Abstract
Objectives:
NR4A1 is overexpressed in many solid tumors, and the objectives of this study were to investigate the expression and functional role of this receptor in endometrial cancer cells and demonstrate that NR4A1 antagonist inhibit mTOR.
Methods:
Ishikawa and Hec-1B endometrial cells were used as models to investigate the parallel effects of NR4A1 knockdown by RNA interference (siNR4A1) and treatment with bis-indole-derived NR4A1 ligands (antagonists) on cell growth and survival by determining cell numbers and effects on Annexin V staining. Western blot analysis of whole cell lysates was used to determine effects of these treatments on expression of growth promoting, survival and apoptotic genes and mTOR signaling. Effects of NR4A1 antagonists on tumor growth were determined in athymic nude mice bearing Hec-1B cells as xenografts.
Results:
siNR4A1 or treatment with bis-indole-derived NR4A1 antagonists inhibited growth of endometrial cancer in vitro and endometrial tumors in vivo and this was accompanied by decreased expression of growth promoting and survival genes and mTOR inhibition.
Conclusions:
NR4A1 exhibited pro-oncogenic activity in endometrial cells due, in part, to regulation of cell growth, survival and mTOR signaling, and all of these pathways and their associated gene products were inhibited after treatment with bis-indole-derived NR4A1 antagonists. Moreover, these compounds also blocked endometrial tumor growth in vivo demonstrating that NR4A1 is a potential novel drug target for treatment of endometrial cancer.
Keywords: NR4A1, Pro-oncogenic antagonists, mTOR inhibition
INTRODUCTION
Endometrial cancer is the fourth most common cancer among women, and in 2018, it was estimated that 63,230 new cases would be diagnosed and 11,350 women would die from this disease in the United States [1]. Moreover, the incidence of endometrial cancer is increasing in most countries and it estimated the number of cases diagnosed by the year 2030 will be doubled [2, 3]. Stage I tumors which are often estrogenic are primarily treated only with surgery and there is a good prognosis for these patients. In contrast, the prognosis for women with later stage tumors is poor and these patients often receive targeted radiation and chemotherapy [4-6]. Several approaches have been used to classify endometrial carcinomas [3, 7-13], including one by the Cancer Genome Atlas Research Network which integrated genomic, transcriptomic and proteomic data from 373 endometrial cancer cases [13]. These approaches have been proposed in order to facilitate development of optimal treatment regimens for different groups of patients, and the success of these classifications are currently being evaluated.
The identification of prominent pro-oncogenic pathways in endometrial cancer has led to development and clinical applications of drugs that target angiogenic, PI3K, mTOR, and Ras signaling [4-6]. Studies in this laboratory have identified and characterized the orphan nuclear receptor 4A1 (NR4A1, Nur77) as a novel drug target in hormone-dependent (i.e. breast) cancer and other solid tumors including lung, pancreatic, colon and kidney cancers and rhabdomyosarcoma [14-20]. Results of RNA interference (RNAi) studies demonstrate that NR4A1 is pro-oncogenic and regulates several genes/pathways that are important for cancer cell proliferation, survival and migration/invasion [21, 22]. NR4A1 plays a key role in activation of mTOR through regulation thioredoxin domain-containing 5 (TXNDC5) and isocitrate dehydrogenase 1 (IDH-1) which keep reductant levels high and reactive oxygen species (ROS) low [14-16]. Knockdown of NR4A1 results in decreased expression of TXNDC5 and IDH1 resulting in the induction of ROS and ROS-dependent induction of sestrin-2 [14-16]. Sestrin-2 is an oxygen sensor that activates AMPK which in turn blocks mTOR and downstream signaling [23, 24]. We have also developed a series of bis-indole-derived analogs (C-DIM) including 1,1-bis(3'-indolyl)-1-(p-hydroxyphenyl) methane [DIM-C-pPhOH (CDIM8)] which is an NR4A1 ligand that acts as an NR4A1 antagonist in cancer cells [14-20](15–21). The effects of DIM-C-pPhOH on cancer cell growth and survival parallel those observed after knockdown of NR4A1 and thus, DIM-C-pPhOH acts as an indirect mTOR inhibitor through inhibiting NR4A1-dependent pro-oncogenic pathways.
In this study, we used both RNA interference (RNAi) to knockdown NR4A1 and NR4A1 ligands (antagonists) to investigate their effects on endometrial cancer cell growth and survival and their impacts on mTOR signaling, a pathway that is currently being targeted for treatment of endometrial cancer [25-28]. The results demonstrate the pro-oncogenic activity of NR4A1 in endometrial cancer cells and also show that the synthetic bis-indole NR4A1 ligands act as antagonists an induce apoptosis, inhibit cell growth and mTOR signaling through an ROS-dependent pathway.
MATERIALS AND METHODS
Cell Lines and Reagents:
Endometrial cancer cell lines, Ishikawa and Hec-1B, were kindly provided by Dr. Russell Broaddus, MD Anderson Cancer Center, Houston, TX. HEC1B cells were derived from an endometrial carcinoma from a 71 year old female and Ishikawa cells were similar but derived from a 39 year old woman. Cells were maintained in DMEM/F12 growth medium supplemented with 10% FBS and 1× antibiotic/antimycotic solution (Sigma-Aldrich, St Louis, MO). All cells were incubated at 37°C in CO2 incubator in an atmosphere of humidified 5% CO2 and 95% air. The two ligands DIM-C-pPhOH and DIM-C-pPhOH-3,5-Br2 were synthesized by condensation of indole with p-hydroxybenzaldehyde or 3,5-dibromo-p-hydroxybenzaldehyde as described [17].
Cell proliferation assay:
Endometrial cancer cells (Ishikawa and Hec-1B) were seed into a 96-well plate and the cells were then treated for 24 hr with either DMSO or different concentrations of CDIM8 and DIM-C-pPhOH 3,5-Br2 and also with siNR4A1 oligonucleotides, the medium was removed, and MTT solution diluted in PBS was added to cell cultures. After 3 hr incubation, the medium was aspirated and washed with PBS. Dimethyl sulfoxide (DMSO) was added and incubated at 37° for 10 min and absorbance was measured at 570 nM.
Western Blotting:
Ishikawa and Hec-1B cells were seeded and allowed to attach for 24 hr and cells were then treated for 24 hr with either DMSO or different concentrations of CDIM8 and DIM-C-pPhOH 3,5-Br2. Cells were then lysed and whole cell lysates were resolved in 10% SDS-PAGE gels and proteins were transferred using PVDF membrane by wet blotting followed by primary and secondary antibody incubation and detected using ECL reagent as described [14-19]. Western blot data were quantitated relative to β-actin and control values were set at 100%
Measurement of ROS:
ROS levels in cells were measured using cell-permeable probe CM-H2DCFDA as described in manufacturer's instructions (Life Technologies, Inc.). Briefly, cells were seeded at a density of 1.0 × 105 cells per well in 6-well plates in DMEM containing 2.5% charcoal-stripped FBS. The cells were pre-treated with GSH for 30 min and treated with vehicle (DMSO, or DIM-C-pPhOH 3,5 Br2 with GSH or with siNR4A1 oligonucleoties with GSH and the ROS were measured using Accuri Flow Cytomter.
Annexin V staining:
Ishikawa and Hec-1B cells were seeded in Nunc chambered coverglass followed by various drug treatments. The cells were then washed with ice cold PBS and 5 μL Alexa Fluor® 488 Annexin V with 100 μg/mL PI (as per the manufacturer instructions) were added to the cells and incubated for 15 min and the cells were observed using a Zeiss confocal fluorescence microscope.
RNA Interference:
Ishikawa and Hec-1B were seeded in six-well plates and allowed to grow to 60% confluence (24 hours), then transfections were performed with Lipofectamine 2000 according to the manufacturer’s protocol. Both siNR4A1 oligonucleotides and nontargeted control small interfering RNAs were used. Six hours after transfection, the medium was replaced with fresh medium and left for 72 hours and the cells were harvested and protein expression was determined.
Luciferase assay:
Cells were plated on 12-well plates at 18×104 (Hec-1B) or 9×104 (Ishikawa cells) per well in DMEM/F12 supplemented with 2.5% charcoal-stripped FBS. After overnight attachment and growth, various amounts of DNA [i.e., UASx5-Luc (500 ng), GAL4-NR4A1 (50 ng), NBREx3-Luc (800 ng), NurREx3-Luc (800 ng) and Flag-NR4A1 (80 ng)] were cotransfected into each well using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. After 6 hr of transfection, cells were treated with plating medium (as above) containing either solvent (DMSO) or indicated concentrations of compound for 18 hr. Cells were then lysed and cell extracts were used for chemiluminescence quantification of luciferase activity. Luciferase activity values were normalized against corresponding protein concentration values determined by Bradford assay. Both GAL4- and Flag-NR4A1 constructs contain full length NR4A1 coding sequence and all the plasmids used in this study were previously described [17, 29].
Xenograft Study:
Female athymic nu/nu mice of 4–6 weeks old were purchased from Harlan Laboratories (Houston, TX). Hec-1B cells were harvested and suspended at a concentration of 1.5 × 106 cells in 100 μl of DMEM with ice-cold Matrigel (1:1 ratio) and injected subcutaneously into either side of the flank area of nude mice. Small tumors developed with 7 days after tumor cell inoculation; mice (6 per treatment group) were then randomized and treated every other day with either vehicle (corn oil) or DIM-C-pPhOH-3,5Br2 (25 mg/kg body weight) in a volume of 100 μl injected intraperitoneally. All mice were weighed once a week over the course of treatment to monitor changes in body weight. All animals were sacrificed after 21 days of treatment, and tumor weights were determined. All animal studies were carried out according to the procedures approved by the Texas A&M University Institutional Animal Care and Use Committee.
Statistical analysis:
All of the experiments were repeated a minimum of three times. The data are expressed as the mean ± standard error (SE). One-way analysis of variance was used to determine statistical significance. P values<0.05 were considered statistically significant.
RESULTS
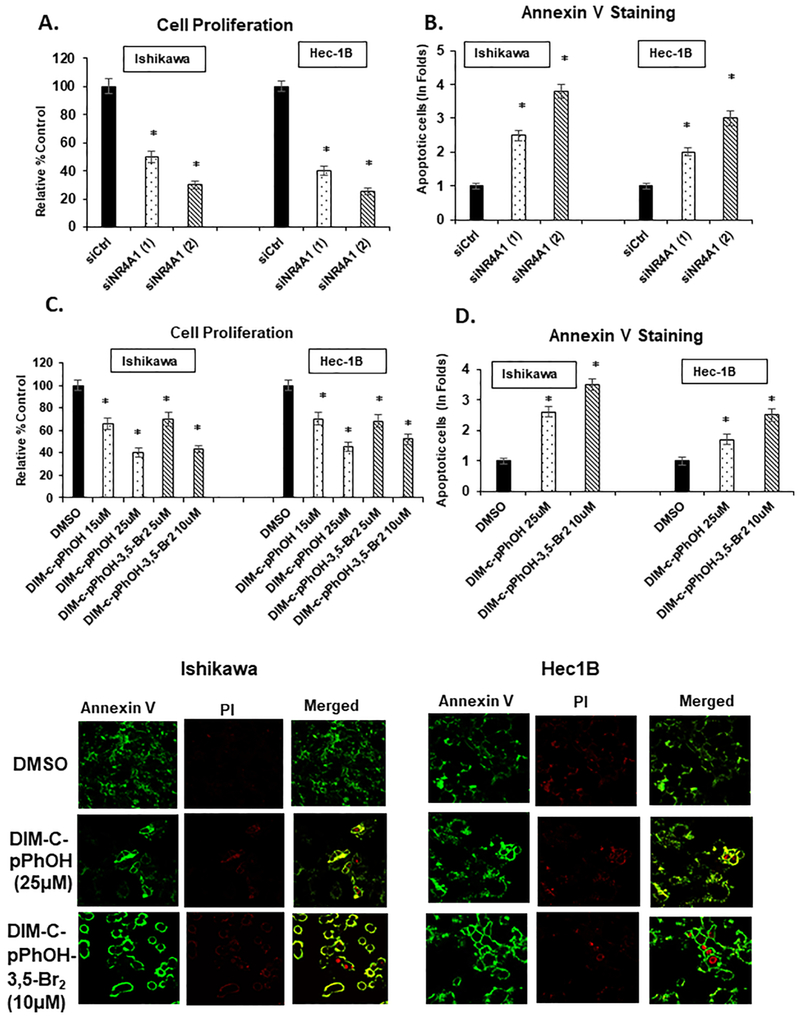
We initially used two oligonucleotides [siNR4A1(1) and siNR4A1(2)] for RNAi studies and transfection of the oligonucleotides into Ishikawa and Hec-1B cells resulted in significant inhibition of cell growth (Fig. 1A), indicating that NR4A1 is a growth-promoting gene in endometrial cancer cells. A similar approach was also used to determine effects of NR4A1 knockdown on Annexin V staining, a marker of apoptosis, and the results showed that loss of NR4A1-induced Annexin V staining in Ishikawa and Hec-1B (Fig. 1B) cells. Studies in this laboratory initially identified DIM-C-pPhOH as an NR4A1 ligand which is an NR4A1 antagonist in cancer cells [17], and subsequent studies showed that buttressed analogs of DIM-C-pPhOH such as the 3,5-dibromo analog (DIM-C-pPhOH-3,5-Br2) are also NR4A1 ligands with enhanced activity [30]. Treatment of Ishikawa and Hec-1B cells with DIM-C-pPhOH and DIM-C-pPhOH-3,5-Br2 inhibited cell growth (Fig 1C) and induced Annexin V staining (Fig.1D) and exhibited NR4A1 antagonist activity in endometrial cancer cells as previously observed in other cancer cell lines [14-20]. The two ligands were used as models for investigating NR4A1- dependent transactivation studies using a GAL4-NR4A1 chimera and a GAL4-response element-luciferase reporter gene (UOS5-luc) (Fig. 2A), an NBRE-luc (Fig. 2B) and an NuRE-luc (Fig. 2C) reporter genes containing 3 binding sites for the NR4A1 monomer and dimer, respectively. Both DIM-C-pPhOH and DIM-C-pPhOH-3,5-Br2 decreased transactivation (luciferase) in cells transfected with all three NR4A1-responsive reporter gene constructs in Ishikawa and Hec-1B cells. These results are similar to those observed in other cancer cell lines treated with DIM-C-pPhOH and transfected with the NR4A1-responsive genes [14-20], indicating that DIM-C-pPhOH and DIM-C-pPhOH- 3,5-Br2 exhibit NR4A1 antagonist activity for these responses.
Figure 1.
Inactivation of NR4A1 inhibits cell growth and survival: Ishikawa and Hec-1B cells were transfected with siCtrl (non-specific oligonucleotide) and two oligonucleotides targeting NR4A1 (siNR4A1(1) and siNR4A1(2) and effects on cell proliferation (A) and Annexin V staining (B) were determined as outlined in the Materials and Methods. Ishikawa and Hec-1B cells were treated with DIM-C-pPhOH (15 and 25 μM) and DIM-C-pPhOH-3,5-Br2 (5 and 10 μM) for 24 hr and effects on cell growth (C) and Annexin V staining (D) were determined as outlined in the Materials and Methods. Results are expressed as mean± SD for atleast 3 determinations per treatment and significant (P<0.05) differences from control treatments are indicated (*).
Figure 2.
Effects of NR4A1 ligands on transactivation. Ishikawa and Hec-1B cells GAL1-4 NR4A1 and UAS-luc reporter gene (A) or NBRE-luc (B) and NuRE-luc (C) reporter genes and FLAG-NR4A1 (D) and after treatment with DIM-C-pPhOH or DIM-C-pPhOH-3,5-Br2 luciferase activity was determined as outlined in the Materials and Methods. Results are expressed as means ± SD for at least 3 replicate determinations for each treatment group and significant (P<0.05) inhibition is indicated (*).
NR4A1 regulates both growth promoting and pro-survival gene products in cancer cells [14-20] and, in Ishikawa and Hec-1B cells transfected with siNR4A1 oligonucleotides, we observed downregulation of the epidermal growth factor receptor (EGFR) and cMyc (Fig. 3A). This same treatment also decreased expression of survivin and bcl-2 and increased expression of bax and cleaved caspase 3 and PARP (Fig. 3B). The cells were also treated with DIM-C-pPhOH and DIM-C-pPhOH-3,5-Br2 and this resulted in decreased expression of EGFR and cMyc (Fig. 3C) and expression of bcl-2/survivin, and increased induction of bax and cleavage of caspase-3 and PARP (Fig. 3D). Thus, the NR4A1 antagonists decreased NR4A1-regulated growth promoting and pro-survival gene products and this paralleled results observed in other cancer cell lines [14-20].
Figure 3.
Role of NR4A1 inactivation on growth promoting and survival gene products Ishikawa and Hec-1B cells were transfected with sictrl or siNR4A1(1)/siNR4A1(2) and whole cell lysates were analyzed for expression of growth promoting (A) and survival/apoptotic (B) gene products by western blots as outlined in the Materials and Methods. Ishikawa and Hec-1B cells were treated with DIM-C-pPhOH (20 and 25 μM) or DIM-C-pPhOH-3,5-Br2 (5 and 10 μM) for 24 hr and whole cell lysates were analyzed for expression of growth promoting (C) and survival/apoptotic gene products by western blots as outlined in the Materials and Methods. Comparable western blots were observed in replicate experiments.
Previous studies show that NR4A1 regulates mTOR signaling in cancer cells and this is primarily due to ROS-dependent induction of sestrin-2 (SES2) and subsequent activation of AMPK [14-16]. Results show that knockdown of NR4A1 induces sestrin-2 and phospho-AMPK (pAMPK) (Fig. 4A). In addition, knockdown of NR4A1 also decreased phospho-mTOR and activation of downstream kinases 70S6K and S6RP (Fig. 4B), confirming that NR4A1 regulates the mTOR pathway. In parallel experiments, treatment of Ishikawa and Hec-1B cells with DIM-C-pPhOH or DIM-C-pPhOH-3,5-Br2 also induced phospho-AMPK and sestrin-2 (Fig. 4C) and downregulated phosphorylation of mTOR 70S6K and S6RP (Fig. 4D).
Figure 4.
NR4A1 inactivation inhibits mTOR signaling. Ishikawa and Hec-1B cells were transfected with siCtrl or siNR4A1(1)/siNR4A1(2) and effects on induction of sestrin-2 and p-AMPKα (A) and mTOR signaling pathway (B) were determined by western blot analysis of whole cell lysates. Ishikawa and Hec-1B cells were treated with DIM-C-pPhOH (20 and 25 uM) and DIM-C-pPhOH-3,5-Br2 (5 and 10 μM) for 24 hr and whole cell lysates were analyzed by western blots for induction of sestrin-2 and p-AMPKα (C) and mTOR signaling (D). Comparable western blots were observed in replicate experiments. Quantitation of the western blots is given in supplemental figures 1 and 2.
NR4A1 knockdown or treatment with NR4A1 antagonists decrease expression of TXNDC5 is several different cancer cell lines and in rhabdomyosarcoma (RMS) cells, knockdown of TXNDC5 induces ROS and oxidative stress [14-16]. Figure 5A demonstrates that both knockdown of NR4A1 or treatment with DIM-CpPhOH-3,5-Br2 decreased TXNDC5 expression and these same treatments induced ROS which was attenuated after cotreatment with GSH in Ishikawa and Hec-1B cells (Figs. 5B and 5C). Knockdown of TXNDC5 by RNA interference (siTXNDC5) also induced ROS (attenuated by cotreatment with GSH) (Fig. 5D) and this is consistent with the effects of NR4A1-knockdown/NR4A1 antagonist effect on TXNDC5 (Fig. 5A) and ROS induction (Figs. 5B). Induction of sestrin-2 and downstream effects were ROS-dependent in RMS, breast and renal cancer cells [14-16]; similar effects were observed in endometrial cancer cells where knockdown of NR4A1 (Fig. 5E) or treatment with DIM-C-pPhOH-3,5-Br2 (Fig. 5F) induced sestrin-2 and pAMPK and cotreatment with GSH attenuate these responses. A direct linkage between TXNDC5 and inhibition of mTOR is illustrated in Fig. 5G where knockdown of TXNDC5 by two oligonucleotides [(1) and (2)] induced sestrin-2 and pAMPK and decreased phosphorylation of mTOR 70S6K and S6RP. We also observed inhibition of tumor growth in athymic nude mice bearing Hec- 1B cells and treated with DIM-C-pPhOH-3,5-Br2 (25 mg/kg/d) (Fig 6A) and this was accompanied by downregulation of TXNDC5, induction of sestrin-2 and activation of AMPK (Fig 6B) and inhibition of mTOR signaling (Fig 6C and 6D) Thus both in vivo and in vitro results were complementary demonstration that NR4A1 antagonists are novel inhibitors of mTOR signaling in endometrial cancer. These results are consistent with the pathway for inhibition of mTOR signaling by DIM-C-pPhOH as illustrated in Figure 5H.
Figure 5.
Role of TXNDC5 in mediating inhibition of mTOR. Ishikawa and Hec-1B cells were transfected with siCtrl or siNR4A1 or treated with DIM-C-pPhOH or DIM-C-pPhOH-3,5-Br2 (5 and 10 μM) for 24hr and effects on TXNDC5 protein expression (A) were determined. A similar treatment protocol (±GSH) was used to determine induction of ROS by RNA interference (B) or treatment with NR4A1 antagonists (C). Ishikawa and Hec-1B cells were transfected with siCtrl or siTXNDC5 and effects on ROS induction (D) were determined as outlined in the Materials and Methods. Ishikawa and Hec-1B cells were transfected with siNR4A1 (±GSH) (E) or treated with DIM-C-pPhOH-3,5-Br2 (F) and effects on NR4A1, sestrin-2 and p-AMPK protein levels were determined by western blot analysis of whole cell lysates. Cells were transfected with siCtrl and siTXNDC5 and whole cell lysates were analyzed for gene products associated with activation of AMPKα and inhibition of mTOR signaling (G). Results (B-D) were expressed as means ± SD for 3 separate determinations and significant (p<0.05) effects (Compared to untreated cells) are indicated (*). The western blot data in Figure 5G is quantitate in supplemental Figure 2.
Figure 6.
In vivo studies. A. Effects of DIM-C-pPhOH-3,5-Br2 (25 mg/kg/d) and vehicle control on growth of tumors in athymic nude mice bearing Hec-1B cells as xenografts. Western blot analysis of tumor lysates from control and DIM-C-pPhOH-3,5-Br2 treated mice on expression of TXNDC5, sestrin-2 and P-AMPKα (B) and mTOR signaling (C). D Quantification of western blot data; results are expressed as means ± SD for 3 separate determinations and significant (p<0.05) treatment-related effects on protein expression are indicated (*)
DISCUSSION
NR4A1 is an immediate early gene induced by multiple stressors and is important for maintaining cellular homeostasis [31, 32]. In addition, this receptor is enhanced and/or a potential drug target for multiple diseases and this is particularly true for solid tumors where NR4A1 exhibits pro-oncogenic activity and for some cancers NR4A1 is a negative prognostic for patient survival or recurrence [14-22, 33-35]. There is evidence that some apoptosis – inducing agents induce nuclear export of NR4A1 to for pro-apoptotic mitochondrial NR4A1-bcl2 complexes [36-38] whereas the C-DIM/NR4A1 antagonists target nuclear NR4A1 [17, 20]. NR4A1 is highly expressed in Ishikawa and Hec-1B endometrial cancer cell lines (Fig.4A) and this is consistent with high expression of this receptor in cell lines derived from many other solid tumors where NR4A1 exhibits pro-oncogenic activities [14-22]. Knockdown of NR4A1 in Ishikawa and Hec-1B cells decreased growth and induced several markers of apoptosis (Figs. 1-3), demonstrating a function for this receptor in the growth and survival of endometrial cancer cells. Moreover, loss of NR4A1 in Ishikawa and Flec-1B cells resulted in downregulation of genes associated with cell growth (EGFR, c-Myc) and survival (bcl-2 and survivin) and induction of apoptosis markers including Annexin V staining, bax and cleaved caspase-3 and PARP. All of these functional responses and effects of gene products, after loss of NR4A1 have previously been reported in other solid tumor-derived cell lines [14-22] and confirm the pro-oncogenic functions of NR4A1 in endometrial cancer.
Bis-indole-derived compounds exhibit structure-dependent activity as peroxisome proliferator-activate receptor γ (PPARγ) ligands [39] and NR4A1 ligands [14-20], and DIM-C-pPhOH was among the most active first generation NR4A1 ligands with a binding Kd of 0.10 μM [17]. In transactivation assays DIM-C-pPhOH decreases NR4A1-dependent luciferase activity (Fig. 2) and effects of this compound of endometrial cancer cell growth and survival paralleled those observed after NR4A1 silencing and confirmed that this NR4A1 ligand exhibited receptor antagonist activity. DIM-C-pPhOH-3,5-Br2 was synthesized as a buttressed analog of DIM-C-pPhOH containing two bromo substituents adjacent to the hydroxyl group to inhibit metabolism (conjugation) and thereby increase potency. The enhanced NR4A1-dependent activity of these buttressed analogs was previously observed in C2C12 muscle cells [30] and in endometrial cancer cells, DIM-C-pPhOH-3,5-Br2 was more potent than DIM-C-pPhOH as an NR4A1 antagonist in most assays.
Endometrial cancer is a complex disease and although cytotoxic drug combination therapies have been commonly used, clinical trials on cytotoxic drug combination and more mechanism-based therapies are in progress [4-6]. Since the mTOR pathway plays a key role in cell growth, metabolism and cell cycle progression, clinical trials with various mTOR inhibitors are ongoing in patients with endometrial and other gynecological cancers [25-28]. There is evidence in multiple cancer cell lines that NR4A1 is upstream from mTOR and plays a role in sustaining mTOR signaling in cancer cells, and in RMS, breast and renal cancer cell lines. Treatment with NR4A1 antagonists or receptor inactivation inhibits mTOR and this has been linked to generation of ROS [14-16]. ROS activates the oxygen sensor sestrin-2 which in turn induces phosphorylation of AMPK resulting in inhibition of mTOR and downstream kinases [14-16]. The major source of ROS in endometrial cancer cells in which NR4A1 is silenced or cells are treated with NR4A1 antagonist is associated with downregulation of TXNDC5 and this is supported by the large increase in ROS after silencing of TXNDC5 by RNA interference (Fig. 5D). Thus, downregulation of TXNDC5 and the subsequent induction of ROS play a key role in NR4A1 antagonist-mediated inhibition of mTOR signaling in endometrial cancer cell lines (Fig. 5E) and we observed comparable effects in vivo (Fig 6). These results illustrate the important pro-oncogenic role of NR4A1 in endometrial cancer and demonstrate for the first time that NR4A1 antagonists represent a novel class of inhibitors of the mTOR signaling pathway which are being developed for future clinical applications.
Supplementary Material
HIGHLIGHTS.
NR4A1 is expressed and is highly pro-oncogenic in endometrial cancer cells
Bis-indole derived NR4A1 antagonists inhibit cell growth and survival
NR4A1 antagonists are novel mTOR inhibitors
Acknowledgments
Financial Support: The financial assistance of the National Institutes of Health (P30-ES023512, S. Safe), [and T32-ESO26568, K. Karki] Texas AgriLife Research (S. Safe), and the Sid Kyle Chair endowment (S. Safe) is gratefully acknowledged.
Footnotes
Conflict of Interest Statement:
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2018, CA Cancer J Clin, 68 (2018) 7–30. [DOI] [PubMed] [Google Scholar]
- [2].Lortet-Tieulent J, Ferlay J, Bray F, Jemal A, International Patterns and Trends in Endometrial Cancer Incidence, 1978-2013, J Natl Cancer Inst, 110 (2018) 354–361. [DOI] [PubMed] [Google Scholar]
- [3].McAlpine JN, Temkin SM, Mackay HJ, Endometrial cancer: Not your grandmother's cancer, Cancer, 122 (2016) 2787–2798. [DOI] [PubMed] [Google Scholar]
- [4].Arend RC, Jones BA, Martinez A, Goodfellow P, Endometrial cancer: Molecular markers and management of advanced stage disease, Gynecol Oncol, 150 (2018) 569–580. [DOI] [PubMed] [Google Scholar]
- [5].Lee YC, Lheureux S, Oza AM, Treatment strategies for endometrial cancer: current practice and perspective, Curr Opin Obstet Gynecol, 29 (2017) 47–58. [DOI] [PubMed] [Google Scholar]
- [6].Rodriguez-Freixinos V, Karakasis K, Oza AM, New Targeted Agents in Endometrial Cancer: Are We Really Making Progress?, Curr Oncol Rep, 18 (2016) 23. [DOI] [PubMed] [Google Scholar]
- [7].Matias-Guiu X, Prat J, Molecular pathology of endometrial carcinoma, Histopathology, 62 (2013) 111–123. [DOI] [PubMed] [Google Scholar]
- [8].Piulats JM, Guerra E, Gil-Martin M, Roman-Canal B, Gatius S, Sanz-Pamplona R, Velasco A, Vidal A, Matias-Guiu X, Molecular approaches for classifying endometrial carcinoma, Gynecol Oncol, 145 (2017) 200–207. [DOI] [PubMed] [Google Scholar]
- [9].Stelloo E, Bosse T, Nout RA, MacKay HJ, Church DN, Nijman HW, Leary A, Edmondson RJ, Powell ME, Crosbie EJ, Kitchener HC, Mileshkin L, Pollock PM, Smit VT, Creutzberg CL, Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative, Mod Pathol, 28 (2015) 836–844. [DOI] [PubMed] [Google Scholar]
- [10].Talhouk A, McConechy MK, Leung S, Li-Chang HH, Kwon JS, Melnyk N, Yang W, Senz J, Boyd N, Karnezis AN, Huntsman DG, Gilks CB, McAlpine JN, A clinically applicable molecular-based classification for endometrial cancers, Br J Cancer, 113 (2015) 299–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Trovik J, Wik E, Stefansson IM, Marcickiewicz J, Tingulstad S, Staff AC, Njolstad TS, MoMaTec Study G, Vandenput I, Amant F, Akslen LA, Salvesen HB, Stathmin overexpression identifies high-risk patients and lymph node metastasis in endometrial cancer, Clin Cancer Res, 17 (2011) 3368–3377. [DOI] [PubMed] [Google Scholar]
- [12].Salvesen HB, Haldorsen IS, Trovik J, Markers for individualised therapy in endometrial carcinoma, Lancet Oncol, 13 (2012) e353–361. [DOI] [PubMed] [Google Scholar]
- [13].Cancer N Genome Atlas Research, Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H, Robertson AG, Pashtan I, Shen R, Benz CC, Yau C, Laird PW, Ding L, Zhang W, Mills GB, Kucherlapati R, Mardis ER, Levine DA, Integrated genomic characterization of endometrial carcinoma, Nature, 497 (2013) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S, Nuclear receptor 4A1 as a drug target for breast cancer chemotherapy, Endocr. Relat. Cancer, 22 (2015) 831–840. [DOI] [PubMed] [Google Scholar]
- [15].Hedrick E, Lee SO, Kim G, Abdelrahim M, Jin UH, Safe S, Abudayyeh A, Nuclear receptor 4A1 (NR4A1) as a drug target for renal cell adenocarcinoma, PLoS One, 10 (2015) e0128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lacey A, Hedrick E, Li X, Patel K, Doddapaneni R, Singh M, Safe S, Nuclear receptor 4A1 (NR4A1) as a drug target for treating rhabdomyosarcoma (RMS), Oncotarget, 7 (2016) 31257–31269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lee SO, Li X, Hedrick E, Jin UH, Tjalkens RB, Backos DS, Li L, Zhang Y, Wu Q, Safe S, Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells, Mol. Endocrinol, 28 (2014) 1729–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lee SO, Jin UH, Kang JH, Kim SB, Guthrie AS, Sreevalsan S, Lee JS, Safe S, The orphan nuclear receptor NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress in pancreatic cancer cells, Mol. Cancer Res, 12 (2014) 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee SO, Andey T, Jin UH, Kim K, Singh M, Safe S, The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53, Oncogene, 31 (2012) 3265–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lee SO, Abdelrahim M, Yoon K, Chintharlapalli S, Papineni S, Kim K, Wang H, Safe S, Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth, Cancer Res, 70 (2010) 6824–6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li QX, Ke N, Sundaram R, Wong-Staal F, NR4A1, 2, 3--an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis, Histol. Histopathol, 21 (2006) 533–540. [DOI] [PubMed] [Google Scholar]
- [22].Safe S, Jin UH, Hedrick E, Reeder A, Lee SO, Minireview: role of orphan nuclear receptors in cancer and potential as drug targets, Mol. Endocrinol, 28 (2014) 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pasha M, Eid AH, Eid AA, Gorin Y, Munusamy S, Sestrin2 as a Novel Biomarker and Therapeutic Target for Various Diseases, Oxid Med Cell Longev, 2017 (2017) 3296294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rhee SG, Bae SH, The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1, Free Radic Biol Med, 88 (2015) 205–211. [DOI] [PubMed] [Google Scholar]
- [25].Husseinzadeh N, Husseinzadeh HD, mTOR inhibitors and their clinical application in cervical, endometrial and ovarian cancers: a critical review, Gynecol Oncol, 133 (2014) 375–381. [DOI] [PubMed] [Google Scholar]
- [26].Kassem L, Abdel-Rahman O, Targeting mTOR pathway in gynecological malignancies: Biological rationale and systematic review of published data, Crit Rev Oncol Hematol, 108 (2016) 1–12. [DOI] [PubMed] [Google Scholar]
- [27].Makker V, Recio FO, Ma L, Matulonis UA, Lauchle JO, Parmar H, Gilbert HN, Ware JA, Zhu R, Lu S, Huw LY, Wang Y, Koeppen H, Spoerke JM, Lackner MR, Aghajanian CA, A multicenter, single-arm, open-label, phase 2 study of apitolisib (GDC-0980) for the treatment of recurrent or persistent endometrial carcinoma (MAGGIE study), Cancer, 122 (2016) 3519–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Slomovitz BM, Lu KH, Johnston T, Coleman RL, Munsell M, Broaddus RR, Walker C, Ramondetta LM, Burke TW, Gershenson DM, Wolf J, A phase 2 study of the oral mammalian target of rapamycin inhibitor, everolimus, in patients with recurrent endometrial carcinoma, Cancer, 116 (2010) 5415–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chen HZ, Liu QF, Li L, Wang WJ, Yao LM, Yang M, Liu B, Chen W, Zhan YY, Zhang MQ, Cai JC, Zheng ZH, Lin SC, Li BA, Wu Q, The orphan receptor TR3 suppresses intestinal tumorigenesis in mice by downregulating Wnt signalling, Gut, 61 (2012) 714–724. [DOI] [PubMed] [Google Scholar]
- [30].Mohankumar K, Lee J, Wu CS, Sun Y, Safe S, Bis-Indole-Derived NR4A1 Ligands and Metformin Exhibit NR4A1-Dependent Glucose Metabolism and Uptake in C2C12 Cells, Endocrinology, 159 (2018) 1950–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Maxwell MA, Muscat GE, The NR4A subgroup: immediate early response genes with pleiotropic physiological roles, Nucl Recept Signal, 4 (2006) e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pearen MA, Muscat GE, Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease, Mol. Endocrinol, 24 (2010) 1891–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wang JR, Gan WJ, Li XM, Zhao YY, Li Y, Lu XX, Li JM, Wu H, Orphan nuclear receptor Nur77 promotes colorectal cancer invasion and metastasis by regulating MMP-9 and E-cadherin, Carcinogenesis, 35 (2014) 2474–2484. [DOI] [PubMed] [Google Scholar]
- [34].Zhou F, Drabsch Y, Dekker TJ, de Vinuesa AG, Li Y, Hawinkels LJ, Sheppard KA, Goumans MJ, Luwor RB, de Vries CJ, Mesker WE, Tollenaar RA, Devilee P, Lu CX, Zhu H, Zhang L, Dijke PT, Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-beta signalling, Nat Commun, 5 (2014) 3388. [DOI] [PubMed] [Google Scholar]
- [35].Wu H, Lin Y, Li W, Sun Z, Gao W, Zhang H, Xie L, Jiang F, Qin B, Yan T, Chen L, Zhao Y, Cao X, Wu Y, Lin B, Zhou H, Wong AS, Zhang XK, Zeng JZ, Regulation of Nur77 expression by beta-catenin and its mitogenic effect in colon cancer cells, FASEB J, 25 (2011) 192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X, Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3, Science, 289 (2000) 1159–1164. [DOI] [PubMed] [Google Scholar]
- [37].Kolluri SK, Zhu X, Zhou X, Lin B, Chen Y, Sun K, Tian X, Town J, Cao X, Lin F, Zhai D, Kitada S, Luciano F, O'Donnell E, Cao Y, He F, Lin J, Reed JC, Satterthwait AC, Zhang XK, A short Nur77-derived peptide converts Bcl-2 from a protector to a killer, Cancer Cell, 14 (2008) 285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK, Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3, Cell, 116 (2004) 527–540. [DOI] [PubMed] [Google Scholar]
- [39].Qin C, Morrow D, Stewart J, Spencer K, Porter W, Smith R 3rd, Phillips T, Abdelrahim M, Samudio I, Safe S, A new class of peroxisome proliferator-activated receptor gamma (PPARgamma) agonists that inhibit growth of breast cancer cells: 1,1- Bis(3'-indolyl)-1-(p-substituted phenyl)methanes, Mol Cancer Ther, 3 (2004) 247–260. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.