Abstract
Objective:
Reports are mixed on the efficacy of omega-3 fatty acids (O3FA) for the treatment of major depressive disorder (MDD), with only limited data in adolescents. The present trial aimed to investigate systematically the efficacy of O3FA as a monotherapy, compared to a placebo, in adolescents with MDD. Secondarily, we explored O3FA effects on anhedonia, irritability, and suicidality—all key features of adolescent MDD.
Methods:
Fifty-one psychotropic medication–free adolescents with DSM-IV-TR diagnoses of MDD (aged 12–19 years; 57% female) were randomized to receive O3FA or a placebo for 10 weeks. Data were collected between January 2006 and June 2013. O3FA and a placebo were administered on a fixed-flexible dose titration schedule based on clinical response and side effects. The initial dose of 1.2 g/d was increased 0.6 g/d every 2 weeks, up to a maximum of 3.6 g/d. Clinician-rated and self-rated depression severity, along with treatment response, served as primary outcome measures. Additionally, we examined O3FA effects on depressionrelated symptoms, including anhedonia, irritability, and suicidality. Treatment differences were analyzed via intent-to-treat analyses.
Results:
O3FA were not superior to a placebo on any clinical feature, including depression severity and levels of anhedonia, irritability, or suicidality. Additionally, response rates were comparable between treatment groups. Within-treatment analyses indicated that both treatments were associated with significant improvement in depression severity on self- (O3FA: t = −4.38, P < .001; placebo: t = −3.52, P = .002) and clinician (O3FA: t = −6.47, P < .001; placebo: t = −8.10, P < .001) ratings.
Conclusions:
In adolescents with MDD, O3FA do not appear to be superior to placebo.
Trial Registration:
ClinicalTrials.gov identifier: NCT00962598
Adolescent major depressive disorder (MDD) incurs serious consequences in all life domains and may lead to suicide,1 the second leading cause of death in adolescents. Although psychotropic medications have proved efficacious for adolescent MDD, many patients fail to respond.2 Additionally, adolescents and their families often reject such treatments,3,4 especially after the 2004 US Food and Drug Administration black-box warning that linked increased “suicidality” to antidepressant use in pediatric patients.5 Therefore, there has been a search for alternative treatments for adolescents with MDD, with omega-3 fatty acids (O3FA) proposed as one such option. O3FA are long-chain polyunsaturated fatty acids, composed of 18 to 22 carbon atoms with the first double bond in the third carbon atom, that are crucial to the dynamic structure of neuronal membranes. In humans, they cannot be synthesized and must be derived from dietary sources. The major forms of O3FA are docosahexaenoic acid (DHA, 22 carbon atoms) and eicosapentaenoic acid (EPA, 20 carbon atoms), which are found primarily in fish.
Several lines of evidence point to the potential of O3FA as a therapeutic agent in MDD. First, O3FA are shown to affect monoamine neurotransmission and to have anti-inflammatory properties,6–9 both of which are implicated in the pathophysiology of MDD.10–13 Additionally, epidemiologic studies have reported inverse correlations between fish consumption and depression across multiple countries.14–18 Moreover, adults with MDD are reported to have lower O3FA in red blood cell (RBC) membranes,19,20 and there is a negative correlation between erythrocyte EPA levels and depression severity.21,22 These observations have inspired over 20 clinical trials in adults with MDD or elevated depressive symptoms, examining the use of O3FA as an alternative or adjunctive antidepressant treatment. Although many studies report clinical benefits of O3FA versus a placebo (particularly those using EPA or combined EPA and DHA, with EPA at relatively high doses),23–25 not all studies concur.26–30 In addition to considerable heterogeneity in study designs, methodological limitations have included low power, insufficient dosage, use of either DHA or EPA (rather than the combination), and inclusion of patients receiving other medications or treatments. A 2016 Cochrane review concluded that there is insufficient evidence supporting O3FA as a treatment for MDD and that additional randomized controlled trials (RCTs) are needed.31
According to the literature to date, only 2 clinical trials32,33 have tested O3FA in young patients with MDD. Nemets et al32 conducted a 16-week RCT in 20 children (aged 6–12 years) with MDD who received 1,000 mg of O3FA (combined EPA + DHA) or placebo. Compared to a placebo group (n = 10), the O3FA group (n = 10) had significantly greater improvement. In the second trial, McNamara and colleagues33 conducted a small open-label trial with youth, aged 8–14 years, with selective serotonin reuptake inhibitorresistant MDD, randomized to receive either a low dose (2.4 g/d) or high dose (16.2 g/d) of O3FA (combined EPA + DHA) for 10 weeks. Both the low-dose (n = 7) and high-dose (n = 7) groups demonstrated significant increases in RBC EPA and DHA composition, as well as decreases in depression severity; however, changes in depression severity were not correlated with changes in erythrocyte EPA and DHA composition. A small number of trials have also been conducted in children with other psychiatric conditions, including Tourette’s disorder,34 bipolar disorder,35–38 borderline personality disorder,39 psychosis,40 attention-deficit/hyperactivity disorder (ADHD),41,42 and externalizing behavior43–45; however, in all, the evidence is mixed.46
The current investigation tested the efficacy of orally administered O3FA (combined EPA + DHA, 2:1 ratio) as a monotherapy for adolescent MDD in a double-blind placebo-controlled trial. Although prior data regarding the effects of O3FA are inconsistent, we hypothesized that O3FA would reduce self- and clinician-reported depression severity significantly when compared to a placebo. In addition, we report on O3FA effects on depression-related symptoms, including anhedonia, irritability, and suicidality, as these symptoms may have distinct neurobiology that may, in turn, be associated with specific responses to O3FA.47–49
METHODS
Participants
Adolescents, aged 12–19 years, with a primary diagnosis of MDD were recruited from the greater New York metropolitan area. Written informed consent was obtained from parents/guardians and from participants aged 18 and 19 years. Participants under 18 years of age provided written assent. The study was approved by the Institutional Review Boards at Mount Sinai, New York University School of Medicine, and the Nathan S. Kline Institute for Psychiatric Research and was registered at ClinicalTrials.gov (identifier: NCT00962598). Data were collected between January 2006 and June 2013.
Eligibility requirements were selected to exclude temporary mood changes commonly seen in adolescents. They consisted of a DSM-IV-TR diagnosis of current MDD of at least 6 weeks’ duration (versus the 2-week criterion per DSM-IV-TR) and a minimum raw score of 40 on the Children’s Depression Rating Scale-Revised (CDRS-R).50 An IQ > 80, assessed with the Kaufman Brief Intelligence Test,51 and the ability to swallow pills were required. Participants were required to be free from immune supplements and psychotropic medications for at least 60 days prior to study participation (a minimum of 90 days for medications with extended half-lives, such as fluoxetine). Females on oral contraceptives were also excluded. Stimulant medication for ADHD was not exclusionary so long as that dosage would remain stable over the study period. Similarly, participants already in psychotherapy were allowed to continue; however, psychotherapy could not be initiated or altered during the study. Other exclusionary criteria included significant medical or neurologic disorders; allergy to seafood/fish; lifetime diagnoses of bipolar disorder, autism, pervasive developmental disorder, or Tourette’s disorder; and current diagnoses of eating disorder, panic disorder, obsessive-compulsive disorder, posttraumatic stress disorder, conduct disorder, and substance-related disorder, other than nicotine dependence.
A baseline medical evaluation consisted of a medical history, physical examination (including vital signs and weight), and laboratory studies (complete blood cell count, haptoglobin test, partial thromboplastin time, prothrombin time, international normalized ratios, as well as metabolic and lipid panels, liver and thyroid function tests, urine toxicology test, and urine pregnancy test for females). Laboratory data were confirmed to be within normal limits for study inclusion.
Study Design
Upon determination of eligibility and the results of medical evaluation, the investigational pharmacist randomly assigned participants to receive O3FA or a placebo. Investigators, participants, and parents were blind to treatment assignment, which lasted 10 weeks. O3FA capsules contained a 2:1 ratio of EPA to DHA. Each participant started with an initial dose of 1.2 g/d. Doses were raised in increments of 0.6 g/d every 2 weeks (maximum possible dose of 3.6 g/d, combined EPA [2.4 g] + DHA [1.2 g]), provided there were no significant adverse events and improvement in depression severity was not satisfactory (see details below). This dosage and ratio were selected based on published clinical data of O3FA in adult and youth mood disorders52–54 and on a previous study conducted by our group34 examining the efficacy of O3FA for Tourette’s disorder. Additionally, a similar fixed-flexible dose titration schedule was utilized in a study of O3FA for pediatric bipolar disorder.38 No significant adverse effects were reported in these trials.
Placebo capsules contained a 50:50 ratio of corn and soybean oils, consisting mainly of omega-6 (50%) and monounsaturated (25%) fatty acids. O3FA and placebo capsules were identical in shade (amber) and scent/taste (vanilla). Capsules were manufactured by Ocean Nutrition Canada Ltd. To monitor adequate levels and stability of O3FA and corn/soybean oil in the capsules, we obtained ongoing tests throughout the study by Lipid Analytic Laboratories. Additionally, an assurance certificate verified that the supplements were free of polychlorinated biphenyl (PCB), lead, and other toxins. Supplementary material provides detailed information about capsule analyses and stability testing.
Participants met weekly for up to 1 hour with a child and adolescent psychiatrist and/or psychologist who assessed clinical status and adverse events. If the Clinical Global Impressions—Improvement (CGI-I) Scale55 score was 1 or 2 (very much improved or much improved), the dose remained stable. If the CGI-I score was 3–7 (minimally improved, no change, minimally worse, much worse, or very much worse), the dose was increased for the following 2 weeks—a duration that provided adequate opportunity to assess clinical response at any one dose.
Measures
Clinical assessment.
DSM-IV-TR Axis I diagnoses were established by child and adolescent psychiatrists and clinical psychologists using the Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version for Children,56 administered to participants and parents/guardians at baseline.
Depression severity.
The clinician-rated CDRS-R was completed at each visit. This scale consists of 17 items reflecting multiple aspects of depression, each rated on a Likert scale of either 1–5 or 1–7. The total raw score, which can range from 17 to 113, was used in the analyses. In addition, participants completed the Beck Depression Inventory–II (BDI-II)57 to self-report depression symptoms at each visit.
Suicidality.
Self-ratings on the Beck Scale for Suicide Ideation58 assessed current suicidal ideation at each visit.
Irritability and anhedonia.
We calculated irritability severity by summing 1 item on the CDRS-R (Item 8: “Irritability,” rated 1–7) and 1 item from the BDI-II (Item 17: “Irritability,” rated 0–3), with total scores ranging from 1 to 10. Anhedonia severity was assessed by the sum of 1 item reflecting anhedonia on the CDRS-R (Item 2: “Difficulty having fun,” rated 1–7) and 2 items from the BDI-II (Item 4: “Loss of pleasure,” rated 0–3, and Item 12: “Loss of interest,” rated 0–3), with the total potential score ranging from 1 to 13. This approach to quantifying anhedonia and irritability has been used in our own as well as others’ investigations.59–62 In a previous investigation, this anhedonia measure was significantly correlated with the Snaith-Hamilton Pleasure Scale63 (r = 0.65, P = .001).59
Dietary intake.
At each visit, participants completed a checklist indicating their weekly consumption of 34 marine, meat, and plant sources known to be high in O3FA. Specifically, participants listed how many times each food was consumed since the prior visit. These numbers were summed for each week, and participants were assigned a mean score for weekly dietary O3FA intake over the course of the study.
Compliance.
To monitor compliance, parents/guardians were asked to supervise each instance of dosing. In addition, adolescents were asked to return medication bottles, and capsule counts were performed at each visit and recorded.
Adverse events.
Treating clinicians interviewed participants and parents weekly about specific complaints using the Safety Monitoring Uniform Report Form.64 These included side effects known to occur with antidepressant treatment (eg, insomnia, activation), physical complaints, and suicidal ideation. Serious adverse events were defined as those that resulted in impairment, threat to life, or emergency care.
Treatment response.
Treatment response, assessed at the end of treatment, was primarily defined as a ≥ 50% improvement from baseline on the CDRS-R total score after subtracting the 17-item base score, or reaching a CDRS-R score of ≤ 28. As a secondary outcome measure, we report final-visit ratings of the CGI-I,55 an estimate of overall change relative to baseline. Treatment response was defined as a rating of 1 or 2 (very much improved or much improved, respectively). These criteria were selected to be consistent with those used in previous treatment studies in adolescent depression.65–67
Statistical Analyses
An intent-to-treat analytic approach was utilized that included all participants who had been randomized and had received at least 1 dose of study treatment.* The final posttreatment measure reflected treatment outcome. Between-group analyses relied on a linear regression model that adjusted for baseline values, thereby providing estimates of treatment effect independent of initial status. The rate of treatment response was analyzed by an odds ratio between the 2 treatment groups and a χ2 test. Paired t tests assessed within-group changes from baseline to last visit. Statistical analyses were performed using SAS (SAS Institute, Cary, North Carolina), Version 9.3.
RESULTS
Sample Characteristics
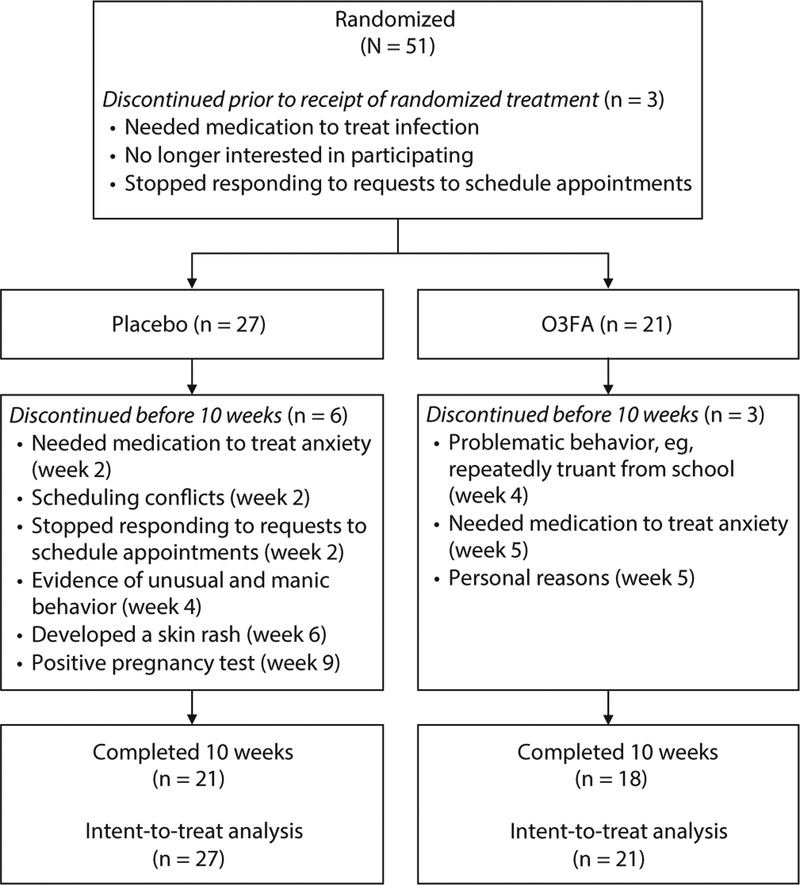
Fifty-one adolescents were randomized, 24 to the O3FA group and 27 to the placebo group. Three participants accepted into the study failed to return prior to receiving treatment and were excluded from analyses. Baseline demographic and clinical data for the 48 participants who entered the study are compiled in Table 1. No one was taking concurrent medication (eg, psychostimulants) during the study. There were no significant differences in baseline data between the O3FA and placebo groups. As detailed in Figure 1, of these 48 participants, 9 (18.75%) did not complete the 10-week treatment (3 taking O3FA, 6 taking placebo). Completers and noncompleters did not differ at baseline, except for a greater proportion of participants with ADHD among the latter (completers: n = 4/39, noncompleters: n = 4/9).
Table 1.
Baseline Demographic and Clinical Characteristics
| Variablea | Placebo (n = 27) |
O3FA (n = 21) |
Total (N=48) |
P Valueb |
|---|---|---|---|---|
| Age, y | 16.34 (1.981) | 15.68 (2.190) | 16.05 (2.079) | .28 |
| Sex | .88 | |||
| Male | 11 (40.7) | 9 (42.9) | 20 (41.7) | |
| Female | 16 (59.3) | 12 (57.1) | 28 (58.3) | |
| Race/ethnicity | .13 | |||
| White | 11 (40.7) | 13 (61.9) | 24 (50.0) | |
| Hispanic | 6 (22.2) | 7 (33.3) | 13 (27.1) | |
| African-American | 6 (22.2) | 1 (4.8) | 7 (14.6) | |
| Asian | 2 (7.4) | 0 (0.0) | 2 (4.2) | |
| Other | 2 (7.4) | 0 (0.0) | 2 (4.2) | |
| Adjunctive therapy | .58 | |||
| None | 20 (74.1) | 13 (61.9) | 33 (68.8) | |
| Cognitive behavioral therapy | 1 (3.7) | 2 (9.5) | 3 (6.2) | |
| Other therapy | 6 (22.2) | 6 (28.6) | 12 (25.0) | |
| Current episode duration, | 28.6 (20.2) | 15.3 (8.6) | 22.5 (17.2) | <.01 |
| moc | ||||
| Total number of episodes | .05 | |||
| 1 | 20 (74.1) | 20 (95.2) | 40 (83.3) | |
| 2 | 7 (25.9) | 1 (4.8) | 8 (16.7) | |
| History of suicide attempt | 5 (18.5) | 1 (4.8) | 6 (12.5) | .15 |
| Current comorbid disorders | ||||
| Anxiety | 13 (48.1) | 11 (52.4) | 24 (50.0) | .77 |
| ADHD | 6 (22.2) | 2 (9.5) | 8 (16.7) | .24 |
| Oppositional defiant disorder | 1 (3.7) | 1 (4.8) | 2 (4.2) | .86 |
Summary statistics presented are mean (standard deviation) for continuous or frequency (%) for categorical variables.
P value according to t test for continuous variables or χ2 test for categorical variables.
Data missing for 2 participants assigned to placebo.
Abbreviations: ADHD = attention-deficit/hyperactivity disorder, O3FA = omega-3 fatty acids.
Figure 1. CONSORT Flow Diagram.
Abbreviation: O3FA = omega-3 fatty acids.
Over the course of treatment, participants reported a mean weekly dietary intake of 8.1 servings of O3FA. There were no significant differences in mean dietary O3FA intake between those prescribed O3FA versus placebo (t = 0.58, P = .57).
Experimental Treatments
Mean end dosage was 3,433.3 mg/d for the 18 completers taking O3FA; responders received 3,200.0 mg/d, and nonresponders received 3,300.0 mg/d. The 21 completers taking placebo received mean end doses of 3,400.0 mg/d; responders received 3,184.6 mg/d, and nonresponders received 3,138.5 mg/d.
Average weekly compliance rate for the full sample ranged from 0.58 to 1.00, with an overall mean compliance rate of 0.89 (SD = 0.11). There were no significant differences in compliance between the 2 groups (t = 1.26, P = .21).
Treatment Effects
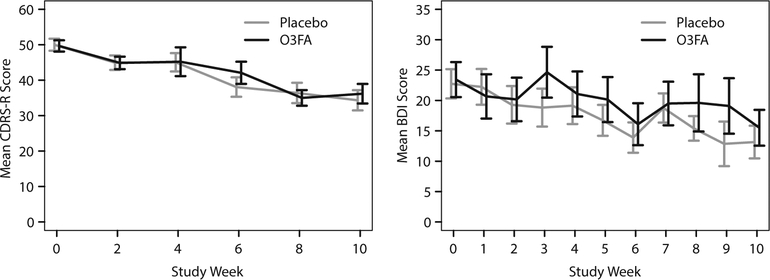
Contrary to our hypothesis, O3FA was not superior to placebo in reducing depression severity, as assessed by the CDRS-R or the BDI (Table 2), or on any other outcome measure. Both treatments were associated with significant improvement in depression severity, as assessed by the CDRS-R (O3FA: t = −6.47, P < .001; placebo: t = −8.10, P < .001) and BDI (O3FA: t = −4.38, P < .001; placebo: t = −3.52, P = .002). Figure 2 presents mean CDRS-R and BDI group scores.
Table 2.
Changes in Assessments From Baseline to Last Visita
| Placebo (n = 27) | O3FA (n = 21) | Baseline-Adjusted Treatment Difference (95% CI)c | Treatment Difference P Valuec | |||
|---|---|---|---|---|---|---|
| Variableb | Baseline, Mean (SD) | Week 10/Last Visit, Mean (SD) | Baseline, Mean (SD) | Week 10/Last Visit, Mean (SD) | ||
| CDRS-R | 50.2 (8.91) | 35.2 (10.57) | 49.5 (8.20) | 36.5 (10.01) | 1.81 (−3.31 to 6.94) | .49 |
| BDI | 22.4 (12.65) | 14.8 (11.97) | 24.2(13.70) | 16.9 (13.21) | 0.79 (−4.27 to 5.84) | .76 |
| Anhedonia | 6.0 (2.09) | 3.5 (2.08) | 6.4 (2.52) | 4.5 (2.20) | 0.86 (−0.21 to 1.94) | .12 |
| BSS | 3.3 (5.62) | 1.9 (5.02) | 5.2 (9.05) | 3.6 (6.93) | 0.39 (−1.47 to 2.25) | .69 |
| Irritability | 5.1 (2.15) | 3.6 (1.94) | 5.2 (2.12) | 3.8 (1.82) | 0.16 (−0.68 to 1.00) | .70 |
| n (%) | n (%) | Odds Ratio (95% Cl) | P Valuef | |||
| Treatment responsed,e | 0.59 (0.16 to 2.14) | .42 | ||||
| Responder | 13 (50.0) | 9 (42.9) | ||||
| Nonresponder | 13 (50.0) | 12 (57.1) | ||||
Results presented for subjects with available pairs of baseline and last visit values for each respective measure.
Mean (standard deviation) presented for baseline, week 10/last visit, and change from baseline.
Baseline-adjusted mean difference between treatment arms and P value from linear regression model adjusted for respective baseline values.
Based on our primary measure of treatment response, defined as ≥ 50% change from baseline relative to baseline value minus 17 base points or reaching a score of ≤ 28 at week 10/last visit on the CDRS-R.
CDRS-R scores were available for only the baseline visit for 1 placebo participant who dropped out after week 2. Therefore, this participant could not be included in the analysis.
P value according to χ2 test.
Abbreviations: BDI = Beck Depression Inventory, BSS = Beck Scale for Suicide Ideation, CDRS-R = Children’s Depression Rating Scale-Revised, O3FA = omega-3 fatty acids.
Figure 2. Mean CDRS-R and BDI Scores Over Time for O3FA and Placebo Treatment Groupsa.
aShown are mean values ± standard error per weekly visit.
Abbreviations: BDI = Beck Depression Inventory, CDRS-R = Children’s Depression Rating Scale-Revised, O3FA = omega-3 fatty acids.
Nine (42.9%) of 21 participants in the O3FA group and 13 (50.0%) of 26 evaluable† participants in the placebo group were classified as treatment responders based on a ≥ 50% decrease from baseline CDRS-R score (after subtracting the 17-item base score), or a CDRS-R score of ≤ 28, at the final visit. There were no significant differences in treatment response between the groups (OR = 0.75, 95% CI, 0.24–2.38, P = .63). Similarly, the CGI-I did not yield differential treatment effects (OR = 0.88, 95% CI, 0.27–2.79, P = .82). Nine participants (42.9%) taking O3FA versus 12 (46.2%) taking placebo were treatment responders (defined as a global improvement of 1 or 2).
Exploratory Analyses of Depressive Symptoms
Most measures showed significant improvement in both groups (eg, irritability [O3FA: t = −4.65, P < .001; placebo: t = −4.22, P < .001]; anhedonia [O3FA: t = −3.89, P = .001; placebo: t = −6.09, P < .001]). However, as was the case for the primary measures, no treatment advantage was found for O3FA over placebo (Table 2).
Adverse Events
There were no significant adverse events among adolescents treated with O3FA. One participant taking placebo developed a mild skin rash during week 5 and was therefore withdrawn from the study. A connection between the rash and the placebo medication was not established. Another participant in the placebo group developed unusual and manic behavior during week 4 and was also subsequently removed. It is unlikely that the placebo was responsible for either of these events.
DISCUSSION
This 10-week randomized placebo-controlled study tested the efficacy of O3FA as a monotherapy for adolescent MDD. Contrary to our hypothesis, O3FA treatment was not superior to placebo on any outcome measure. Thus, there were no treatment differences in overall depression severity, as rated by the participants and clinicians, or in illness remission. Similarly, compared to participants taking the placebo, those taking O3FA did not experience greater improvement on specific symptoms of interest, namely, anhedonia, irritability, and suicidality.
As presented in the introduction, a prior trial by Nemets and colleagues32 was carried out in younger children (aged 6–12 years) with MDD. In contrast to our findings, children treated with O3FA experienced significantly greater improvement than children taking placebo. The younger age in that study may have contributed to these divergent findings. However, in the Nemets et al study,32 no single child responded to the placebo, which is a unique result among clinical trials in pediatric depression that raises questions about generalizing from a highly atypical clinical result. Importantly, the placebo response rate in the present study is in line with the placebo response rates reported in large randomized clinical trials of antidepressant treatment for pediatric MDD68 and was low enough to allow detection of improvement beyond this frequency. Thus, it is most likely that our negative findings are the result of limited efficacy of O3FA in adolescent MDD, rather than being attributable to inadequate clinical procedures or a high placebo response rate.
The current study’s negative findings are consistent with several large RCTs of O3FA as a monotherapy for adults with MDD. A 12-week trial by Rogers et al29 failed to obtain significant superiority for EPA + DHA (1.5 g/d) over placebo in 218 adults with mild to moderate MDD. Another large trial by Lespérance and colleagues69 in 432 adults with MDD (40% of whom were taking antidepressant medications) also failed to find superiority for O3FA over a placebo. More recently, an 8-week study by Mischoulon and colleagues70 obtained negative results from contrasts between EPA (1 g/d), DHA (1 g/d), and a placebo in 196 depressed adults (all groups improved significantly). Similarly, other large trials in adults have failed to detect an advantage for O3FA over a placebo for subclinical depressive symptoms.27,71–73
In follow-up analyses to Mischoulon and colleagues’ 2015 study70 (cited above), the authors74 argued that baseline inflammation may act as a moderator of clinical response to O3FA. If so, a biologically homogenous MDD group with regard to inflammation status might be required to detect therapeutic efficacy of O3FA. Such a possibility awaits rigorous testing. However, in our study, anhedonia, which has been linked specifically with inflammation47,75 and thus would be expected to respond to O3FA, was not significantly improved by O3FA relative to a placebo. At the same time, this negative result may be related to the relatively small number of adolescents with high levels of anhedonia in our study.
Several factors may have contributed to our negative findings. As noted above, not accounting for interindividual differences in clinical/biological phenomena may have reduced the opportunity to detect efficacy for O3FA, given the heterogeneous nature of adolescent MDD. Alternatively, our sample, which consisted of moderate to severe MDD, might be too severe to respond to O3FA as a monotherapy. This factor might be particularly important in light of the selected maximum dose of 3.6 g. McNamara and colleagues33 argued that a high dose of 16.2 g of combined EPA + DHA (2:1) resulted in 100% remission compared to 40% remission with a lower dose of 2.4 g. However, it is important to note that no placebo condition was included, and the trial was limited to only 7 subjects per treatment group.
An additional limitation includes the relatively small sample size, with reduced power to detect subtle group differences. Although no trend in favor of O3FA was noted, a small benefit of O3FA therapy cannot be ruled out. Also, measures of O3FA in blood serum were not available to establish adequate metabolization.
In conclusion, based on our findings, there is no support for O3FA monotherapy at maximum dosages of 3.6 g/d for the treatment of adolescent MDD. Study strengths include the double-blind placebo-controlled design carried out in an academic setting, the stringent inclusion criteria, and minimization of confounding effects that may accompany concurrent therapy or medications. It may be that different results would ensue in studies of biologically homogenous groups of adolescents with MDD who all have abnormally elevated levels of inflammation.
Clinical Points.
Converging data have suggested that omega-3 fatty acids are therapeutic for mood disorders; however, there has been a lack of well-designed clinical studies in youth with major depression.
The present 10-week randomized placebo-controlled study of omega-3 fatty acids in adolescents with major depression did not identify improved response to omega-3 fatty acids compared to placebo in relation to depression severity, anhedonia, irritability, and suicidality.
Funding/support:
This study was funded by grants from the National Institutes of Health (NIH) AT004576 and AT002395 (principal investigator [PI]: Dr Gabbay). Some of the work was also supported by NIH grants MH095807 and MH101479 (PI: Dr Gabbay).
Role of the sponsor: The sponsor had no role in the design, analysis, interpretation, or publication of this study.
Footnotes
Potential conflicts of interest: The authors report no conflicts of interest.
Previous presentation: Poster presented at the annual conference of the American Academy of Child and Adolescent Psychiatry, October 24–29, 2016, New York, NY.
Supplementary material: Available at PSYCHIATRIST.COM.
As shown in Figure 1, 3 participants discontinued following randomization but prior to receiving randomized treatment. These were not included in analyses.
CDRS-R scores were available for only the baseline visit for 1 placebo participant who dropped out after week 2. Therefore, this participant could not be included in these analyses.
REFERENCES
- 1.Asarnow JR, Baraff LJ, Berk M, et al. Pediatric emergency department suicidal patients: two-site evaluation of suicide ideators, single attempters, and repeat attempters. J Am Acad Child Adolesc Psychiatry. 2008;47(8):958–966. [DOI] [PubMed] [Google Scholar]
- 2.March J, Silva S, Petrycki S, et al. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) randomized controlled trial. JAMA. 2004;292(7):807–820. [DOI] [PubMed] [Google Scholar]
- 3.Bradley KL, McGrath PJ, Brannen CL, et al. Adolescents’ attitudes and opinions about depression treatment. Community Ment Health J. 2010;46(3):242–251. [DOI] [PubMed] [Google Scholar]
- 4.Jaycox LH, Asarnow JR, Sherbourne CD, et al. Adolescent primary care patients’ preferences for depression treatment. Adm Policy Ment Health. 2006;33(2):198–207. [DOI] [PubMed] [Google Scholar]
- 5.Libby AM, Brent DA, Morrato EH, et al. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am J Psychiatry. 2007;164(6):884–891. [DOI] [PubMed] [Google Scholar]
- 6.Chalon S, Delion-Vancassel S, Belzung C, et al. Dietary fish oil affects monoaminergic neurotransmission and behavior in rats. J Nutr. 1998;128(12):2512–2519. [DOI] [PubMed] [Google Scholar]
- 7.Li H, Liu D, Zhang E. Effect of fish oil supplementation on fatty acid composition and neurotransmitters of growing rats [in Chinese]. Wei Sheng Yan Jiu. 2000;29(1):47–49. [PubMed] [Google Scholar]
- 8.Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. J Am Coll Nutr. 2002;21(6):495–505. [DOI] [PubMed] [Google Scholar]
- 9.Ellulu MS, Khaza’ai H, Abed Y, et al. Role of fish oil in human health and possible mechanism to reduce the inflammation. Inflammopharmacology. 2015;23(2–3):79–89. [DOI] [PubMed] [Google Scholar]
- 10.Gabbay V, Klein RG, Alonso CM, et al. Immune system dysregulation in adolescent major depressive disorder. J Affect Disord. 2009;115(1–2):177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinclair AJ, Begg D, Mathai M, et al. Omega 3 fatty acids and the brain: review of studies in depression. Asia Pac J Clin Nutr. 2007;16(Suppl 1):391–397. [PubMed] [Google Scholar]
- 12.Gabbay V, Klein RG, Guttman LE, et al. A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. J Child Adolesc Psychopharmacol. 2009;19(4):423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gabbay V, Liebes L, Katz Y, et al. The kynurenine pathway in adolescent depression: preliminary findings from a proton MR spectroscopy study. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beydoun MA, Fanelli Kuczmarski MT, Beydoun HA, et al. ω−3 fatty acid intakes are inversely related to elevated depressive symptoms among United States women. J Nutr. 2013;143(11):1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmire CA, Block RC, Thevenet-Morrison K, et al. Associations between omega-3 polyunsaturated fatty acids from fish consumption and severity of depressive symptoms: an analysis of the 2005–2008 National Health and Nutrition Examination Survey. Prostaglandins Leukot Essent Fatty Acids. 2012;86(4–5):155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horikawa C, Otsuka R, Kato Y, et al. Cross-sectional association between serum concentrations of n-3 long-chain PUFA and depressive symptoms: results in Japanese community dwellers. Br J Nutr. 2016;115(4):672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suominen-Taipale AL, Partonen T, Turunen AW, et al. Fish consumption and omega-3 polyunsaturated fatty acids in relation to depressive episodes: a cross-sectional analysis. PLoS One. 2010;5(5):e10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanskanen A, Hibbeln JR, Tuomilehto J, et al. Fish consumption and depressive symptoms in the general population in Finland. Psychiatr Serv. 2001;52(4):529–531. [DOI] [PubMed] [Google Scholar]
- 19.Peet M, Murphy B, Shay J, et al. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43(5):315–319. [DOI] [PubMed] [Google Scholar]
- 20.Edwards R, Peet M, Shay J, et al. Depletion of docosahexaenoic acid in red blood cell membranes of depressive patients. Biochem Soc Trans. 1998;26(2):S142. [DOI] [PubMed] [Google Scholar]
- 21.Adams PB, Lawson S, Sanigorski A, et al. Arachidonic acid to eicosapentaenoic acid ratio in blood correlates positively with clinical symptoms of depression. Lipids. 1996;31(Suppl):S157–S161. [DOI] [PubMed] [Google Scholar]
- 22.Lin PY, Huang SY, Su KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry. 2010;68(2):140–147. [DOI] [PubMed] [Google Scholar]
- 23.Freeman MP. Omega-3 fatty acids in major depressive disorder. J Clin Psychiatry. 2009;70(suppl 5):7–11. [DOI] [PubMed] [Google Scholar]
- 24.Grosso G, Pajak A, Marventano S, et al. Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS One. 2014;9(5):e96905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mocking RJ, Harmsen I, Assies J, et al. Meta-analysis and meta-regression of omega-3 polyunsaturated fatty acid supplementation for major depressive disorder. Transl Psychiatry. 2016;6(3):e756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grenyer BF, Crowe T, Meyer B, et al. Fish oil supplementation in the treatment of major depression: a randomised double-blind placebo-controlled trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(7):1393–1396. [DOI] [PubMed] [Google Scholar]
- 27.Lucas M, Asselin G, Merette C, et al. Ethyleicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Am J Clin Nutr. 2009;89(2):641–651. [DOI] [PubMed] [Google Scholar]
- 28.Marangell LB, Martinez JM, Zboyan HA, et al. A double-blind, placebo-controlled study of the omega-3 fatty acid docosahexaenoic acid in the treatment of major depression. Am J Psychiatry. 2003;160(5):996–998. [DOI] [PubMed] [Google Scholar]
- 29.Rogers PJ, Appleton KM, Kessler D, et al. No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr. 2008;99(2):421–431. [DOI] [PubMed] [Google Scholar]
- 30.Silvers KM, Woolley CC, Hamilton FC, et al. Randomised double-blind placebo-controlled trial of fish oil in the treatment of depression. Prostaglandins Leukot Essent Fatty Acids. 2005;72(3):211–218. [DOI] [PubMed] [Google Scholar]
- 31.Appleton KM, Sallis HM, Perry R, et al. Omega-3 fatty acids for major depressive disorder in adults: an abridged Cochrane review. BMJ Open. 2016;6(3):e010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nemets H, Nemets B, Apter A, et al. Omega-3 treatment of childhood depression: a controlled, double-blind pilot study. Am J Psychiatry. 2006;163(6):1098–1100 [DOI] [PubMed] [Google Scholar]
- 33.McNamara RK, Strimpfel J, Jandacek R, et al. Detection and treatment of long-chain omega-3 fatty acid deficiency in adolescents with SSRI-resistant major depressive disorder. PharmaNutrition. 2014;2(2):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gabbay V, Babb JS, Klein RG, et al. A double-blind, placebo-controlled trial of omega-3 fatty acids in Tourette’s disorder. Pediatrics. 2012;129(6):e1493–e1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clayton EH, Hanstock TL, Hirneth SJ, et al. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63(8):1037–1040. [DOI] [PubMed] [Google Scholar]
- 36.Fristad MA, Young AS, Vesco AT, et al. A randomized controlled trial of individual family psychoeducational psychotherapy and omega-3 fatty acids in youth with subsyndromal bipolar disorder. J Child Adolesc Psychopharmacol. 2015;25(10):764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wozniak J, Faraone SV, Chan J, et al. A randomized clinical trial of high eicosapentaenoic acid omega-3 fatty acids and inositol as monotherapy and in combination in the treatment of pediatric bipolar spectrum disorders: a pilot study. J Clin Psychiatry. 2015;76(11):1548–1555. [DOI] [PubMed] [Google Scholar]
- 38.Wozniak J, Biederman J, Mick E, et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17(6–7):440–447. [DOI] [PubMed] [Google Scholar]
- 39.Amminger GP, Chanen AM, Ohmann S, et al. Omega-3 fatty acid supplementation in adolescents with borderline personality disorder and ultra-high risk criteria for psychosis: a post hoc subgroup analysis of a double-blind, randomized controlled trial. Can J Psychiatry. 2013;58(7):402–408. [DOI] [PubMed] [Google Scholar]
- 40.Amminger GP, Schafer MR, Papageorgiou K, et al. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch Gen Psychiatry. 2010;67(2):146–154. [DOI] [PubMed] [Google Scholar]
- 41.Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2011;50(10):991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillies D, Sinn J, Lad SS, et al. Polyunsaturated fatty acids (PUFA) for attention deficit hyperactivity disorder (ADHD) in children and adolescents. Cochrane Database Syst Rev. 2012;(7):CD007986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooper RE, Tye C, Kuntsi J, et al. The effect of omega-3 polyunsaturated fatty acid supplementation on emotional dysregulation, oppositional behaviour and conduct problems in ADHD: a systematic review and meta-analysis. J Affect Disord. 2016;190:474–482. [DOI] [PubMed] [Google Scholar]
- 44.Dean AJ, Bor W, Adam K, et al. A randomized, controlled, crossover trial of fish oil treatment for impulsive aggression in children and adolescents with disruptive behavior disorders. J Child Adolesc Psychopharmacol. 2014;24(3):140–148. [DOI] [PubMed] [Google Scholar]
- 45.Raine A, Portnoy J, Liu J, et al. Reduction in behavior problems with omega-3 supplementation in children aged 8–16 years: a randomized, double-blind, placebo-controlled, stratified, parallel-group trial. J Child Psychol Psychiatry. 2015;56(5):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinn N, Milte C, Howe PR. Oiling the brain: a review of randomized controlled trials of omega-3 fatty acids in psychopathology across the lifespan. Nutrients. 2010;2(2):128–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisenberger NI, Berkman ET, Inagaki TK, et al. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry. 2010;68(8):748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brundin L, Bryleva EY, Thirtamara Rajamani K. Role of inflammation in suicide: from mechanisms to treatment. Neuropsychopharmacology. 2017;42(1):271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lotrich FE, Sears B, McNamara RK. Anger induced by interferon-alpha is moderated by ratio of arachidonic acid to omega-3 fatty acids. J Psychosom Res. 2013;75(5):475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Poznanski EO, Mokros H. Children’s Depression Rating Scale-Revised (CDRS-R). Los Angeles, CA: WPS; 1996. [Google Scholar]
- 51.Kaufman AS, Kaufman NL. K-BIT: Kaufman Brief Intelligence Test. Circle Pines, MN: American Guidance Service; 1990. [Google Scholar]
- 52.Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):407–412. [DOI] [PubMed] [Google Scholar]
- 53.Su KP, Huang SY, Chiu CC, et al. Omega-3 fatty acids in major depressive disorder. A preliminary double-blind, placebo-controlled trial. Eur Neuropsychopharmacol. 2003;13(4):267–271. [DOI] [PubMed] [Google Scholar]
- 54.Su KP, Shen WW, Huang SY. Are omega3 fatty acids beneficial in depression but not mania? Arch Gen Psychiatry. 2000;57(7):716–717. [DOI] [PubMed] [Google Scholar]
- 55.Guy W ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976. [Google Scholar]
- 56.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36(7):980–988. [DOI] [PubMed] [Google Scholar]
- 57.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 58.Beck AT, Steer RA, Ranieri WF. Scale for Suicide Ideation: psychometric properties of a self-report version. J Clin Psychol. 1988;44(4):499–505. [DOI] [PubMed] [Google Scholar]
- 59.Gabbay V, Johnson AR, Alonso CM, et al. Anhedonia, but not irritability, is associated with illness severity outcomes in adolescent major depression. J Child Adolesc Psychopharmacol. 2015;25(3):194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McMakin DL, Olino TM, Porta G, et al. Anhedonia predicts poorer recovery among youth with selective serotonin reuptake inhibitor treatment-resistant depression. J Am Acad Child Adolesc Psychiatry. 2012;51(4):404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henderson SE, Johnson AR, Vallejo AI, et al. A preliminary study of white matter in adolescent depression: relationships with illness severity, anhedonia, and irritability. Front Psychiatry. 2013;4:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gabbay V, Ely BA, Li Q, et al. Striatum-based circuitry of adolescent depression and anhedonia. J Am Acad Child Adolesc Psychiatry. 2013;52(6):628–41.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snaith RP, Hamilton M, Morley S, et al. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167(1):99–103. [DOI] [PubMed] [Google Scholar]
- 64.Greenhill LL, Vitiello B, Fisher P, et al. Comparison of increasingly detailed elicitation methods for the assessment of adverse events in pediatric psychopharmacology. J Am Acad Child Adolesc Psychiatry. 2004;43(12):1488–1496. [DOI] [PubMed] [Google Scholar]
- 65.Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Emslie GJ, Kennard BD, Mayes TL, et al. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165(4):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner KD, Jonas J, Findling RL, et al. A double-blind, randomized, placebo-controlled trial of escitalopram in the treatment of pediatric depression. J Am Acad Child Adolesc Psychiatry. 2006;45(3):280–288. [DOI] [PubMed] [Google Scholar]
- 68.Bridge JA, Birmaher B, Iyengar S, et al. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry. 2009;166(1):42–49. [DOI] [PubMed] [Google Scholar]
- 69.Lespérance F, Frasure-Smith N, St-André E, et al. The efficacy of omega-3 supplementation for major depression: a randomized controlled trial. J Clin Psychiatry. 2011;72(8):1054–1062. [DOI] [PubMed] [Google Scholar]
- 70.Mischoulon D, Nierenberg AA, Schettler PJ, et al. A double-blind, randomized controlled clinical trial comparing eicosapentaenoic acid versus docosahexaenoic acid for depression. J Clin Psychiatry. 2015;76(1):54–61. [DOI] [PubMed] [Google Scholar]
- 71.Kiecolt-Glaser JK, Belury MA, Andridge R, et al. Omega-3 supplementation lowers inflammation in healthy middle-aged and older adults: a randomized controlled trial. Brain Behav Immun. 2012;26(6):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mozurkewich EL, Clinton CM, Chilimigras JL, et al. The Mothers, Omega-3, and Mental Health Study: a double-blind, randomized controlled trial. Am J Obstet Gynecol. 2013;208(4):313.e1–313.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van de Rest O, Geleijnse JM, Kok FJ, et al. Effect of fish-oil supplementation on mental well-being in older subjects: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2008;88(3):706–713. [DOI] [PubMed] [Google Scholar]
- 74.Rapaport MH, Nierenberg AA, Schettler PJ, et al. Inflammation as a predictive biomarker for response to omega-3 fatty acids in major depressive disorder: a proof of concept study. Mol Psychiatry. 2016;21(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gabbay V, Mao X, Klein RG, et al. Anterior cingulate cortex gamma-aminobutyric acid in depressed adolescents: relationship to anhedonia. Arch Gen Psychiatry. 2012;69(2):139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]