Abstract
Children with developmental concerns are more likely to be referred to feeding clinics for food selectivity than typically developing (TD) children. However, there is limited research on food selectivity in children with intellectual disabilities (ID). Fifty-nine TD children and 56 children with ID ages 3-8 years participated in the Children’s Mealtime Study to compare food selectivity, conceptualized as food refusal and narrow food repertoire, among TD children and children with ID. Parents completed a 119-item food frequency questionnaire. Food refusal rate was calculated as the number of foods the child refused of those offered. Food repertoire, comprising the number of unique foods eaten, was determined from a 3-day food record. Compared to TD children, among children with ID the food refusal rate was significantly higher (28.5% vs. 15.7%) and mean food repertoire significantly narrower (20.7 vs. 24.2 unique foods) (p<0.01). Approximately 10% of children with ID and approximately 4% of TD children reported eating no fruit on any of the three days of food intake recording, and approximately 10% of children with ID compared to approximately 2% of TD children reported no vegetable intake on any of the three days. In further analyses, we examined the two measures of food selectivity among children with both ID and probable autism spectrum disorder (ASD) (by the Autism Spectrum Rating Scale) compared to children with ID only and to TD children. Food selectivity appeared to be primarily attributable to those children who also had a probable diagnosis of ASD. These findings support the need for screening for food selectivity of children with ID, particularly those who also have ASD. Children who exhibit food selectivity should be referred for further evaluation and intervention.
Keywords: children, intellectual disability, food refusal, nutrient adequacy, food selectivity, vegetables
Introduction
Children with intellectual disabilities (ID) have significant limitations that can substantially restrict their functioning in several major life activities. These limitations include both intellectual functioning (i.e., reasoning, learning, problem-solving), and adaptive behavior, which comprises domains of social, conceptual, and practical skills (American Association on Intellectual and Developmental Disabilities, 2018). The extant literature regarding the feeding problems of children with ID is limited. Several studies have reported that compared to typically developing (TD) children, feeding problems are more common in children with developmental disabilities (Cermak, Curtin, & Bandini, 2010; D. Field, Garland, & Williams, 2003; Sharp et al., 2013), some of whom may have ID, but may also have other developmental conditions such as autism spectrum disorder (ASD) and sensory deficits. For example, Field et al. (2003) published the results of a chart review of 349 children with feeding problems who were referred to an interdisciplinary feeding clinic. Sixty-four percent of the referred children had a developmental disability, which included 21 children with Down syndrome, 26 children with ASD, and 44 children with cerebral palsy. Of these children, 34% exhibited food refusal, 21% were reported to eat a narrow range of foods, and 26% refused foods that were developmentally appropriate.
In contrast to the literature on food selectivity in children with ID, over the past 10 years there has been considerable research documenting food selectivity as a common problem among children with ASD (Bandini et al., 2010; Graf-Myles et al., 2013; Herndon, DiGuiseppi, Johnson, Leiferman, & Reynolds, 2009; Lockner, Crowe, & Skipper, 2008; Sharp et al., 2013; Suarez, Nelson, & Curtis, 2014; Zimmer et al., 2012). Food selectivity is a concern because a diet limited in variety increases the risk for nutrient inadequacy and risk for chronic disease later in life. A recent case study by Ma, Thompson, and Weston (2016) reported on 7 children with a developmental disability and very limited diets who had scurvy. Zimmer et al. (2012) reported that children with ASD who were identified as selective eaters were more likely than TD children to be at risk for inadequate intakes of several nutrients, including zinc and vitamins B12 and D. In our study of children with ASD and TD children, we also found that a narrower food repertoire was associated with greater inadequacy in nutrient intakes (Bandini et al., 2010). While the reasons for food selectivity among children vary, it is thought that sensory sensitivity may be a key factor in food refusal, particularly among children with ASD (Cermak, Curtin, & Bandini, 2010; Chistol et al., 2018; Engel-Yeger, Hardal-Nasser, & Gal, 2016; Suarez, Nelson, & Curtis, 2014).
Although about one-third of children with ASD also have ID (Baio et al., 2018), very little research has focused on the phenomenon of food selectivity in children with ID more generally. In this study, we hypothesized that children with ID would exhibit more food selectivity (i.e., greater food refusal and more limited food repertoire) and would eat fewer fruits and vegetables than TD children. In this report we compare food selectivity in children with ID to TD children and also examine the impact of food selectivity on nutrient adequacy in children with ID.
Methods
Children with ID and TD children aged 3-8 years and their parents were recruited for the Children’s Mealtime Study through communications with local parent support networks, private and public schools, disability-related organizations, list-servs, newspaper advertisements, and online resources (e.g.., Craigslist®). Two hospital-based clinics that care for children with disabilities also assisted with recruiting children with ID. Inclusion criteria required the child to be in good health and free from diseases or disorders that could affect dietary habits and/or engagement in physical activity (e.g., diabetes, cystic fibrosis, chronic GI illness, or cerebral palsy, blindness or significant hearing loss). Children with ID of unexplained etiology as well as those with syndromes such as Down syndrome and Fragile X were included in the study. Children with ASD who also met criteria for ID were also included. Children with Prader-Willi syndrome were excluded due to the genetically-based hypothalamic dysfunction of this syndrome that is associated with dysregulation of food intake (Angulo, Butler, & Cataletto, 2015). Children whose parents could not read or write English were also excluded because the questionnaires were only available in English. The study was approved by the Institutional Review Board at the University of Massachusetts Medical School, and written informed consent was obtained from the parents or guardians of the children who served as participants. Parents and children were compensated with a gift card to selected community and/or online vendors.
The presence of ID was determined by a cognitive assessment and a measure of adaptive functioning. The Differential Abilities Scale (DAS) (Elliott, 1990) was administered to the child by a psychologist to assess cognitive ability. The Vineland Adaptive Behavior Scales (VABS) (Sparrow, Balla, & Cicchetti, 2005) was administered to one parent by a research assistant or trained project staff to characterize the child’s adaptive skills. Children were classified as having ID if they achieved scores ≤75 on both the DAS and VABS. The Autism Spectrum Rating Scale (ASRS) (Goldstein & Naglieri, 2010) was administered to parents to determine if the child had symptoms consistent with ASD.
Study visits were conducted at UMass Medical School sites in Waltham, Charlestown, and Worcester, Massachusetts, and in locations within the community, including libraries, schools, and participants’ homes.
Parents completed a demographic and medical questionnaire, a diet questionnaire, and a modified food frequency questionnaire (FFQ) for their child at the time of the visit. Fifty-seven mothers and two fathers in the TD group and 47 mothers and nine fathers in the ID group completed the questionnaires. After finishing the questionnaires, the parents were instructed by a registered dietitian nutritionist or a nutrition graduate student to keep a 3-day food record on two weekdays and one weekend day. In order to obtain information on the food that the child ate during the school day, parents were given a form on which they listed the foods that they sent to school with their child. The parents were instructed to ask teachers to fill out the form to record how much the child ate and to add any additional foods provided by the school that their child consumed. In instances where school personnel returned the food the child did not eat to the parents at the end of the school day, the parents could amend the food record. Older TD children 6-8 years were included with their parents in the instructions on how to complete a food record so they could inform their parents about foods they ate outside the home.
Diet questionnaire
Parents were asked to complete a diet questionnaire that contained 5-point Likert-type scales and asked parents to report whether their child refused food based on color, shape, consistency or texture, temperature, smell, whether foods were touching each other, whether foods were mixed together, and the brand of the food. Response choices ranged from strongly agree to strongly disagree.
Definition of food selectivity
We defined food selectivity as comprising two distinct domains: food refusal and food repertoire, based on our previous studies of children with ASD (Bandini et al., 2010).
Food refusal was assessed using a modified version of the Youth/Adolescent Food Frequency Questionnaire (YAQ) which was developed for self-administration with children and adolescents aged 9-18 years in the Growing Up Today Study (A. E. Field et al., 1999) and is based on the original Harvard Food Frequency Questionnaire (Willett, 1998). The YAQ has been shown to be reproducible and reliable (Rockett, Wolf, & Colditz, 1995; Rockett et al., 1997). We modified the instructions on the YAQ for parents to complete rather than the children, and we added two response categories to be able to quantify food refusal: 1) N/A Don’t offer and 2) Never/Will not eat. We used this approach in our previous study of children with ASD (Bandini et al., 2010), but made some minor changes to the FFQ based on modifications made to the YAQ in 2012 (Harvard T.H. Chan School of Public Health Nutrition Department, 2013). In addition, we reduced the number of foods listed from 131 to 119 foods, removing items such as coffee, tea, and condiments.
We used data from the 3-day food records to determine food repertoire. Data were entered into the Nutrition Data System for Research (NDSR, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN). Food repertoire was determined by the number of unique foods (including beverages) each child consumed over a three-day period. We developed a coding system to categorize food and to assess the number of unique foods a child ate which comprised 123 unique categories and included 23 fruits and 39 vegetables. Similar to the approach taken by Drewnowski, Henderson, Driscoll, and Rolls (1997), our approach to categorization resulted in a large number of unique fruit and vegetable categories. The other 61 categories included beverages (6), protein-rich foods (18), dairy foods (3), breads/grains/cereals (14), snack foods (8), nuts (3), and sweets (9).
Nutrient inadequacy
Children’s nutrient intakes were derived from the 3-day food records and analyzed for nutrient composition using NDSR software. The average level of each nutrient across the three recording days was determined for each child. We defined nutritional inadequacy relative to the estimated average requirement (EAR) for the specific life stage and sex group; for vitamin K and potassium, for which an EAR has not been defined, we used the adequate intake (AI) (Institute of Medicine, 2006, 2011). These analyses were restricted to food intake and did not include any supplements the child was taking.
Statistical analysis
Differences in characteristics between children with and without ID were assessed for statistical significance using t-tests for comparing means and chi-square tests for comparing proportions. Parental education was defined as the maximum educational attainment by either parent. Mean differences in numbers of foods offered and food repertoire were compared using t-tests. Food refusal rates were estimated from negative binomial regression models using the number of foods refused as the outcome variable and the number of foods offered as the offset variable. The models were adjusted for age, sex, race/ethnicity, and number of foods offered, and the estimated variances of the regression coefficients were scaled by Pearson’s chi-square to adjust the statistical inferences for under-dispersion of the data. Children with ID were further categorized as those with probable ASD and those with ID only. Mean differences in the numbers of foods offered and food repertoire among the resulting three groups were compared using analyses of variance, and food refusal rates were compared across the three groups using the same statistical approach described above. Chi-square tests were used to compare the reasons for food refusal among the three groups by dichotomizing the diet questionnaire’s 5-point Likert-type scales into two categories: (strongly agree, agree) versus (neither agree nor disagree, disagree, strongly disagree). In a subgroup analysis of children with ID who had food repertoire and nutrient data, the relationship between nutrient inadequacy and food repertoire was examined by categorizing their food repertoire into threelevels: 1) < 15 unique foods; 2) 15 to 20 unique foods; and 3) > 20 unique foods consumed over a 3-day period, as we found the relationships to be non-linear. The percentage of children with nutrient inadequacy in each of the three levels was calculated. Multivariate multilevel regression models were used to assess the inadequacy of multiple nutrients relative to food repertoire simultaneously. Model estimates and inferences were derived using a generalized estimation equations approach with a compound symmetry covariance structure to account for the interdependence among the multiple nutrients from the same child. Two sensitivity analyses were performed. The effect of age on food refusal and food repertoire among children with ID was examined by first dichotomizing age into 3-5 years versus 6-8 years categories and then analyzing the data using the corresponding methods described above. The effect of diet type on food refusal was assessed by stratifying the participants as to whether they were eating an unrestricted or eating a restricted diet (gluten-, lactose-, casein-free or vegetarian). Results with p-values < 0.05 were considered to be statistically significant. All analyses were conducted in SAS Version 9.4 (SAS Institute, Cary, NC).
Results
The characteristics of the participants are presented in Table 1. Fifty-six children with ID and 59 TD children completed the study. The children were six years of age, on average. More boys than girls participated in the study, but the proportion by sex in the two groups did not differ significantly. Of the children with ID, 28 (50%) had Down syndrome and 34 (61%) had probable ASD based on the ASRS. Ten of the children with Down syndrome also had probable ASD. Although there was no statistically significant difference between the two groups in the distribution of race/ethnicity categories, 55% of children with ID were non-Hispanic white compared to 37% of TD children. Significantly more parents of children with ID reported their children were following gluten-free or lactose-free diets compared to parents of TD children (p=0.02 for both comparisons).
Table 1.
Participant characteristics
| ID (n=56) |
TD (n=59) |
p-valuea | |
|---|---|---|---|
| Age in years, mean (sd) | 6.0 (1.9) | 5.8 (1.7) | 0.52 |
| Male, n (%) | 38 (68) | 35 (59) | 0.34 |
| Max parental education, n (%) | |||
| High School/Trade School | 9 (16) | 5 (9) | 0.22 |
| Some College | 15 (27) | 12 (20) | |
| Bachelor’s Degree | 17 (30) | 16 (27) | |
| Advanced Degree | 15 (27) | 26 (44) | |
| Race/ethnicity, n (%) | |||
| Non-Hispanic White | 31 (55) | 22 (37) | |
| Non-Hispanic Black | 5 (9) | 9 (15) | |
| Non-Hispanic Asian | 1 (2) | 7 (12) | 0.12 |
| Hispanic | 9 (16) | 11 (19) | |
| Non-Hispanic Other/Multiracial | 10 (18) | 10 (17) | |
| Diagnosis, n (%) | |||
| Down Syndrome | 28 (50) | n/a | |
| Probable Autism Spectrum Disorderb | 34 (61) | n/a | |
| Special diets, n (%) | |||
| Gluten free | 5 (9) | 0 | 0.02 |
| Lactose free | 5 (9) | 0 | 0.02 |
| Casein free | 3 (5) | 1 (2) | 0.35 |
| Vegetarian (1 Vegan in TD group) | 1 (2) | 3 (5) | 0.33 |
t-tests for comparing means and chi-square tests for comparing proportions
as assessed by the Autism Spectrum Rating Scale
The number of foods on the FFQ that parents reported their children would not eat was, on average, significantly higher among children with ID compared to TD children (29.1 vs. 18.5, p=0.014). The mean number of foods offered and food refusal rates are presented in Table 2. On average, children with ID were offered fewer foods than TD children (92.4 vs. 101.8, p=0.002). Of the foods that were offered, the rate of food refusal among children with ID was significantly higher than among TD children (28.5% vs. 15.7%, p=0.001). Examining fruits specifically, the mean number of fruit items offered did not differ significantly between children with ID and TD children, but the rate of fruit refusal was significantly higher among those with ID compared to TD children (29.8% vs. 15.5%, p=0.006). For vegetables, the mean number of vegetable items offered was significantly lower among children with ID compared to TD children (16.8 vs. 19.3, p=0.002). The rate of vegetable refusal was higher among children with ID compared to TD children (42.4% vs. 29.6%, p=0.035).
Table 2.
Comparison of number of foods offered, food refusal, and food repertoire between children with intellectual disabilities (ID) and typically developing (TD) children
| ID | TD | p-value | |
|---|---|---|---|
| Foods Offered and Food Refusal | (n=56) | (n=59) | |
| Total Foodsa | |||
| Number of total foods offered, mean (sd) | 92.4 (20.0) | 101.8 (9.7) | 0.002 |
| Refusal rate, % | 28.5 | 15.7 | 0.001 |
| Refusal rate ratio (95% CI) | 1.81 (1.26 to 2.60) | ||
| Fruitsb | |||
| Number of fruits offered, mean (sd) | 12.8 (2.5) | 13.5 (2.0) | 0.09 |
| Refusal rate, % | 29.8 | 15.5 | 0.006 |
| Refusal rate ratio (95% CI) | 1.92 (1.21 to 3.05) | ||
| Vegetablesc | |||
| Number of vegetables offered, mean (sd) | 16.8 (5.2) | 19.3 (2.7) | 0.002 |
| Refusal rate, % | 42.4 | 29.6 | 0.035 |
| Refusal rate ratio (95% CI) | 1.43 (1.02 to 2.00) | ||
| Food Repertoired | (n=48) | (n=55) | |
| Total foods, mean (sd) | 20.7 (6.9) | 24.2 (5.9) | 0.007 |
| Fruits, mean (sd) | 2.8 (1.8) | 3.2 (1.9) | 0.35 |
| Vegetables, mean (sd) | 3.9 (2.9) | 5.1 (3.5) | 0.07 |
calculated using all 119 items on the FFQ.
calculated using 15 fruit items on the FFQ (raisins, grapes, bananas, strawberries, other berries, melon, apples, applesauce, pears, oranges, grapefruit, peaches, pineapple, plums, apricots).
calculated using 22 vegetable items on the FFQ (tomatoes, string/green beans, baked beans, lentils/soybeans/other beans, broccoli, beets, corn, peas, mixed vegetables, spinach/kale/other greens, red/yellow/orange peppers, green peppers, yams/sweet potatoes, zucchini, summer squash, eggplant, carrots, cauliflower, celery, lettuce/tossed salad, coleslaw, hummus).
the theoretical maximum number of unique foods from the 3-day food record was 123, of which 23 were fruits and 39 were vegetables.
A total of 48 children with ID and 55 TD children completed 3-day food records (Table 2, lower panel). The number of unique foods consumed over a three-day period ranged from 7 to 36 for children with ID and from 12 to 42 for TD children. A small percentage of children did not consume any fruits and vegetables. Among children with ID, 10.4% reported no fruit intake on any of the three days in contrast to 3.6% of TD children (p=0.17). Similarly, among children with ID, 10.4% reported no vegetables on any of the three days compared to 1.8% of TD children (p=0.06). The mean number of different foods that children consumed (i.e., food repertoire) was significantly lower among children with ID compared to TD children (20.7 vs. 24.2, p=0.007, Table 2 lower panel). Whereas the mean number of different types of fruits consumed did not differ significantly between the groups (2.8 for ID vs. 3.2 for TD), the mean number of different types of vegetables was lower among children with ID compared to TD children, but this was not statistically significant (3.9 vs. 5.1, p=0.07).
Contrasting children with ID with and without probable ASD revealed significantly higher rates of food refusal among children with ID who also had ASD (ID+ASD) compared to children with ID only (Table 3). Children with ID+ASD had the highest rate of food refusal (34.6%); those with ID only (i.e., who did not also have probable ASD) had the intermediate rate (22.5%), and TD children had the lowest rate (15.9%). Similar patterns were observed for fruits and for vegetables. Food repertoires were similar between children with ID and TD (24.6 vs. 24.2 unique foods) but was significantly narrower for children with ID+ASD (18.3 unique foods, p<0.001, Table 3 lower panel). Although fruit and vegetable variety were, on average, lower among children with ID, this finding did not reach statistical significance (Table 2, lower panel). However, when we examined the sub-group of participants with ID and probable ASD, we found that this group had the narrowest food repertoire for vegetables (Table 3, lower panel).
Table 3.
Comparison of number of foods offered, food refusal, and food repertoire between children with intellectual disabilities plus probable autism spectrum disorder (ID+ASD), children with intellectual disabilities (ID), and typically developing (TD) children
| ID+ASD | ID | TD | p-value | |
|---|---|---|---|---|
| Foods Offered and Food Refusal | (n=34) | (n=22) | (n=59) | |
| Total Foodsa | ||||
| Number of total foods offered, mean (sd) | 88.8 (23.0) | 98.1 (12.5) | 101.8 (9.7) | 0.001 |
| Refusal rate, % | 34.6 | 22.5 | 15.9 | 0.002 |
| Refusal rate ratio (95% CI): ID+ASD vs. TD | 2.18 (1.41 to 3.36) | |||
| Refusal rate ratio (95% CI): ID vs. TD | 1.42 (0.90 to 2.24) | |||
| Fruitsb | ||||
| Number of fruits offered, mean (sd) | 12.8 (2.6) | 12.8 (2.3) | 13.5 (2.0) | 0.25 |
| Refusal rate, % | 33.8 | 25.9 | 15.6 | 0.019 |
| Refusal rate ratio (95% CI): ID+ASD vs. TD | 2.16 (1.23 to 3.78) | |||
| Refusal rate ratio (95% CI): ID vs. TD | 1.66 (0.92 to 3.00) | |||
| Vegetablesc | ||||
| Number of vegetables offered, mean (sd) | 15.6 (5.6) | 18.5 (4.2) | 19.3 (2.7) | < 0.001 |
| Refusal rate, % | 50.3 | 34.1 | 29.6 | 0.038 |
| Refusal rate ratio (95% CI): ID+ASD vs. TD | 1.70 (1.13 to 2.55) | |||
| Refusal rate ratio (95% CI): ID vs. TD | 1.15 (0.75 to 1.77) | |||
| Food Repertoired | (n=30) | (n=18) | (n=55) | |
| Total foods, mean (sd) | 18.3 (6.7) | 24.6 (5.5) | 24.2 (5.9) | < 0.001 |
| Fruits, mean (sd) | 2.6 (1.8) | 3.3 (1.9) | 3.2 (1.9) | 0.31 |
| Vegetables, mean (sd) | 3.1 (2.8) | 5.3 (2.5) | 5.1 (3.5) | 0.016 |
calculated using all 119 items on the FFQ.
calculated using 15 fruit items on the FFQ (raisins, grapes, bananas, strawberries, other berries, melon, apples, applesauce, pears, oranges, grapefruit, peaches, pineapple, plums, apricots).
calculated using 22 vegetable items on the FFQ (tomatoes, string/green beans, baked beans, lentils/soybeans/other beans, broccoli, beets, corn, peas, mixed vegetables, spinach/kale/other greens, red/yellow/orange peppers, green peppers, yams/sweet potatoes, zucchini, summer squash, eggplant, carrots, cauliflower, celery, lettuce/tossed salad, coleslaw, hummus).
the theoretical maximum number of unique foods from the 3-day food record was 123, of which 23 were fruits and 39 were vegetables.
Parents of children with ID reported that their children were more likely to refuse food based on consistency or texture and temperature compared to parents of TD children (Table 4). In the ID+ASD and ID only groups respectively, 61.8 % and 72.7% of parents agreed or strongly agreed that their child refused food based on texture compared to 45.8% of TD children (p=0.06). Likewise, 42.4% and 31.8% of children with ID+ASD and ID only, respectively refused food based on temperature compared to 16.9% of TD children (p=0.03). We did not find any statistically significant differences between the two groups relative to other characteristics of food such as smell, color, shape, brand, or presentation of the food.
Table 4.
Comparison of reasons for food refusal between children with intellectual disabilities plus probable autism spectrum disorder (ID+ASD), children with intellectual disabilities (ID), and typically developing (TD) children
| ID+ASD (n=34) |
ID (n=22) |
TD (n=59) |
p-valuea | |
|---|---|---|---|---|
| Color, n (%) | 7 (20.6) | 2 (9.1) | 14 (23.7) | 0.34 |
| Shape, n (%) | 5 (14.7) | 0 (0.0) | 6 (10.2) | 0.18 |
| Consistency or texture, n (%) | 21 (61.8) | 16 (72.7) | 27 (45.8) | 0.06 |
| Temperature, n (%) | 14 (42.4) | 7 (31.8) | 10 (16.9) | 0.03 |
| Smell, n (%) | 10 (29.4) | 9 (40.9) | 30 (50.8) | 0.13 |
| Foods touching each other, n (%) | 5 (14.7) | 4 (18.2) | 10 (16.9) | 0.94 |
| Foods that are mixed together, n (%) | 5 (14.7) | 1 (4.6) | 11 (18.6) | 0.28 |
| Brand of the food, n (%) | 6 (17.6) | 1 (4.6) | 4 (6.8) | 0.15 |
chi-square tests for comparing proportion of parents who agreed or strongly agreed that their child refused food based upon particular characteristics of the food.
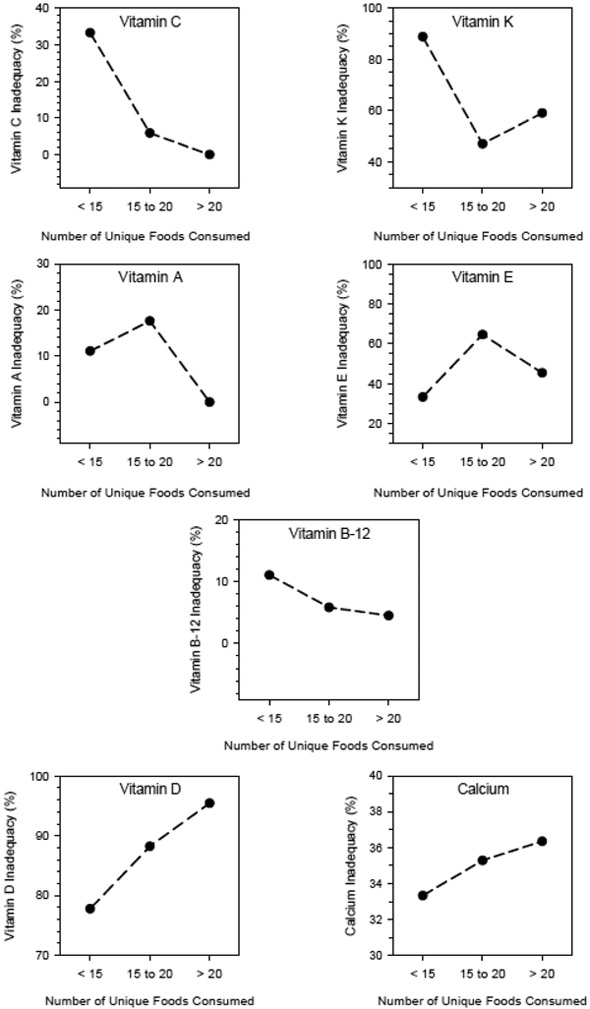
In the 48 children with ID who had completed 3-day food records, we examined the relationship of food repertoire to nutrient inadequacy. Among 17 nutrients assessed, children with ID met their adequacy threshold for 9 nutrients (thiamin, riboflavin, niacin, vitamin B-6, folate, phosphorus, magnesium, iron, zinc) irrespective of the breadth of their food repertoire. In contrast, none of the children met the recommendations for potassium. Among the remaining nutrients, for vitamins C, K, A, and B-12, inadequacy was inversely related to food repertoire breadth, those participants whose diets were low in these vitamins had more narrow food repertoires. In contrast, participants who had inadequate intakes of vitamin D and calcium, had broader food repertoires (Figure). There was no clear relationship of repertoire with Vitamin E. Of particular note, considering vitamins C and K together, 61.1% of children with ID who had the narrowest food repertoire (i.e., < 15 unique foods) had nutrient inadequacy compared to 26.5% and 29.5% of children at moderate (15 to 20 unique foods) and wide repertoire (> 20 unique foods) levels respectively (p=0.019).
Figure. Relationship between the percentage of children with intellectual disabilities (ID) who had inadequate intake of Vitamin C, Vitamin K, Vitamin A, Vitamin E, Vitamin B12, Vitamin D, calcium and food repertoire (< 15, 15-20, and > 20 unique foods consumed)†.
† A total of 9 children with ID consumed < 15 unique foods over a three-day period, 17 consumed between 15 and 20, and 22 consumed > 20.
In the first sensitivity analysis, we did not observe an effect of age on food refusal and food repertoire among children with ID. Specifically, food refusal rates were virtually identical between the 21 children with ID aged 3-5 years and the 35 children with ID aged 6-8 years (24.8% vs. 24.5%, p=0.96). The mean number of unique foods that children consumed was slightly lower among the younger children with ID in comparison to the older children with ID (19.0 vs. 21.8, p=0.17), but the difference was not statistically significant.
In the second sensitivity analysis, the mean number of foods offered and refusal rates among the 104 children eating an unrestricted diet did not differ from the entire study sample of 115 children (Table 5). As expected, the 7 children with ID and 4 TD children eating restricted diets were offered, on average, fewer number of foods than children on unrestricted diets (p<0.05). Even so, the food refusal rate was still twice as high among children with ID compared to TD children eating restricted diets (33.5% vs. 15.9%), despite the difference not reaching statistical significance because of the small sample size.
Table 5.
Comparison of number of foods offered and food refusal between children with intellectual disabilities (ID) and typically developing (TD) children by diet type
| All Dietsa | ID (n=56) |
TD (n=59) |
p-value | Rate Ratio (95% CI) |
|---|---|---|---|---|
| Number of foods offered, mean (sd) | 92.4 (20.0) | 101.8 (9.7) | 0.002 | |
| Refusal rate, %† | 28.5 | 15.7 | 0.001 | 1.81 (1.26 to 2.60) |
| Unrestricted Diet |
ID (n=49) |
TD (n=55) |
p-value | Rate Ratio (95% CI) |
|---|---|---|---|---|
| Number of foods offered, mean (sd) | 94.5 (18.0) | 102.7 (9.1) | 0.004 | |
| Refusal rate, %† | 27.7 | 14.9 | 0.001 | 1.87 (1.28 to 2.72) |
| Restricted Dietsb |
ID (n=7) |
TD (n=4) |
p-value | Rate Ratio (95% CI) |
|---|---|---|---|---|
| Number of foods offered, mean (sd) | 77.8 (28.1) | 89.7 (10.1) | 0.44 | |
| Refusal rate, %† | 33.5 | 15.9 | 0.35 | 2.10 (0.44 to 10.0) |
calculated using all 119 items on the FFQ
gluten-free, lactose-free, casein-free, or vegetarian
Discussion
We hypothesized that children with ID would exhibit more food selectivity (i.e., food refusal and limited food repertoire) and would eat fewer fruits and vegetables than TD children. As hypothesized, we observed that children with ID displayed more food selectivity than their TD peers. Specifically, children with ID refused more foods than TD children and total food repertoire was significantly lower in children with ID than TD children. Although fruit and vegetable variety were, on average, lower among children with ID, the finding did not reach statistical significance. These results did not change when age, sex, and race/ethnicity were accounted for in the analyses. We also did not observe age-related differences in food selectivity in children with ID. Consistent with our findings of greater food refusal among children with ID compared to TD children, more parents of children with ID reported that their children refused food based on texture and temperature.
Food selectivity is a concern because diets limited in variety may put a child at risk for nutrient deficiencies over time. We observed an inverse relationship between nutrient inadequacy and food repertoires for vitamins C, K, A, and B-12. The data suggest that children who consume a limited variety of foods may be at increased likelihood for inadequate nutrient intake.
The findings of an inverse relationship between the percentage of children with inadequate intake of vitamins A, C, and K and food repertoire may reflect the limited fruits and vegetables in the children’s diets. Similarly, the inverse association between the percentage of children with inadequate vitamin B-12 intake and repertoire may be the result of a less varied diet with fewer sources of animal protein. Additionally, the positive association between food repertoire and the percentage of children with inadequate vitamin D and calcium may reflect a diet high in milk intake with few other foods. For example, a child may have a limited repertoire but drink 3-4 glasses of milk per day and thus meet the recommendations for calcium and vitamin D intake. In our previous study of children with and without ASD, we observed an inverse correlation between food repertoire and the number of nutrients for which the AI/EAR was not met (Bandini et al., 2010).
Although this study was not purposely designed to examine children with both ID and ASD, the collected data afforded us the opportunity to assess the impact that the presence of ASD had on the outcomes of interest. When we stratified the group of children with ID into those with and without probable ASD, we only observed differences in overall food refusal and food repertoire between children with ID+ASD and TD children, but not with children who had ID only. Similarly, fruit and vegetable refusal was significantly different between TD children and those with ID+ASD but not different for children with ID only. From a descriptive perspective, the selectivity measures of children with ID only were intermediate between TD children and those with ID+ASD. This suggests that the higher level of food selectivity observed in the group with ID is likely attributable, at least in part, to the presence of ASD. These findings are consistent with our previous findings (Bandini et al., 2010) and the findings of others (Sharp et al., 2013) that document higher levels of food selectivity in children with ASD than in TD children.
We acknowledge several limitations in our study. First, we used a modified food frequency questionnaire to assess food refusal. Two options on the FFQ were “never eat” and “do not offer.” Parents were instructed to indicate foods that their child refused, but it is possible that parents who checked “do not offer” may have stopped offering a food that their child had refused historically. Although we did observe that parents of children with ID reported offering fewer foods than parents of TD children, we do not believe that this had an impact on the findings, as our analyses accounted for the number of foods offered. Another limitation is that the food records were kept for only three days; it is possible that food records of longer duration may have captured more variety. However, the time of recording was the same for both groups; notably, Falciglia, Horner, Liang, Couch, and Levin (2009) found a 3-day food record was an accurate method for capturing variety in children’s diets. At present, no standardized methodology to determine food variety exists. Our food repertoire coding system to capture variety was developed specifically for this project and the same coding rules in our comparative study were used across all records. A third limitation is that these measures were based upon parent report rather than on direct observation. Fourth, the foods offered to children may be affected by family financial resources. We did not ask for parental data on socioeconomic status (SES). However, this was a well-educated sample and while educational level is not a direct measure of SES, we considered it as a proxy measure. There was no statistically significant difference between the TD and ID families relative to education, so it is unlikely that this characteristic accounted for the foods offered children. Finally, comparisons between children with ID with and without co-occurring probable ASD would be strengthened by using more rigorous diagnostic methods to ascertain the presence of ASD, and a larger sample size would afford greater statistical power.
Our study also had several strengths. First, our sample of children with ID was heterogeneous, as we included both children with syndromes (e.g., Down syndrome) as well as children with ID of unknown etiology. Another strength of the study is that we used an operational definition of food selectivity. Many studies that explore food selectivity are based on subjective questions such as “Is your child a picky eater?” and do not provide a quantitative measure of this phenomenon. Third, we recruited a very diverse sample, with both groups representing considerable racial/ethnic diversity: 63% of the TD group and 45% of the ID group comprised children that were from non-white populations. These demographic characteristics of our sample make the findings of our study more likely to be generalizable.
Overall, our findings suggest that children with ID are more food selective than TD children. Greater food selectivity in children with ID appears to be driven, at least in part, by the co-occurrence of a probable ASD diagnosis. Children with ID consumed diets that were limited in fruits and vegetables, and a substantial number did not meet the recommended dietary intakes for several nutrients. These findings support the need for screening for food selectivity of children with ID, particularly those who also have ASD. Children who exhibit food selectivity should be referred to a registered dietitian or an interdisciplinary team for further evaluation.
Acknowledgments
We would like to thank Lucy Lorin, Rosalie Jiang, Alyssa Biller, Barbara Fargnoli, Maresa Weems, and Katlyn Patton for their assistance with the project and the families for their participation in the study.
Funding Sources
Maternal and Child Health (UA3MC25735-01-00); Maternal and Child Health R40MC25678A0; Boston Nutrition Obesity Research Center (DK046200); NICHD Interdisciplinary Research in Intellectual/Developmental Disabilities (2P30HD004147-33A2); Administration for Community Living 90DD-0675.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
Contributor Information
Linda G. Bandini, Eunice Kennedy Shriver Center/University of Massachusetts Medical School, 55 Lake Avenue North, S3-324B, Worcester, MA 01655 USA, and Sargent College of Health and Rehabilitation Sciences, Boston University, 635 Commonwealth Ave., Boston, MA, 02215
Carol Curtin, Eunice Kennedy Shriver Center/University of Massachusetts Medical School, 55 Lake Avenue North S3-317, Worcester, MA 01655 USA.
Misha Eliasziw, Tufts University School of Medicine, Dept. of Public Health and Community Medicine, 136 Harrison Avenue, Boston, MA, 02111 USA.
Sarah Phillips, Tufts University School of Medicine, Dept. of Public Health and Community Medicine, 136 Harrison Avenue, Boston, MA, 02111 USA.
Laura Jay, Center for Nutrition, Boston Children’s Hospital, 333 Longwood Ave, 4th Floor, Boston, MA 02115.
Melissa Maslin, Eunice Kennedy Shriver Center/University of Massachusetts Medical School, 55 Lake Avenue North, S3-324C, Worcester, MA 01655 USA.
Aviva Must, Tufts University School of Medicine, Dept. of Public Health and Community Medicine, 136 Harrison Avenue, Boston, MA, 02111 USA.
References
- American Association on Intellectual and Developmental Disabilities. (2018). Definition of intellectual disability. Retrieved from http://aaidd.org/intellectual-disability/definition#.We4Egkdrx-U [Google Scholar]
- Angulo MA, Butler MG, & Cataletto ME (2015). Prader-Willi syndrome: A review of clinical, genetic, and endocrine findings. Journal of Endocrinological Investigation, 38(12), 1249–1263. doi: 10.1007/s40618-015-0312-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF. (2018). Prevalence of autism spectrum disorder among children aged 8 years - Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries, 67(SS-6), 1–23. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandini LG, Anderson SE, Curtin C, Cermak S, Evans EW, Scampini R, … Must A. (2010). Food selectivity in children with autism spectrum disorders and typically developing children. The Journal of Pediatrics, 157(2), 259–264. doi: 10.1016/j.jpeds.2010.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermak SA, Curtin C, & Bandini LG (2010). Food selectivity and sensory sensitivity in children with autism spectrum disorders. Journal of the American Dietetic Association, 110(2), 238–246. doi: 10.1016/j.jada.2009.10.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistol LT, Bandini LG, Must A, Phillips S, Cermak SA, & Curtin C (2018). Sensory sensitivity and food selectivity in children with autism spectrum disorder. Journal of Autism and Developmental Disorders, 48(2), 583–591. doi: 10.1007/s10803-017-3340-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewnowski A, Henderson SA, Driscoll A, & Rolls BJ (1997). The Dietary Variety Score: Assessing diet quality in healthy young and older adults. Journal of the American Dietetic Association, 97(3), 266–271. [DOI] [PubMed] [Google Scholar]
- Elliott CD (1990). Differential Ability Scales. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Engel-Yeger B, Hardal-Nasser R, & Gal E (2016). The relationship between sensory processing disorders and eating problems among children with intellectual developmental deficits. British Journal of Occupational Therapy, 79(1), 17–25. doi: 10.1177/0308022615586418 [DOI] [Google Scholar]
- Falciglia GA, Horner SL, Liang J, Couch SC, & Levin LS (2009). Assessing dietary variety in children: Development and validation of a predictive equation. Journal of the American Dietetic Association, 109(4), 641–647. doi: 10.1016/j.jada.2008.12.012 [DOI] [PubMed] [Google Scholar]
- Field AE, Camargo CA, Taylor C, Berkey CS, Frazier AL, Gillman MW, & Colditz GA (1999). Overweight, weight concerns, and bulimic behaviors among girls and boys. Journal of the American Academy of Child & Adolescent Psychiatry, 38(6), 754–760. doi: 10.1097/00004583-199906000-00024 [DOI] [PubMed] [Google Scholar]
- Field D, Garland M, & Williams K (2003). Correlates of specific childhood feeding problems. Journal of Paediatrics and Child Health, 39(4), 299–304. [DOI] [PubMed] [Google Scholar]
- Goldstein S, & Naglieri JA (2010). Autism Spectrum Rating Scales: ASRS. North Tonawanda, NY: Multi-Health Systems. [Google Scholar]
- Graf-Myles J, Farmer C, Thurm A, Royster C, Kahn P, Soskey L, … Swedo S. (2013). Dietary adequacy of children with autism compared with controls and the impact of restricted diet. Journal of Developmental and Behavioral Pediatrics, 34(7), 449–459. doi: 10.1097/DBP.0b013e3182a00d17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvard TH Chan School of Public Health Nutrition Department. (2013). File download site. 2012 Youth Adolescent Food Frequency Questionnaire. Retrieved from https://regepi.bwh.harvard.edu/health/KIDS/files
- Herndon AC, DiGuiseppi C, Johnson SL, Leiferman J, & Reynolds A (2009). Does nutritional intake differ between children with autism spectrum disorders and children with typical development? Journal of Autism and Developmental Disorders, 39(2), 212–222. doi: 10.1007/s10803-008-0606-2 [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. (2006). Dietary reference intakes: The essential guide to nutrient requirements. Washington, DC: National Academies Press; [DOI] [Google Scholar]
- Institute of Medicine. (2011). Dietary reference intakes for calcium and vitamin D. Washington, DC: The National Academies Press; 10.17226/13050 [DOI] [PubMed] [Google Scholar]
- Lockner DW, Crowe TK, & Skipper BJ (2008). Dietary intake and parents' perception of mealtime behaviors in preschool-age children with autism spectrum disorder and in typically developing children. Journal of the American Dietetic Association, 108(8), 1360–1363. doi: 10.1016/j.jada.2008.05.003 [DOI] [PubMed] [Google Scholar]
- Ma N, Thompson C, & Weston S (2016). Brief report: Scurvy as a manifestation of food selectivity in children with autism. Journal of Autism and Developmental Disorders, 46(4), 1464–1470. doi: 10.1007/s10803-015-2660-x [DOI] [PubMed] [Google Scholar]
- Rockett HR, Wolf AM, & Colditz GA (1995). Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. Journal of the American Dietetic Association, 95(3), 336–340. doi: 10.1016/S0002-8223(95)00086-0 [DOI] [PubMed] [Google Scholar]
- Rockett HR, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, & Colditz GA (1997). Validation of a youth/adolescent food frequency questionnaire. Preventive Medicine, 26(6), 808–816. doi: 10.1006/pmed.1997.0200 [DOI] [PubMed] [Google Scholar]
- Sharp WG, Berry RC, McCracken C, Nuhu NN, Marvel E, Saulnier CA, … Jaquess DL. (2013). Feeding problems and nutrient intake in children with autism spectrum disorders: A meta-analysis and comprehensive review of the literature. Journal of Autism and Developmental Disorders, 43(9), 2159–2173. doi: 10.1007/s10803-013-1771-5 [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, & Cicchetti DV (2005). Vineland Adaptive Behavior Scales (2nd ed.). Circle Pines, MN: AGS Publishing. [Google Scholar]
- Suarez MA, Nelson NW, & Curtis AB (2014). Longitudinal follow-up of factors associated with food selectivity in children with autism spectrum disorders. Autism, 18(8), 924–32. doi: 10.1177/1362361313499457 [DOI] [PubMed] [Google Scholar]
- Willett W (1998). Nutritional epidemiology. Oxford University Press, USA. [Google Scholar]
- Zimmer MH, Hart LC, Manning-Courtney P, Murray DS, Bing NM, & Summer S (2012). Food variety as a predictor of nutritional status among children with autism. Journal of Autism and Developmental Disorders, 42(4), 549–556. doi: 10.1007/s10803-011-1268-z [DOI] [PMC free article] [PubMed] [Google Scholar]