Abstract
Recent studies have reported structural and functional abnormalities in multiple brain regions of classical trigeminal neuralgia (CTN) patients. Differences in spontaneous neuronal activity between CTN patients and healthy subjects, however, remain unknown. The aim of the present study was to investigate alterations in brain activity by application of amplitude of low frequency fluctuation (ALFF), thus analyzing the correlation between durations of spontaneous pain intensity and ALFF values in CTN patients. A total of 28 CTN patients (male, n=12; female, n=16) and 28 healthy controls (HCs; male, n=12; female, n=16) matched for age and sex were enrolled. All subjects underwent resting-state functional magnetic resonance imaging and changes in spontaneous brain activity were investigated using an ALFF method. Receiver operating characteristic (ROC) curve analysis was applied to differentiate ALFF values of CTN patients from HCs. Altered ALFF values and clinical manifestations were evaluated using Pearson's correlation analysis. ALFF values of the bilateral inferior cerebellum, bilateral fusiform gyrus, right precentral gyrus, left inferior temporal gyrus, right superior cerebellum, left inferior occipital gyrus and right superior occipital gyrus were significantly higher in CTN patients when compared to HCs. ROC curve analysis of each brain revealed a near-perfect AUC accuracy. Pearson's correlation analysis revealed the visual analog scale of the right eye to be positively correlated with both left inferior temporal and occipital gyral findings, while episode duration likewise was positively associated with left inferior temporal gyral findings. CTN patients exhibited abnormal spontaneous activity in multiple brain regions closely related to pain regulation and perception, while VAS and CTN episode duration were positively correlated with ALFF signal values in some brain regions. The present findings provide further insight into the pathological mechanisms underlying CTN.
Keywords: classical trigeminal neuralgia, ALFF, functional magnetic resonance imaging, spontaneous brain activity
Introduction
First described by John Fothergill in 1773, trigeminal neuralgia (TN) is the most common cranial neuralgia (1,2). The incidence of TN in China has been reported to be 182.8/100,000, with a predilection age of 37–67 years and a ratio of 1:3 for males and females. Classical trigeminal neuralgia (CTN) is described by electrical pain sensations not due to intracranial tumors or bone compression in the area where one or more branches of the facial nerve distribute. Pain typically manifests briefly, lasting only a few sec, and patients do not complain of obvious discomfort between episodes. Patients primarily report a fixed pain trigger point at the mouth or nasal wing as well as alleviation or control of pain by oral medication (3).
Multiple theories concerning the etiology of CTN have been suggested, including increased excitability of sensory neurons and abnormal action potentials as a result of neuronal damage (4). Abnormal action potentials or external stimulation excite adjacent resting neurons through direct interneuronal connections, producing abnormally synchronized discharges and resulting in pain. Another theory holds that root exit zone compression caused by local axonal demyelination allows exposed nerve fibers to come into contact, thus producing abnormal conduction. Transmission of minor stimuli elicits efferent central nervous system impulses, which change into afferent impulses via such contact. Such signaling eventually results in pain (5). To date, however, little is known about the pathogenesis of CTN despite its wide prevalence and considerable healthcare burden. However, increasing evidence has suggested that cerebral abnormalities are closely related to pain (6). A prior study which detected brain activity when using an estrogenic stimulation in CTN patients reported that their gray matter in the bilateral superior/middle temporal gyrus, thalamus, putamen, caudate nucleus, forebrain and hindbrain, as well as the primary somatosensory cortex significantly differed from healthy controls (7).
Few studies to date have explored altered brain activity in CTN patients. The aim of the present study was to investigate whether changes in neuronal activity of related brain regions can be considered as a therapeutic target which could help patients alleviate pain and improve their quality of life.
Functional magnetic resonance imaging (fMRI), offering such advantages as convenience and noninvasiveness, is widely used for studying brain functions (8). Based on cerebral blood flow and metabolic analysis, researchers can evaluate specific brain activity and explore brain structure, elucidating how the brain of CTN patients is affected by this condition (9). The active state-MRI captures the brain activity of subjects when they are performing specific tasks, which has the advantage of directly observing which brain regions are activated during tasking state and enabling more direct comparison with psychological behavioral studies. In addition, it highlights the importance of the relationship between tasks and activated brain regions (10). However, this model is greatly influenced by the experimental design and it is also difficult to control the baseline level. Furthermore, the subjects need a strong psychological background. Since certain subjects cannot finish the task or short task time, this pattern has poor clinical practicability (11). Resting-state functional magnetic resonance imaging (RS-fMRI) has attracted wide attention in the field of neuroimaging and is suitable for the study of the central mechanism. It also has become a useful tool for the study of functional connections in the human brain (12). RS-fMRI is considered to be the circuit that forms the information processing and physiological basis of psychological representation (13). It captures the brain activity of subjects when they remain at rest without any other activity, which has the advantage of directly observing the spontaneous neurological brain activity of subjects. It is closer to the physiological state and highlights the importance of the parallelism of human brain neural networks and the interrelation between different brain regions (14). In addition, the resting state can last longer which leaves enough time to collect data. Moreover, this pattern also has the advantage of simple design and easy baseline control. However, the RS-MRI method can be influenced by a variety of physiological noises such as the beating heart and breathing as well as other noises (15).
Amplitude of low-frequency fluctuation (ALFF), a type of resting state fMRI, can be applied to evaluate intrinsic fluctuations of bold signals to reveal local spontaneous static state brain activity (16). Previous studies have demonstrated that ALFF exhibited good to moderate test-retest reliability (17,18). The simple calculation and reliable characterization of the ALFF measurement makes it a suitable and useful tool for resting-state fMRI data analysis in order to investigate a disease trait. Furthermore, it has been demonstrated to be a valuable parameter to reflect the intensity of spontaneous nerve activity (19). In our previous studies, the ALFF method was successfully used to evaluate certain eye diseases (20,21).
The present study, demonstrated that CTN patients had dysfunction of brain activities. These findings may provide important information to explain the underlying neural mechanisms of CTN, which are beneficial to clinical diagnosis. Based on fMRI and ALFF findings, our aim was to explore the local features of spontaneous brain activity in patients with CTN and the relationship between ALFF with the clinical manifestations, thus possibly helping to elucidate the underlying pathological mechanisms involved in CTN and agreed to publish their brain scan images.
Materials and methods
Subjects
The study recruited 28 patients with TN (male, n=12; female, n=16) from the First Affiliated Hospital of Nanchang University. Inclusion criteria were: i) Unilateral pain involving at least one of either the maxillary (V2) or mandibular (V3) branches of the trigeminal nerve; ii) intense, sharp, burning or stabbing paroxysmal facial pain precipitated by trigger factors or zones; iii) conventional MRI T1WI, T2WI sequence examination revealing no evident abnormal brain signals; iv) no clinically evident neurological or sensory dysfunction attributed to other conditions; v) no previous surgical or other invasive treatments for TN; and vi) no contraindications to magnetic resonance scanning. Exclusion criteria were: i) headaches, or other paroxysmal or chronic pain conditions; ii) patient family history of headache or other pain in first degree relatives; iii) other somatic or psychiatric conditions; and iv) contraindications to magnetic resonance scanning. The study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University (no. CDYFY-E-160802), Jiangxi, China.
A total of 28 healthy controls (HCs; male, n=12; female, n=16) matched for age, sex and economic status were also recruited. All subjects were right-handed. The ages of patients and healthy controls were 51.392±9.372 and 51.357±9.302 years, respectively. The range of CTN episode duration was 3.73±4.10 years and visual analogue scale (VAS) scores ranged from 6.32±1.44 (Table I). Inclusion criteria were: i) No TN symptoms; ii) no diagnosed organic conditions including chronic pain; iii) not suffering from neurological or psychiatric disorders; and iv) no contraindications to magnetic resonance imaging (MRI) scanning.
Table I.
Demographics and behavioral results of CTN and HC groups (22).
| CTN | HC | T-value | P-value | |
|---|---|---|---|---|
| Male/female | 12/16 | 12/16 | N/A | >0.99 |
| Age (years) | 51.392±9.372 | 51.357±9.302 | 0.297 | 0.769 |
| Handedness | 28 R | 28 R | N/A | >0.99 |
| Duration (years) | 3.733±4.102 | N/A | N/A | N/A |
| VAS | 6.321±1.442 | N/A | N/A | N/A |
Independent t-tests comparing the age of two groups (P<0.05 represented statistically significant differences). Male/female and Handedness were analyzed using chi-squared test. CTN, classical trigeminal neuralgia; HC, healthy control; N/A, not applicable; R, right; VAS, Visual Analogue Scale.
The present study was approved by the Medical Research Ethics Committee of The First Affiliated Hospital of Nanchang University. All individuals provided signed informed consent to participate in the study.
Pain evaluation
Pain in TN patients was assessed using the VAS. Researchers guided patients in rating their pain on a scale of 0–10 using a 10-cm ruler. A higher score indicated greater pain intensity. A rating of ‘0’ represented no pain, and a ‘10’ meant intolerable pain.
MRI parameters
For the MRI scan, a Trio 3.0T MRI machine (Siemens AG) was used for scanning. All participants were instructed to close their eyes, stay awake, and breathe quietly until the scan was completed. 3D damage-gradient echo sequences to collect functional data were used; parameters were as follows: 176 structural images (repetition time=1,900 msec; echo time=2.26 msec; thickness=1 mm; gap=0.5 mm; acquisition matrix=256×256; field of view=250×250 mm; turning angle=9 degrees), and 240 functional images (repeat time=2,000 msec; echo time=30 msec; thickness=4 mm; gap=1.2 mm; acquisition matrix=64×64; turning angle=90 degrees; field of view=220×220 mm, 29 axial). Each scanning process lasted 15 min.
fMRI data processing
To process fMRI images, MRIcro software (https://www.mccauslandcenter.sc.edu/crnl/mricro) was used to classify functional data and exclude incomplete data. The first fifteen time-points were discarded to maintain magnetization equilibrium. Data Processing Assistant for Advanced Edition of Resting-State fMRI (DPARSF 4.0; http://rfmri.org/DPARSF) software was used for head motion correction, spatial normalization, slice timing, form conversion of digital imaging communications in medicine (DICM), and full-width smoothing with a Gaussian kernel of 6×6×6 mm3 at half-maximum based on the rs-fMRI data analysis toolkit (REST; http://www.restfmri.net) and the Statistical Parametric Mapping software (version 8; http://www.fil.ion.ucl.ac.uk/spm). Subjects were excluded if 1.5 angular motion or an offset exceeding 1.5 mm in either x, y, or z axes were detected during imaging. Friston six-head motion parameters (23) were used to negate any influence of head motion. After correction for head motion, obtained functional images were standardized using a standard echo plane image template, conforming to standards set forth by the Montreal Neurological Institute (MNI).
ALFF processing
First, to calculate ALFF, remaining data were smoothed with a full-width Gaussian kernel of 6×6×6 mm3 at half-maximum. Next, fMRI images were detrended and bandpass-filtered (0.01–0.08 Hz) to reduce effects of low-frequency drift and physiological high-frequency respiratory and cardiac noise. Then, the smoothed signals of each voxel were converted from time to frequency domains using the fast Fourier transform (FFT) algorithm and obtained the power spectrum. Finally, all ALFF maps were divided by the mean value of each ALFF map.
Brain-behavior correlation analysis
For the purposes of brain-behavior correlation analysis, areas with significant ALFF changes were selected as regions of interest (ROIs). Each ROI was calculated by finding each original ALFF value corresponding to each of the original voxels. Correlations among ALFF signals of distinct regions within the cerebrum and clinical manifestations of CTN patients were evaluated using Pearson's correlation analysis.
Data processing
Demographic and clinical variables of CTN and HC groups were statistically analyzed using SPSS 20.0 software (IBM Corp.) and applying an independent sample t-test; P<0.05 was considered to indicate a statistically significant difference. Voxel differences between CTN and HC subjects were studied using REST software and a two-sample t-test. By applying the Gaussian random field theory, the statistical voxel threshold of multiple comparisons was set to P<0.05. Calibration was carried out under the condition of a voxel level of P<0.01 and a cluster size >40 voxels. Mean ALFF values in different brain regions were classified by a receiver operating characteristic (ROC) curve. Pearson correlation coefficient was conducted to assess the relationships between the ALFF values of different brain regions and clinical variables (VAS scores and duration of disease) in CTN using SPSS version 20.0 software.
Results
Differences of ALFF
Compared to HCs, CTN patients had significantly higher ALLF values in the right inferior cerebellum (RIC), left fusiform gyrus (LFG), left inferior cerebellum (LIC), left inferior temporal gyrus (LITG), right fusiform gyrus (RFG), right superior cerebellum (RSC), left inferior occipital gyrus (LIOG), right precentral gyrus (RPG) and right superior occipital gyrus (RSOG), as revealed in Fig. 1 (red) and Table I. The voxel level statistical threshold for multiple comparisons using the Gaussian random field theory was set at a level of P<0.05. Data was corrected to >40 voxel clusters. The histogram in Fig. 2 details mean altered ALFF values of both groups. No correlation was noted between mean ALFF values of relevant brain regions and clinical manifestations in CTN subjects (P>0.05).
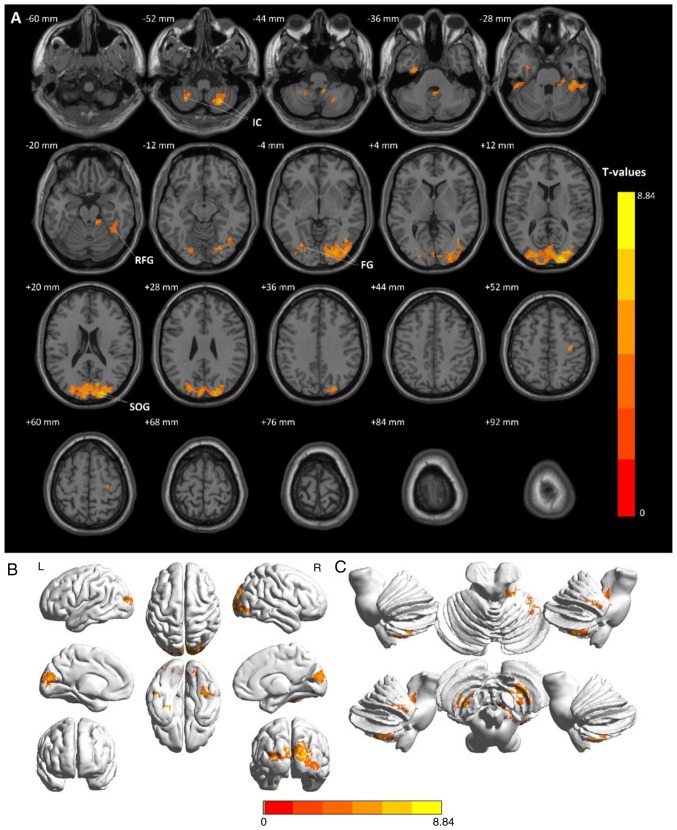
Figure 1.
Comparison of average ALFF values between the patients with CTN and HCs. (A) Significant differences in mean ALFF values between CTNs and HCs were recognized in the bilateral IC, bilateral FG and SOG. The red or yellow regions indicate higher ALFF values (P<0.01 for multiple comparisons using the Gaussian random field theory, z>2.3, P<0.01, cluster >40 voxels, AlphaSim corrected). (B) Stereoscopic form of the cerebrum. (C) Stereoscopic form of the cerebellum and the brain stem. CTN, classical trigeminal neuralgia; HCs, healthy control groups; IC, inferior cerebellum; FG, fusiform gyrus; SOG, superior occipital gyrus; ALFF, amplitude of low frequency fluctuation.
Figure 2.
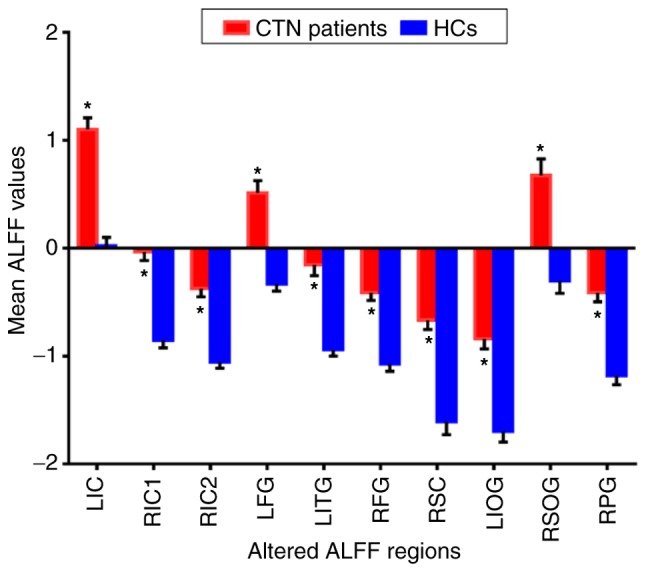
The mean altered ALFF values between CTN and HCs. Compared with the HCs, the ALFF of the following regions were increased to various extents: LIC (t=5.3163); RIC1 (t=5.3491); RIC2 (t=6.6204); LFG (t=6.0603); LITG (t=4.9039); RFG (t=5.8291); RSC (t=5.9398); LIOG (t=5.3456); RSOG (t=8.8391); RPG (t=5.7781) in CTN patients. *P<0.05 vs. HCs. Data are presented as the mean ± standard deviation. ALFF, amplitude of low-frequency fluctuation; CTN, classical trigeminal neuralgia; HCs, healthy control groups; LIC, left inferior cerebellum; RIC1, right inferior cerebellum 1; RIC2, right inferior cerebellum 2; LFG, left fusiform gyrus; LITG, left inferior temporal gyrus; RFG, right fusiform gyrus; RSC, right superior cerebellum; LIOG, left inferior occipital gyrus; RSOG, right superior occipital gyrus; RPG, right precentral gyrus.
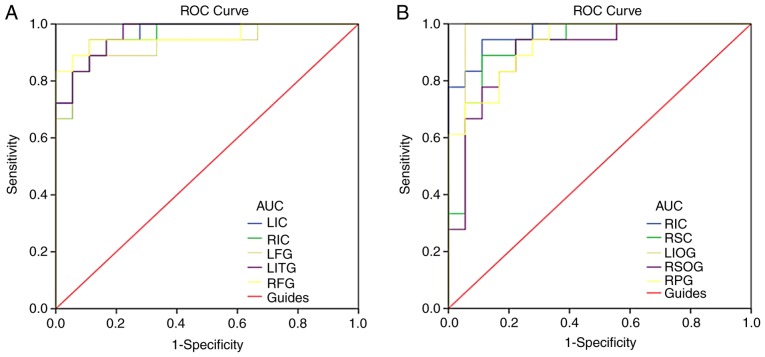
ROC curve
Brain region ALFF values differed between CTN and HC subjects and were thus recognized as differentiating markers. Areas under the curve were as follows and are detailed in Fig. 3: LIC (0.966), RIC (0.957), LFG (0.929), LITG (0.966), RFG (0.957) (Fig. 3A); RIC (0.969), RSC (0.926), LIOG (0.960), RSOG (0.901), and RPG (0.929) (Fig. 3B).
Figure 3.
ROC curve analysis of the mean ALFF values for altered brain regions. (A) The area under the ROC curve was 0.966, (P<0.001; 95% CI: 0.917–1.000) for LIC; 0.957 (P<0.001; 95% CI: 0.899–1.000) for RIC; 0.929 (P<0.001; 95% CI: 0.842–1.000) for LFG; 0.966 (P<0.001; 95% CI: 0.918–1.000) for LITG; 0.957 (P<0.001; 95% CI: 0.887–1.000) for RFG. (B) The area under the ROC curve was 0.969 (P<0.001; 95% CI: 0.923–1.000) for RIC; 0.926 (P<0.001; 95% CI: 0.838–1.000) for RSC; 0.960 (P<0.001; 95% CI: 0.882–1.000) for LIOG; 0.901 (P<0.001; 95% CI: 0.798–1.000) for RSOG; 0.929 (P<0.001; 95% CI: 0.851–1.000) for RPG. ALFF, amplitude of low-frequency fluctuation; ROC, receiver operating characteristic; CTN, classical trigeminal neuralgia; HC, healthy control; LIC, left inferior cerebellum; RIC, right inferior cerebellum; LFG, left fusiform gyrus; LITG, left inferior temporal gyrus; RFG, right fusiform gyrus; RSC, right superior cerebellum; LIOG, left inferior occipital gyrus; RSOG, right superior occipital gyrus; RPG, right precentral gyrus.
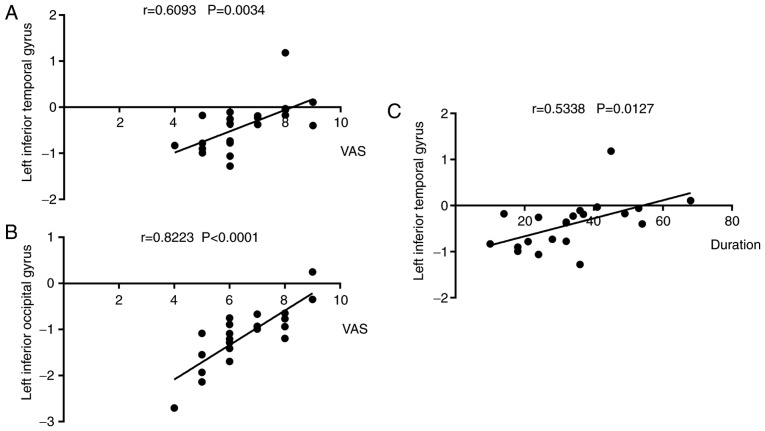
Correlation analysis
Left eye VAS findings were positively correlated with left inferior temporal gyral ALFF signal values in CTN patients (r=0.609, P=0.003; Fig. 4A). Likewise, left eye VAS findings was also positively correlated with left inferior temporal gyral values (r=0.534, P=0.013; Fig. 4C). Right eye pain duration positively was correlated with left inferior occipital gyral values (r=0.822, P<0.0001; Fig. 4B).
Figure 4.
Correlations between mean ALFF signal values and behavioral performance. (A) The VAS of the right eye in the CTN group was positively correlated with the ALFF signal values of the left inferior temporal (r=0.609, P=0.003). (B) The VAS of the right eye in the CTN group was positively correlated with the ALFF signal values of the left inferior occipital gyrus (r=0.822, P<0.0001). (C) CTN duration was positively correlated with the ALFF signal values of the left inferior temporal gyrus (r=0.534, P=0.013). ALFF, amplitude of low-frequency fluctuation; CTN, classical trigeminal neuralgia; VAS, visual analogue scale.
Discussion
ALFF has been successfully applied to the study of a variety of pain-causing conditions (24–29) (Table II) and holds great promise in future research. In the present study, for the first time we studied altered spontaneous brain activity in CTN patients using the ALFF method. ReHo was used to study patients with TN in our previous study (23). Both ReHo and ALFF can be used to study spontaneous brain activity through BOLD signals. ALFF exhibit fluctuation amplitude of time course while ReHo describes local synchronization of neighboring voxels. Therefore, the results obtained by the two methods will be different. Both of them provide complementary information about the regional spontaneous brain activity in CTN patients. No other interventions were imposed and only altered spontaneous brain activity in the resting state was detected in CTN patients compared with healthy controls in the present study. LIC, RIC, LFG, RFG, RPG, LITG, RSC, LIOG and RSOG ALFF values were significantly higher in CTN patients when compared to HCs (Table III; Fig. 5).
Table II.
ALFF method applied in pain-related diseases.
| Author | Year | Disease | (Refs.) |
|---|---|---|---|
| Xue et al | 2013 | Migraine | (24) |
| Wang et al | 2017 | CTN | (25) |
| Pan et al | 2018 | Acute eye pain | (26) |
| Zhang et al | 2017 | Low back pain | (27) |
| Ma et al | 2015 | Visceral pain | (28) |
| Liu et al | 2017 | Dysmenorrhea | (29) |
ALFF, amplitude of low-frequency fluctuation; CTN, classic trigeminal neuralgia.
Table III.
Brain regions with differences in fALFF between CTN patients and HCs.
| CTNs and HCs | MNI coordinates | ||||||
|---|---|---|---|---|---|---|---|
| Brain areas | BA | T-values | Peak voxels | x | y | z | |
| LFG | 20 | 6.0603 | 48 | −30 | −12 | −33 | |
| RFG | 36 | 5.8291 | 93 | 42 | −33 | −27 | |
| LITG | 36,20 | 4.9039 | 22 | −51 | −36 | −27 | |
| LIOG | 18 | 5.3456 | 77 | −24 | −81 | −6 | |
| RSOG | 18 | 8.8391 | 1,310 | 18 | −90 | 15 | |
| RPG | 4 | 5.7781 | 27 | 33 | −18 | 51 | |
| RSC | – | 5.9398 | 28 | 15 | −39 | −24 | |
| RIC1 | – | 5.3491 | 34 | 9 | −45 | −42 | |
| RIC2 | – | 6.6204 | 80 | 24 | −63 | −51 | |
| LIC | – | 5.3163 | 40 | −24 | −51 | −54 | |
The statistical threshold was set at the voxel level with P<0.05 for multiple comparisons using the Gaussian Random Field theory (z>2.3; P<0.01; cluster >40 voxels; alphasim corrected). fALFF, fractional amplitude of low-frequency fluctuations; CTN, classical trigeminal neuralgia; HCs, healthy controls; MNI, Montreal Neurological Institute; BA, Brodmann area; LFG, left fusiform gyrus; RFG, right fusiform gyrus; LITG, left inferior temporal gyrus; LIOG, left inferior occipital gyrus; RSOG, right superior occipital gyrus; RPG, right precentral gyrus; RSC, right superior cerebellum; RIC1, right inferior cerebellum1; RIC2, right inferior cerebellum2; LIC, left inferior cerebellum.
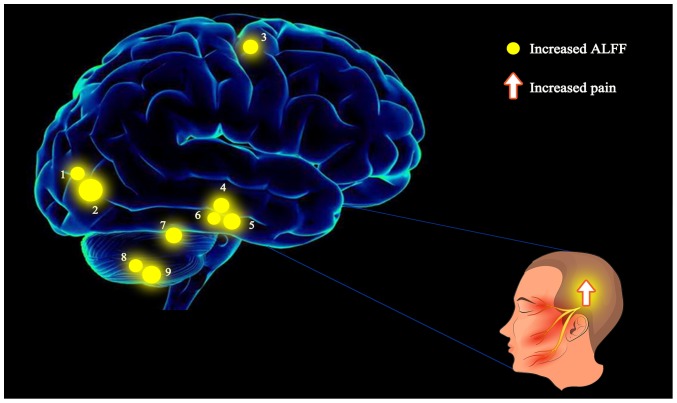
Figure 5.
The ALFF results of the brain activity in the CTN group. The yellow spots represent brain regions in which the ALFF value was significantly higher in CTN patients when compared to HCs: 1-LIOG; 2-RSOG; 3-RPG; 4-RFG; 5-LFG; 6-LITG; 7-RSC; 8-LIC; 9-RIC. The sizes of the spots denote the degree of quantitative changes. ALFF, amplitude of low-frequency fluctuation; CTN, classical trigeminal neuralgia; HCs, healthy controls; LIOG, left inferior occipital gyrus; RSOG, right superior occipital gyrus; RPG, right precentral gyrus; RFG, right fusiform gyrus; LFG, left fusiform gyrus; LITG, left inferior temporal gyrus; RSC, right superior cerebellum; LIC, left inferior cerebellum; RIC, right inferior cerebellum.
The fusiform gyrus is located on the basal surface of the temporal and occipital lobes, and is associated with multiple sensory integration and cognitive processing (30). This region is also a vital component of the marginal system, closely related to psychological faculties such as emotion, behavior, learning and memory (31–33). Schwedt et al (34) revealed that patients suffering migraines exhibited stronger fusiform gyral pain responses compared to controls. It has also been reported that the bilateral fusiform gyrus possesses unique visual processing mechanisms for text and objects, indicating that this region is affected in CTN patients. Further research, however, is required to elucidate precise structural and functional relationships between the fusiform gyrus and CTN. Ter Minassian et al (35) induced pain in healthy adults by electrical stimulation, which revealed evident abnormal activity in the left fusiform gyrus, and was negatively correlated with the pain response level, which revealed that fusiform gyrus plays an important role in acute pain response. Therefore, we inferred that the increase of ALFF signal values and neuronal activity in the bilateral fusiform gyrus of CTN patients may be related to pain to a great extent. In addition, the fusiform gyrus is related to classification and recognition function, which is integrated by connecting with multiple brain regions (36). Researchers speculated that the abnormal increase activity of these brain regions may relate to retrieval of similar feelings in patients with CTN during recurrent electrical-shock-like pain.
The occipital lobe is located in the posterior part of the parietal and temporal lobes. It is a visual processing center responsible for visual communication (33). However, a recent study in rats revealed that the occipital lobe was associated with pain-descending inhibitory mechanisms (37). The occipital cortex of migraine patients was reported to possess decreased left infratemporal gray matter mass and decreased cerebral blood flow (38). In addition, the cortical network activity of this region was revealed to be negatively correlated with intensity of diabetic neuropathic pain (39). In addition, in the present study, increased ALFF signals were revealed in the left supraoccipital gyrus and the right inferior occipital gyrus, which may be related to the activation of pain-descending inhibitory mechanisms. It was also revealed that the ALFF value of the suboccipital gyrus was positively correlated with VAS, which indicated that the occipital lobe is associated with CTN. Higher RSOG ALFF signals indicate that pain may continue to stimulate visual processing networks despite patients keeping eyes closed during scanning.
Inferior temporal gyrus is an important part of the default network of the brain, which plays a key role in self-cognition, emotional processing and regulation. Wang et al (40) revealed that the ReHo value of the right inferior temporal gyrus increased in patients with ITN, indicating that chronic diseases may affect the function of the aforementioned areas. The Gray matter volume of the temporal lobe revealed a negative correlation to disease duration and thus may be an important structure for the development and maintenance of chronic pain in general and TN in particular (41). In the present study, increased ALFF in the inferior temporal gyrus may be associated with the inhibition of pain induced by negative emotions and cognitive degradation. In addition, the ALFF value of the LITG was positively correlated with VAS and duration, and the longer the pain lasted, the stronger the inhibitory effect was. The observed ALFF signal changes in the occipital lobe and temporal lobe further support the assumption that they are related to chronic manifestation of CTN.
It was also revealed that the ALFF signal values of the CTN subjects were abnormally decreased in the LIC and RIC regions. Although the cerebellum has been mainly understood for its role in motor regulation, it also implicated in a range of movement disorders (42,43). Although, the cerebellum has been mainly recognized for its role in motor regulation, a previous study reported that it can receive extensive somatosensory input, underscoring its additional role as a sensory organ (44). Changes in intensity of spontaneous cerebellar and activity in CTN patients provides a basis, on the contrary. In the present study, the brain areas evoked by pain were mainly involved in the cognitive aspects of pain perception, such as pain awareness and memory. Most abnormal brain regions, however, had elevated ALFF signal values. It was speculated that due to chronic and frequent pain input, the flow and integration of information among different brain regions ultimately becomes affected, and spontaneous cerebral activity is strengthened, manifesting in response to pain.
The central prefrontal gyrus is located in the frontal lobe of the cerebral cortex, which is the main movement area cortex (45). It produces outgoing axons in the cortical tract and terminates in the motor neurons of the cranial and spinal cord (45,46). For patients with TN, even simple non-painful movements can elicit attacks of pain, while if facial movements are restricted, the pain would be eased. The precentral gyrus (primary motor cortex) could therefore mirror sensory pain responses to repeated TN, motor inhibition of the maxilla and facial muscle tension (47). In the present study, an increased ALFF value of the RPG was revealed, indicating a link between intrinsic brain activity and pain modulation.
In fact, the ALFF values may be useful as clinical markers to reflect pain severity in CTN patients. A study on obsessive-compulsive disorder (OCD) by Anticevic et al (48), used a global brain connective (GBC) analysis method to reveal an increase in GBC in the cerebellum at rest, indicating an excessive functional connection between the cerebellum and other brain regions; and an increase in cerebellar GBC. The effective treatment of the drug against OCD could alter ligation activity, and break abnormal functional connection, thereby reducing the OCD symptoms of the patient. It suggests that the functional connection between the cerebellum and other important brain regions is related to the occurrence of OCD symptoms, and the cerebellum may participate in the OCD abnormal neural circuit. Although the study is not based on ALFF, it may provide us with inspiration. Perhaps therapeutic goals can be achieved by reducing the spontaneous activity of the CTN-related brain regions at a resting state.
In actuality, TN is divided into several categories according to different criteria, such as, classical and non-classical TN, primary and secondary trigeminal neuralgia. However, there may be a crossover between them. It would be of interest to explore the altered brain activity in patients with non-classical TN, however the diagnosis of non-classical TN is more difficult than that of CTN, which makes it harder to recruit qualified subjects. In future studies, larger sample sizes and detailed grouping of different types of TN are required, however in the present study, conditions were limited.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (grant nos. 81660158, 81460092 and 81400372), the Natural Science Key Project of Jiangxi Province (grant no. 20161ACB21017) and the Health Development Planning Commission Science Foundation of Jiangxi Province (grant no. 20175116).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
YS and LCH conceived and designed the present study. YC, CQX and WFL were responsible for acquiring the data, designing the figures and tables and drafting the manuscript. NJ, PWZ and LY contributed to the acquisition of the data and interpreting the results. BL and QL assisted in the acquisition of the data and drafting the manuscript. YLM and TS assisted in the acquisition and analysis of the data with constructive discussion.
Ethics approval and consent to participate
The present study was approved by the Medical Research Ethics Committee of The First Affiliated Hospital of Nanchang University. Written informed consent was obtained from all individuals enrolled in the study.
Patient consent for publication
Consent was obtained from all individuals enrolled in the study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Heydari M, Shams M, Hashempur MH, Zargaran A, Dalfardi B, Borhani-Haghighi A. The origin of the concept of neuropathic pain in early medieval Persia (9th-12th century CE) Acta Med Hist Adriat. 2015;13:9–21. [PubMed] [Google Scholar]
- 2.Bert C, Engenhartcabillic R, Durante M. Particle therapy for noncancer diseases. Med Phys. 2012;39:1716–1727. doi: 10.1118/1.3691903. [DOI] [PubMed] [Google Scholar]
- 3.Maila SK. Clinical outcome following micro-vascular decompression for trigeminal neuralgia. Int J Res Med Sci. 2015;3:1741–1744. [Google Scholar]
- 4.Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: The ignition hypothesis. Clin J Pain. 2002;18:4–13. doi: 10.1097/00002508-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Love S, Coakham HB. Trigeminal neuralgia. Encyclopedia Neuroscience. 2009;124:1173–1177. doi: 10.1016/B978-008045046-9.00617-3. [DOI] [Google Scholar]
- 6.Wartolowska K, Tracey I. Neuroimaging in Understanding Chronic Pain Mechanisms and the Development of New Therapies. In: Borsook D, Beccera L, Bullmore E, Hargreaves R, editors. Imaging in CNS Drug Discovery and Development. Springer; New York, NY: 2009. pp. 251–261. [Google Scholar]
- 7.Yuan J, Cao S, Huang Y, Zhang Y, Xie P, Zhang Y, Fu B, Zhang T, Song G, Yu T, Zhang M. Altered spontaneous brain activity in patients with idiopathic trigeminal neuralgia: A resting-state functional MRI study. Clin J Pain. 2018;34:600–609. doi: 10.1097/AJP.0000000000000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian L, Jiang T, Liang M, Zang Y, He Y, Sui M, Wang Y. Enhanced resting-state brain activities in ADHD patients: A fMRI study. Brain Dev. 2008;30:342–348. doi: 10.1016/j.braindev.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Moisset X, Villain N, Ducreux D, Serrie A, Cunin G, Valade D, Calvino B, Bouhassira D. Functional brain imaging of trigeminal neuralgia. Neuralgia Eur J Pain. 2011;15:24–131. doi: 10.1016/j.ejpain.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Pilgrim LK, Fadili J, Fletcher P, Tyler LK. Overcoming confounds of stimulus blocking: An Event-Related fMRI design of semantic processing. Neuroimage. 2002;16:713–723. doi: 10.1006/nimg.2002.1105. [DOI] [PubMed] [Google Scholar]
- 11.Josephs O, Turner R, Friston K. Event-related fMRI. Hum Brain Mapp. 1997;5:243–248. doi: 10.1002/(SICI)1097-0193(1997)5:4<243::AID-HBM7>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullmore E, Sporns O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10:186–198. doi: 10.1038/nrn2618. [DOI] [PubMed] [Google Scholar]
- 14.Wu QZ, Li DM, Kuang WH, Zhang TJ, Liu S, Huang XQ, Chan RCK, Kemp GJ, Gong QY. Abnormal regional spontaneous neural activity in treatment-refractory depression revealed by resting-state fMRI. Hum Brain Mapp. 2011;32:1290–1299. doi: 10.1002/hbm.21108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khalili MN, Chang C, van Osch MJ, Veer IM, van Buchem MA, Dahan A, Beckmann CF, van Gerven JM, Rombouts SA. The impact of ‘physiological correction’ on functional connectivity analysis of pharmacological resting state fMRI. Neuroimage. 2013;65:499–510. doi: 10.1016/j.neuroimage.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 16.Xi Q, Zhao X, Wang P, Guo Q, Jiang H, Cao X, He Y, Yan C. Spontaneous brain activity in mild cognitive impairment revealed by amplitude of low-frequency fluctuation analysis: A resting-state fMRI study. Radiol Med. 2012;117:865–871. doi: 10.1007/s11547-011-0780-8. [DOI] [PubMed] [Google Scholar]
- 17.Dai XJ, Liu CL, Zhou RL, Gong HH, Wu B, Gao L, Wang YX. Long-term sleep deprivation decreases the default spontaneous activity and connectivity pattern in healthy male subjects: A resting-state fMRI study. Neuropsychiatr Dis Treat. 2015;11:761–772. doi: 10.2147/NDT.S78335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zuo XN, Martino AD, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP. The oscillating brain: Complex and reliable. Neuroimage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 20.Kang HH, Shu YQ, Yang L, Zhu PW, Li D, Li QH□Min YL, Ye L. Measuring abnormal intrinsic brain activities in patients with retinal detachment using amplitude of low-frequency fluctuation: a resting-state fMRI study. Int J Neurosci. 2019;129:681–686. doi: 10.1080/00207454.2018.1554657. [DOI] [PubMed] [Google Scholar]
- 21.Tan G, Huang X, Ye L, Wu AH, He LX, Zhong YL, Jiang N, Zhou FQ, Shao Y. Altered spontaneous brain activity patterns in patients with unilateral acute open globe injury using amplitude of low-frequency fluctuation: A functional magnetic resonance imaging study. Neuropsychiatr Dis Treat. 2016;12:2015–2020. doi: 10.2147/NDT.S110539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiang CQ, Liu WF, Xu QH, Su T, Yong-Qiang S, Min YL, Yuan Q, Zhu PW, Liu KC, Jiang N, et al. Altered spontaneous brain activity in patients with classical trigeminal neuralgia using regional homogeneity: A resting-state functional MRI study. Pain Pract. 2019;19:397–406. doi: 10.1111/papr.12753. [DOI] [PubMed] [Google Scholar]
- 23.Li HJ, Dai XJ, Gong HH, Nie X, Zhang W, Peng DC. Aberrant spontaneous low-frequency brain activity in male patients with severe obstructive sleep apnea revealed by resting-state functional MRI. Neuropsychiatr Dis Treat. 2015;11:207–214. doi: 10.2147/NDT.S73730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue T, Yuan K, Cheng P, Zhao L, Zhao L, Yu D, Dong T, von Deneen KM, Gong Q, Qin W, Tian J. Alterations of regional spontaneous neuronal activity and corresponding brain circuit changes during resting state in migraine without aura. NMR Biomed. 2013;26:1051–1058. doi: 10.1002/nbm.2917. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Xu C, Zhai L, Lu X, Wu X, Yi Y, Liu Z, Guan Q, Zhang X. Spatial-temporal signature of resting-state BOLD signals in classic trigeminal neuralgia. J Pain Res. 2017;10:2741–2750. doi: 10.2147/JPR.S143734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pan ZM, Li HJ, Bao J, Jiang N, Yuan Q, Freeberg S, Zhu PW, Ye L, Ma MM, Huang X, Shao Y. Altered intrinsic brain activities in patients with acute eye pain using amplitude of low-frequency fluctuation: A resting-state fMRI study. Neuropsychiatr Dis Treat. 2018;14:251–257. doi: 10.2147/NDT.S150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang SS, Wu W, Yang JM, Wang CH. Abnormal spontaneous brain activity in acute Low-back pain revealed by Resting-state functional MRI. Am J Phys Med Rehabil. 2017;96:253–259. doi: 10.1097/PHM.0000000000000597. [DOI] [PubMed] [Google Scholar]
- 28.Ma X, Li S, Tian J, Jiang G, Wen H, Wang T, Fang J, Zhan W, Xu Y. Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: A resting-state fMRI study. Clin Neurophysiol. 2015;126:1190–1119. doi: 10.1016/j.clinph.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Liu P, Liu Y, Wang G, Yang X, Jin L, Sun J, Qin W. Aberrant default mode network in patients with primary dysmenorrhea: A fMRI study. Brain Imaging Behav. 2017;11:1479–1485. doi: 10.1007/s11682-016-9627-1. [DOI] [PubMed] [Google Scholar]
- 30.Parise M, Kubo TT, Doring TM, Tukamoto G, Vincent M, Gasparetto EL. Cuneus and fusiform cortices thickness is reduced in trigeminal neuralgia. J Headache Pain. 2014;15:17. doi: 10.1186/1129-2377-15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starrfelt R, Gerlach C. The visual what for area: Words and pictures in the left fusiform gyrus. Neuroimage. 2007;35:334–342. doi: 10.1016/j.neuroimage.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Lee CU, Shenton ME, Salisbury DF, Kasai K, Onitsuka T, Dickey CC, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Fusiform gyrus volume reduction in first-episode schizophrenia: A magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:775–781. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]
- 33.Mion M, Patterson K, Acosta-Cabronero J, Pengas G, Izquierdo-Garcia D, Hong YT, Fryer TD, Williams GB, Hodges JR, Nestor PJ. What the left and right anterior fusiform gyri tell us about semantic memory. Brain. 2010;133:3256–3268. doi: 10.1093/brain/awq272. [DOI] [PubMed] [Google Scholar]
- 34.Schwedt TJ, Chong CD, Chiang CC, Baxter L, Schlaggar BL, Dodick DW. Enhanced pain-induced activity of pain processing regions in a case-control study of episodic migraine. Cephalalgia. 2014;34:947–958. doi: 10.1177/0333102414526069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ter Minassian A, Ricalens E, Humbert S, Duc F, Aubé C, Beydon L. Dissociating anticipation from perception: Acute pain activates default mode network. Hum Brain Mapp. 2013;34:2228–2243. doi: 10.1002/hbm.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damasio AR, Damasio H. Large Scale Neuronal Theories of the Brain. Cambridge MA: MIT Press; 2011. Cortical systems for the retrieval of concrete knowledge: The convergence zone framework; pp. 61–74. [Google Scholar]
- 37.Reis GM, Dias QM, Silveira JW, Del Vecchio F, Garcia-Cairasco N, Prado WA. Antinociceptive effect of stimulating the occipital or retrosplenial cortex in rats. J Pain. 2010;11:1015–1026. doi: 10.1016/j.jpain.2010.01.269. [DOI] [PubMed] [Google Scholar]
- 38.Cutrer FM, Sorensen AG, Weisskoff RM, Ostergaard L, Sanchez del Rio M, Lee EJ, Rosen BR, Moskowitz MA. Perfusion-weighted imaging defects during spontaneous migrainous aura. Ann Neurol. 1998;43:25–31. doi: 10.1002/ana.410430108. [DOI] [PubMed] [Google Scholar]
- 39.Cauda F, Sacco K, Duca S, Cocito D, D'Agata F, Geminiani GC, Canavero S. Altered resting state in diabetic neuropathic pain. PLoS One. 2009;4:e4542. doi: 10.1371/journal.pone.0004542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Zhang X, Guan Q, Wan L, Yi Y, Liu C. Altered regional homogeneity of spontaneous brain activity in idiopathic trigeminal neuralgia. Neuropsychiatr Dis Treat. 2015;11:2659–2666. doi: 10.2147/NDT.S94877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obermann M, Rodriguez-Raecke R, Naegel S, Holle D, Mueller D, Yoon MK, Theysohn N, Blex S, Diener HC, Katsarava Z. Gray matter volume reduction reflects chronic pain in trigeminal neuralgia. Neuroimage. 2013;74:352–358. doi: 10.1016/j.neuroimage.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 42.Manto M, Bower JM, Conforto AB, Delgado-García JM, Gerwig M, Habas C, Hagura N, Ivry RB, Mariën P, Molinari M, et al. Consensus paper: Roles of the cerebellum in motor control-the diversity of ideas on cerebellar involvement in movement. Cerebellum. 2012;11:457–487. doi: 10.1007/s12311-011-0331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calderon DP, Fremont R, Kraenzlin F. The neural substrates of rapid-onset dystonia-parkinsonism. Nature. 2011;14:357–365. doi: 10.1038/nn.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–547. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- 45.Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–145. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 46.Bigbee J. Springer; New York: 2011. Precentral Gyrus. [DOI] [Google Scholar]
- 47.Ellingson LD, Shields MR, Stegner AJ, Cook DB. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain. 2012;13:195–206. doi: 10.1016/j.jpain.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, et al. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity inobsessive-compulsive disorder. Biol Psychiatry. 2014;75:595–605. doi: 10.1016/j.biopsych.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.