Abstract
Objective:
To evaluate the renoprotective effects of berberine and/or pentoxifylline in reduction of diclofenac-induced acute kidney injury (AKI) in rats.
Material and Methods:
Fifty male Sprague-Dawley rats were allocated into five groups, Group 1: Rats treated with distilled water plus normal saline for 12 days. Group 2: Rats treated with distilled water plus diclofenac for 12 days. Group 3: Rats treated with berberine plus diclofenac for 12 days. Group 4: Rats treated with pentoxifylline plus diclofenac for 12 days. Group 5: Rats treated with berberine + pentoxifylline plus diclofenac 15 mg/kg for 12 days. Blood urea, creatinine, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecules (KIM-1), and cystatin-c were used to measure the severity of AKI.
Results:
Diclofenac led to significant AKI by significant elevation of blood urea, serum creatinine, KIM-1, and NGAL. Treatment with berberine showed no significant effect on all biomarkers level compared to diclofenac group except on serum KIM-1 level which also seen in the pentoxifylline group whereas combination of berberine and pentoxifylline led to more significant effect in the reduction of all renal biomarkers.
Conclusion:
Combination of berberine with pentoxifylline illustrated a synergistic effect in attenuation of diclofenac-induced AKI.
Keywords: Acute kidney injury, berberine, diclofenac, pentoxifylline
INTRODUCTION
Acute kidney injury (AKI) is a sudden episode of acute reduction of kidney function within a short period due to acute kidney damage by different nephrotoxic agents. AKI is a serious clinical condition required immediate intervention and treatment. AKI is often common and reversible clinical complication in patients at intensive care units.[1] Nephrotoxic agents are frequently cause interstitial nephritis, glomerulonephritis, and acute tubular necrosis. It has been verified that proximal renal tubular cells are vulnerable to the toxic effect of diverse drugs due to prolong contact with nephrotoxic agents. Nephrotoxic drugs lead to acute injury of proximal renal tubules through activation of oxidative stress and interference with renal tubular transport processes.[2]
Diclofenac is a nonsteroidal anti-inflammatory drug; belongs to the acetic acid derivatives which used as anti-inflammatory and antipyretic agent.[3]
Diclofenac leads to a dose-dependent reduction of renal vasodilator prostaglandins causing glomerular dysfunction and reduction of glomerular filtration rate (GFR).[4]
Moreover, the nephrotoxic effect of diclofenac depends on its dose- and kidney-related parameters such as plasma coagulation parameters, oxidative damage, and underlying renal damage.[5] Thus, antioxidants play a potential role in prevention of diclofenac-induced AKI through diminution of oxidative stress.[6]
On the other hand, berberine is isoquinoline alkaloids which have antioxidant and anti-inflammatory properties. Berberine inhibits generation of free radical oxygen species and augments the activity of antioxidant enzymes. Beside, berberine improves mitochondrial function through reduction of oxygen consumption rate and increasing of mitochondrial membrane potential against oxidative damage.[7]
The nephroprotective effect of berberine is through prevention of hypoxia/reoxygenation injury of proximal renal tubular through regulation of renal endoplasmic reticulum stress and attenuation of oxidative stress.[8]
Pentoxifylline is a xanthine derivative drug used in treatment of peripheral vascular diseases and nephropathy in diabetic patients. Moreover, pentoxifylline inhibits synthesis of leukotriene and tumor necrosis factor (TNF-α), increases intracellular cAMP and decreases renal inflammation since; pentoxifylline prevents expression of mRNA levels of TNF-α and antagonizes adenosine 2 receptors during AKI.[9]
Likewise, pentoxifylline prevents reduction of GFR due to the renoprotective effect through inhibition of arachidonic acid metabolism, oxidative stress, and glomerular inflammation in diabetic ketoacidosis. As well, pentoxifylline ameliorates renal tissue injury through anti-inflammatory and antioxidant effects.[10,11]
As well, pentoxifylline reduces proteinuria in patients with diabetic nephropathy through improvement of red blood cells deformability which decrease blood viscosity glomerular pressure. Besides, pentoxifylline blocks adenosine receptors which are augmented during AKI since; overproduction of renal adenosine is linked with glomerular hyperfiltration and renal dysfunction.[12]
Therefore, objective of the present study was to evaluate the combined effect of pentoxifylline and berberine in attenuation of diclofenac-induced AKI.
MATERIALS AND METHODS
Fifty Sprague-Dawley Male rats were used; these animals were gained from the Iraqi National Center. Rats age ranged from 3 to 4 months, and their body weight ranged from 250 to 400 g. The animals were isolated as five rats in each sterilized cage and placed with suitable room temperature 22°C–25° with artificial 12/12 light-dark cycle. They left for 1 week for adaptation without any intervention with free access to normal chow pellets and water ad libitum. The study protocol was permitted and approved by Editorial Committee in College of Medicine, Al-Mustansiriya University in accordance with the Guide to the Care and Use of Laboratory Animal.
After acclimatization period, rats were randomly divided into five groups, 10 rats in each group. The study protocol and method for the induction of AKI was according to the previous study.[13]
Group 1 (n = 10): Rats treated with distilled water (5 ml/kg) orally for 12 days, on 6th–12th day they received an intraperitoneal injection of normal saline (5 ml/kg) daily
Group 2 (n = 10): Rats treated with distilled water (5 ml/kg) orally for 12 days, on 6th–12th day they received an intraperitoneal injection of diclofenac (15 mg/kg)
Group 3 (n = 10): Rats treated with berberine (100 mg/kg) orally for 12 days and on 6th–12th day they received an intraperitoneal injection of diclofenac (15 mg/kg)
Group 4 (n = 10): Rats treated with pentoxifylline (100 mg/kg) orally for 12 days and on 6th–12th day they received an intraperitoneal injection of diclofenac (15 mg/kg)
Group 5 (n = 10): Rats treated with pentoxifylline (100 mg/kg) plus berberine (100 mg/kg) orally for 12 days and on 6th–12th day they received an intraperitoneal injection of diclofenac (15 mg/kg).
Sample collection
On the 13th day, rat decapitation was done under chloroform anesthesia. The blood sample was centrifuged for 10 min at 5000 rpm at room temperature which was kept at −20°C for later assessment.
Measurement of biochemical parameters
Blood urea and serum creatinine were estimated using an auto-analyzer (ILab-300-Biomerieux Diagnostic, Milano, Italy) they expressed as mg/dL. Serum neutrophil Gelatinase-associated lipocalin (NGAL), kidney injury molecules (KIM-1), and cystatin-c were measured by ELISA kit methods according to the instruction of the kit manufacturer (Myo-bio source, USA).
Assessment of anthropometric variables
The length was measured by graduated tape measure from nose to the anus (naso-anal length in cm). Rat body weight was measured by specific digital balance in gram. Body mass index (BMI) equal to body weight in grams over the square of length in cm, BMI = BW (grams)/length (cm).[2,14]
Measurement of glomerular filtration rate
Rat GFR was measured indirectly through evaluation of estimated GFR (eGFR). eGFR was measured according to Schwartz formula, eGFR = k × height (cm)/serum creatinine (mg/dL), K = 0.55.[15]
Statistical analysis
Data analysis was done using SPSS (IBM SPSS Statistics for Windows version 20.0, 2014 Armonk, NY, IBM, Corp, USA). Unpaired student t-test was used to test the level of significance between two study groups. The analysis of variance followed by Bonferroni post hoc test was used to compare results of different groups. The level of statistical significance was regarded when P < 0.05.
RESULTS
Anthropometric, biochemical, and inflammatory variables in diclofenac-induced acute kidney injury
During diclofenac-induced AKI, BMI was increased significantly in diclofenac (0.64 ± 0.03) group compared to the control (0.57 ± 0.02) (P = 0.001). Blood urea was raised significantly in diclofenac group (70.5 ± 12.53 mg/dl) compared to the control group (41.83 ± 7.46 mg/dl) (P = 0.0003), serum creatinine in diclofenac group increased significantly (1.52 ± 0.49 mg/dl) compared to the control group (0.7 ± 0.14 mg/dl) (P = 0.001). The eGFR was reduced in diclofenac group (7.59 ± 1.7 ml/min/1.73) compared to the control (16.89 ± 4.21 ml/min/1.73) (P = 0.0001). Moreover, KIM-1 was increased in diclofenac group (269.03 ± 29.61 pg/ml) compared to the control group (73.78 ± 16.29) (P = 0.0001). Nevertheless, NGAL serum levels were insignificantly increased compared to the control group (P = 0.16), [Table 1].
Table 1.
Effect of diclofenac on the anthropometric variables, biochemical and inflammatory biomarkers in diclofenac-induced acute kidney injury
| Variable | Control (n=10) | AKI (n=10) | P |
|---|---|---|---|
| BMI (g/cm2) | 0.57±0.02 | 0.64±0.03 | 0.001* |
| Blood urea (mg/dL) | 41.83±7.46 | 70.50±12.53 | 0.0003* |
| Serum creatinine (mg/dL) | 0.70±0.14 | 1.52±0.49 | 0.0019* |
| eGFR (ml/min/1.73) | 16.89±4.21 | 7.59±1.7 | 0.0001* |
| KIM-1 (pg/mL) | 73.78±16.29 | 269.03±29.61 | 0.0001* |
| NGAL (pg/mL) | 15.78±3.07 | 18.76±4.13 | 0.16 |
*P<0.01. BMI: Body mass index; eGFR: Estimated glomerular filtration rate, KIM-1: Kidney injury molecule-1, NGAL: Neutrophil gelatinase-associated lipocalin, AKI: Acute kidney injury
Effect of berberine on acute kidney injury
Berberine reduced BMI significantly compared to AKI group (P = 0.0003). Blood urea was increased insignificantly in berberine group compared with AKI group (P = 0.85). In while serum creatinine was reduced in berberine group (1.23 ± 0.43 mg/dL) compared with AKI group (1.52 ± 0.49 mg/dL) (P = 0.22). eGFR was improved insignificantly in berberine group (9.55 ± 3.78 ml/min/1.73) compared with AKI group (7.59 ± 1.7 ml/min/1.73), P = 0.20. Furthermore, KIM-1 was significantly decreased in berberine group to (89.00 ± 29.63 pg/mL) compared with AKI group (269.03 ± 29.61 pg/mL) (P = 0.0001). Indeed, NGAL serum level was decreased insignificantly in berberine group (18.13 ± 2.95 pg/mL) compared with AKI group (18.76 ± 4.13 pg/mL) (P = 0.73) [Table 2].
Table 2.
Effect of berberine on the anthropometric variables, biochemical and inflammatory biomarkers in diclofenac-induced acute kidney injury
| Variable | AKI (n=10) | Berberine (n=10) | P |
|---|---|---|---|
| BMI (g/cm2) | 0.64±0.03 | 0.54±0.01 | 0.0003* |
| Blood urea (mg/dL) | 70.50±12.53 | 71.62±10.86 | 0.85 |
| Serum creatinine (mg/dL) | 1.52±0.49 | 1.23±0.43 | 0.22 |
| eGFR (ml/min/1.73) | 7.59±1.7 | 9.55±3.78 | 0.20 |
| KIM-1 (pg/mL) | 269.03±29.61 | 89.00±29.63 | 0.0001* |
| NGAL (pg/mL) | 18.76±4.13 | 18.13±2.95 | 0.73 |
*P<0.01. BMI: Body mass index, KIM-1: Kidney injury molecule-1, NGAL: Neutrophil gelatinase-associated lipocalin, AKI: Acute kidney injury, eGFR: Estimated glomerular filtration rate
Effect of pentoxifylline on acute kidney injury
Pentoxifylline reduced rat BMI compared with AKI group (P = 0.0001). Blood urea was reduced in pentoxifylline group to (64.75 ± 27.48) compared with AKI (70.50 ± 12.53 mg/dL) (P = 0.06). There was insignificant decreased in serum creatinine of pentoxifylline group compared with AKI group (P = 0.06). Besides, pentoxifylline recover eGFR from (7.59 ± 1.7 ml/min/1.73) in AKI group to (12.22 ± 4.33 ml/min/1.73) (P = 0.01). In addition, pentoxifylline reduced KIM-1 significantly (P = 0.0001) compared with AKI. Further, there was insignificant decreased in NGAL serum level in pentoxifylline group compared with AKI group (P = 0.33), [Table 3].
Table 3.
Effect of pentoxifylline on the anthropometric variables, biochemical and inflammatory biomarkers in diclofenac-induced acute kidney injury
| Variable | AKI (n=10) | Pentoxifylline (n=10) | P |
|---|---|---|---|
| BMI (g/cm2) | 0.64±0.03 | 0.55±0.01 | 0.0001* |
| Blood urea (mg/dL) | 70.50±12.53 | 64.75±27.48 | 0.06 |
| Serum creatinine (mg/dL) | 1.52±0.49 | 1.012±0.52 | 0.06 |
| eGFR (ml/min/1.73) | 7.59±1.7 | 12.22±4.33 | 0.01¶ |
| KIM-1 (pg/mL) | 269.03±29.61 | 71.6±31.36 | 0.0001* |
| NGAL (pg/mL) | 18.76±4.13 | 16.78±3.79 | 0.33 |
*P<0.01; ¶P<0.05 unpaired t-test. BMI: Body mass index, eGFR: Estimated glomerular filtration rate, KIM-1: Kidney injury molecule-1, NGAL: Neutrophil gelatinase-associated lipocalin, AKI: Acute kidney injury
Effect of pentoxifylline and berberine combination on acute kidney injury
Combination of berberine with pentoxifylline created a more significant effect than either berberine or pentoxifylline when used alone. This combination reduced BMI to (0.62 ± 0.03 g/cm2) compared with AKI group (0.64 ± 0.03 g/cm2) (P = 0.01). Both blood urea and serum creatinine were reduced in the combination group compared with AKI (P = 0.0001). In addition, eGFR was improved in combination group (22.97 ± 5.33 ml/min/1.73) compared with AKI group (7.59 ± 1.7 ml/min/1.73) (P = 0.0001). Undeniably, this combination established significant reduction in KIM-1 serum levels (42.08 ± 7.57 pg/ml) compared with AKI group (269.03 ± 29.61 pg/ml) (P = 0.0001). In addition, NGAL significantly reduced in combination group (13.37 ± 2.85) compared with AKI group (18.76 ± 4.13) (P = 0.0089) [Table 4].
Table 4.
Effect of pentoxifylline plus berberine on the anthropometric variables, biochemical and inflammatory biomarkers in diclofenac-induced acute kidney injury
| Variable | AKI (n=10) | Pentoxifylline + berberine (n=10) | P |
|---|---|---|---|
| BMI (g/cm2) | 0.64±0.03 | 0.62±0.02 | 0.01¶ |
| Blood urea (mg/dL) | 70.50±12.53 | 29.50±10.60 | 0.0001* |
| Serum creatinine (mg/dL) | 1.52±0.49 | 0.56±0.001 | 0.0001* |
| eGFR (ml/min/1.73) | 7.59±1.7 | 22.97±5.33 | 0.0001* |
| KIM-1 (pg/mL) | 269.03±29.61 | 42.08±7.57 | 0.0001* |
| NGAL (pg/mL) | 18.76±4.13 | 13.37±2.85 | 0.008* |
*P<0.01; ¶P<0.05 unpaired t-test. BMI: Body mass index, eGFR: Estimated glomerular filtration rate, KIM-1: Kidney injury molecule-1, NGAL: Neutrophil gelatinase-associated lipocalin, AKI: Acute kidney injury
Likewise, cystatin-c level was increased significantly in AKI compared with the control (P = 0.000). Coadministration of berberine or pentoxifylline led to a significant reduction of cystatin-c level compared AKI (AKI vs. berberine P = 0.000, AKI vs. pentoxifylline P = 0.000). Furthermore, the combination of berberine and pentoxifylline led to extra reduction in cystatin-c level compared with AKI (P = 0.00001). In addition, cystatin-c level was not significantly differed in combination group compared to the control (P = 0.07), [Figure 1].
Figure 1.
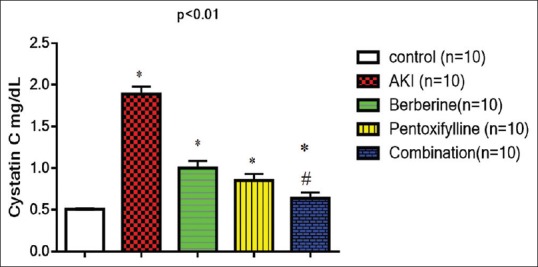
Effects of berberine and/or pentoxifylline on cystatin-c levels in AKI. *P < 0.01 compared to AKI, #P > 0.05 compared to the control
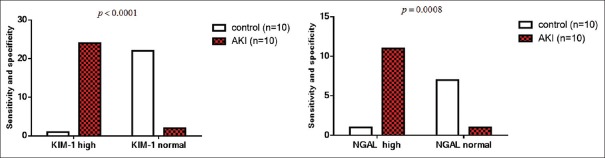
Moreover, the present study confirmed that KIM-1 and NGAL are highly sensitive and specific for AKI with high significant differences compared to the control P < 0.0001 for KIM-1 and P = 0.008 for NGAL [Figure 2].
Figure 2.
Sensitivity and specificity of KIM-1 and NGAL serum levels in AKI
DISCUSSION
The present study demonstrated noteworthy AKI that was induced by diclofenac through the rise of blood urea and serum creatinine compared with control in the experimental rats as maintained by a previous study that showed diclofenac leads to significant renal tubular necrosis and AKI.[16]
Our findings illustrated that induced AKI was associated with a significant reduction in the eGFR due to the development of acute tubular necrosis and glomerular damage since; high dose of diclofenac leads to AKI due to inhibition of renal prostaglandins.[17]
It has been reported that KIM-1serum level is positively correlated with diverse grades of kidney damage in acute nephrotoxicity and AKI because; it is highly sensitive and specific for renal tubular toxicity.[18] However, in vitro and in vivo studies demonstrated that uninterrupted administration of nephrotoxic agents in rats for 7 days' result in time-dependent increase in both KIM-1 and NGAL serum levels before the initiation of renal proximal tubules damage suggesting that both KIM-1 and NGAL are sensitive in the detection of molecular renal damage.[19]
Furthermore, coadministration of berberine with diclofenac led to a significant reduction in rat BMI compared to the AKI group due to the attenuation of diclofenac-induced AKI and amelioration of edematous status through improvement of rat GFR, given that berberine improves renal functions with significant protection of renal proximal tubules against diclofenac-induced tubules injury.[20] Other than blood urea and serum creatinine of the present were not improved adequately which might due to the short duration of therapy or inadequate dose of berberine to counteract the full effect of diclofenac during induction of AKI.
As well, pentoxifylline led to a significant decrease in the rat BMI compared with AKI group due to the amelioration of rat GFR since; pentoxifylline improves renal function with significant protection of renal tubules against diclofenac-induced renal tubules injury.[21] Although blood urea and serum creatinine were not improved sufficiently.
Moreover, the present study illustrated that combined effect of berberine and pentoxifylline demonstrated more important effect than berberine or pentoxifylline alone. This combination may be synergistic depending on the specific parameters that are affected. This combination led to reduction of BMI which may be due to the improvement of GFR and significant nephroprotective effect as reflected by normalization of blood urea and serum creatinine compared with diclofenac group. These findings correspond with a recent study that showed a therapeutic potential effect of berberine in amelioration of diabetic induced-renal dysfunction through inhibition of renal tissue fibrosis.[22] Similarly, pentoxifylline also improves kidney function through attenuation of oxidative stress-induced AKI.[23]
Certainly, cystatin-c jointly with blood urea and serum creatinine is used for the assessment of renal function and GFR. Cystatin-c is a low molecular weight protein excreted by glomerular filtration so; elevated level of cystatin-c is linked with reduction of GFR and glomerular filtration. It has been revealed that cystatin-c serum levels predict the stage and progression of AKI.[24]
Furthermore, cystatin-c is a sensitive biomarker in detection of AKI seeing as; it more sensitive than blood urea and serum creatinine. Consequently, cystatin-c is a surrogate biomarker for AKI and should be incorporated in the estimation of GFR.[25] In the present study, cystatin-c serum levels in AKI were elevated significantly compared with the control group. Thus, coadministration of berberine and/or pentoxifylline led to significant renoprotective effect as disclosed by different studies.[26,27] Finally, the present study is regarded as novel study as this combination was not used previously in the management of AKI.
CONCLUSION
Combination of berberine with pentoxifylline leads to more significant renoprotective than either berberine or pentoxifylline when used alone in diclofenac-induced AKI. Therefore, berberine with pentoxifylline demonstrated a synergistic effect in attenuation of diclofenac-induced AKI.
Research Quality and Ethics Statement
The authors of this manuscript declare that this scientific work complies with reporting quality, formatting and reproducibility guidelines set forth by the EQUATOR Network. The authors also attest that this clinical investigation was determined to require Institutional Ethics Committee review, and was approved by Editorial Committee in College of Medicine, Al-Mustansiriya University in accordance with the Guide to the Care and Use of Laboratory Animal.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to express deep thanks for all under-graduate and post-graduate medical students for their participation in this experimental novel study.
REFERENCES
- 1.Myers RM, Fitzgerald J, Elgarten CW, Getz KD, Li Y, Hogan J, et al. Acute kidney injury after chimeric antigen receptor T-cell therapy for pediatric acute lymphoblastic leukemia. Biol Blood Marrow Transplantation. 2019;25:S168–9. [Google Scholar]
- 2.Al-Kuraishy HM, Al-Gareeb AI, Rasheed HA. Antioxidant and anti-inflammatory effects of curcumin contribute into attenuation of acute gentamicin-induced nephrotoxicity in rats. Asian Journal of Pharmaceutical and Clinical Research. 2019;12:466–8. [Google Scholar]
- 3.Altman R, Bosch B, Brune K, Patrignani P, Young C. Advances in NSAID development: Evolution of diclofenac products using pharmaceutical technology. Drugs. 2015;75:859–77. doi: 10.1007/s40265-015-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bickel M, Khaykin P, Stephan C, Schmidt K, Buettner M, Amann K, et al. Acute kidney injury caused by tenofovir disoproxil fumarate and diclofenac co-administration. HIV Med. 2013;14:633–8. doi: 10.1111/hiv.12072. [DOI] [PubMed] [Google Scholar]
- 5.Al-Kuraishy HM, Al-Gareeb AI, Hussien NR. Betterment of diclofenac-induced nephrotoxicity by pentoxifylline through modulation of inflammatory biomarkers. Asian J Pharm Clin Res. 2019;3:433–7. [Google Scholar]
- 6.Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ. Concept and connotation of oxidative stress in preeclampsia. Journal of laboratory physicians. 2018;10:276. doi: 10.4103/JLP.JLP_26_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imenshahidi M, Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): A clinical review. Phytother Res. 2019;33:504–23. doi: 10.1002/ptr.6252. [DOI] [PubMed] [Google Scholar]
- 8.Visnagri A, Kandhare AD, Bodhankar SL. Renoprotective effect of berberine via intonation on apoptosis and mitochondrial-dependent pathway in renal ischemia reperfusion-induced mutilation. Ren Fail. 2015;37:482–93. doi: 10.3109/0886022X.2014.996843. [DOI] [PubMed] [Google Scholar]
- 9.Donate-Correa J, Tagua VG, Ferri C, Martín-Núñez E, Hernández-Carballo C, Ureña-Torres P, et al. Pentoxifylline for renal protection in diabetic kidney disease. A Model of old drugs for new horizons. J Clin Med. 2019;8 doi: 10.3390/jcm8030287. pii: E287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Morales AM, Goicoechea M, Verde E, Carbayo J, Barbieri D, Delgado A, et al. Pentoxifylline, progression of chronic kidney disease (CKD) and cardiovascular mortality: Long-term follow-up of a randomized clinical trial. J Nephrol. 2019;4:1–7. doi: 10.1007/s40620-019-00607-0. [DOI] [PubMed] [Google Scholar]
- 11.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, Chahin J, Méndez ML, Gallego E, et al. Effect of pentoxifylline on renal function and urinary albumin excretion in patients with diabetic kidney disease: The PREDIAN trial. J Am Soc Nephrol. 2015;26:220–9. doi: 10.1681/ASN.2014010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro-González JF, Muros M, Mora-Fernández C, Herrera H, Meneses B, García J, et al. Pentoxifylline for renoprotection in diabetic nephropathy: The PREDIAN study. Rationale and basal results. J Diabetes Complications. 2011;25:314–9. doi: 10.1016/j.jdiacomp.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Singh AP, Junemann A, Muthuraman A, Jaggi AS, Singh N, Grover K, et al. Animal models of acute renal failure. Pharmacol Rep. 2012;64:31–44. doi: 10.1016/s1734-1140(12)70728-4. [DOI] [PubMed] [Google Scholar]
- 14.Novelli EL, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007;41:111–9. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20:629–37. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alabi QK, Akomolafe RO, Adefisayo MA, Olukiran OS, Nafiu AO, Fasanya MK, et al. Kolaviron attenuates diclofenac-induced nephrotoxicity in male wistar rats. Appl Physiol Nutr Metab. 2018;43:956–68. doi: 10.1139/apnm-2017-0788. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno T, Ito K, Miyagawa Y, Ishikawa K, Suzuki Y, Mizuno M, et al. Short-term administration of diclofenac sodium affects renal function after laparoscopic radical nephrectomy in elderly patients. Jpn J Clin Oncol. 2012;42:1073–8. doi: 10.1093/jjco/hys145. [DOI] [PubMed] [Google Scholar]
- 18.Alkuraishy HM, Al-Gareeb AI, Al-Naimi MS. Pomegranate protects renal proximal tubules during gentamicin induced-nephrotoxicity in rats. J Contemp Med Sci. 2019;5:26. [Google Scholar]
- 19.Al-Kuraishy HM, Al-Gareeb AI, Al-Nami MS. Pomegranate attenuates acute gentamicin-induced nephrotoxicity in Sprague-Dawley rats: The potential antioxidant and anti-inflammatory effects. Asian J Pharm Clin Res. 2019;12:1–3. [Google Scholar]
- 20.Zheng H, Lan J, Li J, Lv L. Therapeutic effect of berberine on renal ischemia-reperfusion injury in rats and its effect on Bax and Bcl-2. Exp Ther Med. 2018;16:2008–12. doi: 10.3892/etm.2018.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sönmez MF, Dündar M. Ameliorative effects of pentoxifylline on NOS induced by diabetes in rat kidney. Ren Fail. 2016;38:605–13. doi: 10.3109/0886022X.2016.1149688. [DOI] [PubMed] [Google Scholar]
- 22.Li HL, Wu H, Zhang BB, Shi HL, Wu XJ. MAPK pathways are involved in the inhibitory effect of berberine hydrochloride on gastric cancer MGC 803 cell proliferation and IL-8 secretion in vitro and in vivo. Mol Med Rep. 2016;14:1430–8. doi: 10.3892/mmr.2016.5361. [DOI] [PubMed] [Google Scholar]
- 23.Ozturk H, Cetinkaya A, Firat TS, Tekce BK, Duzcu SE, Ozturk H. Protective effect of pentoxifylline on oxidative renal cell injury associated with renal crystal formation in a hyperoxaluric rat model. Urolithiasis. 2018;6:1–10. doi: 10.1007/s00240-018-1072-8. [DOI] [PubMed] [Google Scholar]
- 24.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J, Yi Y, Pan R, Zhang C, Han H, Chen J, et al. Berberine protects HK-2 cells from hypoxia/reoxygenation induced apoptosis via inhibiting SPHK1 expression. J Nat Med. 2018;72:390–8. doi: 10.1007/s11418-017-1152-z. [DOI] [PubMed] [Google Scholar]
- 26.Moore PK, Hsu RK, Liu KD. Management of acute kidney injury: Core curriculum 2018. Am J Kidney Dis. 2018;72:136–48. doi: 10.1053/j.ajkd.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Barkhordari K, Karimi A, Shafiee A, Soltaninia H, Khatami MR, Abbasi K, et al. Effect of pentoxifylline on preventing acute kidney injury after cardiac surgery by measuring urinary neutrophil gelatinase – Associated lipocalin. J Cardiothorac Surg. 2011;6:8. doi: 10.1186/1749-8090-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]