciprocally trigger vascular manifestations of PH [161–163], potentially via promoting vascular stiffening and adversely affecting PA compliance. Consequently, it appears crucial to decipher the long-term evolution of cardio-pulmonary interactions in PH, particularly in regard to metabolic cooperation or competition.
At the metabolic level, it has been found that even at an early stage, the chronic RV pressure overloads state results in impaired bioenergetics [16,161]. As in the pulmonary vasculature, much emphasis has been placed on changes in glucose utilization in the RV. In healthy hearts, the RV can modulate its substrate utilization between fatty acids and glucose as needed [12]. In contrast, hypertrophied RV tissue is reliant solely on glucose metabolism. This shift from oxidative phosphorylation to aerobic glycolysis in RVH has been observed in several rodent models of PH as well as in human patients [16]. Consequently, increased glucose consumption and lactate production have been reported, resulting in acidosis, which impairs RV function. Increased glucose consumption has been visualized by FDG-PET scans in RVH of rodent models of PH as well as in human instances of disease [164]. Notably, glutamine metabolism has also been found to be upregulated in the remodeled RV of PH, at least in rodent models [161,165]. 14C-glutamine metabolism was found to be 6-fold higher in the RV of monocrotaline-treated rats compared to controls. In a similar manner as aerobic glycolysis, glutaminolysis is seemingly maladaptive. In accordance with augmented glutaminolysis, RV expression of the glutamine transporters SLC1A5 and SLC7A5 were upregulated in monocrotaline-treated rats [166]. The mechanistic basis for this upregulation looks to be due to the activation of the cMyc-Max pathway, possibly due to RV ischemia. However, when and if true ischemia commonly occurs in the RV of PAH-patients remains controversial. Another appealing hypothesis underlying these metabolic changes invokes the mechanical regulation of RV glutamine metabolism via pressure-volume overload in PH. Such a biomechanical hypothesis may depend upon complex and poorly understood metabolic interactions among RV cardiomyocytes and cardiac fibroblasts – crosstalk that has yet to be described in detail in PH. Such a notion may also suggest that alterations of RV systolic or diastolic function may then directly correlate with glutamine handling. These concepts all await definitive investigation.
In summary, these studies cumulatively illustrate how the overarching control and roles of glutamine handling in PH may conceptually link our understanding of genetics, hypoxia, inflammation, and matrix remodeling into a more comprehensive theory of PH pathogenesis. Such fundamental insights strengthen the notion of therapeutic targeting of glutamine metabolism in both the lung vasculature and RV.
4. Therapeutic targeting of glutamine metabolism in PH
Due to our advancing appreciation of enhanced glutamine metabolism in numerous hyperproliferative pathologies throughout cancer, PH, and other cardiopulmonary diseases, GLS as well as glutamine metabolism in general are being explored as a significant target for therapeutic interventions [39,167]. The emergence of small molecule inhibitors such as BPTES, CB-839, and compound 968 has led to new avenues of metabolism-targeted drugs that block GLS activity and glutaminolysis. However, such strategies are not straightforward, given the toxic side effects of certain glutamine antagonists, for instance, DON (6-diazo-5-oxo-l-norleucine), stemming from its broad inhibition of several other enzymes related to glutamine utilization [168,169]. In addition, recent development of drugs that target the glutamine transporter SLC1A5 as well as GLUD and aminotransferase has emerged. However, while there has been tremendous growth in our understanding of glutamine metabolism, there remain hurdles to overcome before glutamine metabolism pathway inhibitors can be clinically applied.
4.1. GLS inhibitors
4.1.1. BPTES (Bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3)
BPTES is a non-competitive inhibitor of GLS, which functions by driving inactive tetramer formation [170–172]. BPTES binding at an allosteric site induces a conformational change within the key loop (Glu312-Pro329) neighboring the active site. This inhibition is absolute, BPTES can be added prior to or subsequent to phosphorylation of GLS. BPTES represses glutamine uptake, reduces GSH levels and ultimately drives ROS accumulation. Unfortunately, the likelihood for clinical use is limited given its high molecular weight, insufficient solubility, and low bioavailability. Yet, BPTES has been universally used for experimental research studies of glutamine metabolism, due to its high efficiency at least in vitro.
4.1.2. Compound 968 (5-(3-Bromo-4-(dimethylamino)phenyl)-2,2-dimethyl-2,3,5,6-tetrahydrobenzo[a]phenanthridin-4(1H)-one)
Compound 968 (C968) is a selective uncompetitive inhibitor of GLS, and acts to prevent activation of GLS in cells by impeding post-translational modifications of this enzyme [173]. Unlike BPTES, C968 can only inhibit inactivated GLS via binding to the monomeric form of GLS and thus blocking GLS in an inactive conformation. Moreover, C968 has been shown to have distinct effects on transformed/cancer cells, namely on their proliferation, migration, and invasive capabilities, yet has little to no effect on their non-transformed cellular counterparts.
4.1.3. CB-839 (N-(5-(4-(6-((2-(3-(Trifluoromethoxy)phenyl)acetyl)amino)-3-pyridazinyl)butyl)-1,3,4-thiadiazol-2-yl)-2-pyridineacetamide)
CB-839 is another selective GLS inhibitor, carrying greater potency than BPTES [37]. It acts as a non-competitive inhibitor, with slow, time-dependent, reversible kinetics, and potency which is not dependent on glutamine concentration. Preclinical trials of these drugs have shown some promise for metabolic therapies in breast cancer and lymphoma. Levels of glutamate, α-ketoglutarate, aspartate, fumarate, and malate are all reduced during CB-839 treatment. CB-839 is currently being tested in several Phase II clinical trials for advanced kidney cancer (Clinical Trial NCT02071862).
4.2. Glutamine metabolism inhibitors
While promising, a limitation of targeting GLS alone is that such a strategy does not fully address the important extra-mitochondrial roles of glutamine. As such, novel classes of glutamine metabolism inhibitors have been developed and could be considered for applications in PH.
4.2.1. Glutamine transporter inhibitors (SLC1A5 inhibitors)
Benzylserine and L-γ-glutamyl-p-nitroanilide (GPNA), inhibitors of the glutamine transporter SLC1A5, are shown to be effective against glutamine-dependent cancers [174,175]. However, unless precisely aimed within tumor cells, these drugs can induce toxicity by non-selectively blocking essential glutamine pathways in healthy cells. More recently, a competitive inhibitor of the amino acid transporter SLC1A5, V-9302 has been reported [176]. SLC1A5 inhibition by V-9302 ultimately contributes to an antitumor response both in vitro and in vivo by increasing oxidative stress, cell death, and overall attenuation of cancer cell growth and proliferation. This compound also exhibits unique qualities compared with GPNA, which exhibits poor potency and selectivity in human cells. Notably, while V-9302 has demonstrated an impressive ability to inhibit tumor progression, side effects of these molecules have not been assessed and could represent a substantial caveat for clinical therapy.
4.2.2. Others
Given the importance of glutamine-derived glutamate in tumor progression and the rewiring of glutamate to the TCA cycle through either activation of GLUD or transaminase in hyperproliferative cells, the development of inhibitors of these pathways has recently been considered. Specifically, epigallocatechin gallate (EGCG) and R162, inhibitors of GLUD, as well as aminooxyacetic acid (AOA), a transaminase inhibitor, have been found to attenuate tumor growth by disturbing the anaplerotic use of glutamine in the TCA cycle [177,178]. Application to PH remains an untested domain.
5. Conclusion
In summary, increased lung as well as RV glutamine consumption is observed in diverse models of pulmonary hypertension (PH), as well as in human instances of PH. Guided by encouraging results of pharmacologic targeting of glutamine metabolism and especially GLS, in rodent models of PH, the pathways of glutamine metabolism provide novel therapeutic entry points for PH intervention strategies.
Expert opinion
GLS inhibition as promising therapeutic target in PH:
Beyond glycolysis and redox metabolism, targeting glutamine metabolism for therapeutic purpose is a burgeoning idea that is gaining traction recently across multiple human diseases. Indeed, glutamine is a non-essential amino acid that became partially essential in hyperproliferative and transformed cells. The specific requirement of diseased cells for glutaminolysis in order to sustain their anabolic metabolic needs makes GLS a bona fide target for therapy. Combined with the apparent non-toxic effects of certain GLS inhibitors in non-diseased cells, GLS further fits an ideal profile as a specific target for drug development. Moreover, inhibiting GLS not only affects glutamine metabolism but also has a wide-ranging reach of downstream metabolic and proliferative consequences, indicating that targeting glutaminolysis could have more potent effects than even anticipated. Importantly in PH, GLS inhibition appears effective across multiple diseased cell pathophenotypes of proliferation and migration.
Challenges and obstacles for clinical use of GLS inhibitors in PH:
Because the comprehensive extent of influence of glutamine metabolism in pulmonary arterial cells and in PH has not been defined, challenges exist in terms of predicting all consequences of utilizing GLS inhibitors chronically for PH, particularly at the systemic level. To gain such insight into glutamine metabolism during PH development, it appears crucial to perform glutamine tracing experiments in pulmonary vascular cells and tissue both in vitro and in vivo under disease and healthy conditions. Understanding the fate of glutamine in the pulmonary vasculature at such a granular level will provide new insight for both diagnosis and treatment of PH. It also will be important to determine whether other diseases that promote or overlap with PH clinically contribute to the dysregulation of glutamine metabolism in the lung. For example, it has been shown that hepatic fibrosis, which in some cases has been linked to PH via the connection to portopulmonary hypertension, induces glutamine metabolism reprogramming [179]. Whether hepatic secretion or other liver-specific activity of glutamine contributes to PH remains undefined. Furthermore, beyond the importance of glutamine in PAEC and PASMC proliferation, it will be essential to determine the role of glutamine metabolism in the activation of other vascular and vascular associated cells such as pulmonary arterial adventitial fibroblasts, immune and hematopoietic cells important in vascular inflammation such as macrophages and neutrophils, and platelets important in the thrombotic cascade in PH. Indeed, in other diseases beyond PH, glutamine has been shown to play a key role in fibroblast contractility as well as in macrophage activation. Whether glutamine can play a similar role in PH has yet to be determined; but, if so, would reinforce the attractive notion of therapeutic GLS inhibition in PH. Finally, despite extensive pre-clinical testing in rodents and/or large animal models of PH, a vast majority of drugs that enter Phase I clinical trials ultimately fail for PH, indicating a risk for clinical investment of any new drug at that stage. Coupled with the relatively small number of PAH patients available for clinical trial and the still nebulous clinical trial outcomes used to study PH drugs that do not primarily vasodilate, clinical testing of GLS inhibitors will not be without obstacles. Nonetheless, as therapies targeting the metabolic origin of PH continue to emerge, we believe GLS inhibition will remain a key opportunity to develop, either singly or in combination with synergistic drugs, for this historically neglected and still incurable vascular disease.
Article Highlights.
Increased lung glutamine consumption is observed in diverse models of pulmonary hypertension (PH), as well as in human instances of PH.
In PH, glutamine metabolism is rewired to sustain pulmonary vascular cells hyperproliferation.
Glutamine is converted to glutamate to feed the TCA cycle through increased glutaminase (GLS) expression.
A number of PH triggers increase GLS expression in pulmonary vascular cells.
Targeting GLS pharmacologically ameliorates PH in rodent models of PH.
Pathways of glutamine metabolism provide novel therapeutic entry points for PH intervention strategies.
Funding
The authors have received funding from the American Heart Association [18EIA33900027], the U.S. Department of Health and Human Services, National Institutes of Health [HL 122596, HL 138437, R01 HL124021 UH2 TR002073] and the SILAB Foundation and the French National Research Agency
ANR-18-CE14–0025
Declaration of interest
S.Y.C. has served as a consultant for Zogenix, Vivus, Aerpio, and United Therapeutics; S.Y.C. is a director, officer, and shareholder in Numa Therapeutics; S.Y.C. holds research grants from Actelion and Pfizer. S.Y.C. and T.B. have filed patent applications regarding the targeting of metabolism in pulmonary hypertension. D.P. reports no conflicts.
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Rabinovitch M Molecular pathogenesis of pulmonary arterial hypertension. J. Clin. Invest 122, 4306–4313 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan SY & Loscalzo J Pathogenic mechanisms of pulmonary arterial hypertension. J. Mol. Cell. Cardiol 44, 14–30 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoeper MM, Humbert M, Souza R, et al. A global view of pulmonary hypertension. Lancet Respir. Med 4, 306–322 (2016). [DOI] [PubMed] [Google Scholar]; ** This study provides an overview of the global clinical and research landscape relevant to pulmonary hypertension
- 4.Lau EMT, Giannoulatou E, Celermajer DS & Humbert M Epidemiology and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol 14, 603–614 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Simonneau G, Montani D, Celermajer DS, et al. Updated Clinical Classification of Pulmonary Hypertension. J. Am. Coll. Cardiol 62, D34 (2013) [DOI] [PubMed] [Google Scholar]
- 6.Girerd B, Lau E, Montani D & Humbert M Genetics of pulmonary hypertension in the clinic. Curr. Opin. Pulm. Med 23, 386–391 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Austin ED & Loyd JE The Genetics of Pulmonary Arterial Hypertension. Circ. Res 115, 189–202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann. Intern. Med 115, 343–349 (1991). [DOI] [PubMed] [Google Scholar]
- 9.Pullamsetti SS, Savai R, Seeger W & Goncharova EA Translational Advances in the Field of Pulmonary Hypertension. From Cancer Biology to New Pulmonary Arterial Hypertension Therapeutics. Targeting Cell Growth and Proliferation Signaling Hubs. Am. J. Respir. Crit. Care Med 195, 425–437 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study establishes a functional connection between lung cancer and pulmonary hypertension across multiple phenotypic and molecular levels.
- 10.Boucherat O, Vitry G, Trinh I, et al. The cancer theory of pulmonary arterial hypertension. Pulm. Circ 7, 285–299 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rai PR, Cool CD, King JA, et al. The Cancer Paradigm of Severe Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med 178, 558–564 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenmark KR, Tuder RM & El Kasmi KC Metabolic reprogramming and inflammation act in concert to control vascular remodeling in hypoxic pulmonary hypertension. J. Appl. Physiol. Bethesda Md 1985 119, 1164–1172 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Alessandro A, El Kasmi KC, Plecitá-Hlavatá L, et al. Hallmarks of Pulmonary Hypertension: Mesenchymal and Inflammatory Cell Metabolic Reprogramming. Antioxid. Redox Signal. 28, 230–250 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cottrill KA & Chan SY Metabolic dysfunction in pulmonary hypertension: the expanding relevance of the Warburg effect. Eur. J. Clin. Invest 43, 855–865 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Plecitá-Hlavatá L, Tauber J, Li M, et al. Constitutive Reprogramming of Fibroblast Mitochondrial Metabolism in Pulmonary Hypertension. Am. J. Respir. Cell Mol. Biol 55, 47–57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Archer SL, Fang Y-H, Ryan JJ & Piao L Metabolism and bioenergetics in the right ventricle and pulmonary vasculature in pulmonary hypertension. Pulm. Circ 3, 144–152 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan JJ & Archer SL The right ventricle in pulmonary arterial hypertension: disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ. Res 115, 176–188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lunt SY & Vander Heiden MG Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol 27, 441–464 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Vander Heiden MG & DeBerardinis RJ Understanding the Intersections between Metabolism and Cancer Biology. Cell 168, 657–669 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Pavlova NN & Thompson CB Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J 36, 1302–1315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensley CT, Wasti AT & DeBerardinis RJ Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Invest 123, 3678–3684 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertero T, Oldham WM, Cottrill KA, et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Invest 126, 3313–3335 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; **This study offers in vitro and in vivo genetic and pharmacologic proof demonstrating that ECM stiffening sustains vascular cell growth and migration through YAP/TAZ-dependent glutaminolysis and anaplerosis, thereby linking mechanical stimuli to dysregulated vascular metabolism.
- 23.Egnatchik RA, Brittain EL, Shah AT, et al. Dysfunctional BMPR2 signaling drives an abnormal endothelial requirement for glutamine in pulmonary arterial hypertension. Pulm. Circ 7, 186–199 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergström J, Fürst P, Norée LO, Vinnars E, Intracellular free amino acid concentration in human muscle tissue. J. Appl. Physiol 36, 693–697 (1974). [DOI] [PubMed] [Google Scholar]
- 25.Stumvoll M, Perriello G, Meyer C & Gerich J Role of glutamine in human carbohydrate metabolism in kidney and other tissues. Kidney Int 55, 778–792 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Felig P, Wahren J & Räf L Evidence of inter-organ amino-acid transport by blood cells in humans. Proc. Natl. Acad. Sci. U. S. A 70, 1775–1779 (1973). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tischler ME & Goldberg AL Leucine degradation and release of glutamine and alanine by adipose tissue. J. Biol. Chem 255, 8074–8081 (1980). [PubMed] [Google Scholar]
- 28.Patterson BW, Horowitz JF, Wu G, et al. Regional muscle and adipose tissue amino acid metabolism in lean and obese women. Am. J. Physiol. Endocrinol. Metab 282, E931–936 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Dudrick PS, Inoue Y, Espat NJ & Souba WW Na(+)-dependent glutamine transport in the liver of tumour-bearing rats. Surg. Oncol 2, 205–215 (1993). [DOI] [PubMed] [Google Scholar]
- 30.Souba WW, Herskowitz K & Plumley DA Lung glutamine metabolism. JPEN J. Parenter. Enteral Nutr 14, 68S–70S (1990). [DOI] [PubMed] [Google Scholar]
- 31.Lacey JM & Wilmore DW Is glutamine a conditionally essential amino acid? Nutr. Rev 48, 297–309 (1990). [DOI] [PubMed] [Google Scholar]
- 32.Hosios AM, Hecht VC, Danai LV, et al. Amino Acids Rather than Glucose Account for the Majority of Cell Mass in Proliferating Mammalian Cells. Dev. Cell 36, 540–549 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study highlights the critical importance of glutamine consumption to sustain the metabolic needs of proliferating mammalian cells.
- 33.Altman BJ, Stine ZE & Dang CV From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koppula P, Zhang Y, Zhuang L & Gan B Amino acid transporter SLC7A11/xCT at the crossroads of regulating redox homeostasis and nutrient dependency of cancer. Cancer Commun 38, 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matés JM, Segura JA, Martín-Rufián M, et al. Glutaminase isoenzymes as key regulators in metabolic and oxidative stress against cancer. Curr. Mol. Med 13, 514–534 (2013). [DOI] [PubMed] [Google Scholar]
- 36.Gao P, Tchernyshyov I, Chang TC, et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gross MI, Demo SD, Dennison JB, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol. Cancer Ther 13, 890–901 (2014). [DOI] [PubMed] [Google Scholar]
- 38.Wang J-B, Erickson JW, Fuji R, et al. Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18, 207–219 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matés JM, Campos-Sandoval JA & Márquez J Glutaminase isoenzymes in the metabolic therapy of cancer. Biochim. Biophys. Acta Rev. Cancer (2018). doi: 10.1016/j.bbcan.2018.07.007 [DOI] [PubMed] [Google Scholar]
- 40.Loscalzo J Redox Dysregulation in Vascular Pathobiology. Free Radic. Biol. Med 75 Suppl 1, S2 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Panieri E & Santoro MM ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis 7, e2253 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Culley MK & Chan SY Mitochondrial metabolism in pulmonary hypertension: beyond mountains there are mountains. J. Clin. Invest 128, 3704–3715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin L, Li D, Alesi GN, et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell 27, 257–270 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boroughs LK & DeBerardinis RJ Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol 17, 351–359 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiao W, Wang RS, Handy DE, Loscalzo J, NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 28, 251–272 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fessel JP & Oldham WM Pyridine Dinucleotides from Molecules to Man. Antioxid. Redox Signal. 28, 180–212 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tardito S, Oudin A, Ahmed SU, et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat. Cell Biol 17, 1556–1568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lane AN & Fan TW-M Regulation of mammalian nucleotide metabolism and biosynthesis. Nucleic Acids Res 43, 2466–2485 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Birsoy K, Wang T, Chen WW, et al. An Essential Role of the Mitochondrial Electron Transport Chain in Cell Proliferation Is to Enable Aspartate Synthesis. Cell 162, 540–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardaci S, Zheng L, MacKay G, et al. Pyruvate carboxylation enables growth of SDH-deficient cells by supporting aspartate biosynthesis. Nat. Cell Biol 17, 1317–1326 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garcia-Bermudez J, Baudrier L, La K, et al. Aspartate is a limiting metabolite for cancer cell proliferation under hypoxia and in tumors. Nat. Cell Biol 20, 775–781 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sullivan LB, Gui DY, Hosios AM, et al. Supporting Aspartate Biosynthesis Is an Essential Function of Respiration in Proliferating Cells. Cell 162, 552–563 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sullivan LB, Luengo A, Danai LV, et al. Aspartate is an endogenous metabolic limitation for tumor growth. Nat. Cell Biol 20, 782–788 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ben-Sahra I, Howell JJ, Asara JM & Manning BD Stimulation of de novo pyrimidine synthesis by growth signaling through mTOR and S6K1. Science 339, 1323–1328 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben-Sahra I & Manning BD mTORC1 signaling and the metabolic control of cell growth. Curr. Opin. Cell Biol 45, 72–82 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zack TI, Schumacher SE, Carter SL, et al. Pan-cancer patterns of somatic copy number alteration. Nat. Genet 45, 1134–1140 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wise DR, DeBerardinis RJ, Mancuso A, et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. U. S. A 105, 18782–18787 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DeBerardinis RJ, Mancuso A, Daikhin E, et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U. S. A 104, 19345–19350 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu W, Le A, Hancock C, et al. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc. Natl. Acad. Sci. U. S. A 109, 8983–8988 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Terunuma A, Putluri N, Mishra P, et al. MYC-driven accumulation of 2-hydroxyglutarate is associated with breast cancer prognosis. J. Clin. Invest 124, 398–412 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee RC, Feinbaum RL & Ambros V The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75, 843–854 (1993). [DOI] [PubMed] [Google Scholar]
- 62.Bartel DP Metazoan MicroRNAs. Cell 173, 20–51 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ebert MS & Sharp PA Roles for microRNAs in conferring robustness to biological processes. Cell 149, 515–524 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gebert LFR & MacRae IJ Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol 1 (2018). doi: 10.1038/s41580-018-0045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rottiers V & Näär AM MicroRNAs in Metabolism and Metabolic Disorders. Nat. Rev. Mol. Cell Biol 13, 239–250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Condorelli G, Latronico MVG & Cavarretta E microRNAs in Cardiovascular Diseases: Current Knowledge and the Road Ahead. J. Am. Coll. Cardiol 63, 2177–2187 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Farazi TA, Hoell JI, Morozov P & Tuschl T microRNAs in Human Cancer. Adv. Exp. Med. Biol 774, 1–20 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Csibi A, Lee G, Toon SO, et al. The mTORC1/S6K1 pathway regulates glutamine metabolism through the eIF4B-dependent control of c-Myc translation. Curr. Biol. CB 24, 2274–2280 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Craze ML, Cheung H, Jewa N, et al. MYC regulation of glutamine-proline regulatory axis is key in luminal B breast cancer. Br. J. Cancer 2 258–265 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen Z, Wang Y, Warden C & Chen S Cross-talk between ER and HER2 regulates c-MYC-mediated glutamine metabolism in aromatase inhibitor resistant breast cancer cells. J. Steroid Biochem. Mol. Biol 149, 118–127 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cai Y, Han M, Luo L, Song W & Zhou X Increased expression of PDGF and c-myc genes in lungs and pulmonary arteries of pulmonary hypertensive rats induced by hypoxia. Chin. Med. Sci. J. Chung-Kuo Hsueh Ko Hsueh Tsa Chih 11, 152–156 (1996). [PubMed] [Google Scholar]
- 72.Ahmad A, Ahmad S, Malcolm KC, et al. Differential Regulation of Pulmonary Vascular Cell Growth by Hypoxia-Inducible Transcription Factor–1α and Hypoxia-Inducible Transcription Factor–2α. Am. J. Respir. Cell Mol. Biol 49, 78–85 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang W-F, Xiong YW, Zhu TT, et al. MicroRNA let-7g inhibited hypoxia-induced proliferation of PASMCs via G0/G1 cell cycle arrest by targeting c-myc. Life Sci 170, 9–15 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ. Res 104, 236–244, 28 p following 244 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maston LD, Jones DT, Giermakowska W, et al. Interleukin-6 trans-signaling contributes to chronic hypoxia-induced pulmonary hypertension. Pulm. Circ 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Savale L, Tu L, Rideau D, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir. Res 10, 6 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Negi V & Chan SY Discerning functional hierarchies of microRNAs in pulmonary hypertension. JCI Insight 2, e91327 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wei C, Henderson H, Spradley C, et al. Circulating miRNAs as potential marker for pulmonary hypertension. PloS One 8, e64396 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Peng B & Han Y MiR-23a regulates the proliferation and migration of human pulmonary artery smooth muscle cells (HPASMCs) through targeting BMPR2/Smad1 signaling. Biomed. Pharmacother. Biomedecine Pharmacother 103, 1279–1286 (2018). [DOI] [PubMed] [Google Scholar]
- 80.Weinberg F, Hamanaka R, Wheaton WW, et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. U. S. A 107, 8788–8793 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gaglio D, Soldati C, Vanoni M, Alberghina L & Chiaradonna F Glutamine deprivation induces abortive s-phase rescued by deoxyribonucleotides in k-ras transformed fibroblasts. PloS One 4, e4715 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brunelli L, Caiola E, Marabese M, Broggini M & Pastorelli R Capturing the metabolomic diversity of KRAS mutants in non-small-cell lung cancer cells. Oncotarget 5, 4722–4731 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Son J, Lyssiotis CA, Ying H, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pullamsetti SS, Kojonazarov B, Storn S, et al. Lung cancer-associated pulmonary hypertension: Role of microenvironmental inflammation based on tumor cell-immune cell cross-talk. Sci. Transl. Med 9, (2017). [DOI] [PubMed] [Google Scholar]
- 85.Bieging KT, Mello SS & Attardi LD Unravelling mechanisms of p53-mediated tumour suppression. Nat. Rev. Cancer 14, 359–370 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiang L, Shestov AA, Swain P, et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 532, 255–258 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu W, Zhang C, Wu R, et al. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc. Natl. Acad. Sci. U. S. A 107, 7455–7460 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Suzuki S, Tanaka T Poyurovsky MV, et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc. Natl. Acad. Sci. U. S. A 107, 7461–7466 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mizuno S, Bogaard HJ, Kraskauskas D, et al. p53 Gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am. J. Physiol. Lung Cell. Mol. Physiol 300, L753–761 (2011). [DOI] [PubMed] [Google Scholar]
- 90.Jacquin S, Rincheval V, Mignotte B, et al. Inactivation of p53 Is Sufficient to Induce Development of Pulmonary Hypertension in Rats. PloS One 10, e0131940 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mouraret N, Marcos E, Abid S, et al. Activation of lung p53 by Nutlin-3a prevents and reverses experimental pulmonary hypertension. Circulation 127, 1664–1676 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Metallo CM, Gamiero PA, Bell EL, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This article provides critical insight regarding the regulation and utilization of glutamine metabolism in hypoxic cells.
- 93.Sun RC & Denko NC Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab 19, 285–292 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.DeBerardinis RJ, Lum JJ, Hatzivassiliou G & Thompson CB The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab 7, 11–20 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Gameiro PA, Yang J, Metelo AM, et al. In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab 17, 372–385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Le A, Lane AN, Hamaker M, et al. Glucose-Independent Glutamine Metabolism via TCA Cycling for Proliferation and Survival in B Cells. Cell Metab 15, 110–121 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Keith B, Johnson RS & Simon MC HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12, 9–22 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nakazawa MS, Keith B & Simon MC Oxygen availability and metabolic adaptations. Nat. Rev. Cancer 16, 663–673 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ryan J, Bloch K & Archer SL Rodent models of pulmonary hypertension: harmonisation with the world health organisation’s categorisation of human PH. Int. J. Clin. Pract. Suppl 15–34 (2011). [DOI] [PubMed] [Google Scholar]
- 100.Yu AY, Shimoda LA, Iyer NV, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J. Clin. Invest 103, 691–696 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ball MK, Waypa GB, Mungai PT, et al. Regulation of Hypoxia-induced Pulmonary Hypertension by Vascular Smooth Muscle Hypoxia-Inducible Factor-1α. Am. J. Respir. Crit. Care Med 189, 314–324 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kapitsinou PP, Rajendran G, Astleford L, et al. The endothelial PHD2/HIF-2 axis regulates pulmonary artery pressure in mice. Mol. Cell. Biol 36, 1584–1594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hickey MM, Richardson T, Wang T, et al. The von Hippel–Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. J. Clin. Invest 120, 827–839 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kapitsinou PP, Rajendran G, Astleford L, et al. The Endothelial Prolyl-4-Hydroxylase Domain 2/Hypoxia-Inducible Factor 2 Axis Regulates Pulmonary Artery Pressure in Mice. Mol. Cell. Biol 36, 1584–1594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan SY & Loscalzo J MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle Georget. Tex 9, 1072–1083 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chan SY, Zhang YY, Hemann C, et al. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab 10, 273–284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bertero T, Rezzonico R, Pottier N & Mari B Impact of MicroRNAs in the Cellular Response to Hypoxia. Int. Rev. Cell Mol. Biol 333, 91–158 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Bertero T, Robbe-Sermensant K, Le Brigand K, et al. MicroRNA target identification: lessons from hypoxamiRs. Antioxid. Redox Signal. 21, 1249–1268 (2014). [DOI] [PubMed] [Google Scholar]
- 109.White K, Lu Y, Annis S, et al. Genetic and hypoxic alterations of the microRNA-210-ISCU1/2 axis promote iron-sulfur deficiency and pulmonary hypertension. EMBO Mol. Med 7, 695–713 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Favaro E, Ramachandran A, McCormick R, et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PloS One 5, e10345 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Puisségur M-P, Mazure NM, Bertero T, et al. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ 18, 465–478 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grosso S, Doyen J, Parks SK, et al. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis 4, e544 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fasanaro P, Greco S, Lorenzi M, et al. An integrated approach for experimental target identification of hypoxia-induced miR-210. J. Biol. Chem 284, 35134–35143 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Huang X, Ding L, Bennewith KL, et al. Hypoxia-inducible mir-210 regulates normoxic gene expression involved in tumor initiation. Mol. Cell 35, 856–867 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Evans JDW, Girerd B, Montani D, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir. Med 4, 129–137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guignabert C, Bailly S & Humbert M Restoring BMPRII functions in pulmonary arterial hypertension: opportunities, challenges and limitations. Expert Opin. Ther. Targets 21, 181–190 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Antigny F, Hautefort A, Meloche J, et al. Potassium Channel Subfamily K Member 3 (KCNK3) Contributes to the Development of Pulmonary Arterial Hypertension. Circulation 133, 1371–1385 (2016). [DOI] [PubMed] [Google Scholar]
- 118.Ma L, Roman-Campos D, Austin ED, et al. A novel channelopathy in pulmonary arterial hypertension. N. Engl. J. Med 369, 351–361 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gräf S, Haimel M, Bleda M, et al. Identification of rare sequence variation underlying heritable pulmonary arterial hypertension. Nat. Commun 9, 1416 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bernard K, Logsdon NJ, Benavides GA, et al. Glutaminolysis is required for transforming growth factor-β1-induced myofibroblast differentiation and activation. J. Biol. Chem 293, 1218–1228 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soukupova J, Malfettone A, Hyroššová P, et al. Role of the Transforming Growth Factor-β in regulating hepatocellular carcinoma oxidative metabolism. Sci. Rep 7, 12486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo Y, Deng Y, Li X, et al. Glutaminolysis Was Induced by TGF-β1 through PP2Ac Regulated Raf-MEK-ERK Signaling in Endothelial Cells. PLoS ONE 11, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bertero T, Handen AL & Chan SY Factors Associated with Heritable Pulmonary Arterial Hypertension Exert Convergent Actions on the miR-130/301-Vascular Matrix Feedback Loop. Int. J. Mol. Sci 19, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bertero T, Oldham WM, Grasset EM, et al. Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metab 29 124–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harding HP, Zhang Y, Zeng H, et al. An Integrated Stress Response Regulates Amino Acid Metabolism and Resistance to Oxidative Stress. Mol. Cell 11, 619–633 (2003). [DOI] [PubMed] [Google Scholar]
- 126.Jewell JL & Guan K-L Nutrient Signaling to mTOR and Cell Growth. Trends Biochem. Sci 38, 233–242 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pouysségur J, Dayan F & Mazure NM Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature 441, 437–443 (2006). [DOI] [PubMed] [Google Scholar]
- 128.Semenza GL Hypoxia-inducible factors in physiology and medicine. Cell 148, 399–408 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bertero T, Cottrill KA, Lu Y, et al. Matrix Remodeling Promotes Pulmonary Hypertension through Feedback Mechanoactivation of the YAP/TAZ-miR-130/301 Circuit. Cell Rep 13, 1016–1032 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; ** This study provides evidence of pulmonary arterial stiffening as an early and pervasive driver of pulmonary hypertension through the activation of a YAP/TAZ-dependent mechano-feedback molecular circuit.
- 130.Rabinovitch M, Guignabert C, Humbert M & Nicolls MR Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res 115, 165–175 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Pullamsetti SS & Savai R Macrophage Regulation during Vascular Remodeling: Implications for Pulmonary Hypertension Therapy. Am. J. Respir. Cell Mol. Biol 56, 556–558 (2017). [DOI] [PubMed] [Google Scholar]
- 132.Korniszewski L, Kurzyna M, Stolarki B, et al. Fatal primary pulmonary hypertension in a 30-yr-old female with APECED syndrome. Eur. Respir. J 22, 709–711 (2003). [DOI] [PubMed] [Google Scholar]
- 133.Goveia J, Stapor P & Carmeliet P Principles of targeting endothelial cell metabolism to treat angiogenesis and endothelial cell dysfunction in disease. EMBO Mol. Med 6, 1105–1120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kominsky DJ, Campbell EL & Colgan SP Metabolic shifts in immunity and inflammation. J. Immunol. Baltim. Md 1950 184, 4062–4068 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Deidda M, Piras C, Cadeddu Dessalvi C, et al. Distinctive metabolomic fingerprint in scleroderma patients with pulmonary arterial hypertension. Int. J. Cardiol 241, 401–406 (2017). [DOI] [PubMed] [Google Scholar]
- 136.Fernández-Ochoa Á, Quirantes-Piné R, Borrás-Linares I, et al. Urinary and plasma metabolite differences detected by HPLC-ESI-QTOF-MS in systemic sclerosis patients. J. Pharm. Biomed. Anal 162, 82–90 (2018). [DOI] [PubMed] [Google Scholar]
- 137.Li JV, Holmes E, Saric J, et al. Metabolic profiling of a Schistosoma mansoni infection in mouse tissues using magic angle spinning-nuclear magnetic resonance spectroscopy. Int. J. Parasitol 39, 547–558 (2009). [DOI] [PubMed] [Google Scholar]
- 138.Hegedus A, Kavanagh Williamson M & Huthoff H HIV-1 pathogenicity and virion production are dependent on the metabolic phenotype of activated CD4+ T cells. Retrovirology 11, 98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hegedus A, Kavanagh Williamson M, Khan MB, et al. Evidence for Altered Glutamine Metabolism in Human Immunodeficiency Virus Type 1 Infected Primary Human CD4+ T Cells. AIDS Res. Hum. Retroviruses 33, 1236–1247 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu B, Liu J, Zhao R, et al. Glutaminase 1 regulates the release of extracellular vesicles during neuroinflammation through key metabolic intermediate alpha-ketoglutarate. J. Neuroinflammation 15, 79 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Zhao J, Lopez AL, Erichsen D, et al. Mitochondrial glutaminase enhances extracellular glutamate production in HIV-1-infected macrophages: linkage to HIV-1 associated dementia. J. Neurochem 88, 169–180 (2004). [DOI] [PubMed] [Google Scholar]
- 142.Porcheray F Léone C, Samah B, et al. Glutamate metabolism in HIV-infected macrophages: implications for the CNS. Am. J. Physiol. Cell Physiol 291, C618–626 (2006). [DOI] [PubMed] [Google Scholar]
- 143.Huang Y, Zhao L, Jia B, et al. Glutaminase dysregulation in HIV-1-infected human microglia mediates neurotoxicity: relevant to HIV-1-associated neurocognitive disorders. J. Neurosci. Off. J. Soc. Neurosci 31, 15195–15204 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Datta PK, Deshmane S, Khalili K, et al. Glutamate metabolism in HIV-1 infected macrophages: Role of HIV-1 Vpr. Cell Cycle Georget. Tex 15, 2288–2298 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Florentin J, Coppin E, Vasamsetti SB, et al. Inflammatory Macrophage Expansion in Pulmonary Hypertension Depends upon Mobilization of Blood-Borne Monocytes. J. Immunol. Baltim. Md 1950 200, 3612–3625 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dabral S & Pullamsetti SS Vascular Stiffness and Mechanotransduction: Back in the Limelight. Am. J. Respir. Crit. Care Med 196, 527–530 (2017). [DOI] [PubMed] [Google Scholar]
- 147.Schäfer M, Myers C, Brown RD, et al. Pulmonary Arterial Stiffness: Toward a New Paradigm in Pulmonary Arterial Hypertension Pathophysiology and Assessment. Curr. Hypertens. Rep 18, 4 (2016). [DOI] [PubMed] [Google Scholar]
- 148.Sun W & Chan SY Pulmonary Arterial Stiffness: An Early and Pervasive Driver of Pulmonary Arterial Hypertension. Front. Med 5, 204 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Chemla D, Weatherald J, Lau EMT, et al. Clinical and Hemodynamic Correlates of Pulmonary Arterial Stiffness in Incident, Untreated Patients With Idiopathic Pulmonary Arterial Hypertension. Chest. 154, 882–892 (2018). [DOI] [PubMed] [Google Scholar]
- 150.Panciera T, Azzolin L, Cordenonsi M & Piccolo S Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol 18, 758–770 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Halder G, Dupont S & Piccolo S Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat. Rev. Mol. Cell Biol 13, 591–600 (2012). [DOI] [PubMed] [Google Scholar]
- 152.Yu F-X, Zhao B & Guan K-L Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 163, 811–828 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Calvo F, Ege N, Grande-Garcia A, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat. Cell Biol 15, 637–646 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bertero T, Cottrill KA, Annis S, et al. A YAP/TAZ-miR-130/301 molecular circuit exerts systems-level control of fibrosis in a network of human diseases and physiologic conditions. Sci. Rep 5, 18277 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bertero T, Lu Y, Annis S, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. J. Clin. Invest 124, 3514–3528 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lyssiotis CA & Kimmelman AC Metabolic Interactions in the Tumor Microenvironment. Trends Cell Biol 27, 863–875 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bertero T, Oldham WM, Grasset EM, et al. Tumor-Stroma Mechanics Coordinate Amino Acid Availability to Sustain Tumor Growth and Malignancy. Cell Metab 29 124–140 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Humbert M, Montani D, Perros F, et al. Endothelial cell dysfunction and cross talk between endothelium and smooth muscle cells in pulmonary arterial hypertension. Vascul. Pharmacol 49, 113–118 (2008). [DOI] [PubMed] [Google Scholar]
- 159.Naeije R & Manes A The right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev. Off. J. Eur. Respir. Soc 23, 476–487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Noordegraaf AV & Galiè N The role of the right ventricle in pulmonary arterial hypertension. Eur. Respir. Rev 20, 243–253 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ryan JJ & Archer SL The Right Ventricle in Pulmonary Arterial Hypertension: Disorders of metabolism, angiogenesis and adrenergic signaling in right ventricular failure. Circ. Res 115, 176–188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; * This review article provides a discourse on the pathogenic role of the right ventricle in pulmonary hypertension as well as the molecular underpinnings of right ventricle dysfunction in pulmonary hypertension
- 162.Hautefort A, Mendes-Ferreira P, Sabourin J, et al. Bmpr2 Mutant Rats Develop Pulmonary and Cardiac Characteristics of Pulmonary Arterial Hypertension. Circulation. 139, 932–948(2018). [DOI] [PubMed] [Google Scholar]
- 163.Legchenko E, Chouvarine P, Borchert P, et al. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci. Transl. Med 10, eaao0303 (2018). [DOI] [PubMed] [Google Scholar]
- 164.Yang T, Wang L, Xiong CM, et al. The ratio of (18)F-FDG activity uptake between the right and left ventricle in patients with pulmonary hypertension correlates with the right ventricular function. Clin. Nucl. Med 39, 426–430 (2014). [DOI] [PubMed] [Google Scholar]
- 165.Graham BB, Kumar R, Mickael C, et al. Severe pulmonary hypertension is associated with altered right ventricle metabolic substrate uptake. Am. J. Physiol. - Lung Cell. Mol. Physiol 309, L435–L440 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Piao L, Fang YH, Parikh K, et al. Cardiac glutaminolysis: a maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J. Mol. Med. Berl. Ger 91, 1185–1197 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Song M, Kim S-H, Im CY & Hwang H-J Recent Development of Small Molecule Glutaminase Inhibitors. Curr. Top. Med. Chem 18, 432–443 (2018). [DOI] [PubMed] [Google Scholar]
- 168.Ahluwalia GS, Grem JL, Hao Z & Cooney DA Metabolism and action of amino acid analog anti-cancer agents. Pharmacol. Ther 46, 243–271 (1990). [DOI] [PubMed] [Google Scholar]
- 169.Shapiro RA, Clark VM, Curthoys NP, Inactivation of rat renal phosphate-dependent glutaminase with 6-diazo-5-oxo-L-norleucine. Evidence for interaction at the glutamine binding site. J. Biol. Chem 254, 2835–2838 (1979) [PubMed] [Google Scholar]
- 170.Robinson MM, McBryant SJ, Tsukamoto T, et al. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES). Biochem. J 406, 407–414 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cassago A, Ferreira AP, Ferreira IM, et al. Mitochondrial localization and structure-based phosphate activation mechanism of Glutaminase C with implications for cancer metabolism. Proc. Natl. Acad. Sci. U. S. A 109, 1092–1097 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.DeLaBarre B, Gross S, Fang C, et al. Full-length human glutaminase in complex with an allosteric inhibitor. Biochemistry (Mosc.) 50, 10764–10770 (2011). [DOI] [PubMed] [Google Scholar]
- 173.Stalnecker CA, Ulrich SM, Li Y, et al. Mechanism by which a recently discovered allosteric inhibitor blocks glutamine metabolism in transformed cells. Proc. Natl. Acad. Sci. U. S. A 112, 394–399 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hassanein M, Qian J, Hoeksema MD, et al. Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. Int. J. Cancer 137, 1587–1597 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.van Geldermalsen M, Wang Q, Nagarajah R, et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene 35, 3201–3208 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schulte ML, Fu A, Zhao P, et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat. Med 24, 194–202 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Choi Y-K & Park K-G Targeting Glutamine Metabolism for Cancer Treatment. Biomol. Ther 26, 19–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Korangath P, Teo WW, Sadik H, et al. Targeting Glutamine Metabolism in Breast Cancer with Aminooxyacetate. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res 21, 3263–3273 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Du K, Hyun J, Premont RT, et al. Hedgehog-YAP Signaling Pathway Regulates Glutaminolysis to Control Activation of Hepatic Stellate Cells. Gastroenterology 154, 1465–1479.e13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]